Abstract
Mice homozygous for the smallie (slie) mutation lack a collagen receptor, discoidin domain receptor 2 (DDR2), and are dwarfed and infertile due to peripheral dysregulation of the endocrine system of unknown etiology. We used a systems biology approach to identify biological networks affected by Ddr2slie/slie mutation in ovaries using microarray analysis and validate findings using molecular, cellular, and functional biological assays. Transcriptome analysis indicated several altered gene categories in Ddr2slie/slie mutants, including gonadal development, ovulation, antiapoptosis, and steroid hormones. Subsequent biological experiments confirmed the transcriptome analysis predictions. For instance, a significant increase of TUNEL-positive follicles was found in Ddr2slie/slie mutants vs. wild type, which confirm the transcriptome prediction for decreased chromatin maintenance and antiapoptosis. Decreases in gene expression were confirmed by RT-PCR and/or qPCR; luteinizing hormone receptor and prostaglandin type E and F receptors in Ddr2slie/slie mutants, compared with wild type, confirm hormonal signaling pathways involved in ovulation. Furthermore, deficiencies in immunohistochemistry for DDR2 and luteinizing hormone receptor in the somatic cells, but not the oocytes, of Ddr2slie/slie mutant ovaries suggest against an intrinsic defect in germ cells. Indeed, Ddr2slie/slie mutants ovulated significantly fewer oocytes; their oocytes were competent to complete meiosis and fertilization in vitro. Taken together, our convergent data signify DDR2 as a novel critical player in ovarian function, which acts upon classical endocrine pathways in somatic, rather than germline, cells.
Keywords: discoidin domain receptor 2, dwarfism, infertility, luteinizing hormone receptor, mice, ovulation, prostaglandin receptor
although collagen is primarily considered to be an extracellular matrix molecule that supports cell structure and architecture in bodily tissues and bone, it also may act as a signaling molecule, activating specific receptors at the cell surface (3, 9, 15, 27). Over the past decade, collagen has been identified and subsequently confirmed as an endogenous ligand for the two discoidin domain receptors (DDR; 4, 19, 27, 50–52). While both receptors are receptor tyrosine kinases, collagen binding to discoidin domain receptor family, member 1 (DDR1) induces tyrosine kinase phosphorylation and may participate in mammary gland formation and breast cancer, whereas discoidin domain receptor family, member 2 (DDR2) is activated only by fibrillar collagens, which act as its endogenous ligand (4, 19, 22, 27, 50–52). DDR2 binds to and is activated by collagen I, II, III, V, and X, with the notable exception of basement membrane collagen IV (2, 28, 29, 43, 51). DDR2 is expressed in connective tissues arising from embryonic mesoderm, such as skeletal muscle, kidney, heart, lung, and ovary (4, 24, 26, 32). DDR2 regulates cell proliferation, cell adhesion, migration, as well as extracellular matrix remodeling (19, 26, 35, 55). Recently we characterized, mapped, cloned, and identified a spontaneous, homozygous recessive allele, smallie (slie), which contains a deletion in the gene for DDR2 (Ddr2) and causes dwarfism and infertility in mice (24).
The smallie allele, designated as Ddr2slie in genetic nomenclature, is a large intragenic deletion of ∼150 kb encompassing exons 1–17 of Ddr2 gene, leaving its remaining exon 18 intact, along with its proximal gene neighbor (24). Thus, no DDR2 protein is synthesized in Ddr2slie/slie mice. At birth through weaning, Ddr2slie/slie mice are phenotypically indistinguishable from their wild-type littermates; however, the initial phenotype appears soon after weaning, as Ddr2slie/slie do not rapidly gain weight or exhibit a juvenile growth spurt, resulting in a disproportionate dwarfism with mild craniofacial abnormalities (24). The Ddr2slie/slie mice exhibit the same bone mineral density, but decreased total mineral content, compared with their wild-type siblings. While the dwarfism phenotype is not unexpected for a deletion of collagen receptor, given the pivotal functions of collagen for bone matrix formation and elongation (3, 9, 15), an infertility phenotype was not anticipated. Specifically, intercrossing young adult homozygous Ddr2slie/slie mice to other Ddr2slie/slie or heterozygous or wild-type mice failed to produce any progeny. Contrary to expectations, the dwarfism and infertility arise from a peripheral endocrine defect because the hypothalamic-pituitary hormonal axis was found to be intact and functional in Ddr2slie/slie mice (24). In fact, Ddr2slie/slie and control wild-type mice secrete similar levels of anterior pituitary hormones, contain a similar number and distribution of cells containing these hormones within the pituitary, and have comparable levels of mRNA for releasing hormones in the hypothalamus. However, gonadal steroid production is curtailed, even upon stimulation with exogenous pituitary hormones. Together, these data indicate a peripheral, not central, origin.
The purpose of this study was to ascertain cellular and molecular pathways affected by Ddr2 expression and signaling on ovarian function. To identify the molecules affected by the loss of DDR2 signaling in the ovary, we compared ovarian transcriptomes between Ddr2slie/slie mutants and wild-type mice using microarrays to measure relative gene expression across the entire mouse genome. Transcriptome analysis revealed a decrease in the relative expression of several gene categories that likely regulate or affect reproduction in Ddr2slie/slie mice compared with wild type. A subset of predictions derived from the transcriptome analysis and microarray gene expression data was subsequently tested within the context of biology using molecular, cellular, and physiological assays. Contrary to expectations, gonadotropin hormones increased DDR2 immunoreactivity in wild-type ovary, whereas exogenous gonadotropins did not induce luteinizing hormone receptor (LHR) gene expression or immunoreactivity in smallie ovaries, relative to wild-type control. Unexpectedly, DDR2 but did not alter matrix metalloproteinase (Mmp) gene expression in the ovary (12), which is the canonical signaling pathway for discoidin domain receptors (34, 35). Furthermore, a decrease in LHR and prostaglandin type E and F receptors was observed in ovaries lacking DDR2. In summary, these convergent results suggest that DDR2 interfaces with classical endocrine pathways to regulate ovarian function.
MATERIALS AND METHODS
Animals.
Prepubertal and adult wild-type (BKS.HRS/J +/+) and smallie mice (BKS.HRS/J-Ddr2slie/slie) were obtained by mating heterozygous mice carrying the Ddr2slie allele (24). All mice used in this study were bred either at the University of Tokyo or at The Jackson Laboratory, and all procedures in these studies were approved by their respective institutional Animal Care and Use Committees. Mice were housed in groups of two to four, with white pine shavings as bedding, under 12 h:12 h photoperiod (lights on at 07:00), with ad libitum access to water and food.
DNA microarray experiments.
Total RNA was harvested from three adult, 10-wk-old wild-type or Ddr2slie/slie mutant ovaries after gonadotropin treatment [5 IU of pregnant mare serum gonadotropin (PMSG) + 5 IU human chorionic gonadotropin (hCG) 48 h later] as separate samples using RNeasy Mini kit from QIAGEN (Valencia, CA) according to the manufacturer's protocol. We used the small-scale protocol to reproducibly amplify in a linear manner and label total RNA (Affymetrix, Santa Clara, CA). Gene expression was analyzed using GeneChip Mouse Genome 430 2.0 Array covering >39,000 transcripts on a single array (Affymetrix). The procedures of converting RNA to cDNA, labeling, microarray hybridization, and GeneChips scanning were performed at the Microarray Facility at the University of Tokyo (Tokyo, Japan) as described in the Affymetrix protocol. The microarray data have been submitted to the Center for Information Biology gene expression database (accession no. CBX83).
Transcriptome analysis.
Prior to computational analyses, quality control measures were performed on the array data. For each differentially expressed Affymetrix probe set, a corresponding Mouse Genome Informatics (MGI) gene accession number was identified by screening against the ENSEMBL mouse genome assembly (http://www.ensembl.org/Mus_musculus, version 49.37b). This was done to verify that probe sets indeed unambiguously recognized “authentic” genes. Probes that identified retrotransposon sequences and probes mapping to multiple genes were discarded, because these often produce ambiguous and even false gene expression data. In addition, when multiple probe sets for the same gene gave contradictory or opposing signals, the corresponding gene was discarded from further analysis.
VisuaL annotation display analysis.
VisuaL Annotation Display (VLAD) software, developed by the MGI staff (7, 8), searches existing Gene Ontology (GO) annotations (5) for a designated query set of genes. The GO project is a collaborative effort that assigns consistent descriptions for gene products within three structured controlled vocabularies (ontologies: biological processes, cellular components, and molecular functions) for plant, animal, and microbial genomes, in terms of their associated functions in a species-independent manner (5). VLAD software analyzes the data and displays a summary, in graphical and tabular forms, of the GO classifications relevant to the query. VLAD software calculates the hypergeometric distribution statistic and can be used to discover statistically significant overrepresentation of distinct GO categories within three major controlled vocabularies in the query set relative to a specified “universe” gene set. After quality control, microarray probe IDs were matched to their respective MGI accession numbers, and these were used as a query set to compare against all mouse genes, which were set as the universe set.
Reverse-transcriptase PCR and quantitative real-time PCR.
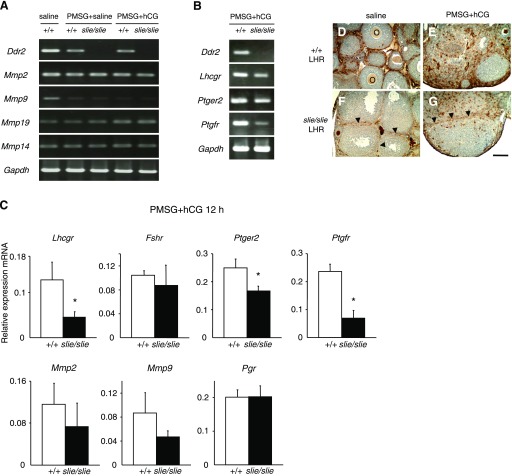
For semiquantitative RT-PCR, total RNAs were isolated from whole ovaries from wild-type (n = 3) and Ddr2slie/slie mutant (n = 5) mice using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA). cDNA was synthesized from total RNA with Superscript First-Strand Synthesis System for RT-PCR (Invitrogen). The primers used to detect genes Ddr2, Mmp2 (matrix metallopeptidase 2), Mmp9 (matrix metallopeptidase 9), Mmp19 (matrix metallopeptidase 19), Mmp14 (matrix metallopeptidase 14), Lhcgr (luteinizing hormone/choriogonadotropin receptor), Fshr (follicle stimulating hormone receptor), Ptger2 (prostaglandin E receptor 2, subtype EP2), Ptgfr (prostaglandin F receptor), and housekeeping gene Gapdh (glyceraldehyde-3-phosphate dehydrogenase, also known as G3PDH) are listed in Table 1. PCR was performed with AccuPrime Pfx DNA Polymerase (Invitrogen). The amplification conditions used were 98°C, 30 s followed by 15–25 cycles of 98°C, 10 s, 58°C, 30 s; 72°C, 30 s. The number of cycles used ensured that the reaction could be quantified within the log phase of the amplification reaction.
Table 1.
Oligonucleotide primer sequences used for RT-PCR or qPCR
| Forward Primer | Reverse Primer | |
|---|---|---|
| Ddr2 | GTCTCAGGCTACGTTCAGATG | GGAATCAAGCCACTCACACAC |
| Mmp2 | CTGGGCAACAAGTATGAGAGC | CCAGTGTCAGTATCAGCATCG |
| Mmp9 | CTTCAAGGACGGTTGGTACTG | GGAAGATGTCGTGTGAGTTCC |
| Mmp19 | GTCTCCAGTGACTGCAAAACC | GAGCCCTTAAACAGGAACACC |
| Mmp14 | CTCCGAGGAGAGATGTTTGTC | TTTAGGGTACTCGCTGTCCAC |
| Lhcgr | GCCCTCCAGAGAAAAATTCAC | CGACTGGTCAGGAGAACAAAG |
| Fshr | TGCCAGGAACTCCTTCATGGGACT | AAGCCATGGTTGGGCAGGGAATAG |
| Ptger2 | CTCCAAGCTAATGGAGGACTG | GATAAGTGGCGCCTGTAGAAG |
| Ptgfr | CACAGAGCACATCGAAGACTG | GAGCTGAGTTCCCAGATATGC |
| Pgr | TCTTTGCCGGAAGAGATGCATCCAG | TCCGAGCTGTCTCGTCTTTTGGTTC |
| Gadph | TGAAGGTCGGTGTCAACGGATTTGGC | GTGGTGGACCTCATGGCCTACATG |
Ddr2, discoidin domain receptor family, member 2; Mmp2, matrix metallopeptidase 2; Mmp9, matrix metallopeptidase 9; Mmp19, matrix metallopeptidase 19; Mmp14, matrix metallopeptidase 14; Lhcgr, luteinizing hormone/choriogonadotropin receptor; Fshr, follicle stimulating hormone receptor; Ptger2, prostaglandin E receptor 2, formerly EP2; Ptgfr, prostaglandin F receptor; Pgr, progesterone receptor; Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
For quantitative real-time PCR (qPCR), total RNAs were isolated using TRIzol reagent from the whole ovaries from wild-type (n = 3) or Ddr2slie/slie (n = 3) mice after treatment with 10 IU PSMG ip followed 48 h later by 10 IU hCG ip (Invitrogen Life Technologies). cDNA was synthesized from total RNA with Superscript First-Strand Synthesis System for RT-PCR (Invitrogen). The amplification conditions used were 95°C, 2 min, followed by 25 cycles of 94°C, 15 s; 58°C, 30 s; 72°C, 30 s. The expression level [threshold cycle (Ct)] of each transcript was detected by counting the number of necessary cycles to yield equivalent expression level of each gene. Ct values were converted to fold differences in expression according to the equation 2[Ct1(slie/slie) − Ct1(18s rRNA)] − [Ct2(+/+) − Ct2(18s rRNA)],where Ct1(18s rRNA) and Ct2(18s rRNA) represent the Ct values for the 18s rRNA gene in the slie and wild-type samples, respectively. The same primers used for RT-PCR were used for qPCR, along with Pgr (progesterone receptor), with the exception of Mmp14 and Mmp19, which failed to yield a reliable signal for qPCR and was excluded from analysis.
Histology, immunohistochemistry, and TUNEL staining.
In morphological experiments, 5 IU of PMSG (Serotropin; Aska Pharmaceutical, Tokyo, Japan) followed 48 h later by 5 IU hCG (Gonatropin 3000, Aska Pharmaceutical) were injected ip into 10-wk-old wild-type or Ddr2slie/slie female mice to induce ovulation. Ovaries were collected 12 h after hCG injection and placed into Bouin's fixative overnight. The tissues were dehydrated, embedded in paraffin, cut in 5 μm sections, and processed for hematoxylin and eosin, terminal deoxynucleotidyl-transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL), or immunohistochemical staining. To localize DDR2 and luteinizing hormone/choriogonadotropin receptor (LHR) in Ddr2slie/slie and wild-type homozygotes, polyclonal antibodies to DDR2 (AF2538), and LHR (LS-A1434) were used (R&D Systems, Minneapolis, MN; Medical & Biological Laboratories, Nagoya, Japan, respectively). Following deparaffinization, the sections of the ovaries were boiled in sodium citrate buffer (pH 6.0) for 1 min in a microwave oven for antigen retrieval. They were preincubated with 5% normal rabbit or goat serum for 30 min, followed by 2 h incubation with the primary antibody (1:500), then with biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA) for 1 h, and next with streptavidin-biotin-horseradish peroxidase (HRP) complex (Vectastain Elite ABC, Vector Laboratories) for 30 min. As a negative control, sections without the primary antibody were processed (data not shown). All the reactions were carried out at room temperature. TUNEL staining was performed according to the manufacturer's protocol (DeadEnd Colorimetric TUNEL System; Promega, Madison, WI).
Western blotting.
Total protein was isolated from whole ovary of wild-type and Ddr2slie/slie mutant mice. The protein samples were separated by electrophoresis using a 10% sodium dodecyl sulfate polyacrylamide gel. Proteins were transferred to a polyvinylidene fluoride membrane (AE-6660; Atto, Tokyo, Japan). After blocking the membrane with 3% skim milk for 20 min, we incubated the membrane with anti-DDR2 polyclonal antibody (AF2538; R&D Systems) and then processed it with 1:5,000 diluted anti-goat IgG antibody conjugated to HRP (Jackson Immuno Research Laboratories, West Grove, PA), and processed using an Enhanced Chemiluminescence detection kit (GE Healthcare UK, Buckinghamshire, UK). Optical density measurements, relative to tubulin levels, were made using Scion Image (beta version 4.3 for Windows XP; Scion, Frederick, MD).
Ovulation and in vitro fertilization.
To induce ovulation, 12–14-wk-old wild-type (n = 8) and Ddr2slie/slie mutant (n = 4) female mice were injected (ip) with 5 IU PMSG and 48 h later with 5 IU hCG. Oviducts were removed 14 h after hCG injection to collect ovulated oocytes. Collected oocytes were incubated with capacitated sperm from ICR males at 37°C and 5% CO2 in air for 4 h. Oocytes were removed, rinsed from sperm, and placed into fresh media. After incubation for 20 h at 37°C and 5% CO2 in air, two-cell embryos were transferred to fresh M16 medium (Sigma) to develop in vitro for 3 days until the expanded blastocyst stage.
Statistical analysis.
Descriptive statistics (means and SE) were reported for number of ovulation oocytes in female wild-type and Ddr2slie/slie mutant mice. To detect significant statistical differences, nonparametric Mann-Whitney or parametric Student's t-tests were used to compare among experimental groups. Differences were considered significant at P < 0.05 (Statview software; SAS Institute, Cary, NC). For VLAD analysis, hypergeometric distribution tests were used to determine significant differences in GO categories (P < 0.05).
RESULTS
Transcriptome analysis of ovarian gene expression.
To circumvent technical bias and unreliable data interpretation, the quality control of microarray data is necessary and essential to yield a reliable analysis of gene expression. It is vitally important in studies involving whole tissues, which by nature contain heterogeneous cell types, such as ovaries. Using the quality control procedures described in the methods, 117 Affymetrix probe sets were discarded, leaving a total of 2,519 probes representing 2,049 expressed genes. Sixty-eight of these probes did not correspond to genes, and most recognizing retrotransposons rather than genes. Comparing Ddr2slie/slie mutants and wild-type gene expression among the remaining quality-controlled set of probes, ∼47% genes had a relative increase in expression levels, whereas ∼52% had a relative decrease in expression. Statistical analysis confirmed an underlying random distribution of genes expressed in germ cells (oocytes) vs. whole ovary when the microarray data were matched against the large representative dataset of known fully-grown oocyte transcripts (18; χ2 = 0.18, P > 0.67). Thus, the quality control procedures yielded a large data set of gene expression appropriate for transcriptome analysis of heterogeneous ovarian tissue because it was statistically shown to be free of bias toward either somatic or germ cell types.
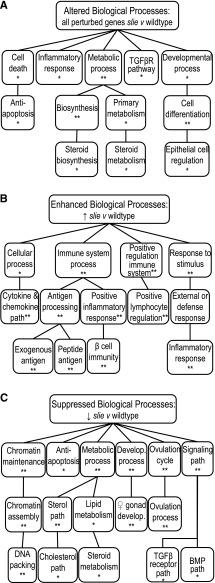
To identify the major functional classes of all differentially expressed genes in Ddr2slie/slie mutants, the VLAD tool for extraction and analysis of GO annotations for large sets of genes was used (7, 8). VLAD analysis revealed perturbations in genes expressed in ovary that belong to major GO categories, such as cell death, inflammatory response, cholesterol biosynthesis and development and differentiation (Fig. 1A). Notably, a narrow set of biologically related gene classes were relatively increased in Ddr2slie/slie mutants, compared with wild type; all of which involved inflammation and immune processes, such as cytokine pathways, antigen production, and inflammatory responses (Fig. 1B). In contrast, the classes of genes that showed a relative decreased expression in Ddr2slie/slie mutants included many classical molecular and cellular pathways known to be involved with ovarian function and regulation, such as antiapoptosis, steroid synthesis, gonadal development, ovulatory cycles, and ovulation (Fig. 1C). Furthermore, decreases in the relative gene expression of antiapoptosis genes corroborated with decreases in other categories, of chromatin maintenance and assembly, as well as DNA packaging genes, which attests to the integrity of the transcriptome data analysis (Fig. 1C). Many of these molecular pathways potentially underlie infertility in Ddr2slie/slie mutants. To confirm genes in these categories are contributing to infertility in Ddr2slie/slie mutants, we conducted several cellular, molecular, histological, and physiological experiments to confirm the predictions from transcriptome analysis.
Fig. 1.
Biological structure of the ovarian transcriptome in Ddr2slie/slie mutant ovaries. Gene Ontology “biological process” annotations common to at least 5% of genes for all expressed genes (A), genes with a significant increase in relative expression (B), and genes with a significant decrease in relative expression (C). The 3rd–5th levels of Gene Ontology hierarchies with a corresponding Gene Ontology term are shown. Connecting lines indicate that a Gene Ontology term is a subset of 2 distinct higher-level terms. DDR2, discoidin domain receptor family, member 2; slie, smallie. *P < 0.01, **P < 0.001.
Confirming DDR2 regulation of ovarian cyclicity and ovulation via gonadotropin hormones.
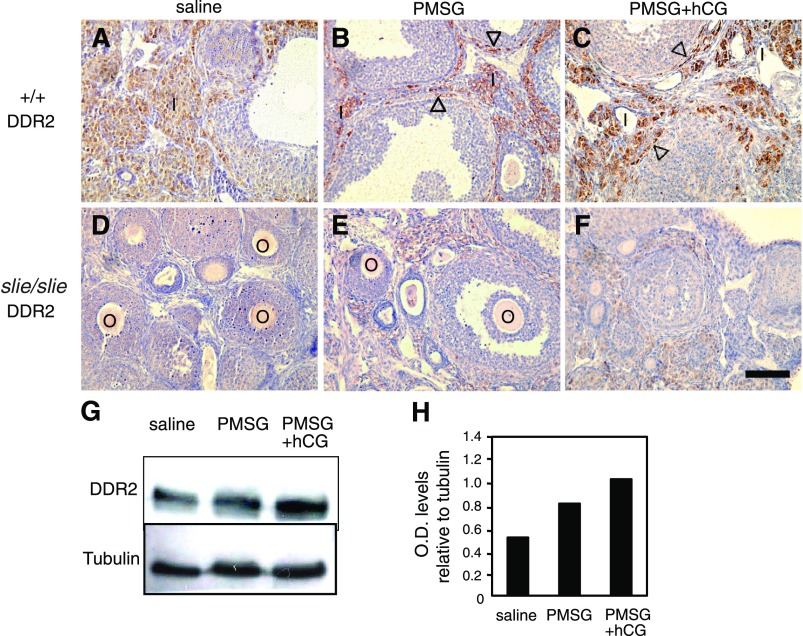
To confirm the prediction from transcriptome analysis that a regulatory feedback for ovulation exists between gonadotropins and DDR2, we tested whether gonadotropin stimulation induces follicular growth and ovulation in adult Ddr2slie/slie mutants relative to wild type. To determine if gonadotropins regulate DDR2 levels in the ovary, PMSG and hCG were injected into wild-type and Ddr2slie/slie mutant female mice. According to indirect immunohistochemistry, DDR2 immunostaining was moderately elevated after PMSG compared with saline alone (Fig. 2 A and B); however, a larger increase occurred after administration of both PMSG and hCG, most notably in somatic cells, such as interstitial and thecal cells (Fig. 2C) but not in the germ cells (i.e., oocytes). Conversely, as expected, DDR2 immunostaining for DDR2 (beyond background signal) was not detected in Ddr2slie/slie mutants after exogenous gonadotropin stimulation (Fig. 2, D–F). In addition, these observations of increased DDR2 protein content after gonadotropin treatment from immunohistochemistry experiments were confirmed by immunoblot studies of whole ovary extracts from wild-type mice (Fig. 2, G and H). These data confirm that a regulatory loop exists between DDR2 and gonadotropins.
Fig. 2.
Gonadotropin regulation of DDR2 expression in ovaries. DDR2 indirect immunoreactivity (brown) in cross-sections of ovaries from adult wild type (+/+) and Ddr2slie/slie mutant, counterstained with hematoxylin (violet), after saline (A, D), PMSG (B, E), or PMSG + hCG (C, F) treatments. DDR2 immunoreactivity was elevated after PMSG alone compared with saline and especially after PMSG + hCG in interstitial cells (open arrowheads) of the ovaries (B, C), whereas ovarian sections form Ddr2slie/slie mutants served as negative control (E, F). Immunoblot (G) and graph of relative optical density for LHR immunoreactive protein (H) harvested from extracts of whole ovaries from wild type upon either PMSG alone or PMSG + hCG treatment compared with saline control. O, oocytes, I, interstitial cells; PMSG, pregnant mare serum gonadotropin; hCG, human chorionic gonadotropin; LHR, luteinizing hormone receptor. Scale bar 100 μm.
Ovulation and embryonic development.
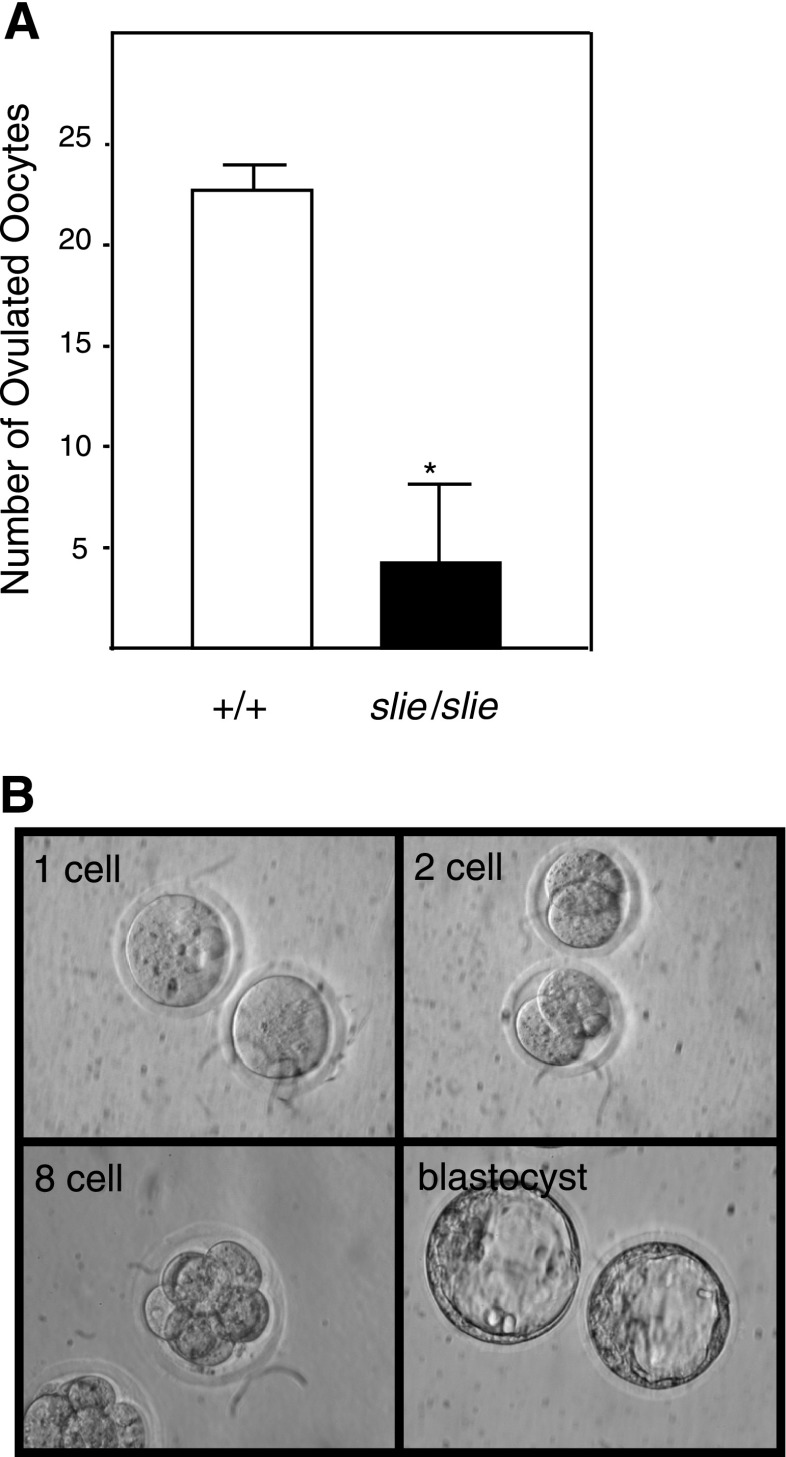
To verify, as predicted by transcriptome analysis, the suppression of gonadal development and ovulation-related genes, but not fertilization and embryo gene categories, we examined ovulation induced by exogenous gonadotropin stimulation and oocytes' potential to complete fertilization and embryonic development in vitro. The number of oocytes ovulated after the superovulation was significantly decreased in adult Ddr2slie/slie mutant mice (Fig. 3A). To investigate whether oocytes from Ddr2slie/slie mutant mice are competent to complete meiosis, fertilization, and development, we performed in vitro fertilization with oocytes from Ddr2slie/slie mutant mice and cultured the fertilized oocytes in vitro. One-cell zygotes did develop to the expanded blastocyst stage (Fig. 3B). Although substantially fewer oocytes were ovulated, these oocytes proved to be competent to complete fertilization and early embryogenesis.
Fig. 3.
Ovulation and developmental capacity of Ddr2slie/slie oocytes. A: significantly fewer oocytes were ovulated in Ddr2slie/slie mutant compared with wild type (+/+) after injection with PMSG + hCG. B: some in vitro fertilization oocytes from Ddr2slie/slie mutant mice developed from one-cell to blastocyst. *P < 0.01.
Apoptosis in Ddr2slie/slie mutant ovaries.
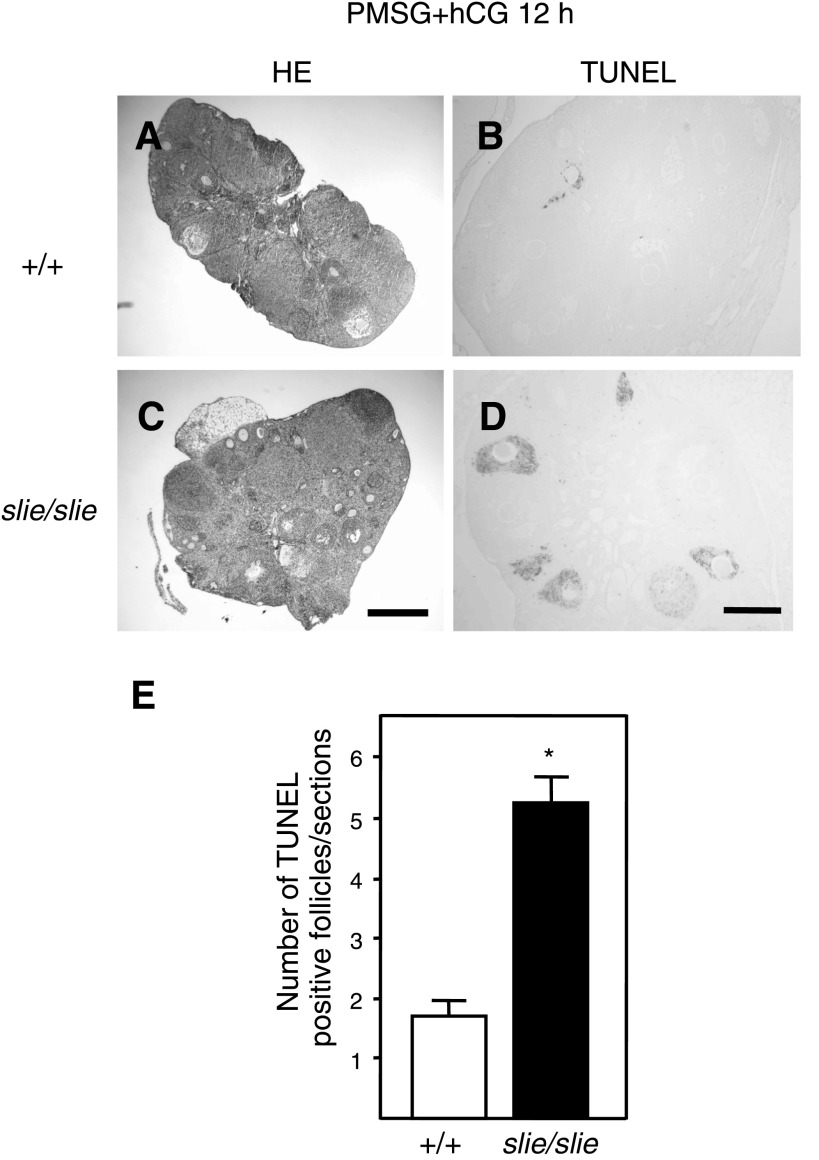
As indicated by transcriptome analysis, many antiapoptotic genes were relatively suppressed in Ddr2slie/slie mutant ovaries (Fig. 1, A and C; Supplemental Table S11) and suggest that an increase in cell death occurs in mice lacking Ddr2. To test the prediction that a greater incidence of follicular apoptosis occurs in Ddr2slie/slie mutant ovaries, we processed ovarian sections for TUNEL assays to illustrate an increase in DNA breakage, a hallmark of programmed cell death, in adult wild-type (Fig. 4, A and B) and Ddr2slie/slie mutant mice (Fig. 4, C and D). Significantly more apoptotic cells were observed in the follicles of 10-wk-old Ddr2slie/slie mutant compared with wild-type ovaries (Fig. 4E).
Fig. 4.
Follicle cell death in ovaries from Ddr2slie/slie mutants. Histological sections of ovaries from wild-type (+/+) and Ddr2slie/slie mutant at 10 wk stained with hematoxylin and eosin (A, C), and TUNEL analyses of ovaries (B, D) after treatment with PMSG + hCG. Higher counts of apoptotic follicles were observed in Ddr2slie/slie mutant mice compared with wild-type per ovarian section (E). HE, hematoxylin and eosin; TUNEL, terminal deoxynucleotidyl-transferase-mediated deoxyuridine triphosphate nick end labeling. *P < 0.01; Scale bar, 200 μm.
Hormonal and molecular pathways affected by Ddr2.
The results from ovarian transcriptome analysis (Fig. 1), the primary microarray data (Supplemental Table S1), along with the data from the immunohistochemistry experiments and published scientific knowledge (1, 6, 12, 17, 23, 31, 36, 39, 42, 55) informed our decision to focus on a subset of genes for gene expression studies. To confirm a subset of prominent molecules regulating ovulation as identified by transcriptome analysis (Fig. 1), by primary expression data by microarray (Supp. Table 1), and by previous published studies, we assessed expression levels of genes Ddr2, Mmp2, Mmp9, Mmp14, Mmp19, Lhcgr, Pgr, Ptger2, Ptgfr, and housekeeping gene Gapdh mRNAs by either RT-PCR or qPCR (or both techniques) following administration with PMSG and hCG. As expected, the expression levels of Ptgfr, Lhcgr, and Ptger2 genes were statistically significantly lower in Ddr2slie/slie ovaries relative to wild type (Fig. 5, A and B). Among these downregulated genes, Lhcgr is known to have important role in ovulation through luteinizing hormone signaling. To assess the alteration of expression and localization after exposure to exogenous gonadotropins, we performed indirect immunohistochemistry for LHR. We observed fewer immunopositive cells for LHR after saline (Fig. 5F), as well as after exogenous PMSG + hCG administration, especially in interstitial cells of the Ddr2slie/slie ovaries at 3 wk of age compared with wild type (Fig. 5G).
Fig. 5.
Gene expression confirmation studies in ovarian tissue from wild-type (+/+) and Ddr2slie/slie mutant mice. Gene expression is affected in Ddr2slie/slie mutant ovaries at 10 wk of age after gonadotropin treatment. Reverse-transcriptase PCR (A, B) and qPCR (C) analysis confirmed some genes identified from transcriptome analysis: Ddr2, Mmp2 (matrix metallopeptidase 2), Mmp9 (matrix metallopeptidase 9), Mmp19 (matrix metallopeptidase 19), Mmp14 (matrix metallopeptidase 14), Lhcgr (luteinizing hormone/choriogonadotropin receptor), Fshr (follicle stimulating hormone receptor), Ptger2 (prostaglandin E receptor 2, subtype EP2), Ptgfr (prostaglandin F receptor), Pgr (progesterone receptor), and housekeeping gene Gapdh (glyceraldehyde-3-phosphate dehydrogenase, also known as G3PDH) in wild-type and Ddr2slie/slie mutant ovaries after injection with PMSG and hCG. All data represent means and SE. Significant differences indicated by *P < 0.05, **P < 0.01. D–G: LHR (brown) protein levels were examined by indirect immunohistochemistry of ovarian tissue from wild type and Ddr2slie/slie mutant after saline (D, F), and PMSG + hCG (E, G) stimulation. LHR immunoreactivity was decreased in interstitial cells of the Ddr2slie/slie ovaries (arrowheads) after treatment to either saline alone or exogenous gonadotropins (F and G). O, oocytes. Scale bar 100 μm.
Contrary to our expectation, transcriptome analyses using VLAD software and primary microarray data, did not predict any significant changes in expression levels for genes Pgr, Fshr, or in two members of the matrix metallopeptidase (Mmp2, Mmp9) family, the canonical downstream pathway activated by DDR2 in somatic cells (7, 8), which are also expressed in ovarian thecal cells (12). To demonstrate a lack of disruption in these pathways in Ddr2slie/slie mutant ovaries, RT-PCR or qPCR experiments were conducted to verify the absence of differential expression. The expression levels of these genes were not significantly different between Ddr2slie/slie mutant and wild-type mice in response to gonadotropin stimulation, which further supports the predications from transcriptome analysis (Fig. 5, A–C).
DISCUSSION
Transcriptome analysis was statistically performed for over-representation of gene categories involved with reproduction, apoptosis and programmed cell death, and inflammation in Ddr2slie/slie mutants compared with wild-type mice. Next, we verified experimentally whether a subset of these gene categories indicated by transcriptome analysis was indeed altered, and the biological significance of this alteration. Treatment with exogenous gonadotropin hormones increased DDR2 immunoreactivity in wild-type ovary, whereas no DDR2 immunoreactivity was detected in ovarian tissue from Ddr2slie/slie mutants. In a similar pattern, exogenous gonadotropin administration did not induce LHR immunoreactivity in ovarian tissue from Ddr2slie/slie mutants relative to wild-type control. Furthermore, gene expression levels were statistically significantly decreased in the genes encoding Lhcgr, Ptger2, and Ptgfr in ovaries from Ddr2slie/slie mutants, relative to wild-type mice, as detected by either RT-PCR or qPCR (or both). Gene expression levels for Mmp genes in ovarian tissue from Ddr2slie/slie mutants did not statistically significant differ from wild-type mice, as measured by RT-PCR and qPCR, which was unexpected as Mmp activity is currently considered the canonical signaling pathway for discoidin domain receptors (12, 34, 35). In summary, these convergent results suggest that DDR2 may ultimately affect fertility in female mice through altering gene expression for classical endocrine pathways to regulate ovarian function.
Ovulation is a complex process initially triggered by LH that culminates in the release of an oocyte, competent to complete meiosis and initiate embryogenesis after fertilization (38, 39, 41). Coordination of autocrine, paracrine, and endocrine pathways affecting associated remodeling of ovarian tissue is essential and critical. To date, little is known about the exact role of extracellular matrix molecules, specifically collagen and its receptors, in ovulation and follicular development. For ovarian pathology and disease, it is known that signaling molecules for the extracellular matrix, specifically members of the Mmp gene family, may be involved with rendering metastatic cancer cells the ability to infiltrate the ovarian epithelium, and thus may be a useful biomarker for diagnosis of ovarian cancer (1, 4, 44, 45, 50, 54). Collagen has been recognized as a component of ovarian tissue in mammals, but it is also known, in other nonmammalian vertebrate species, such as trout, that collagen is present in the ovary (53). It has been proposed that the extracellular matrix, namely collagen, and presumably its receptors, participates in ovarian function as molecule facilitating remodeling of ovarian tissue, which is required for successful ovulation of mature oocytes (49). However, the details about ligands, molecules, receptors, and intracellular signaling pathways of the extracellular matrix involved in ovulation and remodeling of ovarian tissue are not known. Here we show that one of the potential extracellular matrix molecules participating in this process is DDR2.
DDR2 is a fibrillar collagen receptor widely expressed in various tissues and participating in cellular proliferation, adhesion, migration, and tissue remodeling (19, 26, 35, 55). Furthermore, DDR2 is a recently discovered molecule that appears to have a critical role in reproduction, as mice with a homozygous, autosomal deletion of the Ddr2 gene are dwarfed and infertile (24). In addition, humans carrying a homozygous, missense mutation causes SMED-SL, manifesting as disproportionate dwarfism with shortening of the long bones, mild craniofacial abnormalities, and infertility (6). In this current study, we focused on the molecular and cellular pathways underlying infertility in female Ddr2slie/slie mutant mice. Specifically, as a first step to understand how deficient DDR2 signaling leads to an infertility phenotype, we applied bioinformatics tools to analyze global gene expression data from DNA microarrays to identify molecular and cellular pathways in ovarian tissue affected by a loss-of-function allele for Ddr2, known as smallie, compared with wild-type allele, in mice. In summary, we tested a subset of the predictions from our transcriptome analysis with further in vivo and in vitro biological experiments, including quantitative RT-PCR, immunohistochemistry, histological, and physiological studies. These experimental biological approaches confirmed that bioinformatics analyses are a robust “systems biology” tool to identify specific molecular pathways, especially in the perplexity of large gene expression datasets.
The goal of any microarray experiment is to identify the genes that are differentially expressed any samples to yield insight into the complex pathways involved with biological processes. Unfortunately, generating a list of genes by employing an arbitrary relative expression change cutoff value, although still in current use, fails to provide any robust measure of confidence for those genes selected and yields the false positive selection of genes more susceptible to technical artifacts and experimental noise than actual expression changes (10, 11, 14). To circumvent this widespread caveat in microarray studies, we employed the use of publicly available bioinformatics databases, such as GO, and associated analysis platforms, such as VLAD, to examine the relationships of all differentially expressed genes, instead of focusing on a handful of essentially arbitrary chosen “candidate” genes. Indeed, a simple list of differentially expressed genes has two distinct disadvantages: lack of the insight into the complexity of fundamental biological processes, and the human bias due to the experimentalists' temptation to focus on a handful of “favorite” genes. Furthermore, to contain the false discovery under 5% in our VLAD-based transcriptome analysis, we discarded categories of GOs with fewer than five genes members, since inclusion of even only one gene of those members would artificially yield a higher significance in those categories due to few observations (14, 21, 25). Applying these conservative constraints to our microarray data, transcriptome analysis did yield significant insights into pathways affected by disrupting DDR2 signaling in ovarian tissue and led to experimentally testable predications on the pathways indicated by VLAD analysis.
For example, VLAD indicated that the infertility phenotype in Ddr2slie/slie mutant mice may manifest as curtailed ovulation, increased follicular apoptosis, impaired hormone secretion and decreased follicular luteinization. Although cell death is clearly involved, we interpret these data that apoptosis is not the initial trigger underlying female infertility in Ddr2slie/slie mutant mice. Our interpretation is substantiated by experiments demonstrating that absence of DDR2 signaling does not affect apoptosis in other somatic tissues, such as chondrocytes or corneal fibroblasts (26, 32). We interpret these results that DDR2 does not provoke apoptosis directly; instead, apoptosis is a downstream consequence of alteration in other pathways due to the lack of robust DDR2 signaling, such as hormonal signaling via molecules like LHR. Indeed, mice homozygous for null alleles of Lhcgr, liver receptor homolog 1 (Lrh1) and prolactin receptor also display impaired fertility, ovulation and increased follicular apoptosis, a similar phenotype observed in our current and recent studies with Ddr2slie/slie mutant mice (16, 37).
While the specific role of ovarian theca cells is obscure, their putative role in proteolytic events of follicle rupture is undoubtedly critical for ovulation. The extracellular matrix within the theca layer should be altered upon gonadotropin signals to be digested at the ovarian surface (17). This is the first report that documents DDR2 levels are increased by gonadotropins in somatic tissue, with the greatest enhancement in interstitial and thecal cells. Originally, we anticipated that reproductive abnormalities seen in Ddr2slie/slie mutant mice are triggered by disrupted signaling from DDR2 upon Mmp genes and proteins (12), as these have been postulated to act as signal transducers for DDR2 (34). Furthermore, many studies have indicated that Mmp molecules are widely expressed in the ovary during the preovulatory period and thus may play a role in degradation of the follicular wall during ovulation (12, 13, 23, 30, 33, 38–40, 54).
Although it was reasonable to expect that a decrease in expression of Mmp genes underlie infertility in Ddr2slie/slie mutant mice, our data do not support a role for these genes in Ddr2slie/slie ovarian pathology. Instead, by analyzing the ovarian Ddr2slie/slie transcriptome, we did detect other pathways perturbed in Ddr2slie/slie mutant mice. Focusing on the major pathways suppressed in ovaries from Ddr2slie/slie mutant mice, we confirmed that the gene expression for three hormone receptors, Lhcgr, Ptgfr and Ptger2, were not induced upon gonadotropin stimulation in 3-wk-old Ddr2slie/slie mutant mice relative to wild type. This lack of up-regulation for these prostaglandin and luteinizing hormone receptors occurs before the onset of increased apoptosis in adult Ddr2slie/slie mutants.
Decreases in any of the three molecules may contribute to anovulation. For instance, fewer prostaglandin F receptors may contribute to the failed luteinization of Graafian follicles, as it is localized in thecal cells (46, 47). On the other hand, this decrease may simply reflect the presence of fewer corpora lutea. Prostaglandin E receptor 2 (PTGER2, previously called EP2) is expressed in cumulus and granulosa cells and may potentially be the primary trigger underlying the Ddr2slie/slie infertility. However, notable phenotype differences between, mice lacking PTGER2 and Ddr2slie/slie mutant mice (20, 48) and dissimilarity of PTGER2 and DDR2 expression patterns (42) suggest that insufficient PTGER2 might not initiate infertility of Ddr2slie/slie mutant mice. Finally, the localization of LHR on the external theca cells corresponds to, and overlaps with DDR2 expression (31). In addition, the ovarian phenotypes of Ddr2slie/slie mutant mice are similar to genetically engineered mice, homozygous for Lhcgr null allele (36, 56). However, unlike Lhcgr null mutants, Ddr2slie/slie mutants develop deficiencies in ovarian function gradually, during the transition from juvenile to adulthood, and Ddr2slie/slie mutants do retain baseline Lhcgr expression, at least at puberty. The exact molecular and cellular mechanisms and pathways transducing LHR signals to DDR2 are not yet identified, but we speculate that DDR2 may possibly affect somatic cell proliferation during the LH surge. It is also possible that DDR2 and LHR may positively co-regulate each other to gradually induce remodeling of the follicular wall and release of the oocyte.
To summarize, Ddr2 expression is increased by exogenous gonadotropin in wild type, but not Ddr2slie/slie mutant, ovaries. Surprisingly, DDR2 signaling pathways did not activate Mmp genes, as no differences in their expression were found in Ddr2slie/slie mutants compared with wild-type mice. Instead, our findings suggest that overlapping spatial and temporal expression of DDR2 and LHR may permit their mutual co-regulation during specific periods of ovulation. Our results support the interpretation that a lack of DDR2 signals in Ddr2slie/slie mutants most likely triggers anovulation by altering gene expression of Lhcgr, and to a lesser extent, Ptgfr and Ptger2. Reduced expression of these receptors, in particular Lhcgr, may lead to subsequent down regulation of anti-apoptosis genes arising from impaired hormone signaling, and in turn drive follicular apoptosis, anovulation, and ultimately infertility in Ddr2slie/slie mutants.
GRANTS
The research was supported in part by Grants-in-Aid for Scientific Research from Ministry of Education, Science, Sports, and Culture of Japan; the Morinaga Foundation; Foundation for Growth Science (K. Kano, H. Matsumura, K. Naito); and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-46977 and DK-73267 (to C. Marín de Evsikova and J. K. Naggert).
Supplementary Material
ACKNOWLEDGMENTS
Our gratitude is extended to Alexei Evsikov for critique of the manuscript.
Footnotes
1The online version of this article contains supplemental material.
REFERENCES
- 1. Adley BP, Gleason KJ, Yang XJ, Stack MS. Expression of membrane type 1 matrix metalloproteinase (MMP-14) in epithelial ovarian cancer: high level expression in clear cell carcinoma. Gynecol Oncol 112: 319–324, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal G, Kovac L, Radziejewski C, Samuelsson SJ. Binding of discoidin domain receptor 2 to collagen I: an atomic force microscopy investigation. Biochemistry 41: 11091–11098, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Alexopoulos LG, Youn I, Bonaldo P, Guilak F. Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum 60: 771–779, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene 10: 609–618, 1995. [PubMed] [Google Scholar]
- 5. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bargal R, Cormier-Daire V, Ben-Neriah Z, Le Merrer M, Sosna J, Melki J, Zangen DH, Smithson SF, Borochowitz Z, Belostotsky R, Raas-Rothschild A. Mutations in DDR2 gene cause SMED with short limbs and abnormal calcifications. Am J Hum Genet 84: 80–84, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blake JA, Bult CJ, Eppig JT, Kadin JA, Richardson JE. The Mouse Genome Database genotypes::phenotypes. Nucleic Acids Res 37: D712–D719, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bult CJ, Eppig JT, Kadin JA, Richardson JE, Blake JA. The Mouse Genome Database (MGD): mouse biology and model systems. Nucleic Acids Res 36: D724–D728, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carleton SM, McBride DJ, Carson WL, Huntington CE, Twenter KL, Rolwes KM, Winkelmann CT, Morris JS, Taylor JF, Phillips CL. Role of genetic background in determining phenotypic severity throughout postnatal development and at peak bone mass in Col1a2 deficient mice (oim). Bone 42: 681–694, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cui X, Churchill GA. Statistical tests for differential expression in cDNA microarray experiments. Genome Biol 4: 210, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui X, Kerr MK, Churchill GA. Transformations for cDNA microarray data. Stat Appl Genet Mol Biol 2: Article4, 2003. [DOI] [PubMed] [Google Scholar]
- 12. Curry TE, Jr, Osteen KG. Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol Reprod 64: 1285–1296, 2001. [DOI] [PubMed] [Google Scholar]
- 13. Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 24: 428–465, 2003. [DOI] [PubMed] [Google Scholar]
- 14. Draghici S, Khatri P, Eklund AC, Szallasi Z. Reliability and reproducibility issues in DNA microarray measurements. Trends Genet 22: 101–109, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dreier R, Opolka A, Grifka J, Bruckner P, Grassel S. Collagen IX-deficiency seriously compromises growth cartilage development in mice. Matrix Biol 27: 319–329, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Gene Dev 22: 1871–1876, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Espey LL. Ovulation as an inflammatory reaction–a hypothesis. Biol Reprod 22: 73–106, 1980. [DOI] [PubMed] [Google Scholar]
- 18. Evsikov AV, Graber JH, Brockman JM, Hampl A, Holbrook AE, Singh P, Eppig JJ, Solter D, Knowles BB. Cracking the egg: molecular dynamics and evolutionary aspects of the transition from the fully grown oocyte to embryo. Gene Dev 20: 2713–2727, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol 164: 1575–1585, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, Narumiya S, Ichikawa A. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP (2). Proc Natl Acad Sci USA 96: 10501–10506, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosack DA, Dennis G, Jr, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol 4: R70, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ichikawa O, Osawa M, Nishida N, Goshima N, Nomura N, Shimada I. Structural basis of the collagen-binding mode of discoidin domain receptor 2. EMBO J 26: 4168–4176, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jo M, Thomas LE, Wheeler SE, Curry TE., Jr Membrane type 1-matrix metalloproteinase (MMP)-associated MMP-2 activation increases in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Biol Reprod 70: 1024–1032, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Kano K, Marin de Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, Naggert JK. A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Mol Endocrinol 22: 1866–1880, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khatri P, Draghici S. Ontological analysis of gene expression data: current tools, limitations, and open problems. Bioinformatics 21: 3587–3595, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, Bruckner K, Goergen JL, Lemke G, Yancopoulos G, Angel P, Martinez C, Klein R. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep 2: 446–452, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leitinger B, Hohenester E. Mammalian collagen receptors. Matrix Biol 26: 146–155, 2007. [DOI] [PubMed] [Google Scholar]
- 28. Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol 25: 355–364, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Leitinger B, Steplewski A, Fertala A. The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol 344: 993–1003, 2004. [DOI] [PubMed] [Google Scholar]
- 30. Liu K, Wahlberg P, Ny T. Coordinated and cell-specific regulation of membrane type matrix metalloproteinase 1 (MT1-MMP) and its substrate matrix metalloproteinase 2 (MMP-2) by physiological signals during follicular development and ovulation. Endocrinology 139: 4735–4738, 1998. [DOI] [PubMed] [Google Scholar]
- 31. Misrahi M, Beau I, Ghinea N, Vannier B, Loosfelt H, Meduri G, Vu Hai MT, Milgrom E. The LH/CG and FSH receptors: different molecular forms and intracellular traffic. Mol Cell Endocrinol 125: 161–167, 1996. [DOI] [PubMed] [Google Scholar]
- 32. Mohan RR, Mohan RR, Wilson SE. Discoidin domain receptor (DDR) 1 and 2: collagen-activated tyrosine kinase receptors in the cornea. Exp Eye Res 72: 87–92, 2001. [DOI] [PubMed] [Google Scholar]
- 33. Ny T, Wahlberg P, Brandstrom IJ. Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. Mol Cell Endocrinol 187: 29–38, 2002. [DOI] [PubMed] [Google Scholar]
- 34. Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest 108: 1369–1378, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olaso E, Labrador JP, Wang L, Ikeda K, Eng FJ, Klein R, Lovett DH, Lin HC, Friedman SL. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem 277: 3606–3613, 2002. [DOI] [PubMed] [Google Scholar]
- 36. Pakarainen T, Zhang FP, Nurmi L, Poutanen M, Huhtaniemi I. Knockout of luteinizing hormone receptor abolishes the effects of follicle-stimulating hormone on preovulatory maturation and ovulation of mouse graafian follicles. Mol Endocrinol 19: 2591–2602, 2005. [DOI] [PubMed] [Google Scholar]
- 37. Pakarainen T, Zhang FP, Poutanen M, Huhtaniemi I. Fertility in luteinizing hormone receptor-knockout mice after wild-type ovary transplantation demonstrates redundancy of extragonadal luteinizing hormone action. J Clin Invest 115: 1862–1868, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 234: 75–79, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 64: 69–92, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA 97: 4689–4694, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robker RL, Russell DL, Yoshioka S, Sharma SC, Lydon JP, O'Malley BW, Espey LL, Richards JS. Ovulation: a multi-gene, multi-step process. Steroids 65: 559–570, 2000. [DOI] [PubMed] [Google Scholar]
- 42. Segi E, Haraguchi K, Sugimoto Y, Tsuji M, Tsunekawa H, Tamba S, Tsuboi K, Tanaka S, Ichikawa A. Expression of messenger RNA for prostaglandin E receptor subtypes EP4/EP2 and cyclooxygenase isozymes in mouse periovulatory follicles and oviducts during superovulation. Biol Reprod 68: 804–811, 2003. [DOI] [PubMed] [Google Scholar]
- 43. Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell 1: 25–34, 1997. [DOI] [PubMed] [Google Scholar]
- 44. Sodek KL, Brown TJ, Ringuette MJ. Collagen I but not Matrigel matrices provide an MMP-dependent barrier to ovarian cancer cell penetration. BMC Cancer 8: 223, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sodek KL, Evangelou AI, Ignatchenko A, Agochiya M, Brown TJ, Ringuette MJ, Jurisica I, Kislinger T. Identification of pathways associated with invasive behavior by ovarian cancer cells using multidimensional protein identification technology (MudPIT). Mol Biosyst 4: 762–773, 2008. [DOI] [PubMed] [Google Scholar]
- 46. Sugimoto Y, Hasumoto K, Namba T, Irie A, Katsuyama M, Negishi M, Kakizuka A, Narumiya S, Ichikawa A. Cloning and expression of a cDNA for mouse prostaglandin F receptor. J Biol Chem 269: 1356–1360, 1994. [PubMed] [Google Scholar]
- 47. Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S. Failure of parturition in mice lacking the prostaglandin F receptor. Science 277: 681–683, 1997. [DOI] [PubMed] [Google Scholar]
- 48. Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, Coffman TM, Koller BH. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest 103: 1539–1545, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsafriri A. Ovulation as a tissue remodelling process. Proteolysis and cumulus expansion. Adv Exp Med Biol 377: 121–140, 1995. [DOI] [PubMed] [Google Scholar]
- 50. Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J 13, Suppl: S77–S82, 1999. [DOI] [PubMed] [Google Scholar]
- 51. Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1: 13–23, 1997. [DOI] [PubMed] [Google Scholar]
- 52. Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal 18: 1108–1116, 2006. [DOI] [PubMed] [Google Scholar]
- 53. Von Schalburg KR, Cooper GA, Yazawa R, Davidson WS, BFK Microarray analysis reveals differences in expression of cell surface and extracellular matrix components during development of the trout ovary and testis. Comp Biochem Physiol D Genomics Proteomics 3: 78–90, 2008. [DOI] [PubMed] [Google Scholar]
- 54. Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5: 2145–2154, 1991. [PubMed] [Google Scholar]
- 55. Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem 280: 548–555, 2005. [DOI] [PubMed] [Google Scholar]
- 56. Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol 15: 172–183, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.