Abstract
Objectives
Because several experimental studies have demonstrated that cyclic adenosine monophosphate generation following β-adrenoceptor activation can markedly stimulate alveolar fluid clearance, we determined whether the endogenous levels of catecholamines that occur in the pulmonary edema fluid and plasma of patients with acute lung injury are high enough to stimulate alveolar fluid clearance in the human lung.
Design
Observational clinical study.
Setting
Academic university hospital and laboratory.
Patients
Twenty-one patients with acute pulmonary edema plus ex vivo human lungs.
Interventions
Measurements of catecholamine levels in patient samples and controlled laboratory studies of the effects of these catecholamine levels on the rates of alveolar fluid clearance in ex vivo human lungs.
Measurements and Main Results
The concentrations of both epinephrine and norepinephrine in the pulmonary edema fluid and plasma were ~10-9 M (range of 1-8 × 10-9 M) in hydrostatic pulmonary edema (n = 6) and acute lung injury patients (n = 15). We therefore tested whether 10-9 M epinephrine or norepinephrine stimulated alveolar fluid clearance in isolated human lungs and found that these epinephrine or norepinephrine concentrations did not stimulate alveolar fluid clearance. However, higher concentrations of epinephrine (10-7 M), but not norepinephrine (10-7 M), significantly stimulated alveolar fluid clearance by 84% above control. Glibenclamide (10-5 M) and CFTRinh-172 (10-5 M), cystic fibrosis transmembrane conductance regulator inhibitors, completely inhibited the epinephrine-induced stimulation of alveolar fluid clearance.
Conclusions
These results indicate that endogenous catecholamine concentrations in pulmonary edema fluid are probably not sufficient to stimulate alveolar fluid clearance. In contrast, administration of exogenous catecholamines into the distal airspaces can stimulate alveolar fluid clearance in the human lung, an effect that is mediated in part by cystic fibrosis transmembrane conductance regulator. Therefore, exogenous cyclic adenosine monophosphate-dependent stimulation will probably be required to accelerate the resolution of alveolar edema in the lungs of patients with pulmonary edema.
Keywords: pulmonary edema, alveolar epithelium, catecholamine, norepinephrine, cystic fibrosis transmembrane conductance regulator, glibenclamide
The effects of endogenous β-agonists, such as catecholamines, on alveolar fluid clearance have been studied in several animal models (1). Endogenous epinephrine increased alveolar fluid clearance in rats after severe septic or hemorrhagic shock (2) and during neurogenic pulmonary edema in dogs (3). In addition, intravenous epinephrine administration increased alveolar fluid clearance in anesthetized rats (4). Recently, increased plasma catecholamine levels were reported in patients with hydrostatic pulmonary edema (5) and the acute respiratory distress syndrome (6). However, pulmonary edema fluid catecholamine levels were not reported, and it is uncertain whether the catecholamine levels that are present in pulmonary edema fluid can stimulate alveolar fluid clearance in human lungs. This is a key question, since the degree of endogenous stimulation of alveolar fluid clearance by catecholamines in patients with pulmonary edema will determine whether there can be an additional therapeutic effect of exogenous β-agonists. The importance of this issue was raised at a National Heart Lung and Blood Institute conference on pulmonary edema (7).
Although β-adrenergic agonists are known to increase alveolar epithelial sodium transport, the precise role that Cl- transport and Cl- channels play in β-adrenoceptor-stimulated alveolar ion and fluid transport is unclear (8, 9). A series of complementary approaches have suggested an important role for the cystic fibrosis transmembrane conductance regulator (CFTR) in β-adrenoceptor-stimulated alveolar fluid clearance and the resolution of pulmonary edema (10). Interestingly, glibenclamide inhibited cyclic adenosine monophosphate (cAMP)-stimulated alveolar fluid clearance transport in human lungs from lung donors. Recently, CFTRinh-172, a selective inhibitor of the CFTR, has been shown to inhibit the function of CFTR in nasal epithelium in mice (11) and in airway glands from pigs and humans (12). However, it is uncertain whether CFTRinh-172 inhibits the effect of catecholamines on alveolar fluid clearance in the human lung.
Therefore, the first objective in the present study was to measure catecholamine levels in pulmonary edema fluid and plasma of patients with hydrostatic pulmonary edema or with increased permeability pulmonary edema (acute lung injury). Because the majority of epinephrine and norepinephrine levels in the pulmonary edema fluid were in the range of ~10-9 M, the second objective was to determine whether 10-9 M epinephrine or norepinephrine stimulated alveolar fluid clearance in isolated human lungs. Since 10-9 M epinephrine did not stimulate alveolar fluid clearance, we tested 10-7 M epinephrine, which markedly stimulated alveolar fluid clearance. The final objective was to determine whether 10-7 M epinephrine stimulated alveolar fluid clearance via CFTR Cl- channels.
MATERIALS AND METHODS
Materials
Amiloride, epinephrine, glibenclamide, and norepinephrine were obtained from Sigma (St. Louis, MO); 3-[(3-trifluoromethyl)phenyl]-5-[(4-carboxyphenyl)methylene]-2-thioxo-4-thiazolidinone (CFTRinh-172) was obtained from Alan Verkman, MD, PhD (UCSF).
Protocol
Catecholamine Levels in Pulmonary Edema Fluid and Plasma
Undiluted pulmonary edema fluid and simultaneous plasma were obtained, as previously described (6), within 1 hr of endotracheal intubation from patients with hydrostatic pulmonary edema (n = 6) and acute lung injury (n = 15). Patients with hydrostatic edema and acute lung injury were identified using previously published criteria (5, 6). Epinephrine levels were measured in six plasma samples of patients with hydrostatic pulmonary edema, 13 plasma samples of patients with acute lung injury, six edema fluid samples of patients with hydrostatic pulmonary edema, and 14 edema fluid samples of patients with acute lung injury. Norepinephrine levels were measured in six plasma samples of patients with hydrostatic pulmonary edema, 13 plasma samples of patients with acute lung injury, five edema fluid samples of patients with hydrostatic pulmonary edema, and 14 edema fluid samples of patients with acute lung injury collected between 1996 and 2000. Samples were immediately centrifuged at 3000 × g for 10 mins, and supernatants were stored at -70°C until thawed for measurement of catecholamine levels. Concentrations of epinephrine and norepinephrine in human pulmonary edema fluid and plasma were measured by enzyme-linked immunosorbent assay (CatCombi; IBL, Hamburg, Germany). The assays each had a sensitivity of 12 pg/mL and intra- and interassay variabilities of 5% and 12%, respectively. All pulmonary edema fluid samples were collected and assayed at the University of California, San Francisco, and this study was approved by the Committee for Human Research at the University of California, San Francisco.
Ex Vivo Human Lung Study
This study was approved by the Human Research Committee in Kanazawa Medical University, Uchinada, Ishikawa, Japan. Human lungs were obtained from patients who underwent pulmonary resections for bronchogenic carcinoma. There were no fibrous or emphysematous lesions as assessed by preoperative chest radiographs and computed tomograms, and no macroscopic emphysematous changes were found when the lungs were removed from the thorax. Pulmonary function tests before surgery were normal in all patients. The surgical procedure has been described previously (13-15). The segmental bronchus was occluded by a 10-Fr. balloon catheter immediately after removal of the lung. We chose an occluded segment that was located distant from the tumor. A warmed physiologic saline solution (45 mL, 37°C) containing 5% bovine albumin was instilled into the distal air spaces through the catheter. After instillation, the lungs were inflated with 100% oxygen at an airway pressure of 7 cm H2O. Alveolar fluid was aspirated 1 hr after instillation. Aspirated alveolar fluid (1-2 mL) was centrifuged at 3000 × g for 10 mins, and supernatant was obtained for measurement of protein and catecholamine concentrations.
Specific Protocol
Effect of Epinephrine on Alveolar Fluid Clearance
To determine whether epinephrine at levels similar to those measured in the pulmonary edema fluid from patients with acute lung injury stimulated alveolar fluid clearance in isolated human lungs, an albumin solution containing epinephrine (10-9 M, n = 3) was instilled into the isolated human lungs. Since 10-9 M epinephrine did not stimulate alveolar fluid clearance, we determined if a higher epinephrine concentration (10-7 M, n = 5) stimulated alveolar fluid clearance. This concentration was selected because conventional doses of aerosolized β2-adrenergic agonists achieve levels of 10-6-10-7 M in pulmonary edema fluid of ventilated patients with acute respiratory failure (16). To determine whether the epinephrine stimulation was mediated by CFTR Cl- channels, glibenclamide (10-5 M, n = 5) or CFTRinh-172 (10-5 M, n = 4) was added to the albumin solution containing 10-7 M epinephrine and instilled into the isolated human lungs. As control, an albumin solution in the absence of epinephrine was instilled into separate isolated human lungs (n = 7). To determine the effect of glibenclamide alone or CFTRinh-172 alone on basal alveolar fluid clearance, an albumin solution containing glibenclamide (10-5 M, n = 4) or CFTRinh-172 (10-4 M, n = 4) was instilled into the isolated human lungs.
Effect of Norepinephrine on Alveolar Fluid Clearance
To determine whether norepinephrine stimulated alveolar fluid clearance in human lungs, an albumin solution containing norepinephrine (10-7 M, n = 4) was instilled into the isolated human lungs.
Measurement of Alveolar Fluid Clearance
In the human lung study, the protein concentrations in instilled and aspirated solutions were measured by the pyrogallol red protein dye-binding method (SRL, Tokyo, Japan). Alveolar fluid clearance was estimated by the progressive increase in the concentration of albumin as water is absorbed (10, 13-15). Alveolar fluid clearance (AFC) was calculated as follows:
| [1] |
where V is the volume of the instilled albumin solution (i) and the final alveolar fluid (f), and,
| [2] |
where P is the concentration of protein in the instilled albumin solution (i) and the final alveolar fluid (f).
Statistics
The data are summarized as means and standard deviations. The data were analyzed by one-way analysis of variance with Student-Newman-Keuls post hoc test. We regarded as significant those differences with p < .05.
RESULTS
Catecholamine Levels in Pulmonary Edema Fluid and Plasma
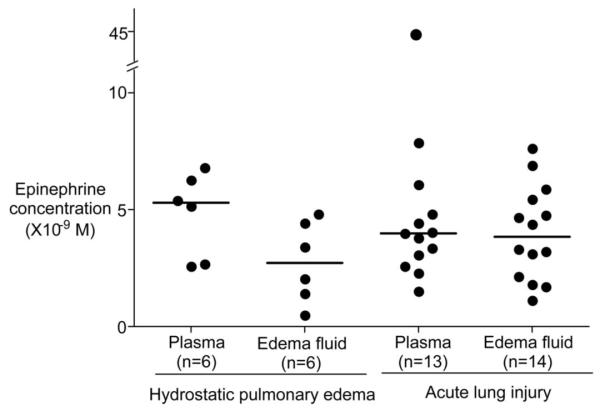
Clinical characteristics of the patients are summarized in Table 1. Epinephrine levels in pulmonary edema fluid (3.7 ± 2.0 × 10-9 M, mean ± sd) and plasma (6.4 ± 9.4 × 10-9 M, mean ± sd) were not significantly different and were in the range of 10-9 M except for one patient with acute lung injury/acute respiratory distress syndrome who had a plasma level of 4.5 × 10-8 M (Fig. 1). Epinephrine levels in edema fluid and plasma were not different when patients with hydrostatic pulmonary edema were compared with patients with acute lung injury/acute respiratory distress syndrome. Norepinephrine levels in pulmonary edema fluid (8.1 ± 5.4 × 10-9 M, mean ± sd) and plasma (22.5 ± 29.1 × 10-9 M, mean ± sd) were not significantly different and were in the range of 10-8-10-9 M except for one patient with acute lung injury/acute respiratory distress syndrome who had a plasma level of 1.4 × 10-7 M (Fig. 2). Again, norepinephrine levels in pulmonary edema fluid and plasma were not different when patients with hydrostatic pulmonary edema were compared with patients with acute lung injury.
Table 1.
Clinical characteristics of 15 patients with acute lung injury (ALI) and six patients with hydrostatic pulmonary edema
| Variable | Hydrostatic (n = 6) |
ALI (n = 15) |
p Value |
|---|---|---|---|
| Age, yrs | 52 ± 26 | 43 ± 15 | .29 |
| Male gender, % | 50 | 60 | .68 |
| Caucasian, % | 50 | 53 | .92 |
| Current smoker, % | 20 | 33 | .57 |
| Pao2/Fio2 ratio | 175 ± 122 | 83 ± 36 | .013 |
| Lung injury score | 2.1 ± 0.7 | 3.1 ± 0.6 | .012 |
| SAPS II score | 32 ± 17 | 45 ± 19 | .16 |
| Any shock, % | 67 | 67 | 1.0 |
| Lowest SBPa | 95 ± 25 | 83 ± 20 | .03 |
| Highest SBPa | 185 ± 28 | 150 ± 26 | .32 |
| Vasoactive medications at time of edema sampling, % |
|||
| Epinephrine | 0 | 0 | |
| Norepinephrine | 33 | 7 | .18 |
| Dopamine | 33 | 47 | .48 |
| Phenylephrine | 33 | 33 | 1.0 |
| Ventilator-free days | 24 ± 6 | 10 ± 10 | .007 |
| Hospital mortality rate, % | 0 | 47 | .040 |
SAPS, Simplified Acute Physiology Score; SBP, systemic blood pressure.
Lowest and highest systolic blood pressure recorded during the 24 hrs after sampling of pulmonary edema fluid. Data are mean ± sd or percent.
Figure 1.
Epinephrine levels in the pulmonary edema fluid and plasma samples from six patients with hydrostatic pulmonary edema and from 15 patients with acute lung injury. Horizontal bar represents a median value. Normal epinephrine levels in plasma are <0.5 × 10-9 M.
Figure 2.
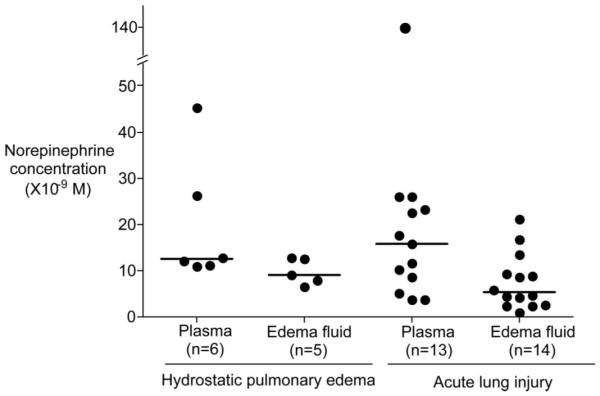
Norepinephrine levels in the pulmonary edema fluid and plasma samples from six patients with hydrostatic pulmonary edema and from 15 patients with acute lung injury. Horizontal bar represents a median value. Normal norepinephrine levels in plasma are 0.6-2.7 × 10-9 M.
Ex Vivo Human Lung Studies
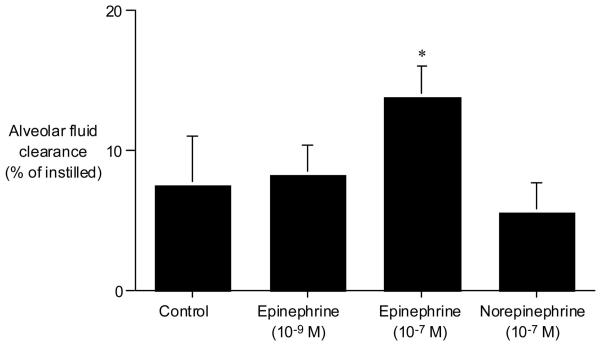
Basal alveolar fluid clearance was 7.5 ± 3.5% of instilled volume over 1 hr in isolated human lungs (Fig. 3). Addition of epinephrine (10-9 M) in the concentration measured in the plasma and pulmonary edema fluid of patients did not stimulate alveolar fluid clearance. However, epinephrine at a higher concentration (10-7 M) stimulated alveolar fluid clearance to 184% of basal alveolar fluid clearance (p < .01). Addition of norepinephrine (10-7 M) to the instillate at levels that were at least a log higher than the levels measured in pulmonary edema fluid or plasma had no effect on alveolar fluid clearance in isolated human lungs.
Figure 3.
Effect of catecholamines on alveolar fluid clearance in the isolated human lung. Epinephrine (10-9 M) did not increase alveolar fluid clearance. However, epinephrine (10-7 M) significantly increased alveolar fluid clearance to 13.8 ± 2.2% from 7.5 ± 3.5% of control clearance over 1 hr in the isolated human lungs. Norepinephrine (10-7 M) had no effect on alveolar fluid clearance. *p < .05 vs. alveolar fluid clearance in control lungs.
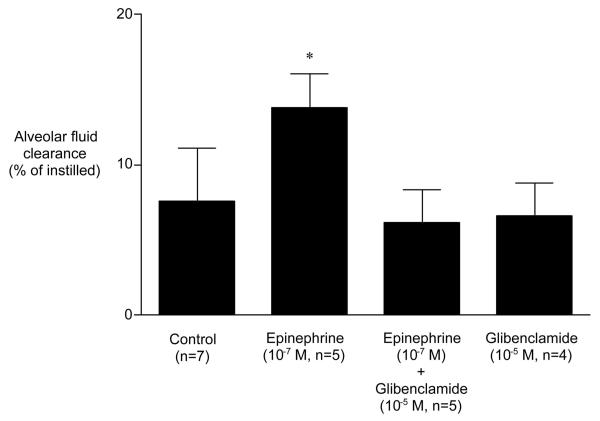
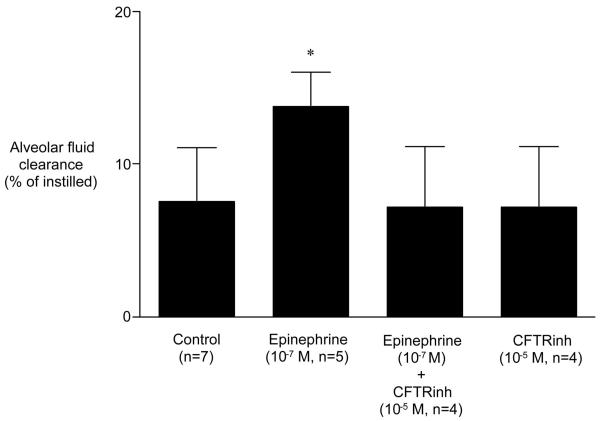
Glibenclamide alone had no inhibitory effect on basal alveolar fluid clearance. However, glibenclamide completely inhibited the epinephrine-stimulation of alveolar fluid clearance (Fig. 4). Similar to glibenclamide, the selective CFTR inhibitor CFTRinh-172 had no inhibitory effect on basal alveolar fluid clearance. However, CFTRinh-172 completely inhibited the epinephrine-induced stimulation of alveolar fluid clearance (Fig. 5).
Figure 4.
Effect of glibenclamide on alveolar fluid clearance in the presence of epinephrine. Glibenclamide (10-5 M) inhibited the ability of 10-7 M epinephrine to stimulate alveolar fluid clearance. Glibenclamide alone had no effect on basal alveolar fluid clearance. *p < .05 vs. alveolar fluid clearance in control. †p < .05 vs. alveolar fluid clearance in the presence of epinephrine.
Figure 5.
Effect of CFTRinh-172 (a selective inhibitor of cystic fibrosis transmembrane conductance regulator) on alveolar fluid clearance in the presence of epinephrine. CFTRinh-172 (10-5 M) inhibited the ability of 10-7 M epinephrine to stimulate alveolar fluid clearance. CFTRinh-172 alone had no effect on basal alveolar fluid clearance. *p < .05 vs. alveolar fluid clearance in control. †p < .05 vs. alveolar fluid clearance in the presence of epinephrine.
DISCUSSION
To determine whether endogenous catecholamine levels are sufficient to stimulate alveolar fluid clearance in patients with pulmonary edema, we measured epinephrine and norepinephrine concentrations in pulmonary edema fluid and plasma obtained from patients with hydrostatic and increased permeability pulmonary edema. The epinephrine concentrations were approximately 10-9 M in pulmonary edema fluid and plasma. Interestingly, we found that 10-9 M epinephrine did not stimulate alveolar fluid clearance in isolated human lungs. This result suggests that the endogenous epinephrine levels measured in edema fluid of patients with acute lung injury or hydrostatic edema are not able to stimulate alveolar fluid clearance. Likewise, norepinephrine at concentrations even higher than endogenous levels had no effect of the rate of alveolar fluid clearance.
In some patients, there may be transient elevations of levels of epinephrine in plasma or edema fluid that exceed the levels we measured. For example, patients with very severe shock might have higher levels (5). However, all patients in this study were critically ill with severe acute respiratory failure and extensive pulmonary edema that required positive pressure ventilation. In addition, 67% of patients in both groups had evidence of shock with a systolic blood pressure <100 mm Hg. Also, the plasma and pulmonary edema fluid catecholamine levels were similar, suggesting that both sides of the alveolar epithelium (apical and basal surfaces) were exposed to these concentrations. From a clinical perspective, the findings (Figs. 1-3) imply that it is unlikely that endogenous epinephrine levels are sufficient to induce a sustained short-term stimulation of alveolar fluid clearance. Severe neurogenic pulmonary edema may be an exception as well as severe shock, although clinicians usually treat severe shock rapidly so it is again unlikely that sufficiently high endogenous catecholamine levels would be maintained for more than a brief period of time. Moreover, cAMP-stimulated alveolar fluid clearance requires ongoing stimulation since the off response in the stimulation of β-adrenoceptors is rapid (1).
These results indicate that endogenous catecholamine concentrations in pulmonary edema fluid are probably not sufficient to stimulate alveolar fluid clearance.
We therefore tested if a higher epinephrine concentration (10-7 M) would stimulate alveolar fluid clearance in isolated human lungs. This concentration was selected because conventional doses of aerosolized β-adrenoceptor agonists delivered through a mechanical ventilator circuit reach concentrations of up to 10-6-10-7 M in pulmonary edema fluid (16). The major finding was that 10-7 M epinephrine, but not 10-7 M norepinephrine, increased alveolar fluid clearance in isolated human lungs. We have previously reported that terbutaline, a hydrophilic β2-adrenergic agonist, and salmeterol, a lipophilic β2-adrenergic agonist, had stimulatory effects on alveolar fluid clearance in isolated human lungs at this concentration range (10, 13-15). In the present study, the experiment was carried out under similar conditions in that the human lungs were obtained from patients with lung cancer and measurements were started within 10 mins after isolation. Although the administered β-adrenergic agonist was different, the stimulatory effect in alveolar fluid clearance by epinephrine was consistent with prior findings.
There has been an inconsistency in the effect of norepinephrine on alveolar fluid clearance. Exogenous norepinephrine did not stimulate alveolar fluid clearance in dog lungs (3). In contrast, we reported that endogenous and exogenous norepinephrine (10-5 M) stimulated β-adrenoceptor-mediated alveolar fluid clearance in rats exposed to hypoxia for 120 hrs (17). Recently, norepinephrine (10-6 M) stimulated Na,K-ATPase activity and protein abundance in alveolar epithelial type II cells via both α1- and β1-adrenergic receptors, but not α2-adrenergic receptors, and also norepinephrine (10-9-10-4 M) increased alveolar fluid clearance in rats (18). Because alveolar fluid clearance was slower in dogs and faster in rats (1), it is likely that the effect of norepinephrine was masked in the dog lungs (17). We did not determine whether high-dose (10-6-10-5 M) norepinephrine increased alveolar fluid clearance in human lungs, because even if exogenous high-dose norepinephrine would increase alveolar fluid clearance in human lungs, high-dose norepinephrine could cause undesirable α-effects on cardiopulmonary circulation and would not be an attractive clinical intervention (18).
We tested two CFTR inhibitors, glibenclamide and CFTRinh-172. Previously, glibenclamide was used to inhibit the effect of YM934, an opener of the adenosine triphosphate-sensitive K+ channel, on alveolar fluid clearance in freshly isolated lungs from lung cancer patients (19). In addition, glibenclamide was used to inhibit the effect of terbutaline on alveolar fluid clearance in isolated and rewarmed human lung from lung donors (10). Recently, CFTRinh-172 was shown to be a potent CFTR inhibitor (20). In the present study, CFTRinh-172 as well as glibenclamide abolished the effect of epinephrine to stimulate alveolar fluid clearance in freshly isolated lungs from lung cancer patients. These results add further support to the hypothesis that CFTR is necessary for cAMP-mediated stimulation of alveolar epithelial fluid transport in the isolated human lung.
CFTRinh-172 and glibenclamide did not change basal alveolar fluid clearance in the human lungs. The results are consistent with previous reports that chloride channel inhibitors had little effect on basal alveolar fluid clearance in normal lungs (8, 10, 21). Since chloride transport occurs in parallel with sodium transport in basal alveolar fluid clearance, there must be an intercellular relationship between sodium and chloride channels (1). Further studies are needed to determine the molecular basis for chloride transport under basal conditions across the alveolar epithelium.
Our findings have some potential clinical relevance. First, endogenous catecholamines may be insufficient to stimulate alveolar fluid clearance in most patients with pulmonary edema. Second, β-adrenergic agonist therapy may be necessary to maximally stimulate alveolar fluid clearance in most patients with pulmonary edema (22). Third, CFTR plays a role in β-agonist-mediated stimulation of alveolar fluid clearance.
There are some limitations to this study. First, the effects of catecholamines on alveolar fluid clearance were measured in the isolated human lung. It is unknown if the effects of catecholamines are similar in in vivo human lungs. Second, we determined the effect of epinephrine and CFTR inhibitors in uninjured human lungs. It is unknown if these agents would have similar effects in injured human lungs. Third, alveolar fluid clearance was measured only for 1 hr. A longer period of treatment with β-adrenergic agonists may be necessary in clinical patients with pulmonary edema.
CONCLUSIONS
This study presents evidence that endogenous epinephrine levels (10-9 M) present in the pulmonary edema fluid and plasma from patients with acute pulmonary edema are probably not sufficient to stimulate alveolar fluid clearance. However, higher epinephrine (10-7 M) concentrations increased the rate of alveolar fluid clearance (184% of control levels). It is likely that CFTR plays a role in epinephrine-stimulated alveolar fluid clearance. This study indicates that administration of exogenous β-adrenoceptor agonists into the distal air spaces of the lung will probably be required to accelerate the resolution of pulmonary edema.
Acknowledgments
Supported, in part, by a Grant for Project Research from the High-Technology Center of Kanazawa Medical University (H2003-7, H2004-7); a Grant for Collaborative Research from Kanazawa Medical University; a Grant-In-Aid for Scientific Research from the MEXT, Japan (14571287); and grants HL51856 (MAM), HL51754 (MAM), and HL 70521 (LBW) from the National Institutes of Health.
Footnotes
The authors have no financial interests to disclose.
REFERENCES
- 1.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 2.Pittet JF, Wiener-Kronish JP, McElroy MC, et al. Stimulation of lung epithelial liquid clearance by endogenous release of catecholamines in septic shock in anesthetized rats. J Clin Invest. 1994;94:663–671. doi: 10.1172/JCI117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane SM, Maender KC, Awender NE, et al. Adrenal epinephrine increases alveolar liquid clearance in a canine model of neurogenic pulmonary edema. Am J Respir Crit Care Med. 1998;158:760–768. doi: 10.1164/ajrccm.158.3.9802031. [DOI] [PubMed] [Google Scholar]
- 4.Charron PD, Fawley JP, Maron MB. Effect of epinephrine on alveolar liquid clearance in the rat. J Appl Physiol. 1999;87:611–618. doi: 10.1152/jappl.1999.87.2.611. [DOI] [PubMed] [Google Scholar]
- 5.Verghese GM, Ware LB, Matthay BA, et al. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol. 1999;87:1301–1312. doi: 10.1152/jappl.1999.87.4.1301. [DOI] [PubMed] [Google Scholar]
- 6.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 7.Crandall ED, Matthay MA. Alveolar epithelial transport. Basic science to clinical medicine. Am J Respir Crit Care Med. 2001;163:1021–1029. doi: 10.1164/ajrccm.163.4.2006116. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X, Ingbar DH, O’Grady SM. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl- channels. Am J Physiol. 1998;275:C1610–C1620. doi: 10.1152/ajpcell.1998.275.6.C1610. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Ingbar DH, O’Grady SM. Adrenergic regulation of ion transport across adult alveolar epithelial cells: Effects on Cl- channel activation and transport function in cultures with an apical air interface. J Membr Biol. 2001;181:195–204. doi: 10.1007/s00232-001-0022-4. [DOI] [PubMed] [Google Scholar]
- 10.Fang X, Fukuda N, Barbry P, et al. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol. 2002;119:199–207. doi: 10.1085/jgp.119.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salinas DB, Pedemonte N, Muanprasat C, et al. CFTR involvement in nasal potential differences in mice and pigs studied using a thiazolidinone CFTR inhibitor. Am J Physiol Lung Cell Mol Physiol. 2004;287:L936–L943. doi: 10.1152/ajplung.00354.2003. [DOI] [PubMed] [Google Scholar]
- 12.Thiagarajah JR, Song Y, Haggie PM, et al. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J. 2004;18:875–877. doi: 10.1096/fj.03-1248fje. [DOI] [PubMed] [Google Scholar]
- 13.Sakuma T, Okaniwa G, Nakada T, et al. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med. 1994;150:305–310. doi: 10.1164/ajrccm.150.2.8049807. [DOI] [PubMed] [Google Scholar]
- 14.Sakuma T, Folkesson HG, Suzuki S, et al. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med. 1997;155:506–512. doi: 10.1164/ajrccm.155.2.9032186. [DOI] [PubMed] [Google Scholar]
- 15.Sakuma T, Suzuki S, Usuda K, et al. Preservation of alveolar epithelial fluid transport mechanisms in rewarmed human lung after severe hypothermia. J Appl Physiol. 1996;80:1681–1686. doi: 10.1152/jappl.1996.80.5.1681. [DOI] [PubMed] [Google Scholar]
- 16.Atabai K, Ware LB, Snider ME, et al. Aerosolized beta(2)-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med. 2002;28:705–711. doi: 10.1007/s00134-002-1282-x. [DOI] [PubMed] [Google Scholar]
- 17.Sakuma T, Hida M, Nambu Y, et al. Effects of hypoxia on alveolar fluid transport capacity in rat lungs. J Appl Physiol. 2001;91:1766–1774. doi: 10.1152/jappl.2001.91.4.1766. [DOI] [PubMed] [Google Scholar]
- 18.Azzam ZS, Adir Y, Crespo A, et al. Norepinephrine increases alveolar fluid reabsorption and Na,K-ATPase activity. Am J Respir Crit Care Med. 2004;170:730–736. doi: 10.1164/rccm.200308-1127OC. [DOI] [PubMed] [Google Scholar]
- 19.Sakuma T, Takahashi K, Ohya N, et al. Effects of ATP-sensitive potassium channel opener on potassium transport and alveolar fluid clearance in the resected human lung. Pharmacol Toxicol. 1998;83:16–22. doi: 10.1111/j.1600-0773.1998.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 20.Sartori C, Allemann Y, Duplain H, et al. Salmeterol for the prevention of high-altitude pulmonary edema. N Engl J Med. 2002;346:1631–1636. doi: 10.1056/NEJMoa013183. [DOI] [PubMed] [Google Scholar]
- 21.Mutlu GM, Adir Y, Jameel M, et al. Interdependency of beta-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res. 2005;96:999–1005. doi: 10.1161/01.RES.0000164554.21993.AC. [DOI] [PubMed] [Google Scholar]
- 22.McAuley DF, Frank JA, Fang X, et al. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32:1470–1476. doi: 10.1097/01.ccm.0000129489.34416.0e. [DOI] [PubMed] [Google Scholar]