Abstract
Individuals who abused alcohol at an early age show decision-making impairments. However, the question of whether maladaptive choice constitutes a predisposing factor to, or a consequence resulting from, alcohol exposure remains open. To examine whether a causal link exists between voluntary alcohol consumption during adolescence and adult decision making the present studies used a rodent model. High levels of voluntary alcohol intake were promoted by providing adolescent rats with access to alcohol in a palatable gel matrix under nondeprivation conditions. A probability-discounting instrumental response task offered a choice between large but uncertain rewards and small but certain rewards to assess risk-based choice in adulthood either 3 weeks or 3 months following alcohol exposure. While control animals' performance on this task closely conformed to a predictive model of risk-neutral value matching, rats that consumed high levels of alcohol during adolescence violated this model, demonstrating greater risk preference. Evidence of significant risk bias was still present when choice was assessed 3 months following discontinuation of alcohol access. These findings provide evidence that adolescent alcohol exposure may lead to altered decision making during adulthood and this model offers a promising approach to the investigation of the neurobiological underpinnings of this link.
Keywords: adolescence, probability discounting
Adolescent alcohol use is a serious public health problem and is associated with an increased risk for development of chronic alcohol use disorders in adulthood (1). Furthermore, an association between a history of alcohol abuse and deficits in decision making has been documented (2–5). However, the question of whether maladaptive choices constitute a predisposing factor to, or a consequence resulting from, alcohol use remains open. Animal models allow for direct testing of causality and for examination of potential neural substrates underlying an association between alcohol use and risky decision making.
Developing rodent models of alcohol abuse has been challenged by the fact that most rat strains do not freely consume significant amounts of ethanol in solution. A method for overcoming the reluctance of nondeprived rats to drink high levels of ethanol in solution was developed by Rowland et al. (6). It utilizes a palatable gel matrix containing ethanol and when made available to rats it stimulates robust and reliable self-administration, without the need for fluid or food deprivation or any training period. Intake of these alcohol “Jello Shots” resulted in significant elevations of blood alcohol concentrations (6) in the range of 5 to 45 mg % with a linear relationship to amount consumed (r = 0.94). Furthermore, alterations in brain chemistry in association with this administration protocol have also been documented (7–8). Since this delivery method does not require training to promote intake, it is particularly appropriate for developmental studies, such as those focused on adolescence, since, in rodents, this period is relatively brief.
Adolescence is a critical period of cortical development that may be disrupted by alcohol use (9). Both limbic and cortical structures, known to be affected by chronic ethanol exposure, undergo active development during the adolescent period (10–13). In addition, adolescent rats consume more alcohol than adults under a variety of conditions, further increasing their vulnerability to adverse consequences (14–15). Chronic, high-dose administration of alcohol during adolescence, but not adulthood, resulted in spatial learning impairments (16) and more evidence of brain damage (17). These data, together with the correlation between early alcohol abuse and later decision-making deficits (18–19) suggest lasting cognitive and behavioral disruptions due to early-life alcohol consumption.
Theories of decision making suggest that individuals analyze potential benefits and costs to guide their actions (20–22). When making decisions with uncertain outcomes, the expected value of an action is represented as the product of the value of an outcome with its relative probability of occurrence. Thus, “probability discounting” represents the extent to which an individual discounts the value of uncertain outcomes as a function of a decreasing probability of receiving them (23) and an individual choosing large but improbable options is said to be risk prone while an individual preferring small, certain ones is termed risk averse (24–25).
In human subjects, early-age binge drinkers (5, 18–19) demonstrated decision-making impairments when exposed to early wins from a large but risky reward option in gambling tasks. It was concluded that these individuals were impaired in their ability to adjust their choice when the decreasing probability of reinforcement for that option rendered it suboptimal. That is, those individuals exhibited risk prone decision making by continuing to pass up a smaller but more certain outcome to chase a larger, but now quite rare, reward. Although intriguing, these findings fall short of supporting a causal link between alcohol use and impairments in choice behavior. Balci et al. (26) recently compared humans and rodents on a complex decision-making task and found remarkable similarities in their performance. This suggests that mechanisms of risk assessment are conserved across mammalian species and that rodents may provide an excellent model for addressing causality and underlying mechanisms. Thus, the present studies used a rat model to test the hypothesis that high alcohol intake during adolescence would impair decision making under conditions of decreasing expected value using a probability-discounting instrumental response task. Specifically, we hypothesized that a history of adolescent alcohol use would lead to increased risk preference and suboptimal choice when animals were tested during adulthood. The present experiments demonstrate adolescent alcohol use resulting in increased risky decision making.
Results
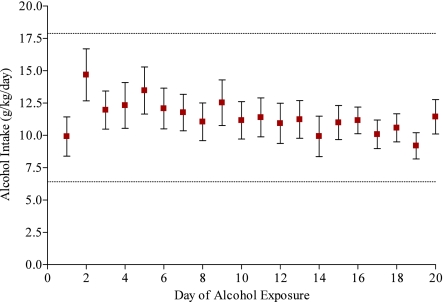
Alcohol Gelatin and Adolescent Intake over 20 Days of Continuous Access.
Adolescent rats (PND 30–49) were provided with continuous access to a 10% EtOH or control gelatin prepared with 10% glucose polymers (Polycose) for 20 days. Adult rats approximately doubled their intake of ethanol when it was presented in a 10% EtOH gel matrix as opposed to solution and previous data from our lab confirm that adult rats consumed approximately 6 g/kg/day of alcohol when presented in a gel, a level of intake comparable to that seen in adult rats selectively bred for alcohol preference (27). In the present study, adolescent alcohol intake averaged 11.4 g/kg/day with a range of 6.4–17.9 g/kg/day (Fig. 1). There were no significant differences in body weight between alcohol-exposed (142.3 ± 22.3 g) and control animals (148.2 ± 26.2 g) over the 20 days [t (20) = 5.69, P = 0.576].
Fig. 1.
Adolescent Alcohol Intake. Average daily alcohol intake for adolescent rats (n = 11) in both experiments over 20 days of exposure. Intake (g/kg) was calculated daily using absolute gelatin intake and daily weights for each animal. Average alcohol intake was 11.39 g/kg/day. The absolute range of daily intake (6.4–17.9 g/kg) for any individual animal throughout the exposure period is represented by the dotted lines. Intake is at asymptote during the first 48 h of alcohol exposure. Data are represented as means ± SEM.
Probability Discounting in Adulthood 3 Weeks Following Gelatin Access.
At PND 50 the alcohol gel was removed and 3 weeks elapsed before training on an instrumental choice task. At this time rats were food restricted to maintain them at approximately 90% of their free-feeding weight. There were no differences in body weight between alcohol-exposed (303.3 ± 34.0 g) and control animals (310.4 ± 43.4 g) [t (20) = 0.43, P = 0.67] at the time training began.
The probability-discounting operant task was designed to assess the influence of uncertainty on choice behavior. Animals began training on an instrumental task involving the presentation of two levers, one associated with the certain delivery of two sucrose pellets and the other associated with four pellets delivered probabilistically. Each daily 45-min session consisted of 24 forced trials, where only one of the levers was extended per trial, followed by 24 choice trials. The forced trials serve to expose the animal to multiple experiences with each lever and its associated expected value for that day while the choice trials assessed animal's preference. Probability of reinforcement for the large reward lever was kept static during a daily session and only reduced across sessions.
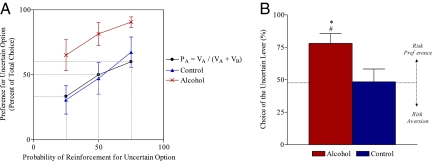
Repeated measures analysis of variance for choice of the larger but uncertain reward option over all conditions generated standard probability discounting curves with decreasing probability of large reward delivery leading to increases in choice of the certain but small reward option for each group [F (2, 40) = 10.80, P < 0.001] (Fig. 2A). However, the two groups differed significantly in their probability discounting curves [F (1, 20) = 6.12, P < 0.05] with animals with a history of alcohol exposure exhibiting a flatter discounting curve and consistently preferring the larger but uncertain option (Fig. 2A).
Fig. 2.
Experiment 1 - Probability Discounting for Treatment and Control Animals Plotted with the Predictions of a Value Matching Model. (A) Percent choice of the uncertain lever as a function of the probability of reward delivery. Animals with a history of alcohol exposure exhibited a shifted discounting curve demonstrating a preference for the larger but uncertain option when compared to controls {P < 0.05; n = 11/group}. (B) Percent choice of the large, uncertain lever averaged across the three uncertain conditions (75%, 50%, and 25% probability of reinforcement). Dotted line denotes the risk neutral choice predictions following a model of value matching: PA = VA/(VA + VB) where VX = MagnitudeX * ProbabilityX. Alcohol exposed animals showed an averaged preference for risk when compared to controls (*, P < 0.05; n = 11/group) and to the predictions of our risk-neutral model (#, P < 0.05; n = 11). Comparisons were made via analysis of variance (ANOVA) (A) and t-tests (B). Data are represented as means ± SEM.
Fig. 2A plots the performance of both groups with the prediction of the risk-neutral value-matching model (see Materials and Methods). For all conditions, the choice behavior of control animals does not deviate from the predictions of the model [75%: t (10) = 6.37, P = 0.54; 50%: t (10) = 0.23, P = 0.83; 25%: t (10) = 1.47, P = 0.17], suggesting relative risk-neutrality. In contrast, the preference of alcohol-exposed animals for the large, uncertain lever was significantly greater than the prediction of the risk-neutral model [75%: t (10) = 7.64, P < 0.001; 50%: t (10) = 3.55, P < 0.01; 25%: t (10) = 6.04, P < 0.001], demonstrating a bias toward the risky option (Fig. 2A).
Of interest is the choice of the uncertain lever when reward is unlikely. Under the 25% large reward probability condition (expected value of one pellet per response on the large, uncertain lever), the small certain option clearly offers greater average reward (2 pellets vs. 1 pellet average). Unlike controls, alcohol-preexposed rats demonstrated a greater preference for the uncertain option and differed significantly from controls [t (20) = 2.14, P < 0.05]. Their choices represented deviation from optimum and yielded them fewer pellets than controls (30.1 ± 3.7 vs. 42.5 ± 2.1 pellets); [t (20) = 2.56, P < 0.01].
Combining performance under all three test conditions (75%, 50%, and 25%) (Fig. 2B) again demonstrates an overall preference for the risky lever in alcohol-exposed animals and a significant difference between them and controls [t (20) = 2.46, P < 0.05].
Probability Discounting in Adulthood 3 Months Following Gelatin Access.
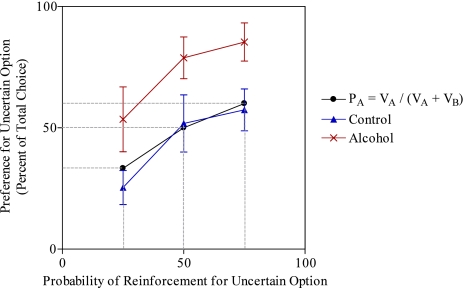
To assess the persistence of risk prone behavior in rats with a history of high levels of alcohol intake in adolescence, a separate group of animals was tested on the same task 3 months after discontinuation of gel access. Results were strikingly similar to the previous experiment. Alcohol-exposed animals consumed a similar daily average (10.62 ± 2.67 g/kg) of alcohol. Furthermore, alcohol-exposed and control groups differed significantly in their rate of probability discounting [F (1, 16) = 5.29, P < 0.05] with alcohol preexposed animals exhibiting a flatter discounting curve (Fig. 3). Confirming and extending the findings of the first experiment, control animals matched the model's prediction [t (9) = 0.35, P = 0.73], while the averaged choice of the risky option for alcohol-exposed animals was significantly greater than the model's prediction [t (8) = 2.79, P < 0.05]. Once again, alcohol-exposed animals acquired fewer pellets than controls [t (16) = 2.14, P < 0.05] when probability of the large reward lever dropped to 25%. Thus, significant risk bias was evident in alcohol-exposed rats even 3 months after adolescent alcohol exposure. Additionally, there appears to be no indication that the magnitude of effect in the two studies (Figs. 2–3) is different, suggesting that the influence of adolescent exposure to alcohol on decision making does not diminish over time.
Fig. 3.
Experiment 2 - Probability Discounting for Alcohol and Control Animals Plotted with the Predictions of a Value Matching Model. (A) Percent choice of the uncertain lever for alcohol (n = 8) and control (n = 9) animals as a function of the probability of reward delivery. Animals with a history of alcohol exposure exhibited a shifted discounting curve demonstrating a preference for the larger but uncertain option when compared to controls (P < 0.05; n = 8–9/group). Comparison made via analysis of variance (ANOVA). Data are represented as means ± SEM.
Discussion
Providing alcohol in a palatable gelatin matrix promoted high alcohol intake, which began within the first 24 h of access. The average intake of alcohol in the gel exceeded intake previously reported for selectively bred alcohol-preferring rats who have been shown to average 5–9 g/kg/day when given access to a single concentration of ethanol in a palatable solution during adolescence (27–29). The alcohol gelatin promotes substantial voluntary alcohol consumption in standard outbred rodents without the need for deprivation. This is seen in adult rats (6) and adolescents (present studies) with adolescents consuming substantially more than adults on a g/kg body weight basis. Despite high voluntary intake for nearly 3 weeks, discontinuation of alcohol access did not appear to trigger outward signs of withdrawal.
Choice behavior of alcohol-exposed and control rats was assessed as the probability of reinforcement for a large reward option in an instrumental paradigm was systematically decreased. This procedure was modeled after studies from the human literature using the Iowa Gambling Task and a systematic reduction in pay-off from a high-reward deck of cards (5). Alcohol use during adolescence led to stable changes in decision making, as reflected in altered probability discounting. These changes were of long duration and were characterized by impairments in decision making and suboptimal choice under conditions of risk. Under all conditions tested the performance of control animals conformed very well to a predictive model of risk neutrality. In contrast, animals exposed to alcohol during adolescence consistently violated this model, statistically preferring the risky option in all conditions.
It is possible that the increases in risk preference observed reflect an increased vulnerability of alcohol-exposed animals to perseveration. The inability or reluctance to adjust behavior in response to changes in associated expected value could represent a means by which alcohol negatively influences choice behavior. That is, the balance between exploration and exploitation strategies used by alcohol-exposed animals may diverge from controls in that experiencing a high probability of large reward delivery early in training leads to a failure to readjust and align subsequent behavior to a situation. In fact, this idea parallels the findings in the human literature and suggests that under some conditions alcohol use has long-term consequences on choice behavior.
The maladaptive bias toward large but unlikely rewards displayed by rats exposed to alcohol during adolescence could suggest that they overweigh the value of a large reward and/or fail to discount that value based on its diminishing probability. A uniform increase in reward signaling as a result of adolescent alcohol exposure would increase the value of the smaller (two-pellet) as well as the larger (four-pellet) reward and this alteration alone could not account for the bias toward the large reward lever. However, if reward signaling, which is known to increase with reward magnitude, grows disproportionately in alcohol exposed animals, then the value of four, relative to two pellets should be considerably greater for them than for controls.
The work presented here provides a model for investigating the neurobiological underpinnings of the link between adolescent ethanol exposure and adult decision making.
The maladaptive bias toward large but unlikely rewards, displayed by rats exposed to alcohol during adolescence, suggests that they overweigh the value of a large reward and/or fail to discount that value based on its diminishing probability. Future studies using this model may clarify the link between risky decisions and a history of alcohol use in adolescence by identifying underlying neural alterations. Determining how adolescent alcohol exposure affects reward signaling to unexpected rewards of varied magnitude, and value computations during probability discounting may increase our understanding the processing of normal and abnormal decisions. Ultimately, such work has the potential to address central challenges relevant to public health and provide insight into the design of behavioral and pharmacological clinical interventions.
Materials and Methods
Animals/Age/Housing.
Male Sprague–Dawley rats aged PND 27 at the start of each experiment, were housed individually in polycarbonate tubs on a 12-h light-dark schedule. Teklad rodent chow and water were available ad libidum except as noted. Animals were weighed and handled daily. All experimental procedures were in accordance with the Institutional Animal Care and Use Committee at the University of Washington.
Alcohol Preparation, Administration, and Withdrawal.
The alcohol gelatin consisted of distilled water, Knox© gelatin, Polycose (10%), and EtOH (10%). This gelatin was made available 24 h/day for 20 days in addition to standard chow and water. To prepare the gel, water was boiled and gelatin powder (Knox; 3 g/100 mL) added. Next, Polycose (10% by weight) was added and the solution was allowed to cool to room temperature. For alcohol gelatin, ethanol (10% by volume) was added to the solution and the mixture was then poured into individual glass jars (approximately 50 mL) (Control = 0.4 kcal/g; Alcohol = 1.1 kcal/g). Jars were sealed and left to refrigerate overnight. This procedure was designed to minimize evaporation of ethanol and has been validated (6) to yield accurate ethanol content. A small amount of evaporation (< 1 g/24 h) occurs from test jars of gelatin placed in the animal room. Procedures for alcohol presentation involve allowing jars of gelatin to warm to room temperature, recording the weight of each and then placing them in animals' cages. Fresh jars were presented every day. During this time rats were weighed and handled. Finally, weights of the jars from the previous day were recorded and consumption of alcohol as a g/kg/bw was calculated for each animal using individual body weights measured that day. Experiments began with 3 days of preexposure to a control gelatin. Subsequently, animals were matched by body weight and baseline gel intake and split into two conditions with one group receiving 24 h access to an alcohol gelatin and the other a control gelatin for 20 days. After 20 days of alcohol exposure, gel access for both groups was discontinued. Animals were monitored daily for changes in weight and changes in behavior during both ethanol exposure and withdrawal. Our threshold for abnormal weight loss and exclusion from the study was a 5% reduction of body weight in any 7-day period. During daily handling we checked for abnormalities, such as excessive locomotor activation, muscle rigidity, clonus, tremors, or convulsions. At no time during these studies did we observe these types of withdrawal symptoms. Animals failing to consume gelatin during the preexposure condition, exhibiting 3 consecutive days of no consumption during the 20-day exposure period or failing to learn operant responding for reward were dropped from the study (two animals in the first experiment and three animals in the second experiment were dropped).
Instrumental Training.
Rats weighed approximately 300 g at the start of instrumental training and were food restricted to maintain them at approximately 90% of their free-feeding weight. Free-feeding weight was based on prerestriction weights and was increased by 1.5% per week. A week before testing, sucrose pellets were sprinkled in the home cages to remove any effect of neophobia when rats first encounter the pellets during training. Once body weights were stable, both groups were trained on an instrumental FR1 schedule for single 45 mg sucrose pellets (Bio Serve) on two separate levers to a criterion of 50 responses in a 30-min session. After acquisition of lever press responding training on the choice task began. Alcohol-exposed rats acquired the instrumental task as quickly (or more quickly) than controls suggesting that general learning impairments are unlikely to explain the decision-making impairments they display in the discounting task.
Probability-Discounting Task.
Animals were tested on a concurrent instrumental response task involving the presentation of two levers, one associated with the certain delivery (100%) of two sucrose pellets and the other associated with the probabilistic delivery (either 75%, 50%, or 25%) of four pellets. Each daily 45-min session consisted of 24 forced choice trials followed by 24 free choice trials. At the start of each session the chamber was in the intertrial interval state, completely dark with no light cues. All trials began with illumination of the house light and a light in the food tray cueing the animal to make a nose-poke into the food tray within 10 s. This ensured that the subject was centered in the chamber at the start of each trial, minimizing position bias. Failure to make a nose-poke response resulted in termination of the trial and the chamber returned to the intertrial interval state. During training animals were exposed to forced choices wherein a successful nose-poke led to the extension of a single lever presented pseudorandomly. A response was required within 10 s or the trial was terminated and the chamber returned to the intertrial interval. A successful response resulted in the illumination of the tray light and the delivery of reward based on the associated probability followed by an intertrial interval of 45 s. Forced choice sessions consisted of 24 trials. These trials served to expose the animal to each option and its associated expected value. During each session, forced choice trials were followed by free choice trials with the same probability in effect for the uncertain lever. Free choice trials followed the guidelines described above but each successful nose-poke resulted in the extension of both levers and the animal was free to choose between the two levers within 10 s. Thus, this session offered the animal a choice between the two levers to assess the animal's preference between options. Similar to recent choice paradigms, probabilities were kept static within a session and only altered between sessions (30). Lever choice was recorded and analyzed using repeated measures ANOVA.
Modeling Risk Neutral Response Allocation.
Although optimal (risk neutral) behavior suggests that an individual should consistently choose the option with the higher expected value, iterative decision making under a variety of conditions is typically much more variable. Assessing behavior in reference to optimality may therefore lead to inaccurate conclusions concerning risk preference or aversion. To account for this variance while modeling risk-neutral behavior we used a variation of the Matching Law, an equation originally formulated to account for the response allocation of animals under concurrent free operant conditions (31), which has been extended to a variety of paradigms and species (32–36). Matching Law posits that under free operant conditions a subject allocates responses in a proportion that matches the relative reinforcement of the available options. Using a derivative of this mathematical framework to account for iterative choices with probabilistic rates of reinforcement, we used the current value matching equation to model risk-neutrality:
where
In the equation above, PA, assuming multiple trials, is the average choice of option A while VA is the expected value associated with option A and VB is the expected value associated with option B. Thus, the equation states that the average choice of option A is equal to the expected value of A (VA) divided by the cumulative expected value of all options (VA + VB). This equation attempts to model the notion that, assuming risk-neutrality, choices averaged over trials will be directly proportional to the expected value of the available options. We used this equation as a model of risk-neutral choice for each probability condition of the discounting task (75%, 50%, and 25%) as well as for the average of all conditions on the probability-discounting task.
Acknowledgments.
We thank Paul Phillips and Mark Walton for their helpful suggestions and Scott Ng-Evans for his considerable technical assistance. This research was supported by National Institutes of Health Grants DA014609, AA014742, and AA018053.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Stansfield KH, Kirstein CL. Chronic cocaine or ethanol exposure during adolescence alters novelty-related behaviors in adulthood. Pharmacol Biochem Behav. 2007;86:637–642. doi: 10.1016/j.pbb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Vuchinich RE, Simpson CA. Hyberbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharm. 1999;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- 3.Bjork JM, Hommer DW, Grant ST, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 5.Bechara A, Damasio H. Decision making and addiction (part I): Impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 6.Rowland NE, Nasrallah N, Robertson KL. Accurate caloric compensation in rats for electively consumed ethanol-beer or ethanol-polycose mixtures. Pharmacol Biochem Behav. 2005;80:109–114. doi: 10.1016/j.pbb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Peris J, et al. Brain ethanol levels in rats after voluntary ethanol consumption using a sweetened gelatin vehicle. Pharmacol Biochem Behav. 2006;85:562–568. doi: 10.1016/j.pbb.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, et al. High temporal resolution of amino acid levels in rat nucleus accumbens during operant ethanol self-administration: Involvement of elevated glycine in anticipation. J Neurochem. 2008;106:170–181. doi: 10.1111/j.1471-4159.2008.05346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crews F, He J, Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 11.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Amer J Psych. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White AM, Swartzwelder HS. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Rec Dev Alcoholism. 2005;17:161–176. doi: 10.1007/0-306-48626-1_8. [DOI] [PubMed] [Google Scholar]
- 13.Gogtay N, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- 14.Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism: Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- 15.Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcoholism: Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: Differential impact on subsequent responsiveness to ethanol. Alcoholism: Clin and Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- 17.Crews F, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism: Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- 18.Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: A longitudinal study. Alcoholism: Clin Exp Res. 2007;31:928–938. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson CA, et al. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia. 2008;46:714–726. doi: 10.1016/j.neuropsychologia.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips PE, Walton ME, Jhou TC. Calculating utility: Preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology. 2007;191:483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- 21.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision making. J Neurosci Aug 1. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loewenstein G, Rick S, Cohen JD. Neuroeconomics Annual Reviews Psychology. 2008;59:647–672. doi: 10.1146/annurev.psych.59.103006.093710. [DOI] [PubMed] [Google Scholar]
- 23.Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psych Bull. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazur JE. Choice between small certain and large uncertain reinforcers. Ani Learn Behav. 1988;16:199–205. [Google Scholar]
- 25.Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neuroscience. 2005;28:37. doi: 10.1186/1471-2202-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balci F, Freestone D, Gallistel CR. Risk assessment in man and mouse. Proc Natl Acad Sci USA. 2009;106:2459–2463. doi: 10.1073/pnas.0812709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinzie DL, McBride WJ, Murphy JM, Lumeng L, Li TK. Rat lines selectively bred for alcohol preference: A potential animal model of adolescent alcohol drinking. In: Hannigan JH, Spear LP, Spear NE, Goodlett CR, editors. Alcohol and Alcoholism: Effects on Brain and Development. Mahweh, NJ: Lawrence Erlbaum Associates; 1999. pp. 135–160. [Google Scholar]
- 28.Bell RL, et al. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol. 2003;29:137–148. doi: 10.1016/s0741-8329(03)00022-3. [DOI] [PubMed] [Google Scholar]
- 29.Bell RL, et al. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on ethanol intake by periadolescent high-alcohol-drinking rats. Alcohol. 2004;33:107–115. doi: 10.1016/j.alcohol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Walton ME, Kennerley SW, Bannerman DM, Phillips PE, Rushworth MF. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Networks. 2006;19:1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrnstein RJ. Relative and absolute strength of responses as a function of frequency of reinforcement. J Exp Anal Behav. 1961;4:267–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Villiers PA, Herrnstein RJ. Toward a law of response strength. Psychol Bull. 1976;83:1131–1153. [Google Scholar]
- 33.Mark TA, Gallistel CR. Kinetics of matching. J Exp Psychol Anim Behav Process. 1994;20:79–95. [PubMed] [Google Scholar]
- 34.Anderson KG, Velkey AJ, Woolverton WL. The generalized matching law as a predictor of choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2002;163:319–326. doi: 10.1007/s00213-002-1012-7. [DOI] [PubMed] [Google Scholar]
- 35.Soltani A, Wang XL. A biophysically based neural model of matching law behavior: Melioration by stochastic synapses. J Neurosci. 2006;26:3731–3744. doi: 10.1523/JNEUROSCI.5159-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallistel CR, et al. Is matching innate? J Exp Anal Behav. 2007;87:161–199. doi: 10.1901/jeab.2007.92-05. [DOI] [PMC free article] [PubMed] [Google Scholar]