Abstract
We have developed a stable isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible-expression plasmid, pIND4, which allows graduated levels of protein expression in the alphaproteobacteria Rhodobacter sphaeroides and Paracoccus denitrificans. pIND4 confers kanamycin resistance and combines the stable replicon of pMG160 with the lacIq gene from pYanni3 and the lac promoter, PA1/04/03, from pJBA24.
Rhodobacter sphaeroides and Paracoccus denitrificans are often used for the study of bacterial metabolism, bioenergetics (8), and signal transduction (11). Although inducible-expression plasmids are available for these organisms, e.g., pRKSK1 (5) and pRECTX (9), these plasmids suffer from one or more of the following problems. First, continuous antibiotic selection is essential for maintaining the plasmid in the population, e.g., plasmid pRK415 (7), which is the vector backbone for most of the available expression vectors for these species, is retained by only approximately 10% of the population after 40 generations without antibiotic selection (6) (segregational instability). Second, the inducer affects the expression of many endogenous genes; for example, several R. sphaeroides vectors use either light- or oxygen-inducible promoters to deliver high levels of protein expression (5). However, light and oxygen affect the expression of over 35% of the endogenous genes in this organism (2), which limifts the use of these vectors in functional studies.
The plasmid developed in this study, pIND4 (Fig. 1), uses the pMG170 vector backbone (6), the lacIq gene from pYanni3 (4), and the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible-expression cassette from pJBA24, which includes the PA1/04/03 promoter, a ribosome binding site, a polylinker, and two transcriptional terminators (1). pMG170 confers kanamycin resistance and is an Escherichia coli shuttle cloning vector derived from naturally occurring plasmid pMG160 of Rhodobacter blasticus, which replicates and is segregationally stable in several other members of the Rhodobacteraceae (6). In E. coli, pMG170 has a high copy number, replicating using the ColE1 origin, while in R. sphaeroides, the pMG160 origin delivers a copy number of 18 to 23 (6).
FIG. 1.
Plasmid map of the pIND4 expression vector. The key features are labeled: the inducible promoter, PA1/04/03 (red); the kanamycin resistance gene, Kmr (purple); the pMG160 sequence (dark green); ColE1 origin of replication, allowing replication of the plasmid in E. coli (pale green); transcriptional terminators (gray); and the lacIq gene (cyan). The vector contains a ribosome binding site (RBS) and provides the option of incorporating a C-terminal hexahistidine tag.
Vector construction.
The construction of pIND4 is described in the supplemental material.
Testing of the expression plasmid.
The coding sequence for the R. sphaeroides cheY6 gene was cloned into pIND4, generating pIND4-Y6. The plasmid was introduced into R. sphaeroides strain JPA1336 (ΔcheY6 derivative of WS8N) and P. denitrificans strain PD1222 (wild type) via conjugation with the E. coli donor strain S17-1 λpir (10). Cells containing the plasmid were grown from single colonies under aerobic conditions with shaking (225 rpm) in succinate medium containing 25 μg/ml kanamycin. The effects of different concentrations of IPTG (Fig. 2) and different induction times (Fig. 3) on CheY6 protein accumulation were investigated.
FIG. 2.
Effect of IPTG concentration on protein expression levels. Cells were grown in the presence of IPTG from single colonies and harvested when the optical density at 700 nm was 0.6 (∼3.8 × 108 cells/ml). The protein content of the cells was estimated by quantitative immunoblotting (3). (A) R. sphaeroides JPA1336 (ΔcheY6) containing pIND4-Y6. (B) P. denitrificans PD1222 containing pIND4-Y6. The zero baseline in each case was cells containing an empty pIND4 vector grown in the presence of 1,000 μM IPTG. Error bars show the standard errors of the means obtained from nine replicates.
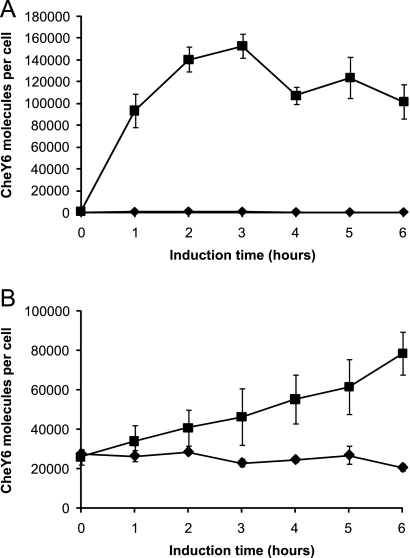
FIG. 3.
Induction time course for cheY6 expression from pIND4-Y6 in R. sphaeroides JPA1336 (ΔcheY6) (A) and P. denitrificans Pd1222 (B). Cells were grown aerobically in succinate medium without IPTG until the optical density at 700 nm reached 0.3. IPTG (1 mM) was then added, and samples were taken at the intervals indicated. ⧫, uninduced cells; ▪, cells induced with 1 mM IPTG. Error bars show the standard errors of the means obtained from nine replicates.
At IPTG concentrations of less than 1 μM, no expression of CheY6 was detectable in R. sphaeroides (the minimum detection limit was ∼500 molecules per cell). When induced with 1,000 μM IPTG, expression levels of CheY6 in R. sphaeroides exceeded 250,000 molecules per cell (equivalent to ∼2.3 mg of protein per liter of culture). This represents at least a 500-fold induction of expression between no induction and maximal induction. The expression levels were sensitive to the concentration of IPTG used, with 10 μM and 100 μM delivering at least 15-fold and 300-fold inductions, respectively.
For P. denitrificans, the maximal induction level achieved with the vector (∼140,000 molecules per cell, corresponding to approximately 1.3 mg of the CheY6 protein per liter of culture) was comparable with that for R. sphaeroides (∼250,000 molecules per cell). However, unlike R. sphaeroides, there was leaky expression of CheY6 in P. denitrificans, with over 45,000 molecules per cell in the absence of IPTG, which limited the level of induction seen in P. denitrificans.
Segregational stability.
R. sphaeroides and P. denitrificans cells were grown in the absence of kanamycin, and the proportion of cells containing the plasmid was estimated by replica plating. After over 85 generations without antibiotic selection, the plasmid was retained in over 97% of bacteria.
Use of pIND4 to analyze an R. sphaeroides chemotaxis mutant.
R. sphaeroides has a complex chemosensory pathway (11). The expression levels of many components of the pathway are affected by light and oxygen levels (14), making previously used expression plasmids reliant on either light or oxygen as their inducer unsuitable for functional studies of the pathway. The cheY6(D56N) mutant of R. sphaeroides has a stopped phenotype, which, due to the stop-start nature of the R. sphaeroides flagellar motor, is analogous to the tumbly phenotype of E. coli chemotaxis mutants. Previously, we have shown that the phosphorylation site mutant protein, CheY6(D56N), can be phosphorylated by CheA3-P on an alternative residue (S83) and that functional CheA3 is required for the stopped phenotype of the cheY6(D56N) mutant, suggesting that phosphorylated CheY6(D56N) causes the stopped phenotype (12). We used pIND4 to test whether we could suppress the stopped phenotype of the cheY6(D56N) mutant by overexpressing each of the eight chemotaxis response regulators (RRs). We found that swimming was restored to this strain by the overexpression of the cognate RRs of CheA3 (CheY1, CheY6, and CheB2) but not by noncognate RRs (CheY2, CheY3, CheY4, CheY5, and CheB1) (Fig. 4 and see the supplemental material). Presumably, this was because the cognate RRs of CheA3 were able to outcompete CheY6(D56N) for phosphorylation by CheA3-P. These results are consistent with our hypothesis that the phosphorylation of CheY6(D56N) by CheA3 causes the stopped phenotype of the cheY6(D56N) mutant.
FIG. 4.
Swarm plate chemotaxis assay comparing the effect of expression of the chemotaxis response regulators on the chemotactic ability of JPA1213 [cheY6(D56N)]. The dotted line shows the swarm diameter produced by a nonmotile strain; swarm diameters significantly larger than this indicate motility. The swarm plates contained 100 μM propionate and 1 mM IPTG and were incubated for 48 h under aerobic conditions. Error bars show the standard errors of the means obtained from nine replicates.
Anticipated uses of pIND4.
The stability of pIND4 combined with its nonendogenous inducible promoter are distinct advantages over previously reported expression plasmids for R. sphaeroides and P. denitrificans. In R. sphaeroides, the plasmid showed minimal levels of leakiness, with greater-than-500-fold induction of CheY6 expression upon the addition of IPTG. In P. denitrificans, the plasmid showed some level of leakiness, which may limit its application in this organism. High levels of protein production were obtained for both R. sphaeroides and P. denitrificans when maximally induced, yielding ∼2.3 and 1.3 mg of the CheY6 protein per liter of culture. We have also successfully used pIND4 to express CheY6 in E. coli, where we obtained a yield of ∼2.5 mg per liter of culture. These high levels of expression suggest that in addition to functional studies, pIND4 may also be useful for the overexpression and subsequent purification of proteins. The large membrane surface area of R. sphaeroides offers the potential for obtaining much higher yields of membrane proteins than with other bacterial hosts (13).
Nucleotide sequence accession number.
The pIND4 sequence has been deposited in the EMBL database under accession number FM164773.
Supplementary Material
Acknowledgments
This work was funded by the MRC-, EPSRC-, BBSRC-, and MoD-funded Bionanotechnology Interdisciplinary Research Collaboration and the BBSRC.
We thank Søren Molin for the kind gift of plasmid pJBA24 used in this study.
Footnotes
Published ahead of print on 14 August 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Andersen, J. B., C. Sternberg, L. K. Poulsen, S. P. Bjorn, M. Givskov, and S. Molin. 1998. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl. Environ. Microbiol. 64:2240-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai, H., J. H. Roh, and S. Kaplan. 2008. Transcriptome dynamics during the transition from anaerobic photosynthesis to aerobic respiration in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 190:286-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Anat Porat, Y., I. Zan-Bar, and A. Ravid. 1995. Quantitative dot-blot assay for low titer anti-lipopolysaccharide antibodies in human plasma. J. Immunol. Methods 180:213-218. [DOI] [PubMed] [Google Scholar]
- 4.Graupner, S., and W. Wackernagel. 2000. A broad-host-range expression vector series including a Ptac test plasmid and its application in the expression of the dod gene of Serratia marcescens (coding for ribulose-5-phosphate 3-epimerase) in Pseudomonas stutzeri. Biomol. Eng. 17:11-16. [DOI] [PubMed] [Google Scholar]
- 5.Hunter, C. N., B. S. Hundle, J. E. Hearst, H. P. Lang, A. T. Gardiner, S. Takaichi, and R. J. Cogdell. 1994. Introduction of new carotenoids into the bacterial photosynthetic apparatus by combining the carotenoid biosynthetic pathways of Erwinia herbicola and Rhodobacter sphaeroides. J. Bacteriol. 176:3692-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inui, M., K. Nakata, J. H. Roh, A. A. Vertes, and H. Yukawa. 2003. Isolation and molecular characterization of pMG160, a mobilizable cryptic plasmid from Rhodobacter blasticus. Appl. Environ. Microbiol. 69:725-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 8.Nicholls, D. G., and S. J. Ferguson. 2009. Bioenergetics 3. Academic Press, London, United Kingdom.
- 9.Pasternak, C., K. Haberzettl, and G. Klug. 1999. Thioredoxin is involved in oxygen-regulated formation of the photosynthetic apparatus of Rhodobacter sphaeroides. J. Bacteriol. 181:100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter, S. L., G. H. Wadhams, and J. P. Armitage. 2007. In vivo and in vitro analysis of the Rhodobacter sphaeroides chemotaxis signaling complexes. Methods Enzymol. 423:392-413. [DOI] [PubMed] [Google Scholar]
- 11.Porter, S. L., G. H. Wadhams, and J. P. Armitage. 2008. Rhodobacter sphaeroides: complexity in chemotactic signalling. Trends Microbiol. 16:251-260. [DOI] [PubMed] [Google Scholar]
- 12.Porter, S. L., G. H. Wadhams, A. C. Martin, E. D. Byles, D. E. Lancaster, and J. P. Armitage. 2006. The CheYs of Rhodobacter sphaeroides. J. Biol. Chem. 281:32694-32704. [DOI] [PubMed] [Google Scholar]
- 13.Roy, A., A. K. Shukla, W. Hasse, and H. Michel. 2008. Employing Rhodobacter sphaeroides to functionally express and purify human G protein-coupled receptors. Biol. Chem. 389:69-78. [DOI] [PubMed] [Google Scholar]
- 14.Shah, D. S. H., S. L. Porter, A. C. Martin, P. A. Hamblin, and J. P. Armitage. 2000. Fine tuning bacterial chemotaxis: analysis of Rhodobacter sphaeroides behaviour under aerobic and anaerobic conditions by mutation of the major chemotaxis operons and cheY genes. EMBO J. 19:4601-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.