Synopsis
Obesity, particularly severe obesity, affects both resting and exercise-related respiratory physiology. Severe obesity classically produces a restrictive ventilatory abnormality, characterized by reduced expiratory reserve volume. However, obstructive ventilatory abnormality may also be associated with abdominal obesity. Decreased peak work rates are usually seen among obese subjects in a setting of normal or decreased ventilatory reserve and normal cardiovascular response to exercise. Weight loss may reverse many adverse physiological consequences of severe obesity on the respiratory system.
Keywords: Obesity, Respiratory physiology, Exercise, Expiratory Reserve Volume, Oxygen consumption.
Introduction
Obesity (Body mass index or BMI ≥ 30 kg/m2) is the most common metabolic disease in the world and its prevalence has risen worldwide, particularly in the United States. Data from the two National Health and Nutrition Examination surveys show that the prevalence of obesity has increased among adults aged 20–74 years in the United States from 15.0% (1976–1980) to 32.9% (2003–2004) 1. Physicians are therefore routinely challenged by the co-morbidities associated with obesity. While the associations between obesity and increased risk of cancer, cardiovascular, endocrine, and rheumatologic diseases are well-described, the respiratory effects of obesity, outside sleep-related disorders, are less well known. It is now clear that respiratory function is impaired in obesity and the magnitude of impairment is more clearly demonstrable in severe obesity 2. This review will focus on the effect of obesity on resting and exercise-related respiratory physiology.
Altered Resting Respiratory Physiology in Obesity
Obesity affects various resting respiratory physiologic parameters such as compliance, neuromuscular strength, work of breathing, lung volumes, spirometric measures, respiratory resistance, diffusing capacity, gas exchange, and airway responsiveness to methacholine (Table 1).
Table 1.
Altered Resting Respiratory Physiology in Obesity
| Physiologic Parameter | Effect of Obesity |
|---|---|
| Respiratory Compliance | Decreased |
| Respiratory Muscle Strength | Decreased |
| Work of Breathing at Rest | Increased |
| Vital Capacity (VC) | Normal or Decreased* |
| Forced Expiratory Volume in One Second (FEV1) |
Normal or Decreased |
| Ratio (FEV1/VC) | Normal, Increased or Decreased |
| Maximal Expiratory Flow Rates at Low Lung Volumes |
Decreased |
| Longitudinal loss in FEV1 and VC | Increased |
| Expiratory Reserve Volume (ERV) | Decreased |
| Functional Residual Capacity (FRC) | Usually Decreased |
| Residual Volume (RV) | Normal |
| Inspiratory Capacity (IC) | Normal or Increased |
| Total Lung Capacity (TLC) | Normal or Slightly Decreased |
| Airway Resistance | Increased |
| Specific Airway Conductance | Normal |
| Diffusing Capacity | Variable |
| Alveolar arterial oxygen tension gradient [P(A-a)O2)] |
Increased |
| Airway Responsiveness to Methacholine |
Often Increased |
The negative association between VC and obesity is better described with abdominal obesity 30.
Respiratory Compliance
Respiratory compliance is the ability of the respiratory system to stretch during a change in volume relative to an applied change in pressure. Total respiratory compliance may be reduced in severe obesity with obesity-hypoventilation syndrome to as little as one-third of the normal 3. In other words, there may be up to a three-fold increase in elastic resistance to respiratory distension in severely obese individuals. This largely results from reduced distensibility of extrapulmonary structures from excess truncal fat 3. However, increased pulmonary blood volume and increased closure of dependent airways also contribute to the low lung compliance seen in severely obese subjects 4. These physiological changes are more pronounced during recumbency in obese subjects, as compared to normal weight subjects, due to the increased gravitational effects of the large abdomen 5.
Respiratory Muscle Strength
Obese subjects may demonstrate inefficiency of respiratory muscles, particularly the diaphragm. Reduced respiratory muscle strength and endurance, as suggested by static maximal inspiratory pressure values of 60-70% of normal subjects, have been described among three severely obese subjects with obesity-hypoventilation syndrome in a 1974 study by Rochester and Enson 4. Recent studies have confirmed that obese subjects are at greater risk for inspiratory muscle fatigue both at rest and with exercise 6, 7. Further, weight loss in severely obese subjects is associated with improved respiratory muscle strength and endurance 8. A possible cause of impaired respiratory muscle function in obesity includes increased elastic load which the respiratory muscles are required to overcome during inspiration 8. An overstretched diaphragm would place this respiratory muscle at a mechanical disadvantage, leading to decreased inspiratory muscle strength and efficiency 9. Additionally, decreased skeletal muscle glycogen synthase activity in obese subjects may be associated with decreased isokinetic skeletal muscle endurance 10, 11, although it is not known if this phenomenon actually occurs in respiratory muscles. Further, fatty infiltration of respiratory and non-respiratory skeletal muscle in obese subjects has been well-documented 12-14, although its clinical significance related to muscle strength is unclear.
Work of Breathing at Rest
To overcome the reduced total respiratory compliance and respiratory muscle inefficiency, severely obese subjects may breathe rapidly and shallowly 6, 15. This pattern of breathing is similar to that seen among patients with neuromuscular and musculoskeletal disorders 16. This pattern of breathing is however associated with increased oxygen cost of breathing 15, 17. The oxygen cost of breathing represents the oxygen consumed by the respiratory muscles per liter of ventilation and is an index of the energy required to breathe. Rochester showed that the oxygen cost of breathing is four-fold and ten-fold higher than normal among subjects with simple eucapnic obesity and obesity-hypoventilation syndrome respectively 15. In a study by Kress et al. of eighteen severely obese patients, a 16% reduction in oxygen consumption was seen after elective intubation, mechanical ventilation and anesthesia from their resting baseline values, as compared to a <1% reduction in among eight controls 17. This relative respiratory inefficiency among obese subjects suggests a decreased ventilatory reserve and a predisposition to respiratory failure in the setting of even mild pulmonary or systemic insults 17.
Lung Volumes
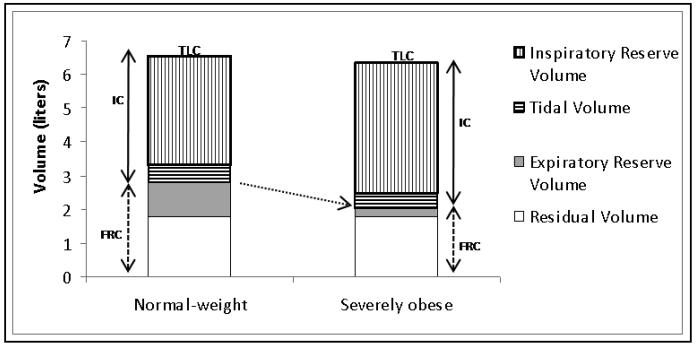
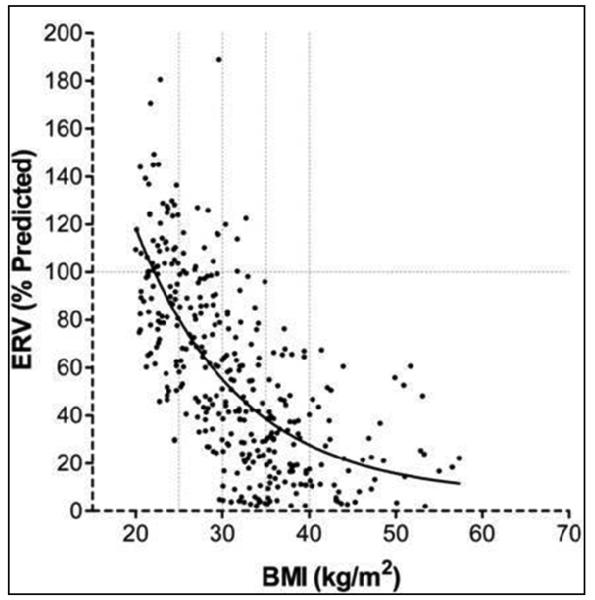
The most common and consistent indicator of obesity is a reduction in expiratory reserve volume (ERV, see Figures 1 and 2) 18. This occurs because of displacement of the diaphragm into the thorax by the obese abdomen and increased chest wall mass 19. Although this association is seen even with modest obesity 20, ERV decreases rapidly in an exponential relationship with increase in BMI (see Figure 2) 21.
Figure 1.
Effect of obesity on lung volumes - Expiratory reserve volume (ERV) is decreased in obesity. Functional residual capacity (FRC), the sum of ERV and residual volume, is usually reduced as well, often approaching residual volume (see arrow). The decline in FRC in obese subjects is primarily the result of reduced ERV. Total lung capacity (TLC, the sum of FRC and inspiratory capacity or IC) is usually preserved. Therefore, in order to compensate for the reduced FRC, inspiratory capacity (IC), the sum of inspiratory reserve volume and tidal volume, may be increased in severe obesity.
Figure 2.
Expiratory reserve volume (ERV) decreases rapidly in an exponential relationship with increase in body mass index (BMI). The best-fit exponential regression equation for ERV is as follows: ERV = 587.8 exp(−0.083 X BMI) + 6.5. The r2 value for ERV was 0.49 (p < 0.01).
Obtained with permission from Chest 21.
On the other hand, obesity has fairly modest effects on the extremes of lung volumes at residual volume (RV) and total lung capacity (TLC) but a relatively larger effect in reducing functional residual capacity (FRC) 21. This reduction is often so marked that FRC approaches RV 22 (see Figure 1). When the reduced FRC is equal to or lower than the closing volume, regional thoracic gas trapping may take place in obese subjects, as suggested by an elevated RV/TLC ratio 23, 24. Further, in order to compensate for the reduced FRC, inspiratory capacity (IC) may be increased in severe obesity (see Figure 1).
As mentioned previously, TLC is usually preserved in most obese subjects, other than those with morbid obesity (weight-to-height ratio of ≥ 0.9 kg/cm) 2, 25, with excessive central adiposity (waist-to-hip ratio of ≥ 0.95) 26, or with obesity hypoventilation syndrome 15. In the absence of the above conditions, a restrictive defect (TLC < lower limit of normal) should not be attributed to obesity, until other causes of restrictive impairment, such as interstitial lung disease, have been excluded.
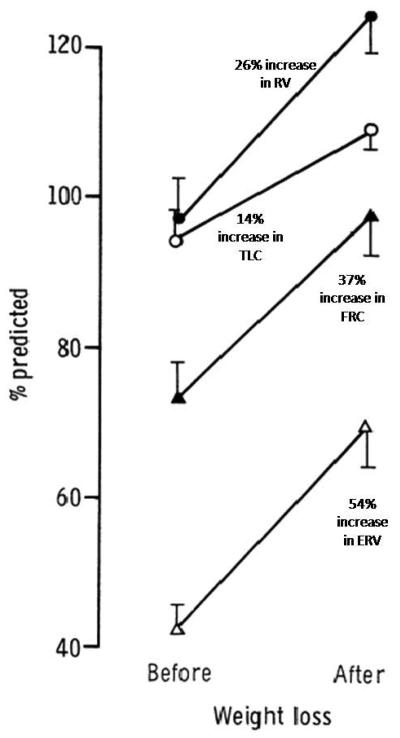
Sequential studies after weight loss, usually in the context of bariatric surgery, usually show a marked improvement in ERV; intermediate improvement in RV and FRC with a more modest improvement in TLC 2, 8, 27, 28 (see Figure 3).
Figure 3.
Vertical banded gastroplasty in a study by Thomas et al. 27 was associated with a mean weight loss of 34.2 kg in 29 morbidly obese subjects. Resulting change in static lung volumes (expressed as change in percent predicted values from baseline) are summarized. Bar lines indicate one standard error of the mean (SEM). Greatest improvement in expiratory reserve volume (ERV); intermediate improvement in residual volume (RV) and functional residual capacity (FRC); and least improvement in total lung capacity (TLC) was seen following surgical weight loss.
Obtained with permission from Thorax 27.
Spirometry
Obesity may be associated with a reduction in vital capacity (VC) and forced expiratory volume in one second (FEV1), depending upon the age, type of body fat distribution (with central fat distribution having a relatively greater effect) 26, and severity of obesity. Previous studies have created the impression that only morbid obesity is associated with this restriction of VC 24 29 but a recent large French population-based study by Leone et al. demonstrated that even mild abdominal obesity, even with a normal BMI, is associated with lower VC and FEV1 in both men and women 30. These findings have prompted a leading authority in this field to recommend the routine measurement of waist circumference prior to spirometry to allow the interpreting physician to take into account the restrictive effect of abdominal obesity on spirometric values 31.
Possible causes of reduced VC in obese subjects may be mechanical and inflammatory. Mechanical causes include decreased respiratory compliance (with consequently decreased lung volumes) and increased gas trapping from premature small airway closure (particularly at the lung bases). In addition, obesity is associated with both increased levels of pro-inflammatory adipokines (such as leptin, interleukin-6, and tumor necrosis factor-alpha 32) and decreased levels of anti-inflammatory adipokines (such as adiponectin 32, 33). The secretion of these adipokines by adipose tissue in chronic respiratory diseases may be regulated by chronic or intermittent hypoxia 34. These adipokines in turn regulate systemic inflammation which is associated with impaired lung function 35-37. Either directly or via systemic inflammation, adipokines may also affect inflammation of small airways, resulting in premature closure of the inflamed and edematous small airway. Additional mechanistic studies are needed to better understand the pathophysiological pathways by which adipokines may affect lung function.
FEV1/VC ratio is usually normal or increased with obesity - the latter is thought to occur because of peripheral airway closure and resulting gas trapping disproportionately reducing the VC 23. The implication is that while obesity may affect small airway function, it may not affect large airways. However, the latter impression may not be entirely true. A recent study by Leone et al. suggests that abdominal obesity may be associated with a reduced FEV1/VC ratio, suggesting an effect on large airway caliber as well 30. Thus, obesity may be associated with obstructive ventilatory abnormality, in addition to its well-known association with restrictive abnormality.
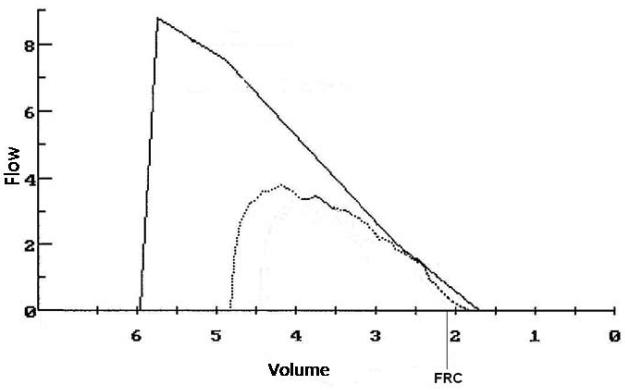
The effect of obesity on forced expiratory flow rates at low-lung volumes is less well-described. A study by Rubinstein et al. in 103 non-smoking morbidly obese men showed reduced maximum expiratory flow rate at 75% of exhaled vital capacity even after normalization for vital capacity, implying peripheral airflow obstruction 23. This phenomenon is illustrated in Figure 4. Possible mechanisms may include obesity-related inflammation or edema of the small airways, thus decreasing their caliber.
Figure 4.
Expiratory flow volume curve (dashed line) from a woman with BMI=50 kg/m2. Solid line shows the predicted curve. Both TLC (79% predicted) and FRC (68% predicted) are reduced, but maximal flows are well preserved and the FEV1/FVC is normal. Nevertheless, expiratory flows at low lung volumes are reduced relative to the predicted values derived from the vital capacity.
Obtained with permission from Cheryl Salome, Ph.D., Woolcock Institute of Research, University of Sydney.
Further, some but not all studies suggest that the effect of obesity on absolute lung function may be greater among men than among women, probably due to greater central fat distribution in men 38-41. Yet, this absolute reduction in lung function may have a relatively greater impact on women, due to their smaller initial lung volume 19. Whether a change in lung volume of this magnitude has a relatively greater impact on dyspnea among obese women, as compared to obese men, is unknown and warrants further research.
Interestingly, while increased fat mass may be negatively associated with spirometric lung function, increased lean mass (i.e. primarily muscle mass) may be positively associated with FEV1 and to a lesser extent with VC, particularly among men 19, 42. This protective effect of lean mass on lung function may be associated with stronger respiratory musculature 42 or larger overall thoracic size 19, although the mechanism remains uncertain.
Several longitudinal studies demonstrate that increasing weight gain is associated with more rapid loss of lung function (both FEV1 and VC) 43-47. Both mechanical and inflammatory mechanisms related to obesity as described above, may contribute to this relatively rapid deterioration in airway function and may predispose obese subjects to long-term adverse effects of cigarette smoking, respiratory infections, and occupational and environmental exposures. Further, obese subjects may improve their lung function by losing weight, suggesting that these detrimental effects of obesity do not involve irreversible structural remodeling of the airways 2, 8, 27, 28, 47, 48.
In addition to the above spirometric changes, severe obesity is associated with a decrease in maximum voluntary ventilation (MVV). This may be explained by respiratory muscle inefficiency, increased upper airway resistance, and inspiratory flow resistance 2. MVV values in obese subjects usually improve following weight reduction 49.
Airway Resistance
An increase in airway resistance (as measured by body plethysmography) is reported in obese subjects 23, 50. However, this may be attributable to breathing at low FRC which in turn results in a relatively decreased airway caliber throughout the tidal breathing cycle. This conclusion is supported by normal values of specific airway conductance 23. Some studies have suggested that the increase in airflow resistance may not be due entirely to the reduced lung volume but have not described the specific cause of the additional resistance 51, 52.
Diffusing Capacity
Although diffusing capacity is usually preserved in obese subjects, both decreased and increased values are reported in the literature 2, 53 27. High values of diffusing capacity may result from increased pulmonary blood volume in obesity. On the other hand, diminished values may result from structural changes in the lung interstitium from lipid deposition and/or decreased alveolar surface area 53.
The effect of weight loss on diffusing capacity has been examined in a few small studies - values remained largely unchanged following surgical weight loss in two separate studies of 16 and 35 morbidly obese subjects by Thomas et al. 27 and Zavorsky et al. 54 respectively and following medical weight loss in 35 obese men in another study by Womack et al. 48
Gas Exchange
Obese subjects have high levels of ventilation-perfusion mismatch from atelectasis of under-ventilated dependent lung units, which continue to be well-perfused. This results in an increased alveolar-arterial oxygen tension gradient [P(A-a)O2)] and reduced partial pressure of oxygen in arterial blood (PaO2). This is worse in recumbent position. Sequential studies demonstrate that weight-reduction may be associated with improved PaO2 27, 28.
Despite their greater carbon dioxide production (V̇ co2), the majority of obese subjects maintain a normal partial pressure of carbon dioxide in arterial blood (PaCO2). In order to maintain normal PaCO2 levels in the face of high V̇ co2, obese subjects generally demonstrate higher minute ventilation (Ve). If they are eucapnic, such individuals have only simple obesity. Patients with obesity hypoventilation syndrome, on the other hand, are unable to adequately augment their Ve and are therefore, hypercapnic. Whether an obese individual demonstrates simple eucapnic obesity or obesity hypoventilation syndrome depends less on the actual BMI value and more on his or her central ventilatory responses to hypoxia and hypercapnia 55. Obesity, genetic predisposition, sleep-disordered breathing, and leptin resistance have all been proposed as possible mechanisms for this blunted ventilatory response to hypercapnia 56 (also see article in this issue by Mokhlesi).
Airway Responsiveness to Methacholine
The association between obesity and asthma has been covered elsewhere in great detail (see article in this issue by Beuther). It is however worth mentioning that the mechanical effects of obesity on the lungs may alter airway smooth muscle contractility and increase airway responsiveness 57. Breathing voluntarily at low lung volumes may increase airway responsiveness to methacholine in lean non-asthmatic subjects 58. In obese subjects breathing at low lung volumes, the airways remain at smaller caliber and the airway smooth muscle is at shorter length throughout the breathing cycle. It is possible that this would change the contractile properties of the airway smooth muscle, either by plastic adaptation to a shorter length 59 or alterations in actin-myosin cross-bridge cycling 60, resulting in an increase in airway smooth muscle contractility and an increase in airway responsiveness. Recent studies also raise the possibility that adipokines (high leptin and low adiponectin concentrations) may increase airway responsiveness 56, 61-64, although the mechanism remains unknown.
Altered Exercise Respiratory Physiology in Obesity (Table 2)
Table 2.
Altered Exercise-Related Respiratory Physiology in Obesity
| Physiologic Parameter | Effect of Obesity |
|---|---|
| V̇ O2 peak | Decreased (for actual weight); Normal or Increased (for ideal weight) |
| V̇ O2 -Work Rate relationship | Displaced Upwards |
| Anaerobic Threshold (percent predicted peakV̇ O2) |
Normal |
| Peak Heart Rate | Normal |
| Peak Oxygen Pulse | Normal |
| Ventilatory Reserve | Normal or Decreased |
| Ventilatory Equivalent for Carbon Dioxide at Anaerobic Threshold |
Normal |
| Dead Space-Tidal Volume Ratio | Normal |
| Arterial Partial Pressure of Oxygen | Normal/may Increase |
| Alveolar-Arterial Oxygen Tension Gradient | May Decrease |
Oxygen consumption
Obesity is associated with increased rates of basal metabolism and oxygen consumption (V̇ o2) at rest 65. However, since adipose tissue has a lower metabolic rate than other tissues, if V̇ o2 is standardized by expressing it per kilogram actual body weight, lower than normal values are obtained in obese individuals 65. Similarly, an active, otherwise healthy, obese subject has reduced peak V̇ o2 if it is correlated to actual body weight, but normal or high if it is correlated to either height,66 predicted body weight, or lean body mass 67.
Exercise-related increase in V̇ o2 is more marked in obese subjects as compared to normal-weight subjects, since additional energy is needed to move heavy body parts 66, 68. Because of the high metabolic cost of performing even modest activity, an otherwise healthy obese subject may have good cardiovascular fitness, despite the reduced work capacity 66, 68.
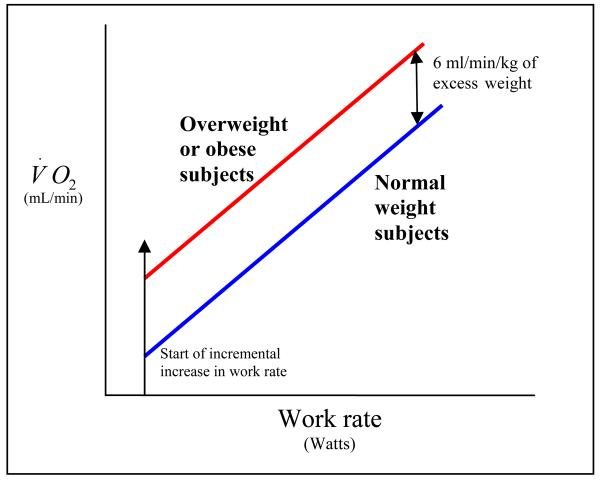
Interestingly, the increased oxygen cost of performing mechanical work is predictable and well-worked out for cycle ergometer 66, 68. The V̇ o2 -work rate relationship is displaced upward by about 6 ml/min/kg. of extra body weight without any discernible change in the slope of the V̇ o2 -work rate relationship (Figure 5) 66, 68. This means that appropriate peak V̇ o2 reference standard for an obese subject can be obtained by increasing the peak V̇ o2 standard obtained from predicted body weight by 6 ml/min for each kilogram above the predicted weight.
Figure 5.
Obesity displaces the V̇ o2 -work rate relationship upward by about 6 ml/min/kg of excess body weight, but the slope itself is unchanged (Adapted from Figure 4.5, Principles of Exercise Testing and Interpretation, Wasserman et al., Fourth edition, Lipincott Williams & Wilkins, Philadelphia, PA, 2005 – copyright permission obtained from Lipincott Williams & Wilkins).
It should also be noted that cardiac and ventilatory reserves in an obese subject are limited in their ability to support the increased muscle oxygen requirement during exercise since the heart and the lungs do not increase in size commensurate with the subject's added weight 69. This imposes physiological constraints on peak exercise performance in obese subjects who cannot attain the same peak work rates as normal-weight subjects 69.
Ventilatory Response to Exercise in Obesity
As discussed previously, obese subjects have increased P(A-a)O2 and reduced PaO2 at rest from atelectasis of peripheral lung units. This usually improves during exercise, because of the effect of deep breathing on expansion of atelectatic lung units. It is thus the only pulmonary condition in which arterial oxygenation improves during exercise 69. Because ventilation-perfusion relationships usually normalize during exercise in the patient with uncomplicated obesity, exercise-related dead space ventilation measures (such as ventilatory equivalent for carbon dioxide or Ve/V̇ co2 ratio at anaerobic threshold and dead space-tidal volume ratio) and exercise-related P(A-a)O2 values are also normal.
Further, FRC is reduced in the resting state in obese subjects from ‘chest strapping’, as discussed previously. However, during heavy-to-peak exercise, while FRC reduces in lean subjects, it may actually increase in obese subjects 70. Both groups, lean subjects and obese subjects, also develop (modest and similar levels of) expiratory flow limitation at peak exercise 70. However, it is important to recognize that unlike lean subjects who easily tolerate this decline in expiratory flow, obese subjects have to increase their FRC (or hyperinflate) during peak exercise to avoid significant levels of expiratory flow limitation 70. This dynamic hyperinflation may contribute to reduced tidal volume and increased respiratory rate and may contribute to a reduced ventilatory reserve during peak exercise 71.
Cardiovascular Response to Exercise in Obesity
Although work capacity is impaired in moderately obese subjects, peak oxygen pulse (a non-invasive determinant of stroke volume) and anaerobic threshold levels (related to percent predicted peak V̇ o2) are usually normal 66, 67, 69, 72. This reflects the ‘training effect’ induced consequent to the demands of performing habitual activities while ‘loaded’ with a greater body mass 72. Although resting heart rate is usually high in obese subjects, peak heart rate is usually normal, resulting in little heart rate reserve 72.
On the other hand, among asymptomatic severely obese subjects, abnormal indices of left ventricular diastolic filling pressures, as measured by pulse Doppler-echocardiography, more frequently develop during exercise, as compared to matched lean controls 73. This may represent a subclinical form of cardiomyopathy in severely obese subjects 73.
Summary
Obesity, particularly severe obesity, affects both resting and exercise-related respiratory physiology. Obesity markedly reduces the expiratory reserve volume (ERV) and respiratory system compliance, classically producing a restrictive ventilatory abnormality. However, reduced FEV1/VC ratio (associated with abdominal obesity) and reduced maximum expiratory flow rates at low lung volumes in obesity may less often produce an obstructive ventilatory abnormality as well. Arterial hypoxemia resulting from ventilation-perfusion mismatch usually improves with exercise. Increased absolute rates of oxygen consumption (V̇ o2) both at rest and with exercise are seen in obese subjects. However, if V̇ o2 is standardized by expressing it per kilogram actual body weight, lower than normal values are obtained. Decreased peak work rates are usually seen in a setting of normal or decreased ventilatory reserve and normal cardiovascular response to exercise in obese subjects. The best treatment of obesity is weight loss which reverses many of the adverse physiological consequences of obesity on the respiratory system.
Acknowledgements
The author wishes to thank Mark Schuyler, M.D., University of New Mexico and Cheryl Salome, Ph.D., Woolcock Institute of Research, University of Sydney for their careful critique of this document.
Funding: This work was supported in part by University of New Mexico Clinical Translational Science Center grant number NIH NCRR M01-RR-00997. The author has no financial relationship with a commercial company that has an interest in the subject matter or materials discussed in the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Center for Health Statistics . Chartbook on Trends in the Health of Americans, Health, United States. Public Health Service; Hyattsville, MD: 2006. 2006. [PubMed] [Google Scholar]
- 2.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983 Sep;128(3):501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 3.Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960 May;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 4.Rochester DF, Enson Y. Current concepts in the pathogenesis of the obesity-hypoventilation syndrome. Mechanical and circulatory factors. Am J Med. 1974 Sep;57(3):402–420. doi: 10.1016/0002-9343(74)90135-1. [DOI] [PubMed] [Google Scholar]
- 5.Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001 Apr;321(4):249–279. doi: 10.1097/00000441-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chlif M, Keochkerian D, Feki Y, Vaidie A, Choquet D, Ahmaidi S. Inspiratory muscle activity during incremental exercise in obese men. Int J Obes (Lond) 2007 Sep;31(9):1456–1463. doi: 10.1038/sj.ijo.0803546. [DOI] [PubMed] [Google Scholar]
- 7.Chlif M, Keochkerian D, Mourlhon C, Choquet D, Ahmaidi S. Noninvasive assessment of the tension-time index of inspiratory muscles at rest in obese male subjects. Int J Obes (Lond) 2005 Dec;29(12):1478–1483. doi: 10.1038/sj.ijo.0803030. [DOI] [PubMed] [Google Scholar]
- 8.Weiner P, Waizman J, Weiner M, Rabner M, Magadle R, Zamir D. Influence of excessive weight loss after gastroplasty for morbid obesity on respiratory muscle performance. Thorax. 1998 Jan;53(1):39–42. doi: 10.1136/thx.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp JT, Druz WS, Kondragunta VR. Diaphragmatic responses to body position changes in obese patients with obstructive sleep apnea. Am Rev Respir Dis. 1986 Jan;133(1):32–37. doi: 10.1164/arrd.1986.133.1.32. [DOI] [PubMed] [Google Scholar]
- 10.Krotkiewski M, Grimby G, Holm G, Szczepanik J. Increased muscle dynamic endurance associated with weight reduction on a very-low-calorie diet. Am J Clin Nutr. 1990 Mar;51(3):321–330. doi: 10.1093/ajcn/51.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Damsbo P, Vaag A, Hother-Nielsen O, Beck-Nielsen H. Reduced glycogen synthase activity in skeletal muscle from obese patients with and without type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991 Apr;34(4):239–245. doi: 10.1007/BF00405082. [DOI] [PubMed] [Google Scholar]
- 12.Lennmarken C, Sandstedt S, von Schenck H, Larsson J. Skeletal muscle function and metabolism in obese women. JPEN J Parenter Enteral Nutr. 1986 Nov-Dec;10(6):583–587. doi: 10.1177/0148607186010006583. [DOI] [PubMed] [Google Scholar]
- 13.Newham DJ, Harrison RA, Tomkins AM, Clark CG. The strength, contractile properties and radiological density of skeletal muscle before and 1 year after gastroplasty. Clin Sci (Lond) 1988 Jan;74(1):79–83. doi: 10.1042/cs0740079. [DOI] [PubMed] [Google Scholar]
- 14.Fadell EJ, Richman AD, Ward WW, Hendon JR. Fatty infiltration of respiratory muscles in the Pickwickian syndrome. N Engl J Med. 1962 Apr 26;266:861–863. doi: 10.1056/NEJM196204262661704. [DOI] [PubMed] [Google Scholar]
- 15.Rochester D. Obesity and pulmonary function. In: Alpert M, Alexander J, editors. The heart and lung in obesity. Futura Publishing Company; Armonk, NY: 1998. pp. 108–132. [Google Scholar]
- 16.Perrin C, Unterborn JN, Ambrosio CD, Hill NS. Pulmonary complications of chronic neuromuscular diseases and their management. Muscle Nerve. 2004 Jan;29(1):5–27. doi: 10.1002/mus.10487. [DOI] [PubMed] [Google Scholar]
- 17.Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (VO(2RESP)) at rest. Am J Respir Crit Care Med. 1999 Sep;160(3):883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 18.Bedell GN, Wilson WR, Seebohm PM. Pulmonary function in obese persons. J Clin Invest. 1958 Jul;37(7):1049–1060. doi: 10.1172/JCI103686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland TJ, Goulding A, Grant AM, et al. The effect of adiposity measured by dual-energy X-ray absorptiometry on lung function. Eur Respir J. 2008 Jul;32(1):85–91. doi: 10.1183/09031936.00112407. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins SC, Moxham J. The effects of mild obesity on lung function. Respir Med. 1991 Jul;85(4):309–311. doi: 10.1016/s0954-6111(06)80102-2. [DOI] [PubMed] [Google Scholar]
- 21.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006 Sep;130(3):827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 22.Gibson GJ. Obesity, respiratory function and breathlessness. Thorax. 2000 Aug;55(Suppl 1):S41–44. doi: 10.1136/thorax.55.suppl_1.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubinstein I, Zamel N, DuBarry L, Hoffstein V. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med. 1990;112(11):828–832. doi: 10.7326/0003-4819-112-11-828. [DOI] [PubMed] [Google Scholar]
- 24.Douglas FG, Chong PY. Influence of obesity on peripheral airways patency. J Appl Physiol. 1972 Nov;33(5):559–563. doi: 10.1152/jappl.1972.33.5.559. [DOI] [PubMed] [Google Scholar]
- 25.Biring MS, Lewis MI, Liu JT, Mohsenifar Z. Pulmonary physiologic changes of morbid obesity. Am J Med Sci. 1999 Nov;318(5):293–297. doi: 10.1097/00000441-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest. 1997 Apr;111(4):891–898. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]
- 27.Thomas PS, Cowen ER, Hulands G, Milledge JS. Respiratory function in the morbidly obese before and after weight loss. Thorax. 1989 May;44(5):382–386. doi: 10.1136/thx.44.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Refsum HE, Holter PH, Lovig T, Haffner JF, Stadaas JO. Pulmonary function and energy expenditure after marked weight loss in obese women: observations before and one year after gastric banding. Int J Obes. 1990 Feb;14(2):175–183. [PubMed] [Google Scholar]
- 29.Sugerman HJ. Pulmonary function in morbid obesity. Gastroenterol Clin North Am. 1987 Jun;16(2):225–237. [PubMed] [Google Scholar]
- 30.Leone N, Courbon D, Thomas F, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009 Mar 15;179(6):509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 31.Enright P. Overindulgence --> overweight --> reduced vital capacity --> reduced longevity. Am J Respir Crit Care Med. 2009 Mar 15;179(6):432–433. doi: 10.1164/rccm.200901-0140ED. [DOI] [PubMed] [Google Scholar]
- 32.Cancello R, Tounian A, Poitou C, Clement K. Adiposity signals, genetic and body weight regulation in humans. Diabetes Metab. 2004 Jun;30(3):215–227. doi: 10.1016/s1262-3636(07)70112-x. [DOI] [PubMed] [Google Scholar]
- 33.Steffes MW, Gross MD, Schreiner PJ, et al. Serum adiponectin in young adults--interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol. 2004 Aug;14(7):492–498. doi: 10.1016/j.annepidem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Franssen FM, O'Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008 Dec;63(12):1110–1117. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 35.Fogarty AW, Jones S, Britton JR, Lewis SA, McKeever TM. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax. 2007 Jun;62(6):515–520. doi: 10.1136/thx.2006.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thyagarajan B, Jacobs DR, Apostol GG, Smith LJ, Lewis CE, Williams OD. Plasma fibrinogen and lung function: the CARDIA Study. Int J Epidemiol. 2006 Aug;35(4):1001–1008. doi: 10.1093/ije/dyl049. [DOI] [PubMed] [Google Scholar]
- 37.Thyagarajan B, Smith LJ, Barr RG, et al. Association of Circulating Adhesion Molecules With Lung Function: The CARDIA Study. Chest. 2009 Feb 18; doi: 10.1378/chest.08-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey IM, Cook DG, Strachan DP. The effects of adiposity and weight change on forced expiratory volume decline in a longitudinal study of adults. Int J Obes Relat Metab Disord. 1999 Sep;23(9):979–985. doi: 10.1038/sj.ijo.0801029. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Rennie D, Cormier YF, Dosman J. Waist circumference is associated with pulmonary function in normal-weight, overweight, and obese subjects. Am J Clin Nutr. 2007 Jan;85(1):35–39. doi: 10.1093/ajcn/85.1.35. [DOI] [PubMed] [Google Scholar]
- 40.Rochester DF. Respiratory muscles and ventilatory failure: 1993 perspective. Am J Med Sci. 1993 Jun;305(6):394–402. doi: 10.1097/00000441-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006 May-Jun;13(4):203–210. doi: 10.1155/2006/834786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wannamethee SG, Shaper AG, Whincup PH. Body fat distribution, body composition, and respiratory function in elderly men. Am J Clin Nutr. 2005 Nov;82(5):996–1003. doi: 10.1093/ajcn/82.5.996. [DOI] [PubMed] [Google Scholar]
- 43.Thyagarajan B, Jacobs DR, Jr., Apostol GG, et al. Longitudinal association of body mass index with lung function: the CARDIA study. Respir Res. 2008;9:31. doi: 10.1186/1465-9921-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wise RA, Enright PL, Connett JE, et al. Effect of weight gain on pulmonary function after smoking cessation in the Lung Health Study. Am J Respir Crit Care Med. 1998 Mar;157(3 Pt 1):866–872. doi: 10.1164/ajrccm.157.3.9706076. [DOI] [PubMed] [Google Scholar]
- 45.Wang ML, McCabe L, Petsonk EL, Hankinson JL, Banks DE. Weight gain and longitudinal changes in lung function in steel workers. Chest. 1997 Jun;111(6):1526–1532. doi: 10.1378/chest.111.6.1526. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax. 1993 Apr;48(4):375–380. doi: 10.1136/thx.48.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bottai M, Pistelli F, Di Pede F, et al. Longitudinal changes of body mass index, spirometry and diffusion in a general population. Eur Respir J. 2002 Sep;20(3):665–673. doi: 10.1183/09031936.02.01282001. [DOI] [PubMed] [Google Scholar]
- 48.Womack CJ, Harris DL, Katzel LI, Hagberg JM, Bleecker ER, Goldberg AP. Weight loss, not aerobic exercise, improves pulmonary function in older obese men. J Gerontol A Biol Sci Med Sci. 2000 Aug;55(8):M453–457. doi: 10.1093/gerona/55.8.m453. [DOI] [PubMed] [Google Scholar]
- 49.Soterakis J, Glennon JA, Ishihara AM, Tyler JM, Iber FL. Pulmonary function studies before and after jejunoileal bypass surgery. Am J Dig Dis. 1976 Jul;21(7):553–556. doi: 10.1007/BF01464762. [DOI] [PubMed] [Google Scholar]
- 50.Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest. 1993 May;103(5):1470–1476. doi: 10.1378/chest.103.5.1470. [DOI] [PubMed] [Google Scholar]
- 51.Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol. 2005 Feb;98(2):512–517. doi: 10.1152/japplphysiol.00430.2004. [DOI] [PubMed] [Google Scholar]
- 52.King GG, Brown NJ, Diba C, et al. The effects of body weight on airway calibre. Eur Respir J. 2005 May;25(5):896–901. doi: 10.1183/09031936.05.00104504. [DOI] [PubMed] [Google Scholar]
- 53.Li AM, Chan D, Wong E, Yin J, Nelson EA, Fok TF. The effects of obesity on pulmonary function. Arch Dis Child. 2003 Apr;88(4):361–363. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zavorsky GS, Kim do J, Sylvestre JL, Christou NV. Alveolar-membrane diffusing capacity improves in the morbidly obese after bariatric surgery. Obes Surg. 2008 Mar;18(3):256–263. doi: 10.1007/s11695-007-9294-9. [DOI] [PubMed] [Google Scholar]
- 55.Gilbert R, Sipple JH, Auchincloss JH., Jr. Respiratory control and work of breathing in obese subjects. J Appl Physiol. 1961 Jan;16:21–26. doi: 10.1152/jappl.1961.16.1.21. [DOI] [PubMed] [Google Scholar]
- 56.Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest. 2007 Oct;132(4):1322–1336. doi: 10.1378/chest.07-0027. [DOI] [PubMed] [Google Scholar]
- 57.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006 Apr;110(1):83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J.Appl.Physiol. 1987;62(3):1324–1330. doi: 10.1152/jappl.1987.62.3.1324. 3/1987. [DOI] [PubMed] [Google Scholar]
- 59.Seow CY. Myosin filament assembly in an ever-changing myofilament lattice of smooth muscle. Am J Physiol Cell Physiol. 2005 Dec;289(6):C1363–1368. doi: 10.1152/ajpcell.00329.2005. [DOI] [PubMed] [Google Scholar]
- 60.Fredberg JJ. Airway smooth muscle in asthma: flirting with disaster. Eur Respir J. 1998 Dec;12(6):1252–1256. doi: 10.1183/09031936.98.12061252. [DOI] [PubMed] [Google Scholar]
- 61.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005 Jan;115(1):103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 62.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006 Aug;118(2):389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Sood A, Camargo CA, Jr, Ford ES. Association between leptin and asthma in adults. Thorax. 2006 Mar 15;61(4):300–305. doi: 10.1136/thx.2004.031468. Epub 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sood A, Cui X, Beckett WS, et al. The effect of serum adiponectin concentration on the association between asthma and adiposity. Proc Am Thor Soc. 2007;175(Abstracts Issue):A962. [Google Scholar]
- 65.Zavala DC, Printen KJ. Basal and exercise tests on morbidly obese patients before and after gastric bypass. Surgery. 1984 Feb;95(2):221–229. [PubMed] [Google Scholar]
- 66.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984 Feb;129(2 Pt 2):S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 67.Buskirk E, Taylor HL. Maximal oxygen intake and its relation to body composition, with special reference to chronic physical activity and obesity. J Appl Physiol. 1957 Jul;11(1):72–78. doi: 10.1152/jappl.1957.11.1.72. [DOI] [PubMed] [Google Scholar]
- 68.Wasserman K, Whipp BJ. Exercise physiology in health and disease. Am Rev Respir Dis. 1975 Aug;112(2):219–249. doi: 10.1164/arrd.1975.112.2.219. [DOI] [PubMed] [Google Scholar]
- 69.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. Fourth ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. [Google Scholar]
- 70.DeLorey DS, Wyrick BL, Babb TG. Mild-to-moderate obesity: implications for respiratory mechanics at rest and during exercise in young men. Int J Obes (Lond) 2005 Sep;29(9):1039–1047. doi: 10.1038/sj.ijo.0803003. [DOI] [PubMed] [Google Scholar]
- 71.Sakamoto S, Ishikawa K, Senda S, Nakajima S, Matsuo H. The effect of obesity on ventilatory response and anaerobic threshold during exercise. J Med Syst. 1993 Aug;17(34):227–231. doi: 10.1007/BF00996950. [DOI] [PubMed] [Google Scholar]
- 72.ATS/ACCP Statement on cardiopulmonary exercise testing Am J Respir Crit Care Med. 2003 Jan 15;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 73.Zarich SW, Kowalchuk GJ, McGuire MP, Benotti PN, Mascioli EA, Nesto RW. Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol. 1991 Aug 1;68(4):377–381. doi: 10.1016/0002-9149(91)90835-9. [DOI] [PubMed] [Google Scholar]