Abstract
Desmosine is a stable breakdown product of elastin that can be reliably measured in urine samples. We tested the hypothesis that higher baseline urine desmosine would be associated with higher mortality in 579 of 861 patients included in the recent Acute Respiratory Distress Syndrome Network trial of lower tidal volume ventilation (1). We also correlated urine desmosine levels with indexes of disease severity. Finally, we assessed whether urine desmosine was lower in patients who received lower tidal volumes. Desmosine was measured by radioimmunoassay in urine samples from days 0, 1, and 3 of the study. The data were expressed as a ratio of urine desmosine to urine creatinine to control for renal dilution. The results show that higher baseline (day 0) urine desmosine-to-creatinine concentration was associated with a higher risk of death on adjusted analysis (odds ratio 1.36, 95% confidence interval 1.02-1.82, P = 0.03). Urine desmosine increased in both ventilator groups from day 0 to day 3, but the average rise was higher in the 12-ml/kg predicted body weight group compared with the 6-ml/kg predicted body weight group (P = 0.053, repeated-measures model). In conclusion, patients with acute lung injury ventilated with lower tidal volumes have lower urine desmosine levels, a finding that may reflect reduced extracellular matrix breakdown. These results illustrate the value of evaluating urinary biological markers that may have prognostic and pathogenetic significance in acute lung injury.
Keywords: acute respiratory distress syndrome, extracellular matrix, stretch injury
acute lung injury (ALI) is a major cause of acute respiratory failure with a high mortality rate despite recent advances in ventilator management (1). Multiple pathological changes occur in ALI, including lung endothelial and alveolar epithelial damage (33) as well as damage to the extracellular matrix (22). Experimental data have shown that high tidal volumes during mechanical ventilation can induce a stretch injury manifested by increased interstitial and alveolar edema (10) and activation of the coagulation and inflammatory cascades leading to vascular endothelial and alveolar epithelial damage (33). Therefore, lower tidal volumes may be protective by reducing stretch injury. The possible relationship between stretch injury in humans and damage to extracellular matrix proteins such as elastin has not been tested in a clinical study. Therefore, we studied levels of urine desmosine, a compound specific to elastin breakdown, in patients with ALI included in the recent Acute Respiratory Distress Syndrome (ARDS) Network trial of lower tidal volume ventilation (1).
The extracellular matrix, the region of the lung between the alveolar epithelium and the vascular endothelium, provides structural support to the lungs through collagens, glycoproteins, and proteoglycans (17). Elevations of extracellular matrix proteins such as procollagen type I, procollagen type III, hyaluronan, and laminin have been reported in the bronchoalveolar lavage and pulmonary edema fluid of patients with ALI implicating damage to the extracellular matrix (4, 15, 18).
The extracellular matrix also facilitates passive recoil of the lungs via the unique stretch properties of elastin (19). The lung is a major source of elastin in the body, and in the absence of some pathological process, elastin is stable over the lifetime of an individual (11, 23, 26, 27, 31). The average adult, however, excretes between 1 and 5 mg of elastin per day in the urine arising from the lung as well as other tissues (26, 30). During lung epithelial and endothelial injury, as in ALI, elastin can be broken down by exposure to proteases such as neutrophil elastase (27). Elastin breakdown results in smaller fragments containing desmosine and isodesmosine, crosslinks unique to elastin (27). Desmosine-containing fragments are eliminated in the urine without further modification as small peptides and up to 15% as free desmosine (27). Formation of the crosslinks is a postribosomal event, and coupled with the fact that there is virtually no dietary absorption, inhalation, or other sources of desmosine in the body (26), urine desmosine is a selective marker of elastin breakdown. Desmosine has been studied as a marker of elastin breakdown in several chronic pulmonary conditions, including chronic obstructive pulmonary disease (COPD), cystic fibrosis, and chronic tobacco use (5, 12, 29). There is some variability in the desmosine levels when measured in certain chronic disease states such as COPD or cystic fibrosis compared with controls (2).
The purpose of the current study was to evaluate urine desmosine as a surrogate marker for elastin breakdown in a large group of patients with ALI. Urine desmosine levels were measured in the subjects enrolled in the recent, multicenter randomized controlled trial of lower tidal volume ventilation in ARDS (1). Three related hypotheses were tested similar to prior studies of biomarkers in patients with ALI included in the ARDS Network trial (8, 20, 21, 32). First, we evaluated whether baseline urine desmosine was higher in patients who died compared with those who survived. Second, we assessed whether urine desmosine levels correlated with clinical markers of disease severity and other outcome measures, such as ventilator-free days and organ failure-free days. Finally, we evaluated whether urine desmosine levels demonstrated an attenuated rise in the group of patients that received the 6-ml/kg predicted body weight ventilation strategy compared with those that received 12-ml/kg predicted body weight reflecting less stretch injury in patients who received the lower tidal volume ventilation strategy.
METHODS
Subjects
All study subjects included in the original ARDS Network trial were evaluated for study participation. Study protocols with informed consent had been approved by the institutional review board at each hospital. Subjects included in the current study were required to have paired urine samples: specifically, a urine sample from study day 0 (baseline) and either study day 1 or study day 3 or both. Of the 861 subjects included in the ARDS Network trial, 579 had paired urine samples as defined above. Baseline characteristics of the subjects included in this study and a comparison to the original ARDS Network trial can be found in Table 1.
Table 1. Demographics of study group.
| Clinical Characteristics | Current Study 6 ml/kg (n = 295) |
Current Study 12 ml/kg (n = 284) |
ARDS Network 6 ml/kg (n = 432) |
ARDS Network 12 ml/kg (n = 429) |
|---|---|---|---|---|
| Age (means ± SD) | 51 ± 17 | 52 ± 18 | 51 ± 17 | 52 ± 18 |
| %Female sex | 43 | 42 | 40 | 41 |
| %Ethnicity/race | ||||
| White | 74 | 73 | 75 | 71 |
| Black | 16 | 17 | 16 | 19 |
| Hispanic | 5 | 7 | 5 | 7 |
| Other or unknown | 4 | 3 | 4 | 3 |
| %ALI risk factor n | ||||
| Sepsis | 23 | 27 | 27 | 26 |
| Pneumonia | 36 | 35 | 33 | 36 |
| Aspiration | 16 | 13 | 15 | 14 |
| Trauma | 14 | 10 | 13 | 9 |
| Other | 11 | 15 | 10 | 11 |
| %Lung injury type n | ||||
| Direct | 52 | 48 | 48 | 50 |
| Indirect | 48 | 52 | 52 | 50 |
ALI, acute lung injury.
Laboratory evaluation
Urine samples were obtained from the National Heart, Lung, and Blood Institute storage facility and aliquoted into 0.5-ml tubes after being assigned sequential integer values to blind the lab investigators to all patient information, including the day of the urine sample. Urine creatinine was determined with a colorimetric kit from Sigma (Sigma Chemical, St. Louis, MO). A lower limit of 0.03 mg/ml was used as a cut-off for urine creatinine since this was the lowest level of dilution for the standard in the colorimetric assay. Desmosine was measured using a previously validated radioimmunoassay (25). This assay is a standard method of measurement of urine desmosine and has been validated in prior work (3, 6, 7, 14, 16, 24-26, 28). Desmosine values <2 pmol/ml were considered below the limits of the assay because at values <2 pmol/ml, interfering substances may provide a false value in this competitive assay. If either the urine desmosine or the urine creatinine was below the detection limit, the results were considered unreliable and therefore these samples were excluded from the statistical analysis. No values above the range of the assays were obtained during this evaluation. Exclusion of unreliable laboratory results meant that 12 patients no longer had paired urine samples. We included the available values from these 12 patients in our final analyses given that they were all part of the original cohort of paired urine samples we selected for evaluation. The analyses were performed in a post hoc fashion although the samples were prospectively collected with the intent to ultimately assess biomarkers that may have importance in ALI.
Statistical analyses
All analyses were done using SAS software. Sample results were reidentified, and information from the ARDS Network database was utilized for analysis. The ratio of urine desmosine to urine creatinine was used for all samples to control for the effect of urine dilution. This ratio has been shown in prior work to provide the same interpretation of the data as determining total urine desmosine in a 24-h urine collection (25). The ratio of urine desmosine-to-creatinine was not normally distributed in our dataset. We presented raw data results in Fig. 1 and Table 5. Because of the abnormal data distribution, we presented the median and interquartile ranges. All statistical analysis was done using log transformation to create a more normally distributed dataset for statistical analysis. We evaluated the affect of the log of the urine desmosine-to-creatinine ratio on the risk of death using logistic regression and then adjusted for ventilator group assignment because ventilator group affected mortality. We compared baseline urine desmosine-to-creatinine ratios in patients with direct vs. indirect pulmonary insults to determine if there were differences based on the category of the medical condition that predisposed to ALI. Pearson correlation coefficients controlling for ventilator group were determined for the baseline log urine desmosine-to-creatinine ratio and other clinical outcomes including ventilator-free days and organ failure-free days. Pearson correlation coefficients were also determined for baseline log urine desmosine-tocreatinine with clinical variables known to correlate with disease severity in ARDS, including PaO2/FiO2, plateau airway pressure, nonpulmonary organ failures, APACHE III score, and age. Finally, the study group was separated by ventilator group assignment, i.e., 6 vs. 12 ml/kg tidal volume, to determine whether tidal volume specifically affected the log urine desmosine level. A repeated measures linear regression model was used to compare the average log of the urine desmosine-to-creatinine ratio on days 1 and 3 while controlling for baseline value. Results were considered to be statistically significant if the P values were <0.05.
Fig. 1.
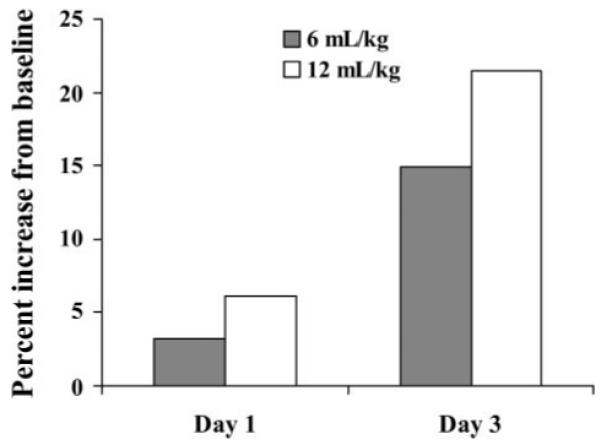
The percent change in the urine desmosine-to-creatinine ratio. The bar graph values show the percentage increase of the median values of the urine desmosine-to-creatinine ratio on day 1 and day 3 of the study compared with the baseline value. The closed bars show the change in the 6-ml/kg group of patients, and the open bars demonstrate the change in the 12-ml/kg group.
Table 5. Urine desmosine-to-creatinine by study day and ventilator group.
| Values By Ventilator Group: Median (interquartile 25-75%) |
Day 0 | Day 1 | Day 3 |
|---|---|---|---|
| 6 ml/kg | 94 (66-134) | 97 (62-136) | 108 (73-159) |
| 12 ml/kg | 98 (74-152) | 104 (72-152) | 119 (82-166) |
Median values and interquartile ranges of the raw urine desmosine-to-creatinine data (not log transformed) are shown. A repeated measures model was used to statistically evaluate the change in the log urine desmosine-to-creatinine over study days 1 and 3, controlling for baseline (day 0) value. This statistical analysis demonstrated a lower mean value of log desmosine-to-creatinine values in the 6-ml/kg group compared with the 12-ml/kg group, P value = 0.053.
RESULTS
Demographics
The baseline characteristics of the current study group are shown in Table 1. The demographics as presented in the original ARDS Network trial are also shown in Table 1. The percentage of women, percentages of each racial background, and risk factors for ARDS were comparable between the current study and the original ARDS Network study groups.
Baseline predictive value
Higher baseline values of the ratio of urine desmosine to urine creatinine were associated with a higher risk of death (odds ratio 1.39 per log increment, 95% confidence interval 1.04-1.85; Table 2). We then tested the baseline predictive value of urine desmosine to urine creatinine in a multivariate analysis that included the ventilator group assignment. This multivariate analysis again demonstrated that the baseline log urine desmosine-to-creatinine ratio still had predictive value for mortality (Table 2). We were unable to compare the urine desmosine results to a control group of ventilated patients without ALI. We did determine that the mean value of desmosine-to-creatinine for all 579 patients in this study was 129 pmol desmosine/mg creatinine. This value was higher than the mean urine values reported in a study that compared COPD patients, COPD patients that continued to smoke, and age-matched normal controls that showed no statistical difference in mean desmosine levels between these three groups (mean 28.4 to 35.5 pmol desmosine/mg creatinine) (2). In addition, five healthy individuals provided urine samples over 3 days for desmosine analysis. The healthy individuals had a much lower concentration of urine desmosine-to-creatinine (mean 27.9 pmol desmosine/mg creatinine) than the mean levels of patients from the ARDS Network.
Table 2. Predictive value of baseline log urine desmosine-to-creatinine ratio for mortality.
| Analysis | Odds Ratio | Confidence Interval | P Value |
|---|---|---|---|
| Unadjusted | 1.39 | 1.04-1.85 | 0.025* |
| Adjusted† | 1.36 | 1.02-1.82 | 0.034* |
Logistic regression analysis of the log of the urine desmosine-to-urine creatinine ratio as a predictor of mortality. Odds ratio is per each log increment.
Adjusted analysis includes ventilator group assignment.
Baseline levels in direct vs. indirect pulmonary insult
Baseline levels of urine desmosine-to-creatinine were compared in study subjects who had a direct pulmonary insult predisposing to ALI vs. an indirect pulmonary insult using t-test comparison. The analysis demonstrated no significant difference in baseline urine desmosine in patients with direct vs. indirect pulmonary insult (P = 0.27).
Correlation with ventilator-free days and organ failure-free days
We evaluated whether urine desmosine would correlate with other outcomes measured in the original ARDS Network trial. Therefore, the correlation coefficients, controlling for ventilator group, were determined between the baseline log urine desmosine-to-creatinine ratio and specific organ failure-free days as well as ventilator-free days (Table 3). All of the correlations in this table were negative. These negative correlations mean that higher urine desmosine-to-creatinine ratios were associated with fewer ventilator-free days or organ failure-free days, i.e., higher log urine desmosine-to-creatinine was associated with worse outcomes. The correlations were weak but statistically significant for all variables except renal failure.
Table 3. Correlation between baseline urine desmosine and clinical outcomes.
| Outcome | Correlation Coefficient | P Value |
|---|---|---|
| Organ failure-free days | ||
| Cardiovascular | -0.16 | 0.0002 |
| Pulmonary | -0.18 | <0.0001 |
| Central nervous system | -0.18 | <0.0001 |
| Coagulation | -0.11 | 0.01 |
| Renal | -0.08 | 0.07 |
| Hepatic | -0.14 | 0.0008 |
| Ventilator-free days | -0.16 | 0.0002 |
Correlations shown were calculated using Pearson correlation coefficients. P value is for the correlation between the log of the baseline urine desmosine-to-creatinine ratio and outcomes controlling for ventilator group assignment.
Correlation with markers of disease severity
Certain clinical characteristics have been identified as markers of disease severity in ALI, whereas others are predictive of mortality in ALI. We tested several of these variables using correlation coefficients to determine whether the baseline log urine desmosine-to-creatinine ratio correlated with these variables. Only two of these clinical variables, age and APACHE III score, showed a significant correlation with the baseline urine desmosine-to-creatinine ratio (Table 4).
Table 4. Correlation of markers of ALI severity with baseline urine desmosine.
| Clinical Variable | Number of Subjects |
Correlation Coefficient |
P Value |
|---|---|---|---|
| Age | 569 | 0.10 | 0.02 |
| APACHE III | 564 | 0.11 | 0.01 |
| Po2 FiO2 | 528 | 0.008 | 0.86 |
| Compliance | 345 | -0.07 | 0.19 |
| Plateau pressure | 447 | 0.08 | 0.11 |
| Nonpulmonary organ failures | 569 | 0.06 | 0.13 |
Correlations shown were calculated using Pearson correlation coefficients. P value is for the correlation between the log of the baseline urine desmosine-to-creatinine ratio and clinical variables.
Modulation of desmosine levels by tidal volume group
A repeated measures model was used to compare the changes in the mean of the log of urine desmosine to creatinine over the first three study days in each ventilator group. The rise in urine desmosine was attenuated by a tidal volume of 6 ml/kg compared with a tidal volume of 12 ml/kg (Table 5). The median values for both the 6- and the 12-ml/kg group increased over the course of the study, but the rise in the 6-ml/kg group was attenuated compared with the 12-ml/kg group (Fig. 1).
DISCUSSION
We studied urine desmosine in ARDS Network patients and found that the baseline log urine desmosine-to-creatinine ratio was significantly higher in patients who died compared with those who survived. This effect persisted even after controlling for the ventilator group assignment. The results provide evidence for the importance of elastin breakdown, an important component of the extracellular matrix, in patients with ALI. These results indicate that damage to the extracellular matrix may influence outcome in the course of ALI.
Prior work has suggested elastin breakdown occurs in ALI. Tenholder and coworkers (31) studied urine desmosine levels in patients with ARDS as a marker of elastin breakdown. The results of that study showed that ARDS patients had a higher ratio of urine desmosine to serum creatinine compared with patients with cardiogenic pulmonary edema. Thus the authors concluded that urine desmosine may be useful to discriminate between different kinds of pulmonary edema when the clinical picture is not clear. Fan and Nagle (9) investigated 100 patients who died with ARDS and in 30 patients found elastin-staining laminar structures within the interstitium that were remnants of elastic fibers. An intriguing finding of this study was the fact that elastin-staining laminar structures were only observed in cases of rapidly developing acute respiratory failure. The results of the current study in ARDS Network patients showed that higher levels of urine desmosine-to-creatinine correlated with worse clinical outcomes, including fewer ventilator-free days and organ failure-free days, suggesting that more severe damage to the extracellular matrix occurred in the most critically ill ALI patients.
There are several reasons why damage to the extracellular matrix might occur. The initial insult that leads to ARDS may directly affect the extracellular matrix. For example, a stimulus may be transmitted by a cytokine cascade leading to breakdown of matrix proteins. Alternatively, the extracellular matrix may be a bystander in ongoing damage to the vascular endothelium and alveolar epithelium that border the extracellular matrix. If this is true, then desmosine may be considered a marker of the severity of endothelial and epithelial injury that occurs in ALI. It is also possible that ventilation with positive pressure may lead to some mechanical damage to the extracellular matrix with stretch injury and breakdown of extracellular matrix proteins. Delivery of a 12-ml/kg tidal volume may overdistend the compliant, less injured alveoli, exacerbating the initial injury with concomitant stretch injury. Because most patients were treated with tidal volumes higher than 6 ml/kg before initiation of the ARDS Network study protocol, subjects may have received tidal volumes high enough to overdistend the functional regions of the lung and thereby may have suffered stretch injury before enrollment in the ARDS Network study.
The second important finding of this study was the attenuation in the rise in urine desmosine over the course of the trial. Subjects ventilated with the lower tidal volume ventilation not only had a lower mortality rate but they also had lower levels of the log urine desmosine-to-creatinine in day 1 and day 3 values as demonstrated by a strong statistical trend that nearly reached significance (P = 0.053). Desmosine is unique to elastin and elastin is in high abundance in the lung. Because the only difference between the groups in this study was the ventilation strategy, it is reasonable to infer that the elastin breakdown occurred in the lung. There is support for this interpretation in the literature. Janoff et al. (13) evaluated urine desmosine in sheep who underwent bronchoalveolar lavage with varying doses of elastase to determine whether urine desmosine could be used as a marker of lung elastin breakdown. Their results showed that baseline urine desmosine concentration in sheep urine was similar to humans. When elastase was instilled directly into the lung, the quantity of desmosine excreted above the baseline level increased linearly with increasing dose of elastase administered. Furthermore, urine desmosine positively correlated with a decrease in perfusion and ventilation in the lung compared with baseline, substantiating that the desmosine measured in the urine originated from injury to the lung. The authors of that study convincingly demonstrated that pathological changes to proteins in the lung could be reliably measured in the urine.
The attenuation in urine desmosine concentrations over 3 days in the current study probably reflected less damage to the extracellular matrix of the lung as a result of the lower tidal volume ventilation coupled with plateau pressure limitation (<30 cmH2O). Pierce et al. (22) studied desmosine levels in preterm lambs and found higher whole lung levels of desmosine and higher mRNA of tropoelastin in ventilated preterm lambs compared with normal term lambs. Furthermore, the preterm lambs with lung injury that received lower tidal volume ventilation (5 ml/kg) had an attenuated rise in desmosine compared with those that had received standard (15 ml/kg) tidal volume ventilation. The results of this experimental study demonstrated both a role for elastin breakdown in lung injury as well as the contribution of stretch injury to the quantity of elastin breakdown.
In the current study, desmosine did not correlate well with markers of disease severity, only showing weak correlations with APACHE III and age. The process of damage to the extracellular matrix may be different than the processes that affect other clinical parameters, such as compliance or oxygenation. Poor oxygenation may be the result of extensive epithelial injury with alveolar filling rather than the result of damage to the extracellular matrix. Alternatively, the urine desmosine measured in this study may reflect elastin breakdown occurring in other parts of the body such as skin or blood vessels. In either case, the results suggest that desmosine may be more useful in understanding the pathogenesis of ALI and less useful as a marker of disease severity.
Although the results of our study implicate a role for damage to the extracellular matrix in ALI patients, there were limitations to consider. First, we were only able to include 579 of the 861 patients included in the original trial. It is possible that the subset we studied was not representative of the whole group. However, the data in Table 1 demonstrate that this large subset was similar in terms of baseline characteristics to the original group included in the ARDS Network trial of lower tidal volume ventilation. Another limitation is that the study design did not allow for discrimination between elastin breakdown that occurred in the extracellular matrix of the lung vs. elastin breakdown that may have occurred in other elastin-rich tissues such as systemic blood vessels or skin tissue. However, since the lung is a major source of elastin in the body (31) and since the intervention in this study was a ventilator strategy, it appears most likely that lower elastin levels represent less damage to the extracellular matrix of the lung. Furthermore, in the Tenholder et al. study (31), the ARDS patients had a significantly higher level of urine desmosine to serum creatinine than critically ill subjects without ARDS and subjects with cardiogenic pulmonary edema. Our results suggest that elevation in urine desmosine, referenced to urine creatinine, is not simply the result of severe illness or pulmonary edema alone but rather is more specific to ALI. One final limitation is that we cannot distinguish association from causation. Damage to the extracellular matrix could be a major factor that worsens ALI and plays a role in ongoing lung damage in ALI. Alternatively, elastin breakdown may simply be a marker of damage that is occurring to tissues surrounding the extracellular matrix, specifically the vascular endothelium and the alveolar epithelium.
In summary, the results of this study provide evidence that worse clinical outcomes are associated with increased elastin breakdown in patients with ALI. In particular, elevated levels of urine desmosine early in the course of ALI are associated with higher mortality rates. Elastin breakdown is attenuated by a lung-protective lower tidal volume ventilation strategy. The results also illustrate the utility of urine samples as a noninvasive source of biological samples for the investigation of matrix breakdown in patients with ALI. Therefore, clinical investigations that utilize urine for biological evaluation should be of value in future studies of ALI.
ACKNOWLEDGMENTS
We acknowledge the National Heart, Lung, and Blood Institute ARDS Network. Network participants: Cleveland Clinic Foundation, Herbert P. Wiedemann, M. D.,* Alejandro C. Arroliga, M. D., Charles J. Fisher, Jr., M. D., John J. Komara, Jr., B. A., R. R. T., Patricia Periz-Trepichio, B. S., R. R. T.; Denver Health Medical Center, Polly E. Parsons, M. D., Denver VA Medical Center, Carolyn Welsh, M. D.; Duke University Medical Center, William J. Fulkerson, Jr., M. D.,* Neil MacIntyre, M. D., Lee Mallatratt, R. N., Mark Sebastian, M. D., John Davies, R. R. T., Elizabeth Van Dyne, R. N., Joseph Govert, M. D.; Johns Hopkins Bayview Medical Center, Jonathan Sevransky, M. D., Stacey Murray, R. R. T.; Johns Hopkins Hospital, Roy G. Brower, M. D., David Thompson, M. S., R. N., Henry E. Fessler, M. D.; LDS Hospital, Alan H. Morris, M. D.,* Terry Clemmer, M. D., Robin Davis, R. R. T., James Orme, Jr., M. D., Lindell Weaver, M. D., Colin Grissom, M. D., Frank Thomas, M. D., Martin Gleich, M. D. (posthumous); McKay-Dee Hospital, Charles Lawton, M. D., Janice D’Hulst, R. R. T.; MetroHealth Medical Center of Cleveland, Joel R. Peerless, M. D., Carolyn Smith, R. N.; San Francisco General Hospital Medical Center, Richard Kallet, M. S., R. R. T., John M. Luce, M. D.; Thomas Jefferson University Hospital, Jonathan Gottlieb, M. D., Pauline Park, M. D., Aimee Girod, R. N., B. S. N., Lisa Yannarell, R. N., B. S. N.; University of California, San Francisco, Michael A. Matthay, M. D.,* Mark D. Eisner, M. D., M. P. H., Brian Daniel, R. C. P., R. R. T.; University of Colorado Health Sciences Center, Edward Abraham, M. D.,* Fran Piedalue, R. R. T., Rebecca Jagusch, R. N., Paul Miller, M. D., Robert McIntyre, M. D., Kelley E. Greene, M. D.; University of Maryland, Henry J. Silverman, M. D.,* Carl Shanholtz, M. D., Wanda Corral, B. S. N., R. N., University of Michigan, Galen B. Toews, M. D.,* Deborah Arnoldi, M. H. S. A., Robert H. Bartlett, M. D., Ron Dechert, R. R. T., Charles Watts, M. D.; University of Pennsylvania, Paul N. Lanken, M. D.,* Harry Anderson III, M. D., Barbara Finkel, M. S. N., R. N., C. William Hanson III, M. D.; University of Utah Hospital, Richard Barton, M. D., Mary Mone, R. N.; University of Washington/Harborview Medical Center, Leonard D. Hudson, M. D.,* Greg Carter, R. R. T., Claudette Lee Cooper, R. N., Annemieke Hiemstra, R. N., Ronald V. Maier, M. D., Kenneth P. Steinberg, M. D.; Utah Valley Regional Medical Center, Tracy Hill, M. D., Phil Thaut, R. R. T.; Vanderbilt University, Arthur P. Wheeler, M. D.,* Gordon Bernard, M. D.,* Brian Christman, M. D., Susan Bozeman, R. N., Linda Collins, Teresa Swope, R. N., and Lorraine B. Ware, M. D.
Clinical Coordinating Center: Massachusetts General Hospital, Harvard Medical School, David A. Schoenfeld, Ph.D.,* B. Taylor Thompson, M. D., Marek Ancukiewicz, Ph.D., Douglas Hayden, M. A., Francine Molay, M. S. W., Nancy Ringwood, B. S. N., R. N., Gail Wenzlow, M. S. W., M. P. H., and Ali S. Kazeroonin, B. S.
NHLBI Staff: Dorothy B. Gail, Ph.D., Andrea Harabin, Ph.D.,* Pamela Lew, and Myron Waclawiw, Ph.D.
*Steering Committee: Gordon R. Bernard, M. D., Chair, Principal Investigator from each center as indicated by an asterisk.
Data and Safety Monitoring Board: Roger G. Spragg, M. D., Chair, James Boyett, Ph.D., Jason Kelley, M. D., Kenneth Leeper, M. D., Marion Gray Secundy, Ph.D., and Arthur Slutsky, M. D.
Protocol Review Committee: Joe G. N. Garcia, M. D., Chair, Scott S. Emerson, M. D., Ph.D., Susan K. Pingleton, M. D., Michael D. Shasby, M. D., and William J. Sibbald, M. D.
GRANTS
This work was supported in part by National Institutes of Health Contract NO1-HR46059 and National Heart, Lung, and Blood Institute Grants P50-HL-74006 and RO1-HL-51856.
REFERENCES
- 1.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Bode DC, Pagani ED, Cumiskey WR, von Roemeling R, Hamel L, Silver PJ. Comparison of urinary desmosine excretion in patients with chronic obstructive pulmonary disease or cystic fibrosis. Pulm Pharmacol Ther. 2000;13:175–180. doi: 10.1006/pupt.2000.0245. [DOI] [PubMed] [Google Scholar]
- 3.Bruce MC, Schuyler M, Martin RJ, Starcher BC, Tomashefski JF, Jr, Wedig KE. Risk factors for the degradation of lung elastic fibers in the ventilated neonate. Implications for impaired lung development in bronchopulmonary dysplasia. Am Rev Respir Dis. 1992;146:204–212. doi: 10.1164/ajrccm/146.1.204. [DOI] [PubMed] [Google Scholar]
- 4.Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997;156:840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 5.Cocci F, Miniati M, Monti S, Cavarra E, Gambelli F, Battolla L, Lucattelli M, Lungarella G. Urinary desmosine excretion is inversely correlated with the extent of emphysema in patients with chronic obstructive pulmonary disease. Int J Biochem Cell Biol. 2002;34:594–604. doi: 10.1016/s1357-2725(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 6.Cohen AB, Girard W, McLarty J, Starcher B, Davis D, Stevens M, Rosenbloom J, Kucich U. A controlled trial of colchicine to reduce the elastase load in the lungs of ex-cigarette smokers with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;143:1038–1043. doi: 10.1164/ajrccm/143.5_Pt_1.1038. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AB, Girard W, McLarty J, Starcher B, Stevens M, Fair DS, Davis D, James H, Rosenbloom J, Kucich U. A controlled trial of colchicine to reduce the elastase load in the lungs of cigarette smokers with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;142:63–72. doi: 10.1164/ajrccm/142.1.63. [DOI] [PubMed] [Google Scholar]
- 8.Eisner MD, Parsons P, Matthay MA, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan K, Nagle WA. Amyloid associated with elastin-staining laminar aggregates in the lungs of patients diagnosed with acute respiratory distress syndrome. BMC Pulm Med. 2002;2:5. doi: 10.1186/1471-2466-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA. High tidal volume ventilation induces NOS2 and impairs cAMP-dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2003;284:L791–L798. doi: 10.1152/ajplung.00331.2002. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein RA, Starcher BC. Urinary excretion of elastin peptides containing desmosin after intratracheal injection of elastase in hamsters. J Clin Invest. 1978;61:1286–1290. doi: 10.1172/JCI109045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb DJ, Stone PJ, Sparrow D, Gale ME, Weiss ST, Snider GL, O’Connor GT. Urinary desmosine excretion in smokers with and without rapid decline of lung function: the Normative Aging Study. Am J Respir Crit Care Med. 1996;154:1290–1295. doi: 10.1164/ajrccm.154.5.8912738. [DOI] [PubMed] [Google Scholar]
- 13.Janoff A, Chanana AD, Joel DD, Susskind H, Laurent P, Yu SY, Dearing R. Evaluation of the urinary desmosine radioimmunoassay as a monitor of lung injury after endobronchial elastase instillation in sheep. Am Rev Respir Dis. 1983;128:545–551. doi: 10.1164/arrd.1983.128.3.545. [DOI] [PubMed] [Google Scholar]
- 14.King GS, Starcher BC, Kuhn C. The measurement of elastin turnover by the radioimmunoassay of urinary desmosine excretion. Bull Eur Physiopathol Respir. 1980;16(Suppl):61–64. doi: 10.1016/b978-0-08-027379-2.50006-5. [DOI] [PubMed] [Google Scholar]
- 15.Kropf J, Grobe E, Knoch M, Lammers M, Gressner AM, Lennartz H. The prognostic value of extracellular matrix component concentrations in serum during treatment of adult respiratory distress syndrome with extracorporeal CO2 removal. Eur J Clin Chem Clin Biochem. 1991;29:805–812. doi: 10.1515/cclm.1991.29.12.805. [DOI] [PubMed] [Google Scholar]
- 16.Luisetti M, Sturani C, Sella D, Madonini E, Galavotti V, Bruno G, Peona V, Kucich U, Dagnino G, Rosenbloom J, Starcher B, Grassi C. MR889, a neutrophil elastase inhibitor, in patients with chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled clinical trial. Eur Respir J. 1996;9:1482–1486. doi: 10.1183/09031936.96.09071482. [DOI] [PubMed] [Google Scholar]
- 17.McGowan SE. Extracellular matrix and the regulation of lung development and repair. FASEB J. 1992;6:2895–2904. [PubMed] [Google Scholar]
- 18.Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998;158:1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 19.Negri EM, Hoelz C, Barbas CS, Montes GS, Saldiva PH, Capelozzi VL. Acute remodeling of parenchyma in pulmonary and extrapulmonary ARDS. An autopsy study of collagen-elastic system fibers. Pathol Res Pract. 2002;198:355–361. doi: 10.1078/0344-0338-00266. [DOI] [PubMed] [Google Scholar]
- 20.Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230-232. [DOI] [PubMed] [Google Scholar]
- 21.Parsons PE, Matthay MA, Ware LB, Eisner MD. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 22.Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol Lung Cell Mol Physiol. 1997;272:L452–L460. doi: 10.1152/ajplung.1997.272.3.L452. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208–1218. [PubMed] [Google Scholar]
- 24.Rosenbloom J, Campbell EJ, Mumford R, Saldeen T, Senior RM, Starcher B, Stone P. Biochemical/immunologic markers of emphysema. Ann NY Acad Sci. 1991;624(Suppl):7–12. doi: 10.1111/j.1749-6632.1991.tb55333.x. [DOI] [PubMed] [Google Scholar]
- 25.Starcher B, Green M, Scott M. Measurement of urinary desmosine as an indicator of acute pulmonary disease. Respiration. 1995;62:252–257. doi: 10.1159/000196458. [DOI] [PubMed] [Google Scholar]
- 26.Starcher B, Scott M. Fractionation of urine to allow desmosine analysis by radioimmunoassay. Ann Clin Biochem. 1992;29:72–78. doi: 10.1177/000456329202900111. [DOI] [PubMed] [Google Scholar]
- 27.Starcher BC. Lung elastin and matrix. Chest. 2000;117:229S–234S. doi: 10.1378/chest.117.5_suppl_1.229s-a. [DOI] [PubMed] [Google Scholar]
- 28.Starcher BC, Goldstein RA. Studies on the absorption of desmosine and isodesmosine. J Lab Clin Med. 1979;94:848–852. [PubMed] [Google Scholar]
- 29.Stone PJ, Konstan MW, Berger M, Dorkin HL, Franzblau C, Snider GL. Elastin and collagen degradation products in urine of patients with cystic fibrosis. Am J Respir Crit Care Med. 1995;152:157–162. doi: 10.1164/ajrccm.152.1.7599816. [DOI] [PubMed] [Google Scholar]
- 30.Stone PJ, Lucey EC, Snider GL, Franzblau C. Effect of diet on urinary excretion of desmosine and hydroxylysyl pyridinoline. Am J Respir Crit Care Med. 1994;149:174–177. doi: 10.1164/ajrccm.149.1.8111578. [DOI] [PubMed] [Google Scholar]
- 31.Tenholder MF, Rajagopal KR, Phillips YY, Dillard TA, Bennett LL, Mundie TG, Tellis CJ. Urinary desmosine excretion as a marker of lung injury in the adult respiratory distress syndrome. Chest. 1991;100:1385–1390. doi: 10.1378/chest.100.5.1385. [DOI] [PubMed] [Google Scholar]
- 32.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170:766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 33.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1134–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]


