Abstract
T cell receptor (TCR) variable region exons are assembled from germline V, (D), and J gene segments, each of which is flanked by recombination signal (RS) sequences that are composed of a conserved heptamer, a spacer of 12 or 23 bp, and a characteristic nonamer. V(D)J recombination only occurs between V, D, and J segments flanked by RS sequences that contain, respectively, 12(12-RS)- and 23(23-RS)-bp spacers (12/23 rule). Additional mechanisms can restrict joining of 12/23 RS matched segments beyond the 12/23 rule (B12/23). The TCRδ locus is contained within the TCRα locus; TCRα variable region exons are encoded by TRAV and TRAJ segments and those of TCRδ by TRDV, TRDD, and TRDJ segments. On the basis of the 12/23 rule, both TRAV and TRDV gene segments are compatible to rearrange with TRDD gene segments; however, TRAV-to-TRDD joins are not observed in vivo. Absence of TRAV-to-TRDD rearrangement might be explained either by B12/23 restriction or by differential accessibility of the TRDV versus TRAV gene segments for rearrangement to TRDD. We used in vitro substrate analysis to reveal that both TRAV and TRDV 23-RSs mediate rearrangements to the 5′TRDD1 12-RS, demonstrating that B12/23 restriction does not explain these rearrangement biases. However, targeted replacement of TRDD1 and its 12-RSs with TRAJ38 and its 12-RS showed that TRDV gene segments rearrange with the ectopic TRAJ38, whereas TRAV segments do not. Our results demonstrate that sorting of TRAV and TRDV gene segments is determined by differential locus accessibility during T cell development.
Keywords: epigenetic regulation, T cell development, VDJ recombination
Assembly of antigen receptor variable gene segments through V(D)J recombination generates the remarkable diversity and specificity of the immune system. To initiate this reaction, the RAG-1 and RAG-2 proteins (RAGs) introduce a DNA double-strand break between a pair of V, D, or J coding gene segments and their flanking recombination signal sequences (RSs) (1, 2). RSs are composed of a conserved heptamer and nonamer, separated by a relatively nonconserved 12- or 23-bp spacer. V(D)J recombination only occurs between a pair of gene segments flanked by, respectively, a 12-RS and a 23-RS, a restriction known as the 12/23 rule (3). Successful V(D)J recombination leads to production of a functional B cell receptor in B cells, and either an α/β or γ/δ receptor in T cells. As expression of appropriate antigen receptor chains is closely associated with the general program of lymphocyte development, control of V(D)J recombination is important to ensure that only appropriate rearrangement events occur in a developing B or T cell (4–6). Regulation of V(D)J recombination, therefore, takes place on several levels and involves factors both intrinsic and extrinsic to the respective antigen receptor loci (7, 8).
The T cell receptor (TCR)α/δ locus has a unique genomic organization and pattern of V(D)J recombination. V gene segments for both loci are found in 1 large cluster, with exclusive TRAV gene segments generally found toward the distal end of the cluster, TRADV gene segments (that rearrange efficiently with both TRDD and TRAJ gene segments) located in the middle of the cluster, and largely TRDV gene segments lying toward the proximal end of the cluster (9). Two TRDD gene segments are linked closely to 2 TRDJ segments, and TCRδ locus rearrangements typically employ both TRDD segments to form V(DD)J rearrangements on both alleles. Therefore, in developing thymocytes, the TCRδ locus undergoes 3 distinct joining events: TRDV-5′TRDD1, 3′TRDD1–5′TRDD2, and 3′TRDD2-TRDJ rearrangements (10). Approximately 60 TRAJ gene segments are arrayed downstream of the TRDD/TRDJ segments and rearrange directly with the TRAV gene segments to generate TRAV-TRAJ joins (Fig. 1A) (11). As a result of the genomic architecture of the α/δ locus, TRAV-TRAJ rearrangements excise the TCRδ gene segments; therefore, the developmental timing of TCRα/δ rearrangements must be tightly linked to the α/β versus γ/δ T cell fate decision (12). The repertoire of particular TRAV and TRDV gene segments remains restricted in the absence of selection, strongly suggesting that these restrictions occur at the level of V(D)J recombination (13). However, it remains unclear whether exclusive TRAV and TRDV gene segments are identified as such only on the basis of differential accessibility of RS DNA to the RAGs, or whether there are unique structural features, such as intrinsic RS preferences, which distinguish them with respect to rearrangement potential.
Fig. 1.
TRDV 23-RS rearrangements with TRDD 12-RSs are B12/23 restricted. (A) Schematic representation of the TCRαδ locus with its various gene segments and flanking RSs. Edelta and Ealpha represent the δ and α enhancers, whereas Cδ and Cα refer to the constant region of the TCRδ and TCRα chains. (B) Diagram representing the primers and probe used for the in vitro substrate analysis. (C) TRDV 23-RS rearrangements to the distal- and proximal-positioned TRDD1 12-RS. (D) Preferential rearrangements of various TRDV 23-RSs to the 5′TRDD1 12-RS over the TRDJ1 12-RS, by using 3-fold serial dilution of the input substrate DNA.
Differential accessibility of RSs to the RAGs as a mechanism of controlling V(D)J recombination was first described for the Ig heavy chain (IgH) locus (14). Much subsequent work has gone toward studying the relationship between transcriptional control elements and V(D)J recombination at the antigen receptor loci. With respect to the TCRα/δ locus, the TCRα enhancer (Eα) (15) and the TCRδ enhancer (Eδ) (16) are required for efficient rearrangement of the TCRα and TCRδ loci, respectively. Further, various markers of accessible chromatin such as histone modification have also been associated with TCRα/δ gene segments undergoing V(D)J recombination in different stages of T cell development, suggesting differential accessibility as potential mechanism for determining TRAV versus TRDV sorting (17, 18). Differential accessibility would define TRAV gene segments as inaccessible to the RAGs during earlier TRDV-TRDD rearrangement but accessible later to rearrange with the Jα gene segments (17, 18).
RS sequences also can play a major role in determining which gene segments recombine with one another beyond 12/23 restriction. An RS-based restriction beyond the 12/23 rule (B12/23) governs rearrangements at the endogenous TCRβ locus to ensure Dβ gene segment utilization (19). With respect to the murine TCRα/δ locus, TRAV/TRDV gene segments are flanked by 23-RSs and TRAJ/TRDJ gene segments are flanked by 12-RSs, whereas the 2 TRDD gene segments are flanked 5′ by a 12-RS and 3′ by 23-RS (Fig. 1A). Thus, B12/23 conceivably might contribute to the tight developmental regulation of TCRα/δ locus rearrangements. In this regard, TRDD gene segments have not been described to rearrange with downstream TRAJ gene segments, and the TRAV and TRDV repertoires are highly restricted and largely mutually exclusive in peripheral T cells (9, 20).
To evaluate the potential contribution of B12/23 restriction to TRAV/TRDV sorting during V(D)J recombination at the TCRα/δ locus, we have assessed the native preference of TCRα/δ RS pairs in V(D)J recombination substrates. We have also replaced the TRDD1 gene segment with an ectopic 12/23-compatible TRAJ gene segment in the native TCRα/δ locus, which allows experiments that definitively determine whether TRAV and TRDV gene segments rearrange with TCRδ gene segments on the basis of differential accessibility.
Results
B12/23 Restriction Mediates the Preference of TRDV 23-RSs for the 5′TRDD1 12-RS Over the TRDJ1 12-RS.
We have previously shown that the B12/23 restriction of Vβ 12-RSs drives significant preference for a 5′Dβ 12-RS over Jβ 12-RSs in nonlymphoid cells by using extrachromosomal recombination substrates (21). To directly assess whether a similar mechanism might account for the observed preference of TRDV gene segments to rearrange with a 5′TRDD 12-RS over a TRDJ 12-RS, we inserted the various RSs into our competitive rearrangement substrates. The substrates were cotransfected with RAG expression plasmids into CHO cells and PCR was used to analyze the recombination products as shown in Fig. 1B. We found that the TRDV4 23-RS strongly prefers a 5′TRDD1 12-RS to a TRDJ1 12-RS in either the proximal or distal position (Fig. 1C). As a control for position within the substrate, we placed 2 identical 5′TRDD1 12-RS at both the proximal and distal position and found there is a slight preference for the distal position (Fig. 1C). To mitigate this potential bias in further experiments, we either placed the 5′TRDD1 12-RS in the proximal position or tested the various 12-RSs in both positions.
To determine whether the observed B12/23 restriction of TRDV gene segments could be recapitulated for a range of TRDV 23-RSs, we assembled a further series of extrachromosomal competitive rearrangement substrates. We chose several TRDV 23-RSs for analysis, on the basis of their native position within the TCRα/δ locus. TRDV4 is located most proximal to the TRDD gene segments at the end of the Vα/δ gene cluster, TRDV5 lies downstream of the Cδ exon and rearranges by inversion, and TRADV15–1 is found within a small island of TRDV gene segments roughly in the middle of the TRDV/AV gene cluster (Fig. 1A). These TRDV gene segments are also among the most frequently used TRDV genes (22, 23). None of these TRDV gene segments rearrange exclusively with TRDD gene segments, and we observed them in participation with TRAJ gene segment rearrangements as well, consistent with previous reports (9, 22, 24). When competing for either a 5′TRDD1 or a TRDJ1 12-RS, all 3 of the tested TRDV 23-RSs strongly preferred the 5′TRDD1 12-RS (Fig. 1D). Therefore, as in the TCRβ locus, we conclude that RS differences beyond the 12/23 rule limit TRDV 23-RSs to preferentially rearrange with the 5′TRDD1 12-RS over the TRDJ1 12-RS.
TRDD1 Is a Preferential Substrate for both TRDV and TRAV in Substrate Assays.
Next we asked whether B12/23 restriction at the level of RS sequence might account for the observed in vivo preference of TRAV gene segments to rearrange with TRAJ gene segments and of TRDV gene segments to rearrange with TRDD gene segments. To test this possibility, we used the competition substrate analysis to see whether various TRAV 23-RSs prefer various TRAJ 12-RSs to the 5′TRDD1 12-RS. TRAV1 and TRAV2 are found at the extreme distal end of the TRAV/DV cluster, whereas TRAV3–4 is located at the opposite proximal end of the cluster, just distal to several exclusive TRDV gene segments. The TRAJ56, -38, and -27 gene segments we chose were selected from within the Jα gene segment cluster (Fig. 1A). All of the TRAV 23-RSs tested showed significant rearrangement to both the 5′TRDD1 12-RS and to the TRAJ27 12-RS, with slight preference for the 5′TRDD1 12-RS in both proximal and distal positions (Fig. 2A). In Fig. 2 B and C, it is clear that all of the TRAV 23-RS tested showed similar significant levels of rearrangements to the 5′TRDD1 12-RS when competing with either the TRAJ38 12-RS or the TRAJ56 12-RS. Therefore, we conclude that RS-specific differences cannot account for the observed in vivo restriction of TRAV 23-RS gene segments to rearrange with TRAJ 12-RS gene segments and not with the 12/23-compatible TRDD1 12-RS gene segment.
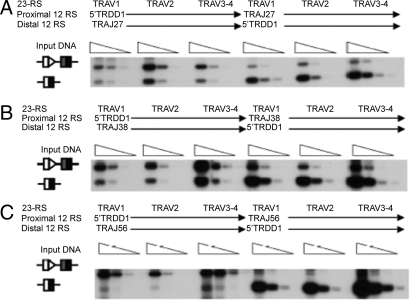
Fig. 2.
TRAV 23-RSs prefer the TRDD1 12-RS over a range of TRAJ 12-RSs. Representative blot showing various TRAV 23-RS rearrangements with the TRDD1 12-RS and the (A) TRAJ27, (B) TRAJ38, and (C) TRAJ56 12-RSs in competitive substrates.
Replacement of the TRDD1 Gene Segment with a TRAJ Gene Segment.
To directly determine whether differential accessibility regulates rearrangements of TRAV and TRDV to the TRDD1 gene segment, we precisely replaced the TRDD1 gene segment and its 12-RSs with TRAJ38 and its 12-RS (Fig. 3A). ES cells were screened by using the 5′ probe, which allows distinction between the 10-kb wild-type band and the 3-kb targeted band by using an EcoRV digest (Fig. 3B). The positive ES clones were infected with Cre-expressing adenovirus to delete the neomycin cassette, leaving a single loxP site (Fig. 3C). We used TRDD1-TRAJ38ect ES cells, heterozygous for the ectopic TRAJ38 allele, via RAG-2-deficient blastocyst complementation (25) to generate chimeric TRDD1-TRAJ38ect mice in which all lymphocytes are derived from the TRDD1-TRAJ38ect ES cells. Thymocyte numbers and the distribution of DN, DP, and SP populations as determined by flow cytometry (FACS) analysis of multiple chimeric TRDD1-TRAJ38ect mice were comparable to control mice. The development of T cells therefore appeared to be grossly normal in chimeric mice harboring lymphocytes heterozygous for the TRDD1-TRAJ38ect allele.
Fig. 3.
Generation of the TRAJ38ect replacement allele. (A) Schematic diagram showing the targeting strategy in which TRAJ38 and its RS were targeted to replace TRDD1 and its RS. This targeting results in 1 wild-type allele and 1 TRDD1- TRAJ38ect allele. (B) Southern blot showing a positive targeted ES clone, in which the 10-kb wild-type germline band drops to a 3-kb band when the 5′ probe is used to evaluate EcoRV-digested genomic DNA. (C) Southern blot confirming neomycin deletion by using StuI digest and the 3′ probe. Neomycin positive clones show 2 bands at 6 kb for the wild-type allele and 3.8 kb from the targeted allele, whereas neomycin-deleted ES clones show one 6-kb band.
Differential Rearrangement of TRDV Versus TRAV Gene Segments with the Ectopic TRAJ38 Gene Segment.
By using total thymic genomic DNA from the TRDD1-TRAJ38ect knock-in mice, we assessed possible rearrangements of different TRAV and TRDV gene segments with the endogenous TRDD1 and with the targeted TRAJ38ect by PCR. To increase the specificity of the assay, we used Southern blotting to analyze the resulting PCR products, employing an oligonucleotide probe against the region downstream of the TRDD1 gene segment shared by the wild-type and the targeted alleles (Fig. 4A). We chose forward primers for the same TRAV1, TRAV2, and TRAV3–4 gene segments tested in our in vitro assays, each paired with a reverse primer ≈400 bp downstream of the native TRDD1 gene segment and outside the targeted region (Fig. 4A). Initially, we carried out several control experiments to validate the integrity of our primers. First, we tested these primers and probes on wild-type thymic DNA to ensure the detection of different TRAV/DV rearrangements with the TRDD1 gene segment. Next, to determine the detection threshold of our assay, we generated plasmids containing the various TRAV sequences and sequence around TRDD1. We then diluted limiting amounts of these plasmids into nonlymphocyte mouse genomic DNA to reach very low copy number (≈40 copies) relative to the number of rearrangement events expected in the amount of thymic genomic DNA used in the experimental PCR reactions (≈40,000 copies). The PCR with the control plasmid showed a distinct band (Fig. 4B), allowing us to conclude that, if seen, absence of PCR products corresponding to TRAV-to-TRDD1 region rearrangements would indeed be the result of the absence of the target DNA (TRAV-to-TRAJ38ect joins) in the experimental sample, and not the result of inefficient priming during the PCR reactions.
Fig. 4.
Absence of TRAV gene segment rearrangement with the ectopic TRAJ38 gene segment in vivo. (A) Schematic representation of the PCR strategy to detect specific rearrangements of various Vα/δ rearrangements on the TRDD1/TRAJ38ect alleles. Threefold dilution of genomic DNA from total thymocytes derived from wild-type control and 2 chimeric mice harboring the TRDD1-TRAJ38ect allele were amplified by using: (B) TRAV forward primers (FP) and TRDD1 reverse primers (RP) to demonstrate absence of TRAV rearrangement to TRAJ38ect (Top panels); TRAV forward primers (FP) and TRAJ38 reverse primers (RP) to demonstrate presence of TRAV rearrangement to the endogenous TRAJ (bottom panels); and (C) TRDV forward primers (FP) and TRDD1 reverse primer (RP) to detect rearrangements of various TRDVs to both TRDD1 and TRAJ38ect. Amplified PCR products were subjected to Southern blot analysis by using 32P-labeled oligonucleotide probes. Rearrangements with the TRAJ38ect gene segment result in a larger PCR product relative to the wild-type allele because of the retained loxP site on the targeted allele. The control panel shows detection thresholds of various TRAV and TRDD1 primer pairs as diagrammed in A and described in the text.
With our experimental conditions established, we then assayed for the presence of the various TRDV and TRAV gene segment rearrangements with the ectopically placed TRAJ38 gene segment in heterozygous chimeric animals, by using the detection strategy outlined above. Strikingly, we did not detect rearrangements of the various TRAV gene segments with either the wild-type TRDD1 gene segment or the ectopically placed TRAJ38 gene segment (Fig. 4B). Conversely, however, we found that each of the tested TRDV gene segments readily rearrange with both the native TRDD1 and the TRAJ38ect gene segments (Fig. 4C). Furthermore, in accordance with our in vitro rearrangement substrate data, rearrangements to the TRDD1 gene segment on the wild-type allele appeared to be favored relative to the TRAJ38ect gene segment on the targeted allele (Fig. 4C). This finding is further underscored by the fact that a sizable fraction of TRDV-TRDD1 rearrangements on the wild-type allele would undergo further rearrangements to TRDD2 and not be detected in our analysis. We conclude from these findings that targeted ectopic placement of the TRAJ38 gene segment at the native TRDD1 gene segment position allows for rearrangement with TRDV gene segments but does not support rearrangement with TRAV gene segments (Fig. 1A).
TRDV-to-Ectopic TRAJ38 Rearrangements Occur Intrachromosomally.
Because of the complex nature of the TCRα/δ locus (Fig. 1A) thymocytes that have undergone TCRα rearrangement excise the TCRδ locus within a chromosomal circle. These extrachromosomal DNA circles are present both in thymocytes and naive T cells (26–28), but are lost with cell division as they migrate to secondary lymphoid tissues (27). It was therefore possible that the TRDV-to-TRAJ38ect rearrangements detected in total thymocytes (Fig. 4C) might have occurred within excised DNA circles and, as a result, outside the controls enforcing intrachromosomal TCRα/δ rearrangements. To address this potential confounding factor, we generated and analyzed different γδ T cell hybridoma lines from the spleen and screened for the rearrangement of different TRDV to the targeted TRAJ38ect, because the large number of cell divisions required for hybridoma generation would have diluted out the extrachromosomal circles. As shown in Fig. 5, PCR products corresponding to TRDV-TRAJ38ect rearrangements were detected on the nonproductive allele in a number of hybridoma lines. This finding strongly supports the idea that TRDV-to-TRAJ38ect rearrangements occur intrachromosomally and are thus regulated in the context of TCRα/δ locus V(D)J recombination.
Fig. 5.
Hybridoma analysis of TRDV rearrangements with TRAJ38ect. Thirty-four γδ T cell hybridoma lines, positive for TCRγδ surface expression and generated from 4 chimeric animals, were screened for TRDV4, TRDV5, and TRDV15–1 rearrangement with the TRAJ38ect by using TRDV4, TRDV5, and TRDV15–1 forward primers (FP) in conjunction with a common TRDD1 reverse primer. The PCR products were then Southern blotted and probed with a TRAJ38-specific oligo probe. Various hybridoma lines positive for the various rearrangements are shown. The control lanes are as follows: BW, hybridoma fusion partner cell line; T-wt, total thymus from wild-type animals; T-Ch, total thymus from chimeric animals; H2O, no input DNA.
Discussion
Differential accessibility has been proposed to mediate differential sorting of TRAV versus TRDV gene segments in the TCRα/δ locus (18). B12/23 RS restriction was also an attractive mechanism by which TRAV and TRDV gene segments might have been distinguished. We now have used 2 complementary approaches to definitively resolve these possible mechanisms. First, we evaluated the relative ability of the TRDV and TRAV 23-RSs to rearrange with either the 5′TRDD1 12-RS or a variety of TRAJ 12-RSs in V(D)J rearrangement substrates transfected into nonlymphoid cells. These studies showed that the various TRDV 23-RSs and TRAV 23-RSs all prefer the 5′TRDD1 12-RS to either the TRDJ1 12-RS or a range of TRAJ 12-RSs. Second, we used gene targeting to replace the TRDD1 gene segment and its associated RS within the endogenous TCRα/δ locus with a TRAJ gene segment and its 12-RS to directly test whether its position in the locus influenced its ability to rearrange with TRDV versus TRAV segments. Analysis of rearrangement events on this allele, as detected both in total thymus and in hybridomas generated from single T cells, demonstrated that TRDV gene segments rearrange with this ectopic TRAJ gene segment, whereas TRAV gene segments do not. Our studies therefore demonstrate that location of the TRAJ versus the TRDD segments within the TCRα/δ locus, rather than RS sequence, is the critical determinant of their preference to rearrange, respectively, with TRAV versus TRDV gene segments. Because B12/23 restriction does not control TRAV/DV sorting during V(D)J rearrangement at the TCRα/δ locus, this process must rather be controlled by differential developmental accessibility of TCRα versus TCRδ variable region gene segments.
Our current findings are consistent with a model in which the TCRδ locus variable region gene segments become accessible first during rearrangement at the TCRα/δ locus, and TCRα gene segments become competent for rearrangements later in development. In this regard, the TCRδ locus initiates V(D)J recombination at an earlier stage of thymocyte development (double negative II-III) than does the TCRα locus (17). Additional functional evidence that this process relies on regulated, differential accessibility comes from studies showing that excision of the TCRδ gene segments is not required for TRAV-TRAJ rearrangements to efficiently occur (28). This finding strongly suggests that accessibility is actively retargeted from the TCRδ gene segments to the TCRα gene segments, as opposed to a terminal rearrangement process that spreads outward from the TCRδ gene segments. Previous studies have further implicated differential accessibility in control of TRAV versus TRDV sorting on the basis of analyses of correlative markers of accessibility. Thus, both hyperacetylation and germline transcription in double-negative thymocytes are enriched among TRDV gene segments as opposed to TRAV gene segments (29). Further work is necessary to define the specific elements required for differential developmental assembly of TCRα versus TCRδ gene segments, perhaps in the context of TCRα/δ loci simplified via gene targeting mutation.
Our studies show that B12/23 restriction in D gene segment utilization is conserved among the TCRβ and TCRδ gene segments during V(D)J recombination (21). The IgH locus encompasses the only other known antigen receptor gene family with D gene segments. However, because the IgH variable (VH) and joining (JH) gene segments are both flanked by 23-RSs, the 12/23 rule dictates that the IgH diversity (DH) gene segments, which are flanked on both sides by 12-RSs, are included as linkers between the VH and JH gene segments in complete V(D)J rearrangements. Thus, in the IgH locus, 12/23 restriction ensures DH gene segment utilization. TRDV gene segments also are flanked by a 23-RS, but the TRDD and TRDJ gene segments are both flanked by 12-RSs, thereby theoretically allowing direct TRDV to TRDJ rearrangements. In this regard, our current work shows that B12/23-restricted preference of the TRDV gene segments for the 5′TRDD1 12-RS promotes TRDD gene segment utilization and thereby increases TCRδ chain diversity.
Materials and Methods
V(D)J Recombination Substrate Construction.
We modified the V(D)J recombination substrate pJH290 (30) to generate our competitive rearrangement substrate as previously described (21).
Transient Transfection Assays.
Assays were performed in CHO cells as previously described (31), with the following modifications: 3 μg of substrate were included in each transfection, and SuperFect (Qiagen) was used according to the manufacturer's protocol to introduce the substrates and RAG expression plasmids into cells. Recovered substrate DNA was digested with NotI to uniformly decrease the amplification of unrearranged substrates and then analyzed via PCR and Southern blotting as previously described (21). Threefold dilution of the digested substrate DNA was used for the amplification.
Generation of Targeting Construct and Probes.
We used the pLNTK parent targeting vector (32) to replace the TRDD1 gene segment with the TRAJ38 gene segment. We first generated pBS/SK-TRDD1, in which the 3.1 5′ BglII fragment flanking the TRDD1 region was cloned into NotI and XhoI sites of an altered pBS/SK that had deleted the region between SacI and BstXI. The TRDD1 fragment was altered by using overlapping primers, which replaced precisely the TRDD1 gene segment with the TRAJ38 gene segment. The TRAJ38 fragment with flanking TRDD1 region sequence inclusive of the BstXI and SacI sites was then cloned into pGEM to generate pGEM-TRAJ38. Next, the BstXI and SacI fragment of the pGEM-TRAJ38 was cloned into the BstXI and SacI site of the pBS/SK-TRDD1. The 5′ homology arm was subcloned into the SalI site of the pLNTK. The 3′ homology arm, a 1.6-kb genomic fragment spanning the SacI and BglII site located 3′ of TRDD1, was subcloned into the XhoI site of pLNTK.
Gene-Targeting and Generation of ES Cells.
The TRAJ38 knock-in targeting vector was electroporated into TC1 ES cells as previously described (19) to generate TRDD1-TRAJ38ectNeo ES cells. Two targeted clones out of 800 screened clones were identified by Southern blot analysis with the 5′ probe on EcoRV-digested genomic DNA (5.8 kb WT, 3.8 kb TRDD1-TRAJ38ectNeo) and confirmed with the 3′ probe on StuI-digested genomic DNA (6 kb WT, 4.7 kb TRDD1-TRAJ38ectNeo). The neomycin-resistance cassette with flanking loxP sites was deleted from the targeted ES clones by using adenoviral vector expressing Cre-recombinase, leaving 1 loxP site. The correct Cre-deleted TRDD1-TRAJ38ect ES subclones were identified by Southern blot analysis by using the 3′ probe on the StuI-digested genomic DNA (4.7 kb TRAJ38ectNeo, 6 kb TRDD1-TRAJ38ect).
Generation and Analysis of Chimeric Mice.
TRDD1-TRAJect chimeric mice were generated through Rag2-deficient blastocyst complementation (25). Cells from the thymuses and spleens of 4- to 6-week-old mice were isolated, counted, and stained with FITC-conjugated anti-CD8 and PE-conjugated anti-CD4 or FITC-conjugated anti-IgM and PE-conjugated anti-B220 antibodies (PharMingen). Data acquisition was conducted on a FACSCalibur equipped with CellQuest and data analysis was performed with FlowJo software. More than 5 mice of each genotype were analyzed.
Analysis of TCRα/δ Gene Segment Rearrangements.
The genomic DNA of total thymuses and γδ T cell hybridomas were prepared as previously described (33). We used PCR to detect rearrangements of specific TRDV and TRAV gene segments to TRDD1, native TRAJ38, or TRDD1-TRAJ38ect within genomic DNA. The primers that were used are as follows: TRDV4F, 5′-AAT CAG CAA CCC TGG ACT GCA CCT AT -3′; TRDV5F, 5′-CAG ATC CTT CCA GTT CAT CC-3′; TRDV15–1F, 5′-GCA GGA TCT AAT GTG GCC CAG AAA GTG ATT CA -3′; TRAV1F, 5′-CCA TCA TCT CCT GTG GAT AG-3′; TRAV2F, 5′-AGA AAG TAG CCG CTA CCA CT-3′; TRAV3–4F, 5′-AGG TGA TCA CAG AGG CAT CCT-3′; TRDD1R, 5′-TGC TTT GTT ATG CAC AAG GC-3′; TRAJ38R, 5′-CAA AGA CGA CTT TGT TGG-3′; TRAJ27R, 5′-TTA AGA GCC CAA GCA GAT GCA TAA G-3′; TRAJ56R, 5′-ACG TAC CTG GTA TAA CAC TCA GAA C-3′; and TRAJ38TRDD1, 5′-GAG TCA CAG CTG CTT GGT AAT GC-3′. The PCR products were subjected to Southern blot analysis by using highly specific oligo probes as per standard techniques. The oligo probes that were used are as follows: TRDD1P, 5′-GCA GTG ACA ATA CAG ACC-3′; TRAJ38P, 5′-CAA AGA CGA CTT TGT TGG-3′; TRAJ27P, 5′-AAA GGT TAA TTT GCC TGT ATT GGT GTT A-3′; and TRAJ56P, 5′-TCA GCC TTA TTA TTG CCT CCA GTA GCC A-3′. Threefold dilution of the digested substrate DNA was used for the amplification.
Acknowledgments.
We thank Michael Krangel and Barry Sleckman for critical review of this manuscript. Y.N.L. was supported by the Human Frontiers Science Program. D.J. received support from the Medical Scientist Training Program, Harvard Medical School. F.W.A. is an investigator of the Howard Hughes Medical Institute and is supported by National Institutes of Health Grant AI-20047.
Footnotes
The authors declare no conflict of interest.
References
- 1.Mombaerts P, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 2.Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: Complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 3.Early P, Huang H, Davis M, Calame K, Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D, and JH. Cell. 1980;19:981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- 4.Sleckman BP, Gorman JR, Alt FW. Accessibility control of antigen-receptor variable-region gene assembly: role of cis-acting elements. Annu Rev Immunol. 1996;14:459–481. doi: 10.1146/annurev.immunol.14.1.459. [DOI] [PubMed] [Google Scholar]
- 5.Sleckman BP, et al. Accessibility control of variable region gene assembly during T-cell development. Immunol Rev. 1998;165:121–130. doi: 10.1111/j.1600-065x.1998.tb01235.x. [DOI] [PubMed] [Google Scholar]
- 6.Pereira P, et al. Developmentally regulated and lineage-specific rearrangement of T cell receptor Valpha/delta gene segments. Eur J Immunol. 2000;30:1988–1997. doi: 10.1002/1521-4141(200007)30:7<1988::AID-IMMU1988>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 8.Schatz DG. V(D)J recombination. Immunol Rev. 2004;200:5–11. doi: 10.1111/j.0105-2896.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang K, et al. Organization of the V gene segments in mouse T-cell antigen receptor alpha/delta locus. Genomics. 1994;20:419–428. doi: 10.1006/geno.1994.1196. [DOI] [PubMed] [Google Scholar]
- 10.Chien YH, et al. T-cell receptor delta gene rearrangements in early thymocytes. Nature. 1987;330:722–727. doi: 10.1038/330722a0. [DOI] [PubMed] [Google Scholar]
- 11.Capone M, et al. TCR beta and TCR alpha gene enhancers confer tissue- and stage-specificity on V(D)J recombination events. EMBO J. 1993;12:4335–4346. doi: 10.1002/j.1460-2075.1993.tb06118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson SD, Manzo AR, Pelkonen J, Larche M, Hurwitz JL. Developmental T cell receptor gene rearrangements: Relatedness of the alpha/beta and gamma/delta T cell precursor. Eur J Immunol. 1991;21:1939–1950. doi: 10.1002/eji.1830210824. [DOI] [PubMed] [Google Scholar]
- 13.Migone N, et al. Restriction of the T-cell receptor V delta gene repertoire is due to preferential rearrangement and is independent of antigen selection. Immunogenetics. 1995;42:323–332. doi: 10.1007/BF00179393. [DOI] [PubMed] [Google Scholar]
- 14.Yancopoulos GD, Alt FW. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–281. [PubMed] [Google Scholar]
- 15.Sleckman BP, Bardon CG, Ferrini R, Davidson L, Alt FW. Function of the TCR alpha enhancer in alphabeta and gammadelta T cells. Immunity. 1997;7:505–515. doi: 10.1016/s1074-7613(00)80372-6. [DOI] [PubMed] [Google Scholar]
- 16.Monroe RJ, et al. Developmental regulation of TCR delta locus accessibility and expression by the TCR delta enhancer. Immunity. 1999;10:503–513. doi: 10.1016/s1074-7613(00)80050-3. [DOI] [PubMed] [Google Scholar]
- 17.Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: Current perspectives on the control of V(D)J recombination at the murine T-cell receptor alpha/delta locus. Immunol Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 18.Krangel MS. T cell development: Better living through chromatin. Nat Immunol. 2007;8:687–694. doi: 10.1038/ni1484. [DOI] [PubMed] [Google Scholar]
- 19.Bassing CH, et al. Recombination signal sequences restrict chromosomal V(D)J recombination beyond the 12/23 rule. Nature. 2000;405:583–586. doi: 10.1038/35014635. [DOI] [PubMed] [Google Scholar]
- 20.Winoto A, Baltimore D. Separate lineages of T cells expressing the alpha beta and gamma delta receptors. Nature. 1989;338:430–432. doi: 10.1038/338430a0. [DOI] [PubMed] [Google Scholar]
- 21.Jung D, et al. Extrachromosomal recombination substrates recapitulate beyond 12/23 restricted VDJ recombination in nonlymphoid cells. Immunity. 2003;18:65–74. doi: 10.1016/s1074-7613(02)00507-1. [DOI] [PubMed] [Google Scholar]
- 22.Elliott JF, Rock EP, Patten PA, Davis MM, Chien YH. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988;331:627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- 23.Ezquerra A, et al. T cell receptor delta-gene expression and diversity in the mouse spleen. J Immunol. 1990;145:1311–1317. [PubMed] [Google Scholar]
- 24.Bluestone JA, Cron RQ, Cotterman M, Houlden BA, Matis LA. Structure and specificity of T cell receptor gamma/delta on major histocompatibility complex antigen-specific CD3+, CD4-, CD8- T lymphocytes. J Exp Med. 1988;168:1899–1916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: An assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimoto S, Yamagishi H. Isolation of an excision product of T-cell receptor alpha-chain gene rearrangements. Nature. 1987;327:242–243. doi: 10.1038/327242a0. [DOI] [PubMed] [Google Scholar]
- 27.Livak F, Schatz DG. T-cell receptor alpha locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol Cell Biol. 1996;16:609–618. doi: 10.1128/mcb.16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khor B, Wehrly TD, Sleckman BP. Chromosomal excision of TCRdelta chain genes is dispensable for alphabeta T cell lineage commitment. Int Immunol. 2005;17:225–232. doi: 10.1093/intimm/dxh202. [DOI] [PubMed] [Google Scholar]
- 29.Hawwari A, Krangel MS. Regulation of TCR delta and alpha repertoires by local and long-distance control of variable gene segment chromatin structure. The J Exp Med. 2005;202:467–472. doi: 10.1084/jem.20050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieber MR, et al. The defect in murine severe combined immune deficiency: Joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988;55:7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- 31.Taccioli GE, et al. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993;260:207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- 32.Gorman JR, et al. The Ig(kappa) enhancer influences the ratio of Ig(kappa) versus Ig(lambda) B lymphocytes. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]
- 33.Sleckman BP, Khor B, Monroe R, Alt FW. Assembly of productive T cell receptor delta variable region genes exhibits allelic inclusion. J Exp Med. 1998;188:1465–1471. doi: 10.1084/jem.188.8.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]