Abstract
Ca2+-dependent activator protein for secretion (CAPS) is an essential factor for regulated vesicle exocytosis that functions in priming reactions before Ca2+-triggered fusion of vesicles with the plasma membrane. However, the precise events that CAPS regulates to promote vesicle fusion are unclear. In the current work, we reconstituted CAPS function in a SNARE-dependent liposome fusion assay using VAMP2-containing donor and syntaxin-1/SNAP-25-containing acceptor liposomes. The CAPS stimulation of fusion required PI(4,5)P2 in acceptor liposomes and was independent of Ca2+, but Ca2+ dependence was restored by inclusion of synaptotagmin. CAPS stimulated trans-SNARE complex formation concomitant with the stimulation of full membrane fusion at physiological SNARE densities. CAPS bound syntaxin-1, and CAPS truncations that competitively inhibited syntaxin-1 binding also inhibited CAPS-dependent fusion. The results revealed an unexpected activity of a priming protein to accelerate fusion by efficiently promoting trans-SNARE complex formation. CAPS may function in priming by organizing SNARE complexes on the plasma membrane.
Keywords: vesicle exocytosis, priming, PIP2, contents mixing
Vesicle fusion in the secretory pathway employs SNARE proteins, members of a conserved family of membrane-associated proteins that contain approximately 60-aa heptad repeat motifs (1). A donor membrane SNARE protein associates in trans with acceptor membrane SNARE proteins to generate a 4-helix bundle (2, 3). Vesicle exocytosis in neuronal and endocrine cells utilizes VAMP2 (also known as synaptobrevin 2) on the vesicle (v-SNARE) and the target membrane SNAREs (t-SNAREs) syntaxin-1 and SNAP-25. The structure of a 4-helix bundle of neuronal SNAREs suggested how an assembled trans-SNARE complex could promote fusion through close membrane apposition (3). Evidence that neuronal SNARE complexes might directly catalyze membrane fusion was provided by lipid mixing studies with SNAREs reconstituted into proteoliposomes (4). The function of several key SNARE regulatory proteins, including synaptotagmin and Munc18–1, was successfully reconstituted in the lipid-mixing assay (5, 6).
Dense-core vesicle exocytosis in cells proceeds through several stages before Ca2+-triggered fusion, consisting of docking/tethering and priming steps (7). Vesicles engage the plasma membrane in docking/tethering interactions that involve syntaxin-1 and SNAP-25 (8, 9). However, not all docked/tethered vesicles are competent for fusion, and priming reactions are needed to convert docked vesicles to a fusion-ready state (10). Priming involves the progressive assembly of trans-SNARE complexes, likely through the initial assembly of t-SNARE heterodimers with subsequent incorporation of VAMP2 by zippering N-terminal to C-terminal regions of the SNAREs (11–14). SNARE-binding proteins likely catalyze SNARE complex assembly during priming but little is known about how this is achieved.
Molecular studies of factors that act in priming are needed to elucidate the pathway of SNARE complex assembly before fusion. Munc13 proteins, which are required for vesicle priming (15), may function through interactions with SNAREs (16, 17). A Munc-13–1 C-terminal domain (18, 19) binds pre-assembled SNARE complexes (20, 21), but the effect of Munc13–1 on SNARE complex assembly is unknown. Ca2+-dependent activator protein for secretion (CAPS) proteins, which exhibit sequence similarity to a C-terminal domain of Munc13–1 (22), also function in vesicle priming (23), but the mechanism used remains to be defined. CAPS-1 reconstitutes late stages of Ca2+-triggered dense-core vesicle exocytosis in permeable PC12 cells (24) and is required for optimal Ca2+-triggered vesicle exocytosis in endocrine and neural cells (25, 26). CAPS proteins contain a central PH domain and are recruited to membrane PI 4,5-P2 (27), which itself is essential for priming vesicle exocytosis (28).
To characterize the mechanism of CAPS-1 function in priming, we reconstituted its activity in a minimal liposome assay employing neuronal SNARE proteins in PI 4,5-P2-containing liposomes (29). As we report here, CAPS dramatically enhanced rates of non-leaky full fusion in liposomes that contained limiting densities of t-SNAREs, and promoted trans-SNARE complex formation on the liposomes. CAPS interacted directly with membrane t-SNAREs, and a t-SNARE-binding CAPS C-terminal Munc13 homology domain protein inhibited CAPS stimulation of SNARE-dependent fusion. The results suggest that CAPS is a regulator of t-SNAREs that functions in priming by promoting SNARE complex assembly for vesicle exocytosis.
Results
CAPS Accelerates SNARE-Dependent Liposome Fusion.
Studies in permeable PC12 cells indicate that CAPS functions after vesicle docking to prime Ca2+-dependent dense-core vesicle exocytosis (23, 24). To determine the mechanism of CAPS function, we reconstituted its activity in a minimal SNARE-dependent liposome fusion assay (4). Because lipid mixing in the liposome assay is strongly dependent on SNARE surface densities [supporting information (SI) Table S1], we used VAMP2 densities on donor and syntaxin-1/SNAP-25 densities on acceptor liposomes in the physiological range (see Materials and Methods) (30, 31). The lipid composition of membranes also affects liposome fusion (29). Because CAPS associates with membranes through interactions with PI 4,5-P2, this lipid was included at 5 to 10 mol% in the t-SNARE-containing acceptor liposomes (29).
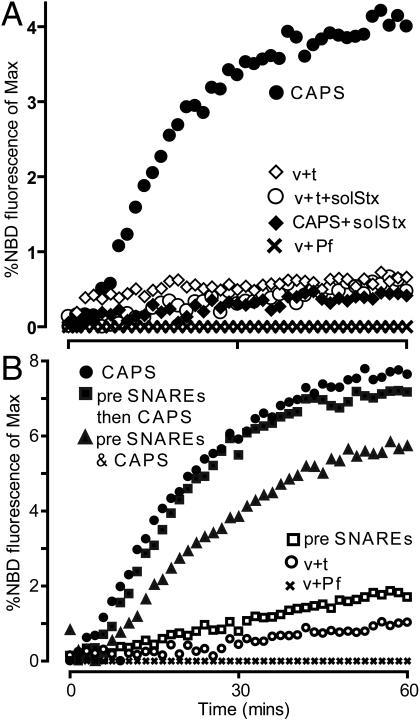
CAPS markedly stimulated the rates and extent of lipid mixing measured by the de-quenching of N-4-nitrobenzo-2-oxa-1,3-diazole-phosphatidylethanolamine (NBD-PE) fluorescence (Fig. 1A). The increased lipid mixing with CAPS was completely inhibited by inclusion of a soluble syntaxin fusion protein that competitively inhibits trans-SNARE complex formation, which indicated that CAPS stimulation was SNARE-dependent. Munc18–1 was reported to stimulate SNARE-dependent lipid mixing with preincubation (6). By contrast, CAPS stimulation of lipid mixing occurred immediately upon addition of the protein (Fig. 1B), and the preincubation of liposomes with CAPS did not further enhance the stimulation of lipid mixing (Fig. 1B). This indicated that CAPS promoted SNARE-dependent lipid mixing directly rather than through a SNARE complex intermediate that formed during pre-incubation. Overall, the results showed that aspects of CAPS function were successfully reconstituted in a minimal SNARE-dependent liposome assay.
Fig. 1.
CAPS stimulates SNARE-dependent liposome fusion. (A) Lipid mixing between v-SNARE and t-SNARE(10% PIP2) liposomes was determined in the absence of additions (open circles), with 5 μM soluble syntaxin (open diamonds), or with 1 μM CAPS in the absence (filled circles) or presence (filled diamonds) of 5 μM soluble syntaxin. Lipid mixing is shown as a percent of maximal NBD fluorescence corrected for protein-free (Pf, x) liposomes. (B) v- and t-SNARE liposomes were incubated overnight at 0 °C to 4 °C in the absence (open squares, filled squares) or presence (filled triangles) of 1 μM CAPS and compared with freshly assembled (open circles, filled circles) liposomes in lipid mixing reactions after acute addition of 1 μM CAPS to freshly assembled (filled circles) or preincubated (filled squares) liposomes.
Because CAPS is a Ca2+ binding protein (32) and acts at a late Ca2+-triggered stage of exocytosis in permeable PC12 cells (23), we tested the effects of Ca2+ over a broad concentration range. We found that CAPS-dependent lipid mixing was not affected by Ca2+ (Fig. S1A) but that Ca2+ regulation was conferred by introducing a synaptotagmin C2AB protein (Fig. S1B) (5). In the absence of Ca2+, C2AB inhibited CAPS stimulation of lipid mixing, but in the presence of Ca2+, the rates of lipid mixing were restored to those with CAPS alone (Fig. S1B). The results indicated that CAPS function was not directly Ca2+-regulated, and they were consistent with CAPS function at a priming step that precedes synaptotagmin-dependent, Ca2+-triggered fusion. The results revealed that a priming factor could promote SNARE-dependent lipid mixing in the absence of downstream regulators.
CAPS Stimulates Full Fusion and Contents Mixing in Liposomes.
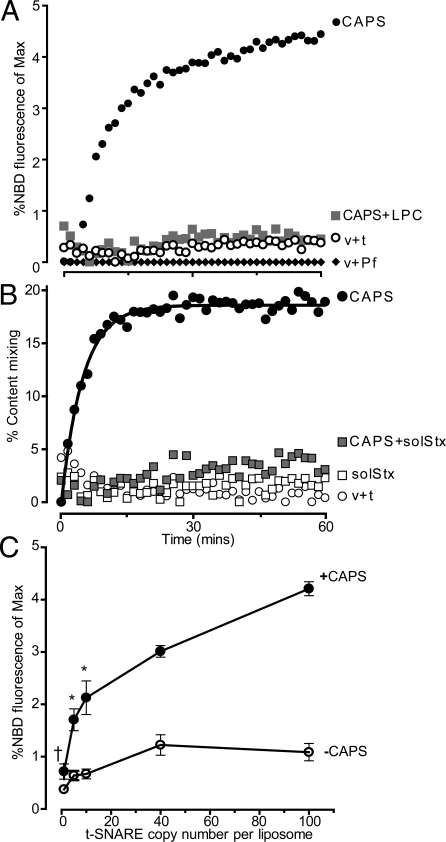
Vesicle fusion with the plasma membrane occurs by the initial merger of contacting cytoplasmic leaflets, which results in formation of a stalk intermediate that leads to fusion pore formation upon merger of non-contacting leaflets (33). Inverted cone-shaped lipids that induce positive curvature inhibit a wide variety of membrane fusion processes because they antagonize the high negative curvature needed to form a stalk intermediate. LysoPC, an inverted cone lipid, fully inhibited CAPS-stimulated lipid mixing (Fig. 2A), which suggested that CAPS promoted a physiological form of membrane merger.
Fig. 2.
CAPS stimulates full fusion at low t-SNARE densities. (A) v-SNARE and t-SNARE(10% PIP2) liposomes were incubated with 0.1 mM LPC for 10 min (filled squares) before lipid mixing reactions were conducted without additions (open circles) or with 1 μM CAPS (filled circles, filled squares). (B) Tb3+-loaded v-SNARE and DPA-loaded t-SNARE(10% PIP2) liposomes were prepared with 640 and 340 SNARE molecules/μm2, respectively, and incubated without additions (open circles), with 5 μM soluble syntaxin (open squares), with 1 μM CAPS (filled circles), or with CAPS plus soluble syntaxin (filled squares). Mixing of contents was expressed as percentage of complete mixing following detergent lysis. (C) Lipid mixing reactions were conducted with v-SNARE liposomes and t-SNARE(5% PIP2) liposomes that contained 1 to 100 copies of syntaxin-1/SNAP-25 in the absence (open circles) or presence of 1 μM CAPS (filled circles) (mean ± SD, n = 3; †Not different; *, P > 0.05 for with vs. without CAPS).
Low SNARE densities were reported to enable hemi-fusion rather than full fusion (34). Because we used similar SNARE densities (lipid:protein ratios of 225:1 for v-SNARE and 500:1 for t-SNARE liposomes), it was possible that CAPS acted to promote the transition of a hemi-fused intermediate to full fusion. To assess this, we measured the mixing of contacting and non-contacting leaflets with an assay (35) that oxidizes the NBD fluorophore on the solvent-exposed leaflet. Liposomes were treated with dithionite to fully bleach the exposed NBD, which corresponded to approximately 60% of the fluorescence (Fig. S2A), and were purified by gradient centrifugation. Parallel fusion assays conducted with the bleached and unbleached liposomes revealed that little total or inner leaflet mixing occurred in the absence of CAPS (Fig. S2B). By contrast, CAPS promoted rapid increases in both total and inner leaflet mixing (Fig. S2B). Rate constants derived for total versus inner leaflet mixing with CAPS (0.09 min−1 ± 0.004 and 0.08 min−1 ± 0.01, respectively; Fig. S2B) did not differ significantly. The results indicated that CAPS did not act by accelerating the transition of a hemi-fused intermediate to full fusion but rather initiated full bilayer fusion.
FRET-based assays that rely on NBD-PE and Rh-PE dilution do not uniquely indicate whether increased fluorescence results from fusion of donor and acceptor liposomes or from lipid transfer between membranes (36). To determine whether CAPS promoted bona fide non-leaky membrane fusion, we used a classical assay to detect contents mixing (37). Donor and acceptor liposomes were loaded with terbium (Tb3+) and dipicolinic acid (DPA), respectively, to monitor contents mixing by the unique fluorescence emission of the Tb3+-DPA complex (Fig. S2C). Our attempts to entrap low molecular weight Tb3+ and DPA in extruded 100-nm liposomes failed at commonly used higher SNARE densities. However, with VAMP2 densities at 640 molecules/μm2 in donor liposomes and t-SNAREs at 318 heterodimers/μm2 in acceptor liposomes, successful Tb3+ and DPA incorporation was achieved. No contents mixing occurred in liposome incubations in the absence of CAPS at these SNARE densities (Fig. 2B). By contrast, CAPS promoted robust contents mixing corresponding to consumption of approximately 20% of the donor liposomes (Fig. 2B). CAPS-dependent contents mixing was fully blocked by inclusion of a soluble syntaxin fusion protein, indicating that it was SNARE-dependent. NBD fluorescence assays conducted under the same conditions also showed virtually no lipid mixing in the absence of CAPS but robust mixing in the presence of CAPS (Fig. S3). Calibration of the NBD fluorescence to rounds of fusion indicated that 1 μM CAPS stimulated fusion equivalent to approximately 20% of the donor liposomes undergoing one round of fusion (Fig. S4A). Considering that we used less than the half-maximally effective concentration of CAPS (29) (Fig. S4B), these results indicated that CAPS promoted efficient full fusion of the liposomes.
CAPS Drives Fusion at Low t-SNARE Densities.
PI 4,5-P2 is required for CAPS activity in fusion but is effective only when present in the acceptor liposomes containing SNAP-25 and syntaxin-1 (29), which suggested that CAPS acts in cis with t-SNAREs. By generating acceptor liposomes with varying t-SNARE content, we found that CAPS promoted efficient liposome fusion at extremely low t-SNARE densities. Whereas fusion was not achieved with as many as 100 copies of t-SNARE proteins per 50 nm liposome in the absence of CAPS, CAPS effectively accelerated fusion of liposomes that contained as few as 5 to 10 copies of t-SNAREs (Fig. 2C). This corresponds to 3 to 8 outward-oriented t-SNAREs in the liposomes (38), which is close to estimates for the number of trans-SNARE complexes needed for a Ca2+-triggered exocytic event in cells (39, 40). The results were compatible with an action of CAPS on t-SNARE proteins (as described later).
It was suggested that the kinetics of SNARE-dependent lipid mixing may be artificially slow because of the presence of non-functional 1:2 SNAP-25:syntaxin-1 t-SNARE complexes (13). Such non-functional complexes, which have been demonstrated to form as soluble counterparts, would require displacement of a syntaxin by VAMP2 to form fusogenic heterotrimeric complexes, which would slow the kinetics of liposome fusion. To determine the role, if any, played by such potentially dysfunctional t-SNARE complexes in the liposome fusion assay, we systematically varied the ratio of SNAP-25 to syntaxin-1 in acceptor liposomes. Whereas fusion without CAPS was negligible over the full range of ratios we tested, CAPS accelerated rates and increased the extent of fusion optimally at a SNAP-25 to syntaxin-1 ratio of approximately 1:1 (Fig. S5). The results indicated that CAPS operated on liposomes that contain functional 1:1 SNAP-25/syntaxin-1 heterodimers rather than on potentially dysfunctional 1:2 complexes.
CAPS Drives trans-SNARE Complex Formation in Liposomes.
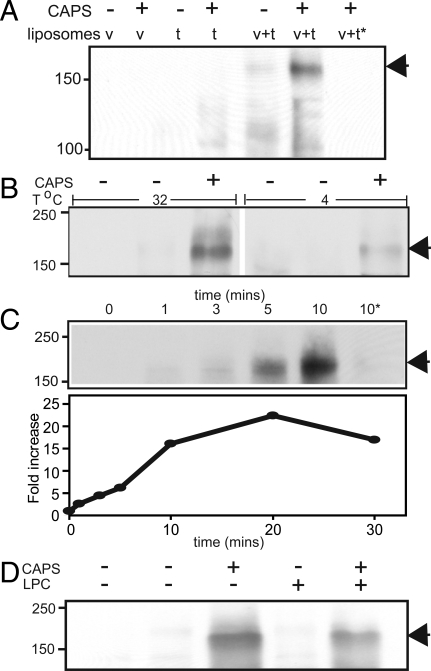
Membrane fusion requires the formation of heterotrimeric SNARE complexes in trans between donor and acceptor membranes (4, 38). Heterotrimeric SNARE complexes are unusually stable and persist in SDS gel electrophoresis (41). To determine whether CAPS promoted SNARE complex formation, we analyzed liposome fusion reactions for SDS-resistant SNARE complexes. Liposome incubations conducted without CAPS, which exhibited little fusion, contained only very low levels of high molecular weight SDS-resistant SNARE complexes (Fig. 3 A and B). By contrast, incubations with CAPS strongly stimulated the formation of high molecular weight SNARE complexes, the most prominent of which was approximately 165 kDa (Fig. 3 A and B, arrowhead). Detergent-soluble full-length SNAREs readily form complexes of approximately 165 kDa upon mixing (Fig. S6A). Formation of the ≈165 kDa complex is impaired when SNAREs are inserted into liposomes but readily form when CAPS is incubated with v- and t-SNARE liposomes (Fig. 3A). The SDS-resistant SNARE complex of approximatey 165 kDa contained the 3 SNAREs as detected by antibody probing for each (Fig. S6C). The ≈165 kDa complex likely consisted of dimers of heterotrimers because equimolar amounts of syntaxin-1 and VAMP2 were detected by quantitative Western blotting when the ≈165 kDa complex was re-run on SDS/PAGE after boiling (Fig. S7). As expected for heterotrimeric SNARE complexes, boiling in SDS sample buffer eliminated the ≈165 kDa complex (Fig. 3A), with half of it eliminated at 70 °C (not shown).
Fig. 3.
CAPS stimulates SNARE complex formation. (A) CAPS stimulates formation of a ≈165 kDa SNARE complex. Incubations were assembled with v- or t-SNARE(5% PIP2) liposomes or with both in the absence or presence of 1 μM CAPS and incubated for 30 min at 32°. One incubation (asterisk) was boiled before SDS/PAGE. Complexes of ≈165 kDa (arrowhead) were detected with syntaxin antibody. (B) Incubations were conducted with VAMP2- and t-SNARE(5% PIP2) liposomes for 0 time (lanes 1 and 4) or for 30 min at 32 °C (lanes 2 and 3) or 4 °C (lanes 5 and 6). One micrometer of CAPS was present as indicated (lanes 3 and 6). Complexes of ≈165 kDa (arrowhead) were detected with syntaxin antibody. (C) Incubations with CAPS as in B were conducted for indicated times at 32 °C were analyzed for SDS-resistant SNARE complexes with syntaxin antibody. Intensities of the ≈165 kDa band (arrowhead) were quantified and expressed as fold increase. A representative blot is shown for the 0- to 10-min incubations. One 10-min sample (10*) was boiled before SDS/PAGE. (D) Liposome incubations as in B were conducted for 0 time (lane 1) or 30 min (lanes 2–5) with 1 μM CAPS and 0.1 mM LPC as indicated. SNARE complexes of ≈165 kDa (arrowhead) were detected with syntaxin antibody.
CAPS promoted a rapid accumulation of the approximate 165 kDa complex under typical liposome fusion assay conditions (Fig. 3C) with a time course that was similar to that of fusion itself (see Fig. 2A). From these results, it was unclear whether CAPS promoted trans-SNARE complex formation or whether cis SNARE complexes accumulated as the result of CAPS-stimulated fusion. To distinguish these 2 alternatives, we incubated liposomes with CAPS under conditions in which fusion did not occur. Inclusion of lysoPC in liposome incubations, which abolished CAPS-dependent fusion (Fig. 2A), reduced but did not abolish levels of the ≈165 kDa complex (Fig. 3D). Similarly, incubations at 0 °C to 4 °C, in which CAPS-dependent fusion did not occur, reduced but did not abolish formation of the ≈165 kDa complex (Fig. 3B). The formation of ≈165 kDa SNARE complexes, driven by CAPS under conditions in which fusion did not occur, implied that these were trans rather than cis SNARE complexes. Consistent with this, in liposome incubations at 0 °C to 4 °C, CAPS strongly stimulated the amount of VAMP2 that co-immuno-isolated with syntaxin-1 (Fig. S6B). The results indicated that CAPS promoted trans-SNARE complex formation under non-fusion conditions. The greater accumulation of the ≈165 kDa complex promoted by CAPS under fusion conditions (Fig. 3 B and D) likely represented the accumulation of cis SNARE complexes as fusion proceeded.
To determine the number of SNARE proteins driven into trans-SNARE complexes by CAPS at 0 °C to 4 °C, we analyzed incubations by 2D gel electrophoresis and quantified the immunoreactive SNAREs (Fig. S7). CAPS (1 μM) stimulated the incorporation of 1.65% of syntaxin-1 and 0.75% of VAMP2 into high molecular weight complexes. Based on the SNARE content of the proteoliposomes, this would correspond to approximately 9 to 10 trans-SNARE complexes per fusion event stimulated by CAPS assuming a single round of fusion that consumed 20% of the v-SNARE liposomes (as described earlier), which is similar to that estimated by the t-SNARE titration study (Fig. 2C).
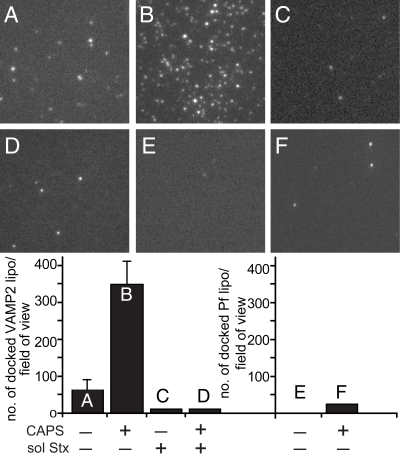
To further characterize the ability of CAPS to promote trans-SNARE complex formation, we used a liposome docking assay. v-SNARE (i.e., VAMP2-containing) liposomes labeled with Rh-PE were incubated with supported planar bilayers that contained SNAP-25, syntaxin-1, and PI 4,5-P2. We monitored v-SNARE liposome encounters with the t-SNARE bilayer by total internal reflection fluorescence microscopy (TIRF) and determined their dwell time. In the absence of CAPS, few v-SNARE liposomes persisted on the supported bilayer (Fig. 4). By contrast, addition of CAPS markedly enhanced v-SNARE liposome docking, which depended on SNARE complex formation based on the ability of soluble syntaxin to eliminate it (Fig. 4). We found that v-SNARE liposome docking was similarly enhanced regardless of whether CAPS was directly added to incubations or first added to bilayers followed by washing. In the absence of CAPS, most v-SNARE liposome encounters with the t-SNARE bilayer were brief, lasting no more than 0.15 sec. In the presence of CAPS, v-SNARE encounters exhibited long dwell times, with many lasting 37.5 s or longer, which were completely inhibited by soluble syntaxin (Fig. S6D). The results indicated that CAPS could drive SNARE protein associations in trans by acting on t-SNARE-containing membranes.
Fig. 4.
CAPS promotes stable docking of VAMP2 liposomes to t-SNARE supported bilayers. Bilayers containing t-SNAREs and 5% PIP2 were incubated with Rh-PE labeled VAMP2 liposomes (A) or additionally with 1 μM CAPS (B), 5 μM soluble syntaxin (C), or CAPS with soluble syntaxin (D). Protein-free Rh-PE liposomes were incubated with bilayers (E) or co-incubated with 1 μM CAPS (F). Bilayers were washed and imaged by TIRF microscopy, and the number of docked liposomes per field (6,300 μm2) was quantified for 7 different regions for each condition (means with range of duplicates shown for 2 experiments).
CAPS Binds Membrane-Associated Syntaxin.
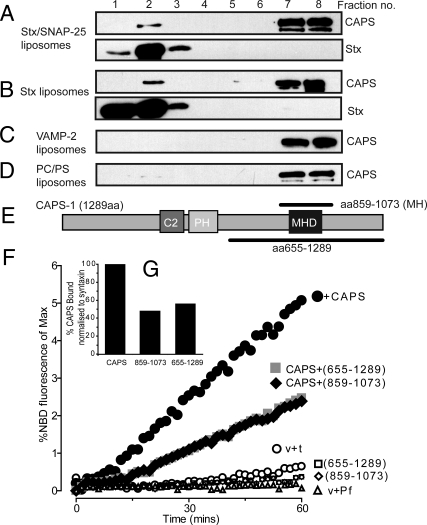
The preceding results implied that CAPS may interact directly with t-SNARE proteins. To determine whether a stable CAPS interaction with a component on liposomes could be detected, we subjected liposome fusion reactions to buoyant density gradient separation. We found that soluble CAPS added to liposome fusion reactions was retained on floated liposomes. Subsequent studies indicated that CAPS was retained on liposomes that contained syntaxin-1/SNAP-25 but not on liposomes that contained VAMP2 (Fig. 5 A and C). Liposomes containing only syntaxin-1 were also found to retain CAPS (Fig. 5B). By contrast, protein-free liposomes (Fig. 5D) failed to retain CAPS. The results indicated that CAPS is a syntaxin-1-binding protein.
Fig. 5.
CAPS interacts with syntaxin-1. CAPS (1 μM) was incubated with phosphatidylcholine/phosphatidylserine liposomes containing t-SNAREs (A), syntaxin-1 (B), or VAMP2 (C), or with protein-free liposomes (D) and bound CAPS (fractions 1–3) was separated from free CAPS (fractions 7 and 8) by Accudenz gradient centrifugation. Fractions were analyzed by immunoblotting for CAPS or syntaxin (Stx). Results representative of 3 experiments. (E) Schematic representation of CAPS domains and fusion proteins containing MH domain. (F) MH domain-containing CAPS proteins inhibited liposome fusion. v-SNARE and t-SNARE/5% PIP2 liposomes were incubated without additions (open circles) or with 1 μM CAPS (filled circles), 10 μM CAPS(655–1289) (open squares), 10 μM CAPS(859–1073) (open diamonds), CAPS plus CAPS(655–1289) (filled squares), and CAPS plus CAPS(859–1073) (filled diamonds). Representative results of 3 experiments are shown as percentages of maximal NBD fluorescence corrected for protein-free liposomes. (G) CAPS binding to t-SNARE liposomes. CAPS bound to t-SNARE liposomes was adjusted for protein-free binding and normalized to syntaxin intensity. CAPS(859–1073) and CAPS(655–1289) inhibited CAPS binding to t-SNARE liposomes by 52% and 46%, respectively.
To determine whether CAPS binding to t-SNAREs was responsible for the fusion-promoting activity of CAPS in SNARE-dependent liposome fusion, we tested 2 CAPS fusion proteins that contain the CAPS Munc13 homology (MH) domain (Fig. 5E). The CAPS MH domain encompasses DUF1041 and MHD1 homology domains (22) that correspond to a portion of a Munc13–1 domain that binds SNARE complexes (20). A fusion protein that contained the core of the MH domain (residues 859–1,073) along with a larger overlapping C-terminal peptide (residues 655–1,289) were generated. Neither of the 2 fusion proteins stimulated liposome fusion (Fig. 5F), which is consistent with a requirement for additional domains (e.g., PH domain) for CAPS stimulation of SNARE-dependent fusion (29). However, both fusion proteins were similarly effective in inhibiting CAPS-stimulated fusion (Fig. 5F). Each of the fusion proteins bound to t-SNARE liposomes (Fig. S8) and inhibited CAPS binding to t-SNARE liposomes (Fig. 5G). Thus, CAPS protein truncations that contained the MH domain exerted dominant interfering effects on CAPS-stimulated fusion. This indicated that CAPS promoted SNARE-dependent liposome fusion through direct interactions with t-SNAREs.
Discussion
CAPS, a priming factor for regulated vesicle exocytosis, exhibited robust activity in accelerating SNARE-dependent liposome fusion. Future studies will need to determine the relationship of the in vitro fusion-promoting properties of CAPS discovered here to the priming activity of CAPS in neural and endocrine cells. The current work provides several major conclusions about the in vitro properties of CAPS that are likely relevant to its role in priming vesicle exocytosis in cells. First, the fusion-promoting activity of CAPS was evident in a minimal liposome fusion assay, which implied that CAPS interacts with SNARE proteins and/or phospholipids. Previous work characterized CAPS interactions with PI 4,5-P2 and phosphatidylserine (23) whereas the current work revealed direct interactions of CAPS with membrane syntaxin-1. Second, the fusion-promoting activity of CAPS was rapid, SNARE-dependent, and accompanied by trans-SNARE complex formation. The rapid effects of CAPS with no discernible latency are consistent with a direct action of CAPS on SNARE proteins to enhance trans-SNARE complex formation. Third, CAPS promoted full fusion at physiological SNARE densities that, in the absence of CAPS, had little ability to mediate fusion. This implied that CAPS accelerated a rate-limiting step in SNARE complex assembly, which is likely important for its priming activity in vesicle exocytosis. Future studies are needed to identify the precise step in SNARE complex assembly that CAPS affects through syntaxin-1 binding. Last, CAPS, which functions in a Ca2+-regulated vesicle fusion process, accelerated SNARE-dependent liposome fusion in the absence of Ca2+ regulation. Here a priming factor of the CAPS/Munc13 family was shown to possess fusion-promoting activity, which indicates that priming factors can accelerate upstream steps in vitro that lead to fusion in the absence of downstream regulators that operate in vivo (e.g., synaptotagmin). Our results provide a basis for elucidating the mechanisms by which priming factors catalyze SNARE complex assembly for vesicle exocytosis.
At all stations of membrane trafficking, a common set of factors comprised of members of the Rab, Sec-1/Munc-18, and SNARE protein families operate to enable specific donor-acceptor membrane pairing and fusion (42). In addition, a diverse set of tethering factors characteristic of each transport step operate (directly or indirectly) as Rab effectors to activate SNAREs for fusion (43). Tethering factors such as p115 (44) and the HOPS complex (45) were shown to promote trans-SNARE complex formation. For regulated vesicle exocytosis, Rab effectors such as rabphilin, RIM, Slp4a/granuphilin, and Munc18–1 interact with the t-SNAREs SNAP-25 and syntaxin-1 (9, 46, 47). These factors signal vesicle arrival at the plasma membrane and mediate vesicle tethering. However, not all docked/tethered vesicles are competent to undergo triggered fusion and other factors are needed to “prime” vesicles for triggered fusion. Munc18–1 promotes trans-SNARE complex formation in preincubations in vitro (6), but in vivo, additional factors are needed. Munc13–1, a well characterized priming factor, was proposed to convert syntaxin-1 from a “closed” state incapable of engaging SNARE partners into an “open” state that interacts to assemble SNARE complexes (48). However, the pathway for SNARE protein complex assembly during priming catalyzed by Munc13–1 is poorly understood and remains to be directly studied.
CAPS proteins, which exhibit sequence similarity to Munc13–1 in a C-terminal MH domain (22), also function in vesicle priming. The phenotype of a strongly reduced pool of primed vesicles in neurons from CAPS-1/CAPS-2-knockout mice (26) was similar to that characterized in neurons from Munc13-knockout mice (15), which indicates closely related but non-redundant functions for CAPS and Munc13 proteins. To characterize the potential priming mechanism of CAPS, we used a SNARE-dependent liposome fusion assay (4). CAPS activity was reconstituted in this assay when physiological levels of PI 4,5-P2 were included in t-SNARE liposomes (29). CAPS rapidly and efficiently stimulated SNARE-dependent liposome fusion without a requirement for preincubation, which distinguishes the activity of CAPS from that observed for Munc18–1 (6).
The rate-limiting step for fusion in the liposome assay at high SNARE densities is the collision-limited formation of fusogenic trans-SNARE complexes between VAMP2 and SNAP-25/syntaxin-1 (38). How might CAPS accelerate trans-SNARE complex formation and SNARE-dependent liposome fusion? We considered the possibility that CAPS activity in promoting SNARE complex formation and membrane fusion could be a result of liposome aggregation mediated by the PI 4,5-P2- and phosphatidylserine-binding properties of CAPS (27). However, light scattering studies failed to detect any liposome aggregation promoted by CAPS (Fig. S9). The current evidence suggests that CAPS is directed to t-SNARE liposomes through low-affinity interactions with PI 4,5-P2 (29). On the t-SNARE liposomes, CAPS engages syntaxin-1 in high affinity interactions. An important clue on how CAPS promotes trans-SNARE complex formation through t-SNARE interactions was provided by studies in which t-SNARE densities were systematically varied. At physiological t-SNARE densities (1,273–5,095 molecules/μm2), there was little if any spontaneous fusion because collisional encounters with v-SNARE liposomes under our assay conditions would have a low probability of leading to trans-SNARE complex formation. It is significant that our direct estimate of plasma membrane syntaxin-1 densities in the PC12 cell plasma membrane is in this range (2,280 molecules/μm2). CAPS accelerated SNARE-dependent fusion at t-SNARE densities even as low as 10 copies/100 nm liposome (318 molecules/μm2). This suggests that CAPS might function to “cluster” t-SNARE complexes to promote high-density regions on the membrane, which would increase the probabilities of trans-SNARE complex formation in collisional encounters with v-SNARE liposomes. The number of SNARE complexes needed for a fusion event has previously been indirectly estimated to be 3 to 8 (39, 40). Our analysis of SNARE complexes promoted by CAPS estimated that complexes with 9 or 10 copies of syntaxin-1 and VAMP2 form per fusion event, which is close to our minimal estimates of the number of t-SNAREs per liposome required for CAPS-dependent fusion. Additional studies will be needed to fully elucidate the mechanism by which CAPS accelerates SNARE-dependent fusion. In conclusion, our studies suggest that CAPS functions directly on t-SNAREs to increase the likelihood of forming fusion-competent trans-SNARE complexes. This provides an important step toward obtaining a molecular description of the function of priming factors in regulated vesicle exocytosis.
Materials and Methods
v-SNARE Liposome Docking on Planar-Supported t-SNARE Bilayers.
Planar supported bilayers were formed as described (29). v-SNARE or protein-free liposomes (85:12 mol% DOPC:DOPS) labeled with 3 mol% Rh-PE diluted to 50 μM lipid in reconstitution buffer were added to 5 mol% PI 4,5-P2-, t-SNARE-containing planar supported bilayers. Bilayers were incubated with 1 μM CAPS and or 5 μM GST-syntaxin-1A (1–265) in reconstitution buffer without glycerol for 10 min before addition of v-SNARE liposomes. Bilayers were imaged on a Nikon TE2000-U inverted microscope by TIRF using an Apo TIRF ×100, NA 1.45 objective lens. Images were acquired at 75-ms intervals with a CoolSNAP-ES camera (Photometrics). Bilayers were washed 3 times with 1 mL reconstitution buffer to remove free liposomes, and images of docked v-SNARE liposomes were acquired. Image analysis used Metamorph software (Universal Imaging).
t-SNARE Liposome Binding and SNARE Complex Formation.
Protein-free liposomes or liposomes containing VAMP2, syntaxin-1A, or syntaxin-1A/SNAP-25 were mixed with 1 μM CAPS and incubated at room temperature for 30 min. For competition studies, 1 μM of either CAPS(859–1073) or CAPS(655–1289) proteins were incubated with 300 nM CAPS and syntaxin-1A/SNAP-25 liposomes. Binding reactions were subject to buoyant density flotation on Accudenz gradients by centrifugation at 192,000 × g at 4 °C for 4 h. Fractions were collected, analyzed by SDS/PAGE, and immunoblotted with CAPS antibody and syntaxin-1A (HPC-1; Sigma-Aldrich) antibody. For SNARE complex formation studies, incubations with VAMP2 liposomes, t-SNARE liposomes, or both were conducted in reconstitution buffer without glycerol at 0 °C to 4 °C or 37 °C for the indicated times in the presence or absence of CAPS or 0.1 mM LPC. To resolve SDS-resistant complexes, reactions were separated by SDS/PAGE as described (41). SDS-resistant complexes were identified by immunoblot detection using monoclonal syntaxin1A/HPC-1, polyclonal SNAP-25 (LifeSpan Biosciences), or VAMP2 polyclonal antibodies (Synaptic Systems).
Additional methods are described in the SI Text.
Supplementary Material
Acknowledgments.
This work was funded by National Institutes of Health grant DK40428 (to T.F.J.M.) and by an American Heart Association fellowship award (to D.J.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900755106/DCSupplemental.
References
- 1.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poirier MA, et al. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- 3.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 4.Weber T, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 5.Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435–438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- 6.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Martin TF. Tuning exocytosis for speed: fast and slow modes. Biochim Biophys Acta. 2003;1641:157–165. doi: 10.1016/s0167-4889(03)00093-4. [DOI] [PubMed] [Google Scholar]
- 8.de Wit H, Cornelisse LN, Toonen RF, Verhage M. Docking of secretory vesicles is syntaxin dependent. PLoS ONE. 2006;1:e126. doi: 10.1371/journal.pone.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuboi T, Fukuda M. The C2B domain of rabphilin directly interacts with SNAP-25 and regulates the docking step of dense core vesicle exocytosis in PC12 cells. J Biol Chem. 2005;280:39253–39259. doi: 10.1074/jbc.M507173200. [DOI] [PubMed] [Google Scholar]
- 10.Wojcik SM, Brose N. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron. 2007;55:11–24. doi: 10.1016/j.neuron.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Hanson PI, Heuser JE, Jahn R. Neurotransmitter release - four years of SNARE complexes. Curr Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen YA, Scales SJ, Patel SM, Doung YC, Scheller RH. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 13.Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen JB, et al. Sequential N- to C-terminal SNARE complex assembly drives priming and fusion of secretory vesicles. EMBO J. 2006;25:955–966. doi: 10.1038/sj.emboj.7601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 16.Madison JM, Nurrish S, Kaplan JM. UNC-13 interaction with syntaxin is required for synaptic transmission. Curr Biol. 2005;15:2236–2242. doi: 10.1016/j.cub.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 17.Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue Munc13–1 with the N terminus of syntaxin. J Biol Chem. 1997;272:2520–2526. doi: 10.1074/jbc.272.4.2520. [DOI] [PubMed] [Google Scholar]
- 18.Stevens DR, et al. Identification of the minimal protein domain required for priming activity of Munc13–1. Curr Biol. 2005;15:2243–2248. doi: 10.1016/j.cub.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 19.Basu J, et al. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol. 2005;12:1017–1018. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- 20.Guan R, Dai H, Rizo J. Binding of the Munc13–1 MUN domain to membrane-anchored SNARE complexes. Biochemistry. 2008;47:1474–1481. doi: 10.1021/bi702345m. [DOI] [PubMed] [Google Scholar]
- 21.Weninger K, Bowen ME, Choi UB, Chu S, Brunger AT. Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin/SNAP-25 complex. Structure. 2008;16:308–320. doi: 10.1016/j.str.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J. 2000;349:247–253. doi: 10.1042/0264-6021:3490247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grishanin RN, et al. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Walent JH, Porter BW, Martin TF. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, et al. CAPS facilitates filling of the rapidly releasable pool of large dense-core vesicles. J Neurosci. 2008;28:5594–5601. doi: 10.1523/JNEUROSCI.5672-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jockusch WJ, et al. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Grishanin RN, et al. Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. J Biol Chem. 2002;277:22025–22034. doi: 10.1074/jbc.M201614200. [DOI] [PubMed] [Google Scholar]
- 28.Hay JC, et al. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- 29.James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 31.Sieber JJ, et al. Anatomy and dynamics of a supramolecular membrane protein cluster. Science. 2007;317:1072–1076. doi: 10.1126/science.1141727. [DOI] [PubMed] [Google Scholar]
- 32.Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J Biol Chem. 1997;272:19637–19640. doi: 10.1074/jbc.272.32.19637. [DOI] [PubMed] [Google Scholar]
- 33.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 34.Lu X, Zhang F, McNew JA, Shin YK. Membrane fusion induced by neuronal SNAREs transits through hemifusion. J Biol Chem. 2005;280:30538–30541. doi: 10.1074/jbc.M506862200. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre JC, Sleight RG. Fluorescence assay for phospholipid membrane asymmetry. Biochemistry. 1991;30:11819–11827. doi: 10.1021/bi00115a012. [DOI] [PubMed] [Google Scholar]
- 36.Wilschut J, Scholma J, Bental M, Hoekstra D, Nir S. Ca2+-induced fusion of phosphatidylserine vesicles: mass action kinetic analysis of membrane lipid mixing and aqueous contents mixing. Biochim Biophys Acta. 1985;821:45–55. doi: 10.1016/0005-2736(85)90151-8. [DOI] [PubMed] [Google Scholar]
- 37.Duzgunes N, Wilschut J. Fusion assays monitoring intermixing of aqueous contents. Methods Enzymol. 1993;220:3–14. doi: 10.1016/0076-6879(93)20069-f. [DOI] [PubMed] [Google Scholar]
- 38.Schuette CG, et al. Determinants of liposome fusion mediated by synaptic SNARE proteins. Proc Natl Acad Sci USA. 2004;101:2858–2863. doi: 10.1073/pnas.0400044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han X, Wang CT, Bai J, Chapman ER, Jackson MB. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science. 2004;304:289–292. doi: 10.1126/science.1095801. [DOI] [PubMed] [Google Scholar]
- 40.Hua Y, Scheller RH. Three SNARE complexes cooperate to mediate membrane fusion. Proc Natl Acad Sci USA. 2001;98:8065–8070. doi: 10.1073/pnas.131214798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi T, et al. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lang T, Jahn R. Core proteins of the secretory machinery. Handb Exp Pharmacol. 2008;184:107–127. doi: 10.1007/978-3-540-74805-2_5. [DOI] [PubMed] [Google Scholar]
- 43.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shorter J, Beard MB, Seemann J, Dirac-Svejstrup AB, Warren G. Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J Cell Biol. 2002;157:45–62. doi: 10.1083/jcb.200112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins KM, Wickner WT. Trans-SNARE complex assembly and yeast vacuole membrane fusion. Proc Natl Acad Sci USA. 2007;104:8755–8760. doi: 10.1073/pnas.0702290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuboi T, Fukuda M. The Slp4-a linker domain controls exocytosis through interaction with Munc18–1. syntaxin-1a complex. Mol Biol Cell. 2006;17:2101–2112. doi: 10.1091/mbc.E05-11-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham ME, et al. A gain-of-function mutant of Munc18–1 stimulates secretory granule recruitment and exocytosis and reveals a direct interaction of Munc18–1 with Rab3. Biochem J. 2008;409:407–416. doi: 10.1042/BJ20071094. [DOI] [PubMed] [Google Scholar]
- 48.Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10:303–311. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.