Abstract
The self-organization of multicomponent supramolecular systems involving a variety of two-dimensional (2-D) polygons and three-dimensional (3-D) cages is presented. Nine self-organizing systems, SS1–SS9, have been studied. Each involving the simultaneous mixing of organoplatinum acceptors and pyridyl donors of varying geometry and their selective self-assembly into three to four specific 2-D (rectangular, triangular, and rhomboid) and/or 3-D (triangular prism and distorted and nondistorted trigonal bipyramidal) supramolecules. The formation of these discrete structures is characterized using NMR spectroscopy and electrospray ionization mass spectrometry (ESI-MS). In all cases, the self-organization process is directed by: (1) the geometric information encoded within the molecular subunits and (2) a thermodynamically driven dynamic self-correction process. The result is the selective self-assembly of multiple discrete products from a randomly formed complex. The influence of key experimental variables – temperature and solvent – on the self-correction process and the fidelity of the resulting self-organization systems is also described.
Keywords: Multicomponent Supramolecular Systems, Self-Organization, Supramolecular polygons, Supramolecular Cages, Self-Assembly
Introduction
The implementation of molecular information to direct self-assembly based on specific noncovalent interactions1 (e.g., hydrogen bonding, metal ligand coordination or electrostatic forces, etc) has facilitated the rapid development of supramolecular chemistry from the pioneering work of Lehn, Cram, and Pederson2 to the present detailed investigations of constructing individual well-defined functionalized inorganic3 or organic supramolecular structures.4 Closely related fields, such as material science and biochemistry, have also benefited from merging with supramolecular chemistry. Examples include the development of supramolecular polymers,5 DNA nanofabrication,6 chemical sensing ensembles,7 drug delivery,8 and membrane transport agents.9 With the continual development of supramolecular chemistry over the last three decades one thing has remained largely constant: self-assembly has focused primarily on the formation of a single supramolecular product. This observation contrasts with biological systems in that complex biological systems and their corresponding functions are achieved by the coexistence and collaboration of multiple biological supramolecular components rather than single discrete supramolecular species. Examples include DNA replication,10 RNA transcription11 and posttranslational modification etc,12 all of which are accomplished by the collaboration of various enzymes within complex biological systems. With the aim of obtaining insight into multicomponent biological systems, it is necessary to explore the controllable and organized self-assembly of multicomponent supramolecular systems.
Self-organization13 is a natural phenomenon capable of spontaneously forming multiple ordered structures from within a system composed of many individual subunits. By designing molecular subunits with specific geometric and/or electronic properties, self-organization can enable dynamic self-assembly that extends beyond the formation of a single supramolecular entity to the simultaneous assembly of multiple ordered supramolecular structures within one complex system.13 Insight into such supramolecular behaviour may open avenues towards the efficient construction of multicomponent supramolecular systems for synthetic chemists. Indeed, in the last decade, numerous efforts have been made by pioneers such as Lehn,14a Raymond,14b and Isaacs13c to obtain insight into self-organization. Following the first example demonstrated by Lehn and cowokers14a wherein multiple different supramolecular helicates were simultaneously self-assembled, synthetic self-organized systems have been developed based on metal ligand coordination bonding,14 hydrogen bonding,15 solvophobic effects,16 and dynamic covalent interactions.17
Upon inspection of these pioneering studies, it is found that the geometric features of molecular subunits play a significant role in self-organization. Geometric features enable the success of the synthetic self-organization and often simultaneously dictate the structural features of the assembled supramolecular entities.14a,b As a representative example of geometrically directed self-organization, Raymond et al. have demonstrated the simultaneous self-assembly of three metallo-supramolecular triple helicates that relied solely on the length of a rigid spacer separating two catecholate binging sites.14b It is clear that the use of geometric information within molecular subunits to drive self-organization not only enables the assembly of multicomponent supramolecular systems but also provides a means of controlling the structural features of the resulting systems, which is key to manipulating the multicomponent supramolecular systems. Exploration of such geometry directing self-organization is necessary to obtain a greater understanding and better control of the synthesis of multicomponent supramolecular systems. To the best of our knowledge, however, reports focusing on such self-organization are still relatively few.14a,b
In the last few years, the metal ligand coordination-driven self-assembly of supramolecular polygons and polyhedrons has been well developed.18 Since the development of such self-assembly provides multiple building blocks for achieving geometry directing coordination-driven self-organization systems, we have based our entry into multicomponent supramolecular systems on self-organization that depends on the geometric features of directional molecular building blocks.19 Recently, we have demonstrated the two-component self-organization of either 2-D or 3-D supramolecular structures of varying size or dimensionality driven by one specific type of geometric information (angle19a,b or size19c). The high-fidelity self-organization of the discrete supramolecular species can be achieved, over time, through a thermodynamically determined self-correction process by excluding thermodynamically unfavored oligomeric species that are derived from the random combination of different ligands.19
As a further step into ordered multicomponent supramolecular systems of higher complexity, it is necessary to explore the geometry selective self-organization of more supramolecular components and higher structural diversity. Increasing the diversity of molecular components in the mixtures will amplify the complexity of the initially formed oligomeric structures.19 Self-correction processes in such mixtures require an even greater degree of selectivity to regulate disordered oligomeric species into discrete supramolecular species. Many different geometric features (such as size, angle, and number of binding sites) must be encoded within the molecular building blocks to conduct such self-organization. Thus, the development of self-organization toward higher diversity and complexity involving multiple 2-D and 3-D supramolecular structures is a considerable challenge.
With the aim of developing multicomponent supramolecular systems of high structural diversity, we have carried out a detailed study of nine self-organization systems, SS1–SS9. Each system shows selective self-assembly of three to four 2-D and/or 3-D supramolecular components comprising six different structural motifs (rectangular,20 triangular,21 rhomboid,22 distorted and nondistorted triangular prism23 and triangular bipyramidal24). Through these nine self-organization systems, geometric differences encoded within the rigid and directional molecular components are the major driving force directing the self-organization process. Such geometric information both efficiently directs these self-organization processes while excluding the thermodynamically less favoured oligomeric assemblies, and also reveals the generality of the coordination-driven self-organization of supramolecular structures. To obtain insight into the dynamic nature of such complicated supramolecular self-organization processes, we also explored the effects of temperature and solvent choice.
Results and Discussion
Self-organization with “molecular clip” 1
Di-platinum(II) molecular clip 1 has been previously used to self-assemble individual supramolecular 2-D rectangles20 and 3-D triangular prisms.23 Self-organization studies involving molecular clip 1 with pyridyl donors 2–6 may provide an efficient approach toward the construction of multicomponent supramolecular systems involving various 2-D structures and 3-D assemblies.
The self-organization system SS1 was produced by mixing molecular clip 1 with different sized linear bipyridyl linkers 2 and 3 and a tripyridyl donor 4 in a 7:2:2:2 ratio as shown in Scheme 1. After heating at 65–70 °C in an aqueous acetone solution (v/v 1:1) for 24 h, the 2-D supramolecular rectangles RS and RL and 3-D distorted triangular prism DTPS were formed as the dominant products. The self-organization process was monitored by 31P and 1H multinuclear NMR spectroscopy as shown in Figures 1 and 2, respectively.
Scheme 1.
Graphical representation of self-organization systems SS1–SS4 involving diverse coordination-driven self-assembly: small rectangle (RS), large rectangle (RL), small distorted triangular prism (DTPS), large distorted triangular prism (DTPL), and nondistorted triangular prism (NTP).
Figure 1.

31P{1H} NMR spectra (Acetone-d6/D2O 1:1) recorded at 1 h, 6 h and 24 h time intervals during the formation of supramolecular rectangles RS and RL and distorted triangular prism DTPS.
Figure 2.

1H NMR spectra (Acetone-d6/D2O 1:1) recorded at 1 h, 6 h and 24 h time intervals during the formation of supramolecular rectangles RS and RL and distorted triangular prism DTPS.
After 1 h of heating at 65–70 °C, SS1 changed from a suspension to a clear solution. The 31P{1H} NMR spectrum (Figure 1) of the reaction mixture showed a highly complex pattern of signals that are representative of disordered oligomeric species as the result of random combinations of molecular subunits (1, 2, 3, and 4). Similarly, broad peaks belonging to oligomeric structures were found in the 1H NMR spectrum (Figure 2). Within the ESI mass spectrum (Figure 3) of the mixture, signals corresponding to fragments of the randomly formed oligomers can be found at 1256.6 for [2(1) + 2(2) − 2NO3]2+, 899.5 for [2(1) + 2(3) − 3NO3]3+, 888.8 for [2(1) + 2(4) − 3NO3]3+, 858.2 for [2(1) + (2) + (3) − 3NO3]3+, 1310.0 for [2(1) + (2) + (4) − 2NO3]2+, and 1372.4 for [2(1) + (3) + (4) − 2NO3]2+, which further confirm the initially random assembly of these building blocks shortly after mixing.
Figure 3.

Full ESI mass spectrum (Acetone-d6/D2O 1:1) recorded after 1 h and 24 h during the self-organization of supramolecular rectangles RS and RL and distorted triangular prism DTPS.
After 24 h, however, only three sets of sharp signals (consistent with those previously reported for RS,20 RS20, and DTPS 23a) can be observed in the NMR spectra as shown in Figures 1 and 2, indicating that the initial randomly formed oligomeric species have been dynamically and selectively converted to specific supramolecular assemblies (RS, RL, and DTPS) in the equilibrated mixture. The selective formation of supramolecuar rectangles RS and RL and distorted triangular prism DTPS was further confirmed by ESI mass spectrometry as shown in Figure 3. The ESI mass peaks corresponding to the consecutive loss of nitrate anions from the prism DTPS: m/z = 1944.8 [M - 2NO3]2+ and m/z = 1275.9 [M - 3NO3]3+ are observed, as are those corresponding to the formation of the different sized rectangles, RS: m/z = 1256.7 [M - 2NO3]2+ and m/z = 816.9 [M - 3NO3]3+, and RL: m/z = 1380.3 [M - 2NO3]2+ and m/z = 899.2 [M - 3NO3]3+, each of which is in agreement with those reported previously.19c
The multinuclear NMR and mass spectral results described above support the self-organization of RS, RL and DTPS and also present a typical geometry selective self-organization process that regulates disordered oligomeric species into discrete supramolecular species (Scheme 2).19 Upon mixing, disordered oligomeric structures can be formed as major species by random combinations of the molecular subunits. This is observed in the NMR and ESI-MS spectra recorded after 1 h. However, the kinetically liable nature of the Pt-N coordination bond allows a self-correction process within the mixture and the ultimate product distribution is determined by the thermodynamic stability of each species. Disordered oligomeric species of lower stability can be dynamically self-corrected to the thermodynamically preferred discrete supramolecular entities,19 as witnessed by the simplified NMR and ESI-MS spectra recorded after 24 h. Such thermodynamically driven self-correction results in the exclusive formation of multiple discrete supramolecular entities.
Scheme 2.

Graphical representation of the self-organization process in SS1 regulating randomly formed and disordered oligomeric structures into an ordered multicomponent supramolecular system of RS, RL, and DTPS.
The results of one such study, however, are not able to definitively show whether such geometry directed self-organization is an exceptional case or a general behavior for the coordination driven self-assembly of supramolecular polygons and 3-D cages. To further understand such geometry directing self-organization processes, the investigation of mixed self-assemblies incorporating multiple 2-D and 3-D superstructures RS, RL, DTPS, DTPL, and NTP was carried out by examining their self-organization behavior.
Three different self-organization systems SS2–SS4, each involving three of the five species RS, RL, DTPS, DTPL, and NTP, were prepared by mixing multiple molecular subunits in an aqueous acetone solution (Scheme 1). Disordered oligomeric species were initially formed in all mixtures as indicated by the 31P and the 1H NMR spectra recorded periodically for these self-organization systems (see Supporting Information, Figures S5–S7). These disordered mixtures slowly (24–48 h) converted into the discrete supramolecular structures via a dynamic self-correction process, as evidenced by the sharpening of their NMR spectra over a 24–48 h period of heating at 65–70 °C. As a result, the assemblies RS, RL,20 DTPS, DTPL23a, and NTP23b were formed as the predominant species within the self-organization systems SS2–SS4, as supported by the sharp and identifiable spectroscopic signals in the 31P{1H} NMR spectra (SS2: 8.32 ppm for RS; 8.83 ppm for DTPL; 9.81 ppm for DTPS, SS3: 8.32 ppm for RS; 8.48 ppm for RL; 8.65 ppm for NTP, and SS4: 8.32 ppm for RS; 8.65 ppm for NTP; 9.81 ppm for DTPS; Figure 4). In both SS3 and SS4 a small peak at 9.01 ppm appears and is believed to indicate a small amount of disordered byproduct. The 1H NMR spectra (Figures 5) show intense identifiable signals corresponding to the different discrete supramolecular species, each of which is consistent with that reported previously,20,23 despite partial overlapping of some signals.
Figure 4.

Equilibrated 31P{1H} NMR spectra (Acetone-d6/D2O 1:1) recorded for self-organization systems (a) SS2 (RS, DTPS, and DTPL), (b) SS3 (RS, RL, and NTP), and (c) SS4 (RS, DTPS, and NTP).
Figure 5.

Partial 1H NMR spectra (Acetone-d6/D2O 1:1) recorded for equilibrated self-organization systems (a) SS2 (RS, DTPS, and DTPL), (b) SS3 (RS, RL, and NTP), and (c) SS4 (RS, DTPS, and NTP).
The formation of discrete supramolecular rectangles and 3-D cages in the complex mixtures SS2–SS4 is further characterized by ESI mass spectrometry (Figure 6). Identifiable signals for the consecutive loss of nitrate anions from RS, RL, DTPS, DTPL and NTP can be found in the ESI mass spectra of SS2–SS4: e.g. intense peaks for RS: m/z = 1256.7 [M - 2NO3]2+ and m/z = 816.9 [M - 3NO3]3+, RL: m/z = 1380.3 [M - 2NO3]2+ and m/z = 899.2 [M - 3NO3]3+, DTPS: m/z = 1944.8 [M - 2NO3]2+ and m/z = 1275.9 [M - 3NO3]3+, DTPL: m/z = 1474.5 [M - 3NO3]3+ and m/z = 1090.4 [M - 4NO3]4+, and NTP: m/z = 1354.1[M - 3NO3]3+ and m/z = 1000.4 [M - 4NO3]4+. Each of these peaks is consistent with those previously reported.19b,c The combined evidence from 31P and 1H multinuclear NMR spectroscopy as well as ESI mass spectrometry clearly indicates that high-fidelity self-organization has been achieved in systems SS2–SS4.
Figure 6.
Full ESI mass spectrum (Acetone-d6/D2O 1:1) of equilibrated self-organizing systems (a) SS2 (RS, DTPS, and DTPL), (b) SS3 (RS, RL, and NTP), and (c) SS4 (RS, DTPS, and NTP).
The geometric information encoded within the size, angle and number of binding sites of various pyridyl donors is sufficient to direct structurally diverse self-organization involving different sized 2-D supramolecular rectangles (RS and RL) as well as a variety of 3-D triangular prisms (DTPS, DTPL, and NTP). The successful achievement of self-organization in systems SS1–SS4 indicates the generality of self-organization behavior for the self-assembly of these structural motifs – rectangles and distorted and nondistorted triangular prisms – which is the first step toward the construction of highly diverse multicomponent supramolecular systems via self-organization.
Self-organization with 60° organoplatinum acceptor 7
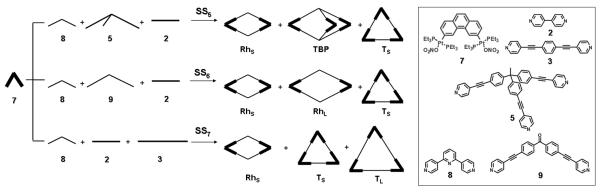
To further demonstrate the generality of geometry selective self-organization in coordination-driven self-assembly, detailed investigations into supramolecular species assembled by additional molecular components are necessary. Thus a 60° organoplatinum acceptor,21 a widely utilized molecular subunit capable of assembling 2-D supramolecular rhomboids25 and triangles21 as well as 3-D triangular bipyramids,24 has been investigated.
Three different self-organization systems SS5–SS7, each involving three of the five different assemblies TS, TL, RhS, RhL, and TBP, were prepared as shown in Scheme 3. The 60° organoplatinum acceptor 7 was mixed with complementary ditopic and tritopic pyridyl donors 2, 3, 5, 8, and 9 in an aqueous acetone solution (v/v 1:1) and heated at 65–70 °C. The 31P and 1H multinuclear NMR spectra of these mixtures were used to follow these self-organization processes. Significant sharpening over time of both the 31P{1H} and 1H NMR spectra was observed for each mixture during continued heating (24–48 h) (see Figures S8–S10 in the Supporting Information). These results indicate that the previously described dynamic self-correction process occurs in these self-organization systems to regulate the randomly formed disordered oligomeric species into discrete multicomponent assemblies. Figures 7 and 8 present the 31P{1H} and the 1H NMR spectra of self-organization systems SS5–SS7 after full equilibration. As expected, the 31P{1H} NMR spectra for each of SS5–SS7 (Figures 7a–c) show peaks that are consistent with those of the individually prepared assemblies (SS5: 14.31 ppm for TS; 14.40 ppm for TBP; 14.43 ppm for RhS, SS6: 14.31 ppm for TS; 14.38 ppm for RhL; 14.43 ppm for RhS, and SS7: 14.31 ppm for TS; 14.43 ppm for RhS), though overlap of signals from TL and RhS of SS7 at 14.41 ppm can be seen. In addition, three sets of signals for discrete self-assembled aggregates19c,21,24,25 in each self-organization system can be clearly identified in each 1H NMR spectrum (Figure 8a–c), though some small broad peaks associated with minor amounts of disordered structures can also be found in the 1H NMR spectra (SS5: 7.12 ppm, 7.82 ppm, and 8.12 ppm; SS6: 7.48 ppm; SS7: 7.48 ppm, 8.12 ppm, and 8.54 ppm).
Scheme 3.
Graphical representation of self-organization systems SS5–SS7 involving diverse coordination-driven self-assembly: small rhomboid (RhS), large rhomboid (RhL), small triangle (TS), large triangle (TL), and triangular bipyramid (TBP).
Figure 7.
Equilibrated 31P{1H} NMR spectra (Acetone-d6/D2O 1:1) recorded for self-organizing systems (a) SS5 (TS, RhS, and TBP), (b) SS6 (TS, RhS, and RhL), and (c) SS7 (TS, TL, and RhS).
Figure 8.

Partial 1H NMR spectra (Acetone-d6/D2O 1:1) recorded for equilibrated self-organizing systems (a) SS5 (TS, RhS, and TBP), (b) SS6 (TS, RhS, and RhL), and (c) SS7 (TS, TL, and RhS).
The formation of discrete supramolecular species TS, TL, RhS, RhL, and TBP in complex mixtures SS5–SS7 is further supported by ESI mass spectrometry as shown in Figure 9. Signals for the consecutive loss of nitrate anions from TS, TL, RhS, RhL, and TBP (TS: m/z = 1916.6 [M - 2NO3]2+ and m/z = 927.06 [M - 4NO3]4+, TL: m/z = 1381.5 [M - 3NO3]3+ and m/z = 1020.6 [M - 4NO3]4+, RhS: m/z = 1333.4 [M - 2NO3]2+ and m/z = 868.3 [M - 3NO3]3+, RhL: m/z = 1484.5 [M - 2NO3]2+ and m/z = 969.0 [M - 3NO3]3+, and TBP: m/z = 1474.5[M - 3NO3]3+ and m/z = 1090.4 [M - 4NO3]4+) are observed in the ESI mass spectra and are consistent with previously reported results.19c,21,24,25 The results of both multinuclear NMR spectroscopy and ESI mass spectrometry indicate successful self-organization within systems SS5–SS7, which incorporate a high degree of structural diversity (triangles, rhomboids and triangular bipyramids).
Figure 9.
Full ESI mass spectrum (Acetone-d6/D2O 1:1) of equilibrated self-organizing systems (a) SS5 (TS, RhS, and TBP), (b) SS6 (TS, RhS, and RhL), and (c) SS7 (TS, TL, and RhS).
Self-organization with both molecular clip 1 and the 60° organoplatinum acceptor 7
Additional investigations exploring geometry directed self-organization that involves the combination of two organic donors and two organoplatinum acceptors have also been performed. Self-organization system SS8, for example, involves mixing molecular clip 1 and the 60° organoplatinum acceptor 7 with different sized linear dipyridyl donors 2 and 3 in a 1:1:1:1 ratio in the aqueous acetone solution (v/v 1:1). The complex mixture is capable of simultaneously self-assembling four supramolecular metallacycles of varying size as shown in Scheme 4. The self-organization process was followed by 31P and 1H multinuclear NMR spectroscopy (see Figure S11 of the Supporting Information). Disordered oligomeric structures were initially formed as demonstrated by signals at 7.54 ppm, 8.94 ppm, 9.11 ppm 14.03 ppm, and 15.80 ppm in the 31P{1H} NMR spectra as well as unidentifiable broad peaks in the 1H NMR spectra recorded at 6 h and 24 h time intervals. A longer heating time of 72 h at 65–70 °C was necessary to accomplish self-organization. The 31P{1H} and the 1H NMR spectra of the resulting mixture are shown in Figure 10. Four peaks corresponding to the different sized supramolecular rectangles and triangles at 8.32 ppm (RS 20), 8.48 ppm (RL 20), 14.32 ppm (TS 21), and 14.40 ppm (TL 19c) are seen in the 31P{1H} NMR spectrum (Figure 10a). Likewise, four sets of signals for these different sized rectangles and triangles can be clearly identified within the 1H NMR spectrum as shown in Figure 10b, the chemical shifts of which are consistent with those previously reported.19c,20,21 Certain unassignable signals that are representative of a small amount of disordered byproducts, however, can still be found as small peaks in the 1H NMR spectrum such as those at 7.45 ppm and 8.90 ppm. These peaks cannot be eliminated even with longer heating. ESI mass spectrometry was also used to characterize the products of SS8. Intense ESI mass peaks corresponding to the consecutive loss of nitrate anions from four self-organized supramolecular structures can be observed as shown in Figure S13 of the Supporting Information.
Scheme 4.
Graphical representation of self-organization systems SS8 and SS9 involving diverse coordination-driven self-assembly: small rectangle (RS), large rectangle (RL), large distorted triangular prism (DTPL), small triangle (TS), large triangle (TL), and triangular bipyramid (TBP).
Figure 10.

31P{1H} (a) and Partial 1H (b) NMR spectra (Acetone-d6/D2O 1:1) recorded for equilibrated self-organizing system SS8 (RS, RL, TS, and TL).
The use of two organoplatinum acceptors and two organic donors in such complex self-organization systems can be extended to three-dimensions by mixing molecular clip 1 and the 60° acceptor 7 with ditopic and tritopic pyridyl donors 2 and 5 in a 6:6:3:2 ratio, SS9. This self-organization system is capable of forming 2-D supramolecular polygons (rectangle RS and triangle TS) as well as 3-D cages (distorted triangular prism DTPL and triangular bipyramid TBP) as shown in Scheme 4. 31P and 1H multinuclear NMR spectroscopy were used to study the self-organization of SS9 as shown in Figure S12 of the Supporting Information. The mixture remained highly disordered after 6 h of heating, as indicated by unassignable signals at 8.83 ppm, 13.98 ppm, and 15.97 ppm in the 31P{1H} NMR spectra (Figure S12a). Over time and with continued heating, these oligomeric peaks decreased as the peaks of RS, TS, DTPL, and TBP sharpened indicating that the mixture slowly approached equilibrium. After 96 h of heating at 65–70 °C, four different 2-D and 3-D supramolecular structures (RS, TS, DTPL, and TBP) were generated from SS9 as supported by 31P and 1H multinuclear NMR spectroscopy (Figure 11) as well as ESI mass spectrometry (Figure S14 in the Supporting Information). In the 31P{1H} NMR spectrum (Figure 11a), peaks at 8.33 ppm (RS 20), 8.83 ppm (DTPL 23a), 14.32 ppm (TS 21), and 14.39 ppm (TBP24) clearly indicate the formation of the four discrete supramolecular species as the predominant products of the reaction. Similarly, the 1H NMR spectrum (Figure 11b) displayed identifiable signals belonging to RS,20 TS,21 DTPL,23a and TBP24, along with small broad peaks attributable to disordered structures (7.88 ppm and 9.05 ppm). Data from ESI-MS studies also indicated the formation of the four supramolecular entities (Figure S14).
Figure 11.

31P{1H} (a) and Partial 1H (b) NMR spectra (Acetone-d6/D2O 1:1) recorded for equilibrated self-organizing system SS9 (RS, TS, DTPL, and TBP).
SS8 and SS9 indicate that self-organization can be achieved in complex mixtures of two different organoplatinum acceptors as well as two organic donors. Compared with self-organization systems SS1–SS7 discussed previously, SS8 and SS9 require longer reaction times (72 h and 96 h) to reach equilibrium, presumably because of the increased complexity of the disordered byproducts encountered during assembly. Furthermore, the use of four molecular subunits in SS8 and SS9 allows for the simultaneous assembly of four supramolecular species of high structural diversity, while only three entities can be formed within SS1–SS7. Such self-organization provides a more efficient way to assemble multicomponent supramolecular systems of high structural diversity.
Variables that affect the fidelity of self-organization processes
In addition to differences in geometry, it has been shown that both temperature and solvent can and do affect self-organization, and the effects of varying these two parameters have been investigated in the cases of SS1 and SS6.
Temperature
To investigate the effect of temperature changes on self-organization processes, the molecular subunits of SS1 were mixed in an aqueous acetone solution (v/v 1:1) and heated at 65–70 °C for 1 h. As a result, disordered oligomeric species were formed via the initial random combination of acceptors and donors as implied by the 31P{1H} (Figure 12a) and the 1H (Figure S15a of the Supporting Information) NMR spectra. This mixture was then separated into three samples that were kept at 25–30 °C, 45–50 °C, and 65–70 °C respectively until each reached thermodynamic equilibrium. 31P and 1H multinuclear NMR spectroscopy were used to monitor the self-organization process within each mixture. Results from the 31P{1H} NMR spectra of these three mixtures (Figure 12b–d) as well as the 1H NMR spectra (Figure S15b–d in the Supporting Information) indicate that a decrease of temperature causes a significant decrease in the rate of the self-organization process. Decreasing the reaction temperature from 65–70 °C to 45–50 °C increased the time required for self-organization from 24 h to 120 h. At even lower temperatures of 25–30 °C the self-organization process occurs extremely slowly and did not reach equilibrium after 20 d. Similar temperature effects can be found within self-organization system SS6 as indicated by the multinuclear NMR spectral results shown in Figures S16 and S17.
Figure 12.

31P{1H} NMR spectra (Acetone-d6/D2O 1:1) recorded for mixtures of SS1 (RS, RL, and DTPS) heated at (a) 65–70 °C for 1 h, (b) 65–70 °C for 24 h, (c) 45–50 °C for 120 h, and (d) 25–30 °C for 20 d.
Solvent
Self-assembly is also sensitive to changes in solvent due to the different thermodynamic stabilities of species intermediate and final formed in different media.20 To test how solvent affects self-organization, studies of both SS1 and SS6 were carried out in two different solvents: CD2Cl2 and Acetone-d6/D2O (20:1). The self-organized mixtures were obtained by: (1) initially heating an aqueous acetone solution (v/v 1:1) for 24 h, then (2) concentrating the solution to dryness and redissolving in either CD2Cl2 or Acetone-d6/D2O (20:1), and finally (3) heating each (CD2Cl2: 45–50 °C; Acetone-d6/D2O (20:1): 60–65 °C) until achieving equilibrium. As indicated by 31P and 1H multinuclear NMR spectroscopy (Figures 13, S18, S19, and S20), the change of solvent shows little effect in SS6 but significantly effects the self-organization system SS1.
Figure 13.
31P{1H} NMR spectra of SS1 in various solvents: (a) Acetone-d6/D2O (1:1), (b) CD2Cl2, (c) Acetone-d6/D2O (20:1), (d) Acetone-d6/D2O (1:1) after removal of CD2Cl2, and (e) Acetone-d6/D2O (1:1) after removal of Acetone-d6/D2O (20:1).
Mixtures of SS6 in either CD2Cl2 or Acetone-d6/D2O (20:1) were shown capable of self-organization after 36 h of heating as indicated by identifiable signals corresponding to the discrete supramolecular species in the 31P{1H} and the 1H NMR spectra (Figure S19 and S20 of the Supporting Information). However, in the case of SS1, initially organized supramolecular mixtures of RS, RL, and DTPS that were transferred to CD2Cl2 and Acetone-d6/D2O (20:1) solutions were significantly disordered after 36 h heating. The three original intense peaks with were replaced by multiple unassignable signals within the 31P{1H} NMR spectra (Figure 13b–c) and the appearance of broad signals in the 1H NMR spectra (Figure S18b–c of the Supporting Information). Thus, destruction of the ordered supramolecular species occurred as a result of the change of solvent for SS1. However, it was found that the solvent effects on SS1 were reversible. Upon concentrating the disordered CD2Cl2 and Acetone-d6/D2O (20:1) mixtures of SS1 to dryness and re-dissolving in Acetone-d6/D2O (1:1), the self-organized mixture can be achieved again as shown by the 31P{1H} (Figure 13d–e) and the 1H (Figure S18d–e of the Supporting Information) NMR spectra. The combined evidence indicates that the choice of solvent is critical to the efficiency of self-organization in the coordination-driven self-assembly of organoplatinum supramolecules.
Conclusion
In summary, the thermodynamically driven self-organization of nine different complex mixtures (SS1–SS9) has been clearly demonstrated. Each mixture is capable of spontaneously and selectively producing multiple discrete supramolecular structures at the exclusion of less stable disordered assemblies. The selective formation of discrete self-organized products is supported by multinuclear NMR spectroscopy and ESI mass spectrometry. Various supramolecular species, from the self-assembly of 2-D supramolecular polygons to 3-D polyhedra, can be self-organized within complex mixtures containing a variety of complementary molecular subunits. Self-organization proceeds via a thermodynamically determined self-correction process that is largely dependent on the geometric information pre-coded within the subunits. Additionally, the experimental variables of temperature and solvent have been investigated and shown capable of affecting these self-organization systems. As expected, temperature changes have a dramatic effect on the rate of the self-organization process. Different solvents present a significant, yet reversible, change in the fidelity of self-organization.
More importantly, the successful self-organization in systems SS1–SS9, including ten different 2-D and 3-D supramolecular structures (RS, RL, DTPS, DTPL, NTP, TS, TL, RhS, RhL, and TBP) and six different structural motifs (rectangle, triangle, rhomboid, normal and distorted triangular prism, and triangular bipyramid), reveals the generality of geometry directed self-organization behavior in coordination-driven self-assembly of supramolecular polygons and cages. The results also represent a promising approach toward the build-up of structurally diverse multicomponent supramolecular systems. The implications of this research go beyond the comprehensive study of geometry selective self-organization in coordination driven self-assembly as many recent reports have been focused on the synthesis and self-assembly of functionalized supramolecules.26 Provided that high-fidelity self-organization can be extended to these new functionalized systems, the current research can be extended to the development of muti-funcitonalized multicomponent supramolecular systems. The spontaneous self-assembly and self-organization of multiple, discrete, functionalized supramolecules could lead to integrated systems wherein many functional molecules are able to collaborate to achieve functions in a manner akin to natural biological systems. Thus, we envision that detailed studies of the self-organization of multicomponent supramolecular systems can pave the way toward the construction of complex, functional systems and have implications in the related processes that govern the assembly of more complex biological structures.
Experimental Section
Methods and Materials
Molecular building blocks 1,20 3,19c 4,23a 5,23a 6, 23b and 721 were prepared according to literature procedures. Deuterated solvents were purchased from Cambridge Isotope Laboratory (Andover, MA). NMR spectra were recorded on a Varian Unity 300 spectrometer and 500 spectrometer. The 1H NMR chemical shifts are reported relative to residual solvent signals, and 31P NMR resonances are referenced to an external unlocked sample of 85% H3PO4 (δ 0.0). Mass spectra for SS1–SS9 were recorded on a Micromass Quattro II triple-quadrupole mass spectrometer using electrospray ionization with a MassLynx operating system.
General procedure for self-organization
Appropriate organoplatinum acceptors and pyridyl donors were placed in a 2-dram vial, followed by the addition of the mixed solvent Acetone-d6/D2O (v/v 1:1), which was then sealed with Teflon tape and immersed in an oil bath at 65–70 °C. The clear solution was periodically transferred to an NMR tube for analysis. After three or four sets of signals corresponding to discrete supramolecular structures were clearly presented in the NMR spectra with no further changes, the reaction mixture was characterized by ESI-MS.
Self-organization system SS1 (RS, RL, and DTPS)
Molecular “Clip” 1 (10.56 mg, 0.00908 mmol), 4,4'-dipyridyl 2 (0.40 mg, 0.0026 mmol), 1,4-bis(4-pyridylethynyl)benzene 3 (0.71 mg, 0.0025 mmol) and tritopic tecton 4 (0.70 mg, 0.0026 mmol) were added to 1.1 mL of mixed solvent. After 24 h heating, RS, RL and DTPS were formed predominantly. 1H NMR (Acetone-d6/D2O: 1/1, 300 MHz) for RS: δ 9.53 (s, 2H, H9), 9.21 (d, 3J = 5.8 Hz, 4H, Hα-Py), 9.18 (d, J = 5.4 Hz, 2H, Hα'-Py), 8.71 (d, 3J = 5.4 Hz, 4H, Hβ-Py), 8.53 (d, 3J = 5.4 Hz, 4H, Hβ'-Py), 8.37 (s, 2H, H10), 7.71 (d, 3J = 10.5 Hz, 4H, H4,5), 7.62 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; RL: δ 9.45 (s, 2H, H9), 9.03 (d, 4H, 3J = 5.1 Hz, Hα-Py), 8.95 (d, 4H, 3J = 5.4 Hz, Hα'-Py), 8.36 (s, 2H, H10), 8.03 (m, 8H, Hβ-Py), 7.74 (s, 8H, Hphenylene), 7.71 (d, 4H, 3J = 10.5 Hz, H4,5), 7.64 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; DTPS: δ 9.14 (d, 6H, 3J = 6.3 Hz, Hα-Py), 8.98 (m, 9H, Hα'-Py,9), 8.36 (s, 3H, H10), 8.25 (d, 6H, 3J = 4.2 Hz, Hβ-Py), 7.72 (m, 12H, H 3β'-Py, H4,5), 7.68 (d, 6H, 3J = 9.9 Hz, H2,7), 7.14 (m, 6H, H3,6), 1.40 (m, 72H, PCH2CH3), 0.82 (m, 108H, PCH 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) for RS: δ 8.32 (195Pt satellites, 1JPt-P = 2640 Hz) ppm; RL: δ 8.48 (195Pt satellites, 1JPt-P = 2640 Hz) ppm; DTPS: δ 9.81 (195Pt satellites, 1JPt-P = 2651 Hz) ppm. MS (ESI) for RS (C96H152N8O12P8Pt4): m/z: 1256.7 [M - 2NO3]2+, 816.9 [M - 3NO3]3+; R1 (C116H160N8O12P8Pt4): m/z: 1380.3 [M - 2NO3], 899.2 [M - 3NO3]3+; DTPS (C146H230N12O20P12Pt6): m/z: 1944.8 [M - 2NO3]2+, 1275.9 [M - 3NO3]3+.
Self-organization system SS2 (RS, DTPS, and DTPL)
Molecular “Clip” 1 (11.98 mg, 0.0103 mmol), 4,4'-dipyridyl 2 (0.43 mg, 0.0028 mmol), tritopic tectons 4 (0.68 mg, 0.0026 mmol) and 5 (1.35 mg, 0.0024 mmol) were added to 1.1 mL of mixed solvent. After 24 h heating, RS, DTPS and DTPL were formed predominantly. 1H NMR (Acetone-d6/D2O: 1/1, 300 MHz) for R : δ 9.53 (s, 2H, H9, 9.21 (d, 3J = 5.8 Hz, 4H, Hα-Py), 9.18 (d, J = 5.4 Hz, 2H, Hα'-Py), 8.71 (d, 3J = 5.4 Hz, 4H, Hβ-Py), 8.53 (d, J = 5.4 Hz, 4H, Hβ'-Py), 8.37 (s, 2H, H10), 7.71 (d, 3J = 10.5 Hz, 4H, H4,5), 7.62 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; DTPS: δ 9.14 (d, 6H, 3J = 6.3 Hz, Hα-Py), 8.98 (m, 9H, Hα'-Py,9), 8.36 (s, 3H, H10), 8.25 (d, 6H, J = 4.2 Hz, Hβ-Py), 7.72 (m, 12H, Hβ'-Py, H4,5), 7.68 (d, 6H, 3J = 9.9 Hz, H2,7), 7.14 (m, 6H, H3,6), 1.40 (m, 72H, PCH2CH3), 0.82 (m, 108H, PCH2CH3) ppm; DTPL: δ 9.32 (s, 3H, H), 9.01 (d, 6H, 3 3J = 5.1 Hz, Hα-Py), 8.91 (d, 6H, 3J = 4.8 Hz, Hα'-Py), 8.36 (s, 3H, H3), 8.00 (d, 6H, 3J = 6.0 Hz, H 3 β-Py), 7.95 (d, 6H, J = 6.0 Hz, Hβ Py), 7.73 (m, 12H, H 3 – 4,5,2,7), 7.57 (d, 12H, J = 8.4 Hz, H3,5-phenylene), 7.17 (d, 12H, J = 8.1 Hz, H2,6-phenylene), 7.14 (m, 6H, H3,6), 1.40 (m, 72H, PCH2CH3), 0.82 (m, 108H, PCH2CH) ppm. 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) for RS: δ 8.32 (195Pt satellites, 1JPt-P = 2636 Hz) ppm; DTPS: δ 9.81 (195Pt satellites, 1JPt-P = 2649 Hz) ppm; DTPL: δ 8.83 (195Pt satellites, 1JPt-P = 2655 Hz) ppm. MS (ESI) for RS (C96H152N8O12P8Pt4): m/z: 1256.7 [M - 2NO3]2+, 816.9 [M - 3NO3]3+; DTPS (C146H230N12O20P12Pt6): m/z: 1944.8 [M - 2NO3]2+, 1275.9 [M - 3NO3]3+; DTPL (C196H258N12O18P12Pt6): m/z: 1474.5 [M - 3NO3]+3, 1090.4 [M - 4NO3]4+.
Self-organization system SS3 (RS, RL, and NTP)
Molecular “Clip” 1 (16.09 mg, 0.01383 mmol), 4,4'-dipyridyl 2 (0.60 mg, 0.0038 mmol), 1,4-bis(4-pyridylethynyl)benzene 3 (1.16 mg, 0.00414 mmol) and tritopic tecton 6 (1.49 mg, 0.00391 mmol) were added to 2.0 mL of mixed solvent. After 48 h heating, RS, RL and NTP were formed predominantly. 1H NMR (Acetone-d6/D2O: 1/1, 300 MHz) for RS: δ 9.53 (s, 2H, H9), 9.21 (d, 3J = 5.8 Hz, 4H, Hα-Py), 9.18 (d, 3J = 5.4 Hz, 2H, Hα'-Py), 8.71 (d, 3J = 5.4 Hz, 4H, Hβ-Py), 8.53 (d, 3J = 5.4 Hz, 4H, Hβ'-Py), 8.37 (s, 2H, H10), 7.71 (d, 3J = 10.5 Hz, 4H, H4,5), 7.62 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; RL: δ 9.45 (s, 2H, H9), 9.02 (m, 4H, Hα-Py), 8.94 (d, 4H, J = 5.4 Hz, Hα'-Py), 8.36 (s, 2H, H10), 8.03 (m, 8H, Hβ-Py), 7.74 (s, 8H, Hphenylene), 7.71 (d, 4H, 3J = 10.5 Hz, H4,5), 7.64 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; NTP: δ 9.41 (s, 3H, H9), 9.02 (m, 6H, Hα-Py), 8.90 (d, 6H, J = 5.4 Hz, Hα'-Py), 8.36 (s, 3H, H), 7.92 (m, 12H, Hβ-Py), 7.69 (d, 6H, J = 9.9 Hz, H2,7), 7.85 (s, 6H, HBenzene), 7.62 (d, 6H, 3J = 9.9 Hz, H2,7), 7.15 (m, 6H, H3,6), 1.40 (m, 72H, PCH2CH3), 0.82 (m, 108H, PCH2CH3) ppm. 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) for R : δ 8.33 (195Pt satellites, 1JPt-P = 2648 Hz) ppm; RL : δ 8.48 (195Pt satellites, 1JPt-P = 2648 Hz) ppm; NTP δ 8.65(195Pt satellites, 1JPt-P = 2648 Hz) ppm. MS (ESI) for RS (C96H152N8O12P8Pt4): m/z: 1256.7 [M - 2NO3]2+, 816.9 [M - 3NO3]3+; RL (C116H160N8O12P8Pt4): m/z: 1380.3 [M - 2NO3]2+, 899.2 [M - 3NO3]3+; NTP (C168H234N12O18P12Pt6): m/z: 1354.1[M - 3NO3]3+, 1000.4 [M - 4NO3]4+ .
Self-organization system SS4 (RS, DTPS, and NTP)
Molecular “Clip” 1 (13.43 mg, 0.01155 mmol), 4,4'-dipyridyl 2 (0.49 mg, 0.0031 mmol), tritopic tectons 4 (0.73 mg, 0.0028 mmol) and 6 (1.08 mg, 0.00283 mmol) were added to 2.0 mL of mixed solvent. After 48 h heating, RS, DTPS and NTP were formed predominantly. 1H NMR (Acetone-d6/D2O: 1/1, 300 MHz) for RS: δ 9.53 (s, 2H, H9), 9.21 (d, 3J = 5.8 Hz, 4H, Hα-py), 9.18 (d, 3J = 5.4 Hz, 2H, Hα'-Py), 8.71 (d, 3J = 5.4 Hz, 4H, Hβ-Py), 8.53 (d, 3J = 5.4 Hz, 4H, Hβ'-Py), 8.37 (s, 2H, H10), 7.71 (d, 3J = 10.5 Hz, 4H, H4,5), 7.62 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; DTPS: δ 9.14 (d, 6H, 3J = 6.3 Hz, Hα-Py), 8.98 (m, 9H, Hα'-Py,9), 8.36 (s, 3H, H10), 8.25 (d, 6H, 3J = 4.2 Hz, Hβ-Py), 7.72 (m, 12H, Hβ'-Py, H4,5), 7.68 (d, 6H, 3J = 9.9 Hz, H2,7), 7.14 (m, 6H, H3,6), 1.40 (m, 72H, PCH2CH3), 0.82 (m, 108H, PCH2CH3) ppm; NTP: δ 9.41 (s, 3H, H9), 9.02 (m, 6H, Hα-py), 8.90 (d, 6H,3J = 5.4 Hz, Hα'-Py), 8.36 (s, 3H, H10), 7.92 (m, 12H, Hβ-Py), 7.69 (d, 6H, 3J = 9.9 Hz, H2,7), 7.85 (s, 6H, HBenzene), 7.62 (d, 6H, 3J = 9.9 Hz, H2,7), 7.15 (m, 6H, H3,6), 1.40 (m, 72H, PCH2CH3), 0.82 (m, 108H, PCH2CH3) ppm. 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) for RS: δ 8.33 (195Pt satellites, 1JPt-P = 2651 Hz) ppm; DTPS: δ 9.81 (195Pt satellites, 1JPt-P = 2654 Hz) ppm; NTP: δ 8.65 (195Pt satellites, 1JPt-P = 2651 Hz) ppm. MS (ESI) for RS (C96H152N8O12P8Pt4): m/z: 1256.7 [M - 2NO3]2+, 816.9 [M - 3NO3]3+; DTPS (C146H230N12O20P12Pt6): m/z: 1944.8 [M - 2NO3]2+, 1275.9 [M - 3NO3]3+; NTP (C168H234N12O18P12Pt6): m/z: 1354.1[M - 3NO3]3+, 1000.4 [M - 4NO3]4+.
Self-organization system SS5 (TS, RhS, and TBP)
60° organoplatinum acceptor 7 (13.01 mg, 0.01119 mmol), 4,4'-dipyridyl 2 (0.51 mg, 0.0033 mmol), 2,6-dipyridyl pyridine 8 (0.76 mg, 0.0033 mmol) and tritopic tecton 5 (1.75 mg, 0.00312 mmol) were added to 1.2 mL of mixed solvent. After 24 h heating, TS, RhS and TBP were formed predominantly. 1H NMR (Acetone-d6 /D2O: 1/1, 300 MHz) for TS: δ 9.16 (d, 3J = 5.7 Hz, 6H, Hα-Py), 9.09 (d, 3J = 5.7 Hz, 6H, Hα'-Py), 8.65 (s, 6H, H4,5), 8.35 (m, 12H, Hβ-Py), 7.54 (m, 18H, H1,2,7,8,9,10), 1.33 (m, 72H, PCH2CH3), 1.05 (m, 108H, PCH2CH3) ppm; RhS: δ 9.09 (d, 4H, 3J = 5.7 Hz, Hα-Py), 8.87 (m, 4H, Hα'-Py), 8.62 (s, 4H, H4,5), 8.41 (m, 12H, Hβ-Py, 3,5-Py), 8.25 (m, 2H, H4-Py), 7.54 (m, 12H, H1,2,7,8,9,10), 1.33 (m, 48H, PCH2CH3), 1.05 (m, 72H, PCH2CH3) ppm; TBP: δ 8.91 (m, 12H, Hα-Py), 8.61 (s, 6H, H4,5), 7.78 (m, 12H, Hβ-Py), 7.54 (m, 30H, H1,2,7,8,9,10, α-Phenylene), 7.15 (d, 12H, 3J = 8.7 Hz, H1,2,7,8,9,10, β-Phenylene), 1.33 (m, 72H, PCH2CH3), 1.05 (m, 108H, PCH2CH3) ppm. 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) for Ts : δ 14.31 (195Pt satellites, 1JPt-P = 2695 Hz) ppm; Rhs : δ 14.42 (195Pt satellites, 1JPt-P = 2695 Hz) ppm.; TBP: δ 14.38 (195Pt satellites, 1JPt-P = 2695 Hz) ppm. MS (ESI) for TS (C144H228N12O18P12Pt6): m/z: 1916.6 [M - 2NO3]2+, 927.06 [M - 4NO3]4+; RhS (C106H158N10O12P8Pt4): m/z: 1333.4 [M - 2NO3]2+, 868.3 [M - 3NO3]; TBP (C146H230N12O20P12Pt6): m/z: 1474.5[M - 3NO3]3+, 1090.4 [M - 4NO3]4+.
Self-organization system SS6 (TS, RhS, and RhL)
60° organoplatinum acceptor 7 (9.96 mg, 0.00856 mmol), 4,4'-dipyridyl 2 (0.45 mg, 0.0029 mmol), 2,6-dipyridyl pyridine 8 (0.67 mg, 0.0029 mmol) and bis(4-(pyridin-4-ylethynyl)phenyl) methanone 9 (1.08 mg, 0.00281 mmol) were added to 1.4 mL of mixed solvent. After 24 h heating, TS, RhS and RhL were formed predominantly. 1H NMR (Acetone-d6/D2O: 1/1, 300 MHz) for TS: δ 9.16 (d,3J = 5.7 Hz, 6H, Hα-Py), 9.06 (m, 6H, Hα'-Py), 8.65 (s, 6H, H4,5), 8.35 (m, 12H, Hβ-Py), 7.54 (m, 18H, H1,2,7,8,9,10), 1.33 (m, 72H, PCH2CH3), 1.05 (m, 108H, PCH2CH3) ppm; RhS: δ 9.06 (m, 4H, Hα-Py), 8.87 (m, 4H, Hα'-Py), 8.62 (s, 4H, H4,5), 8.41 (m, 12H, Hβ-Py, 3,5-Py), 8.25 (m, 2H, H4-Py), 7.54 (m, 12H, H1,2,7,8,9,10), 1.33 (m, 48H, PCH2CH3), 1.05 (m, 72H, PCH2CH3) ppm; RhL: δ 8.92 (d, 8H, 3J = 6.0 Hz, Hα-Py), 8.58 (s, 4H, H4,5),7.84 (m, 8H, Hβ-Py), 7.81 (s, 8H, HPhenylene), 7.54 (m, 16H, H1,2,7,8,9,10), 1.33 (m, 48H, PCH2CH3), 1.05 (m, 72H, PCH2CH3) ppm. 31P{1H} NMR (Acetone-d6/D2O: 1/1, 121.4 MHz) for TS: δ 14.32 (195Pt satellites, 1JPt-P = 2690 Hz) ppm; Rhs : δ 14.43 (195Pt satellites, 1JPt-P = 2690 Hz) ppm; RhL : δ 14.39 (195Pt satellites, 1JPt-P = 2690 Hz) ppm. MS (ESI) for TS (C144H228N12O18P12Pt6): m/z: 1916.6 [M - 2NO3]2+, 927.06 [M - 4NO3]4+; RhS (C106H158N10O12P8Pt4): m/z: 1333.4 [M - 2NO3]2+, 868.3 [M - 3NO3]3+; RhL (C130H168N8O14P8Pt4): m/z: 1484.5 [M - 2NO3]2+, 969.0 [M - 3NO3]3+.
Self-organization system SS7 (TS, TL, and RhS)
60° organoplatinum acceptor 7 (9.21 mg, 0.00792 mmol), 4,4'-dipyridyl 2 (0.42 mg, 0.0027 mmol), 1,4-bis(4-pyridylethynyl)benzene 3 (0.71 mg, 0.0025 mmol) and 2,6-dipyridyl pyridine 8 (0.63 mg, 0.0027 mmol) were added to 1.2 mL of mixed solvent. After 48 h heating, TS, TL, and RhS were formed predominantly. 1H NMR (Acetone-d6 /D2O: 1/1, 300 MHz) for TS: δ 9.16 (d, 3J = 5.7 Hz, 6H, Hα-Py), 9.06 (m, 6H, Hα'-Py), 8.65 (s, 6H, H4,5), 8.35 (m, 12H, Hα-Py), 7.54 (m, 18H, H1,2,7,8,9,10), 1.33 (m, 72H, PCH2CH3), 1.05 (m, 108H, PCH2CH3) ppm; TL: δ 8.90 (m, 12H, Hα-Py), 8.62 (s, 6H, H4,5), 7.84 (d, 12H, 3J = 6.6 Hz, Hα-Py), 7.73 (s, 12H, Hphenylene), 7.54 (m, 18H, H1,2,7,8,9,10), 1.30 (m, 72H, PCH2CH3), 1.07 (m, 108H, PCH2CH3) ppm; RhS: δ 9.06 (m, 4H, Hα-Py), 8.87 (m, 4H, Hα'-Py), 8.62 (s, 4H, H4,5), 8.41 (m, 12H, Hβ-Py, 3,5-Py), 8.25 (m, 2H, H4-Py), 7.54 (m, 12H, H1,2,7,8,9,10), 1.33 (m, 48H, PCH2CH3), 1.05 (m, 72H, PCH2CH) ppm. 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) for TS: δ 14.32 (195Pt satellites, 1JPt-P = 2687 Hz) ppm; TL: δ 14.42 (195Pt satellites, 1JPt-P = 2687 Hz) ppm; RhS: δ 14.42 (195Pt satellites, JPt-P= 2687 Hz) ppm. MS (ESI) for TS (C144H228N12O18P12Pt6): m/z: 1916.6 [M - 2NO3]2+, 927.06 [M - 4NO3]4+; TL (C174H240N12O18P12Pt6): m/z: 1381.5 [M - 3NO3]3+, 1020.6 [M - 4NO3]4+; RhS (C106H158N10O12P8Pt4): m/z: 1333.4 [M - 2NO3]2+, 868.3 [M - 3NO3]3+.
Self-organization system SS8 (RS, RL, TS, and TL)
Molecular “Clip” 1 (11.61 mg, 0.00998 mmol), 60° organoplatinum acceptor 7 (11.67 mg, 0.0100 mmol), 4,4'-dipyridyl 2 (1.56 mg, 0.00998 mmol) and 1,4-bis(4-pyridylethynyl)benzene 3 (2.81 mg, 0.0100 mmol) were added to 1.7 mL of mixed solvent. After 72 h heating, RS, RL, TS, and TL were formed predominantly. 1H NMR (Acetone-d6 /D2O: 1/1, 500 MHz) for RS: δ 9.53 (s, 2H, H9), 9.21 (d, 3J = 5.8 Hz, 4H, Hα-Py), 9.18 (d, J = 5.4 Hz, 2H, Hα'-Py), 8.71 (d, 3J = 5.4 Hz, 4H, Hβ-Py), 8.53 (d, 3J = 5.4 Hz, 4H, Hβ'-Py), 8.37 (s, 2H, H10), 7.71 (d, 3J = 10.5 Hz, 4H, H4,5), 7.62 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; RL: δ 9.45 (s, 2H, H9), 9.02 (m, 4H, Hα-Py), 8.94 (d, 4H, J = 5.4 Hz, Hα'-Py), 8.36 (s, 2H, H10), 8.03 (m, 8H, Hβ-Py), 7.74 (s, 8H, Hphenylene), 7.71 (d, 4H, J = 10.5 Hz, H4,5), 7.64 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; TS: δ 9.16 (d, 3J = 5.7 Hz, 6H, Hα-Py), 9.06 (m, 6H, Hα'-Py), 8.65 (s, 6H, H4,5), 8.35 (m, 12H, Hβ-Py), 7.54 (m, 18H, H1,2,7,8,9,10), 1.33 (m, 72H, PCH2CH3), 1.05 (m, 108H, PCH2CH3) ppm; TL: δ 8.90 (m, 12H, Hα-Py), 8.62 (s, 6H, H4,5), 7.84 (d, 12H, 3J = 6.6 Hz, Hα-Py), 7.73 (s, 12H, Hphenylene), 7.54 (m, 18H, H1,2,7,8,9,10), 1.30 (m, 72H, PCH2CH3), 1.07 (m, 108H, PCH2CH3) ppm. 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) for RS: δ 8.33 (195Pt satellites, 1JPt-P = 2639 Hz) ppm; RL : δ 8.48 (195Pt satellites, 1JPt-P = 2639 Hz) ppm; TS: δ 14.32 (195Pt satellites, 1JPt-P = 2673 Hz) ppm; TL: δ 14.40 (195Pt satellites, 1JPt-P = 2673 Hz) ppm. MS (ESI) for RS (C96H152N8O12P8Pt4): m/z: 1256.7 [M - 2NO3]2+, 816.9 [M - 3NO3]3+; RL (C116H160N8O12P8Pt4): m/z: 1380.3 [M - 2NO3]2+, 899.2 [M - 3NO3]3+; TS (C144H228N12P12O18P12Pt6): m/z: 1916.6 [M - 2NO3]2+, 927.06 [M - 4NO3]4+; TL (C174H240N12O18P12Pt6): m/z: 1381.5 [M - 3NO3]3+, 1020.6 [M - 4NO3]4+.
Self-organization system SS9 (RS, DTPL, TS, and TBP)
Molecular “Clip” 1 (7.52 mg, 0.00647 mmol), 60° organoplatinum acceptor 7 (7.50 mg, 0.00645 mmol), 4,4'-dipyridyl 2 (1.00 mg, 0.00640 mmol) and tritopic tectons 5 (2.43 mg, 0.00433 mmol) were added to 1.2 mL of mixed solvent. After 96 h heating, RS, DTPL, TS, and TBP were formed predominantly. 1H NMR (Acetone-d6 /D2O: 1/1, 500 MHz) for RS: δ 9.53 (s, 2H, H9), 9.21 (d, 3J = 5.8 Hz, 4H, Hα-Py), 9.18 (d, 3J = 5.4 Hz, 2H, Hα'-Py), 8.71 (d, 3J = 5.4 Hz, 4H, Hβ-Py), 8.53 (d, 3J = 5.4 Hz, 4H, Hβ'-Py), 8.37 (s, 2H, H10), 7.71 (d, 3J = 10.5 Hz, 4H, H4,5), 7.62 (m, 4H, H2,7), 7.14 (t, 3J = 7.2 Hz, 4H, H3,6), 1.45 (m, 48H, PCH2CH3), 0.84 (m, 72H, PCH2CH3) ppm; DTPL: δ 9.32 (s, 3H, H9), 9.01 (d, 6H, 3J = 5.1 Hz, Hα-Py), 8.91 (d, 6H, 3J = 4.8 Hz, Hα'-Py), 8.36 (s, 3H, H10), 8.00 (d, 6H, 3J = 6.0 Hz, Hβ-Py), 7.95 (d, 6H, 3J = 6.0 Hz, Hβ-Py), 7.73 (m, 12H, H4,5,2,7), 7.57 (d, 12H, 3J = 8.4 Hz, H3,5-phenylene), 7.17 (d, 12H, 3J = 8.1 Hz, H2,6-phenylene), 7.14 (m, 6H, H3,6), 1.40 (m, 72H, PCH2CH3), 0.82 (m, 108H, PCH2CH3) ppm; TS: δ 9.16 (d, 3J = 5.7 Hz, 6H, Hα-Py), 9.06 (m, 6H, Hα'-Py), 8.65 (s, 6H, H4,5), 8.35 (m, 12H, Hα-Py), 7.54 (m, 18H, H1,2,7,8,9,10), 1.33 (m, 72H, PCH2CH3), 1.05 (m, 108H, PCH2CH3) ppm; TBP: δ 8.91 (m, 12H, Hα-Py), 8.61 (s, 6H, H4,5), 7.78 (m, 12H, Hβ-Py), 7.54 (m, 30H, H1,2,7,8,9,10, α-Phenylene), 7.15 (d, 12H, 3J = 8.7Hz, H1,2,7,8,9,10, β-Phenylene), 1.33 (m, 72H, PCH2CH3), 1.05 (m, 108H, PCH2CH3) ppm. 31P{1H} NMR (Acetone-d6 /D2O: 1/1, 121.4 MHz) for RS: δ 8.33 (195Pt satellites, 1JPt-P = 2638 Hz) ppm; DTPL: δ 8.83 (195 Pt satellites, 1JPt-P = 2637 Hz) ppm; TS: δ 14.32 (195Pt satellites, 1JPt-P = 2667 Hz) ppm; TBP: δ 14.39 (195Pt satellites, 1JPt-P = 2667 Hz) ppm. MS (ESI) for RS (C96H152N8O12P8Pt4): m/z: 1256.7 [M - 2NO3]2+, 816.9 [M - 3NO3]3+; DTPL (C196H258N12O18P12Pt6): m/z: 1474.5 [M - 3NO3]3+, 1090.4 [M - 4NO3]4+; TS (C144H228N12O18P12Pt6): m/z: 1916.6 [M - 2NO3]2+, 927.06 [M - 4NO3]4+; TBP (C146H230N12O20P12Pt6): m/z: 1474.5[M - 3NO3]3+, 1090.4 [M - 4NO3]4+.
Supplementary Material
Acknowledgements
P.J.S. thanks the NIH (Grant GM-057052) for financial support. We thank Dr. Brian H. Northrop for helpful discussions and assistance with revisions.
References
- [1].a) Lehn J-M. Science. 2002;295:2400–2403. doi: 10.1126/science.1071063. [DOI] [PubMed] [Google Scholar]; b) Lehn J-M. Rep. Prog. Phys. 2004;67:249–265. [Google Scholar]; c) Lehn J-M. Chem. Soc. Rev. 2007;36:151–160. doi: 10.1039/b616752g. [DOI] [PubMed] [Google Scholar]
- [2].a) Lehn J-M. Angew. Chem. Int. Ed.. Engl. 1988;27:89–112. [Google Scholar]; b) Cram DJ. Angew. Chem. Int. Ed.. Engl. 1988;27:1009–1020. [Google Scholar]; c) Pederson CJ. Angew. Chem. Int. Ed.. Engl. 1988;27:1021–1027. [Google Scholar]
- [3].a) Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001:509–518. [Google Scholar]; b) Ruben M, Rojo J, Romero-Salguero FJ, Uppadine LH, Lehn J-M. Angew. Chem. Int. Ed. 2004;43:3644. doi: 10.1002/anie.200300636. [DOI] [PubMed] [Google Scholar]; c) Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc. Chem. Res. 2005;38:349. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]; d) Gianneschi NC, Masar MS, III, Mirkin CA. Acc. Chem. Res. 2005;38:825. doi: 10.1021/ar980101q. [DOI] [PubMed] [Google Scholar]
- [4].a) Prins LJ, Reinhoudt DN, Timmerman P. Angew. Chem. Int. Ed. 2001;40:2382–2426. doi: 10.1002/1521-3773(20010702)40:13<2382::aid-anie2382>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; b) Corbin PS, Lawless LJ, Li Z, Ma Y, Witmer MJ, Zimmerman SC. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5099–5104. doi: 10.1073/pnas.062641199. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) B. Purse W, Rebek J., Jr. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10777–10782. doi: 10.1073/pnas.0501731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pollino JM, Weck M. Chem. Soc. Rev. 2005;34:193–207. doi: 10.1039/b311285n. [DOI] [PubMed] [Google Scholar]
- [6].a) Seeman NC. Mol. Biotechnol. 2007;37:246–257. doi: 10.1007/s12033-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) He Y, Ye T, Su M, Zhang C, Ribbe AE, Jiang W, Mao C. Nature. 2008;452:198–202. doi: 10.1038/nature06597. [DOI] [PubMed] [Google Scholar]
- [7].Wright AT, Anslyn EV. Chem. Soc Rev. 2006;35:14–28. doi: 10.1039/b505518k. [DOI] [PubMed] [Google Scholar]
- [8].Li J, Loh XJ. Adv. Drug. Delivery Rev. 2008;60:1000–1017. doi: 10.1016/j.addr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- [9].Davis AP, Sheppard DN, Smith BD. Chem. Soc. Rev. 2007;36:348–357. doi: 10.1039/b512651g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. Garland Science; New York: 2002. [Google Scholar]
- [11].Berg J, Tymoczko JL, Stryer L. Biochemistry. 6th ed. W. H. Freeman; San Francisco: 2006. [Google Scholar]
- [12].a) Edde B, Rossier J, Le Caer JP, Desbruyeres E, Gros F, Denoulet P. Science. 1990;247:83. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]; b) Walker CS, Shetty RP, Clark K, Kazuko SG, Letsou A, Olivera BM, Bandyopadhyay PK. J. Biol. Chem. 2001;276:7769–7774. doi: 10.1074/jbc.M009576200. [DOI] [PubMed] [Google Scholar]; c) Glozak MA, Sengupta N, Zhang X, Seto E. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]; d) Yang XJ, Seto E. Mol. Cell. 2008;31:449–61. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Lehn J-M. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4763–4768. doi: 10.1073/pnas.072065599. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hollingsworth MD. Science. 2002;295:2410–2413. doi: 10.1126/science.1070967. [DOI] [PubMed] [Google Scholar]; c) Wu A, Isaacs L. J. Am. Chem. Soc. 2003;125:4831–4835. doi: 10.1021/ja028913b. [DOI] [PubMed] [Google Scholar]
- [14].a) Krämer R, Lehn J-M, Marquis-Rigault A. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5394–5398. doi: 10.1073/pnas.90.12.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Caulder DL, Raymond KN. Angew. Chem. Int. Ed.. Engl. 1997;36:1440–1442. [Google Scholar]; c) Masood MA, Enemark EJ, Stack TDP. Angew. Chem. Int. Ed. 1998;37:928–932. doi: 10.1002/(SICI)1521-3773(19980420)37:7<928::AID-ANIE928>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]; d) Enemark EJ, T. D. P. Angew. Chem. Int. Ed. 1998;37:932–935. doi: 10.1002/(SICI)1521-3773(19980420)37:7<932::AID-ANIE932>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]; e) Stiller R, Lehn J-M. Eur. J. Inorg. Chem. 1998:977–982. [Google Scholar]; f) Taylor PN, Anderson HL. J. Am. Chem. Soc. 1999;121:11538–11545. [Google Scholar]; g) Albrecht M, Schneider M, Röttele H. Angew. Chem. Int. Ed. 1999;38:557–559. doi: 10.1002/(SICI)1521-3773(19990215)38:4<557::AID-ANIE557>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]; h) Kondo T, Oyama K-I, Yoshida K. Angew. Chem. Int. Ed. 2001;40:894–897. doi: 10.1002/1521-3773(20010302)40:5<894::AID-ANIE894>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]; i) Pinalli R, Cristini V, Sottili V, Geremia S, Campagnolo M, Caneschi A, Dalcanale E. J. Am. Chem. Soc. 2004;126:6516–6517. doi: 10.1021/ja038694+. [DOI] [PubMed] [Google Scholar]; j) Kamada T, Aratani N, Ikeda T, Shibata N, Higuchi Y, Wakamiya A, Yamaguchi S, Kim KS, Yoon ZS, Kim D, Osuka A. J. Am. Chem. Soc. 2006;128:7670–7678. doi: 10.1021/ja0611137. [DOI] [PubMed] [Google Scholar]; k) Saur I, Scopelliti R, Severin K. Chem. Eur. J. 2006;12:1058–1066. doi: 10.1002/chem.200500621. [DOI] [PubMed] [Google Scholar]; l) Langner A, Tait SL, Lin N, Rajadurai C, Ruben M, Kern K. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17927–17930. doi: 10.1073/pnas.0704882104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Jolliffe KA, Timmerman P, Reinhoudt DN. Angew. Chem. Int. Ed. 1999;38:933–937. doi: 10.1002/(SICI)1521-3773(19990401)38:7<933::AID-ANIE933>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; b) Cai M, Shi X, Sidorov V, Fabris D, Lam Y-F, Davis JT. Tetrahedron. 2002;58:661–671. [Google Scholar]; c) Corbin PS, Lawless LJ, Li Z, Ma Y, Witmer MJ, Zimmerman SC. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5099–5104. doi: 10.1073/pnas.062641199. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Ma Y, Kolotuchin SV, Zimmerman SC. J. Am. Chem. Soc. 2002;124:13757–13769. doi: 10.1021/ja0202006. [DOI] [PubMed] [Google Scholar]; e) Wu A, Chakraborty A, Fettinger JC, Flowers RA, II, Isaacs L. Angew. Chem. Int. Ed. 2002;41:4028–4031. doi: 10.1002/1521-3773(20021104)41:21<4028::AID-ANIE4028>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]; f) Mukhopadhyay P, Zavalij PY, Isaacs L. J. Am. Chem. Soc. 2006;128:7670–7678. doi: 10.1021/ja063390j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Bilgicüer B, Xing X, Kumar K. J. Am. Chem. Soc. 2001;123:11815–11816. doi: 10.1021/ja016767o. [DOI] [PubMed] [Google Scholar]; b) Schnarr NA, Kennan AJ. J. Am. Chem. Soc. 2003;125:667–671. doi: 10.1021/ja027489b. [DOI] [PubMed] [Google Scholar]
- [17].a) Rowan SJ, Hamilton DG, Brady PA, Sanders JKM. J. Am. Chem. Soc. 1997;119:2578–2579. [Google Scholar]; b) Rowan SJ, Reynolds DJ, Sanders JKM. J. Org. Chem. 1999;64:5804–5814. [Google Scholar]; c) Rowan SJ, Cantrill SJ, Cousins GRL, Sanders JKM, Stoddart JF. Angew. Chem. Int. Ed. 2002;41:898–952. doi: 10.1002/1521-3773(20020315)41:6<898::aid-anie898>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]; d) Corbett PT, Leclaire J, Vial L, West KR, Wietor J-L, Sanders JKM, Otto S. Chem. Rev. 2006;106:3652–3711. doi: 10.1021/cr020452p. [DOI] [PubMed] [Google Scholar]
- [18].a) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502–518. [Google Scholar]; b) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853–907. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; c) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972–983. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]
- [19].a) Addicott C, Das N, Stang PJ. Inorg. Chem. 2004;43:5335–5338. doi: 10.1021/ic049326p. [DOI] [PubMed] [Google Scholar]; b) Yang H-B, Ghosh K, Northrop BH, Stang PJ. Org. Lett. 2007;9:1561–1564. doi: 10.1021/ol070371l. [DOI] [PubMed] [Google Scholar]; c) Zheng Y-R, Yang H-B, Northrop BH, Ghosh K, Stang PJ. Inorg. Chem. 2008;47:4706–4711. doi: 10.1021/ic800038j. [DOI] [PubMed] [Google Scholar]
- [20].Kuehl CJ, Huang SD, Stang PJ. J. Am. Chem. Soc. 2001;123:9634–9641. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]
- [21].Kryschenko YK, Seidel SR, Arif AM, Stang PJ. J. Am. Chem. Soc. 2003;125:5193–5198. doi: 10.1021/ja030018k. [DOI] [PubMed] [Google Scholar]
- [22].Addicott C, Oesterling I, Yamamoto T, Muellen K, Stang PJ. J. Org. Chem. 2005;70:797–801. doi: 10.1021/jo048239b. [DOI] [PubMed] [Google Scholar]
- [23].a) Kuehl CJ, Kryschenko YK, Radhakrishnan U, Seidel SR, Huang SD, Stang PJ. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4932–4936. doi: 10.1073/pnas.012540799. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kuehl CJ, Yamamoto T, Seidel SR, Stang PJ. Org. Lett. 2002;4:913–915. doi: 10.1021/ol017296d. [DOI] [PubMed] [Google Scholar]
- [24].Yang H-B, Ghosh K, Arif AM, Stang PJ. J. Org. Chem. 2006;71:9464–9469. doi: 10.1021/jo061779j. [DOI] [PubMed] [Google Scholar]
- [25].The supramolecular rhomboids RhS and RhL have been characterized by 31P and 1H multinuclear NMR spectroscopy and ESI mass spectrometry, which are shown in Supporting Information.
- [26].a) Yang H-B, Das N, Huang F, Hawkridge AM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2006;128:10014–10015. doi: 10.1021/ja063377z. [DOI] [PubMed] [Google Scholar]; b) Yang H-B, Hawkridge AM, Huang SD, Das N, Bunge SD, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:2120–2129. doi: 10.1021/ja066804h. [DOI] [PubMed] [Google Scholar]; c) Yang H-B, Ghosh K, Northrop BH, Zheng Y-R, Lyndon MM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:14187–14189. doi: 10.1021/ja073744m. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Yang H-B, Ghosh K, Zhao Y, Northrop BH, Lyndon MM, Muddiman DC, White HS, Stang PJ. J. Am. Chem. Soc. 2008;130:839–841. doi: 10.1021/ja710349j. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Ghosh K, Yang H-B, Northrop BH, Lyndon MM, Zheng Y-R, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2008;130:5320–5334. doi: 10.1021/ja711502t. [DOI] [PubMed] [Google Scholar]; f) Northrop BH, Yang H-B, Stang PJ. Chem. Commun. 2008:5896–5908. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.