Abstract
Patients with critical limb ischemia (CLI) are a heterogeneous population with respect to risk for mortality and limb loss, complicating clinical decision-making. Endovascular options, as compared to bypass, offer a tradeoff between reduced procedural risk and inferior durability. Risk stratified data predictive of amputation-free survival (AFS) may improve clinical decision making and allow for better assessment of new technology in the CLI population.
METHODS
This was a retrospective analysis of prospectively collected data from patients who underwent infrainguinal vein bypass surgery for CLI. Two datasets were used: the PREVENT III randomized trial (n=1404) and a multicenter registry (n=716) from 3 distinct vascular centers (2 academic, 1 community-based). The PREVENT III cohort was randomly assigned to a derivation set (n=953) and to a validation set (n=451). The primary endpoint was AFS. Predictors of AFS identified on univariate screen (inclusion threshold, p<0.20) were included in a stepwise selection Cox model. The resulting 5 significant predictors were assigned an integer score to stratify patients into 3 risk groups. The prediction rule was internally validated in the PREVENT III validation set and externally validated in the multicenter cohort.
RESULTS
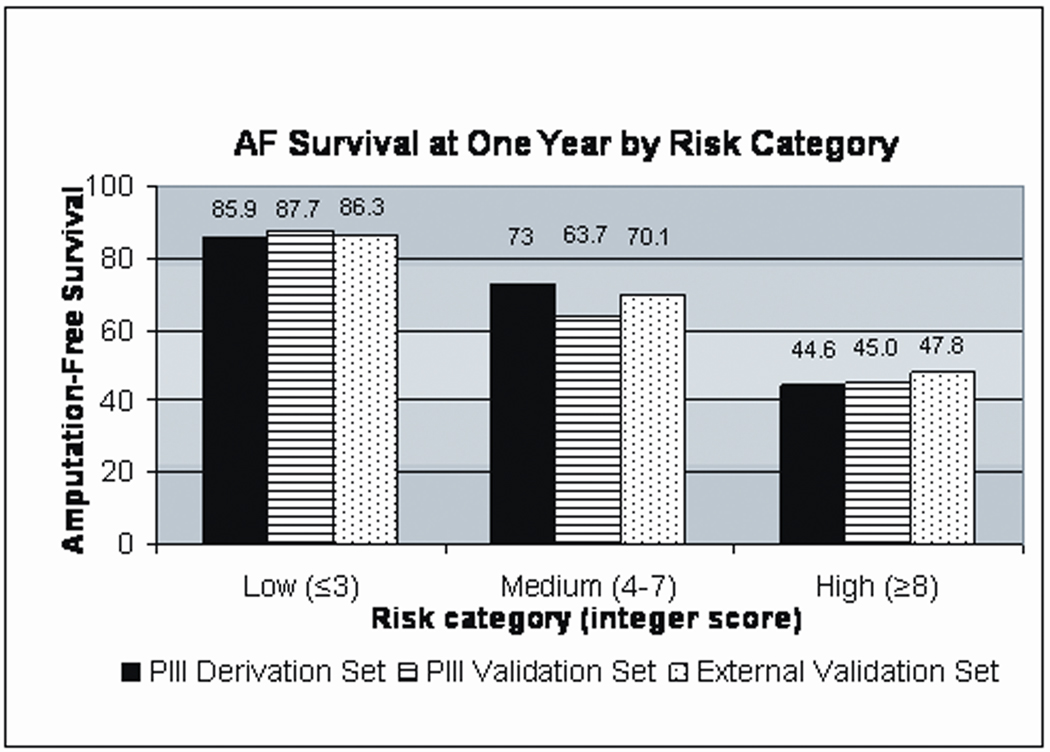
The estimated 1 year AFS in the derivation, internal validation, and external validation sets were 76.3%, 72.5%, and 77.0%, respectively. In the derivation set, dialysis (HR 2.81, p<.0001), tissue loss (HR 2.22, p=.0004), age ≥75 (HR 1.64, p=.001), hematocrit ≤30 (HR 1.61, p=.012), and advanced CAD (HR 1.41, p=.021) were significant predictors for AFS in the multivariable model. An integer score, derived from the β coefficients, was used to generate 3 risk categories (low ≤ 3 [44.4% of cohort], medium 4–7 [46.7% of cohort], high ≥8 [8.8% of cohort]). Stratification of the patients, in each dataset, according to risk category yielded 3 significantly different Kaplan-Meier estimates for one year AFS (86%, 73%, and 45% for low, medium, and high risk groups respectively). For a given risk category, the AFS estimate was consistent between the derivation and validation sets.
CONCLUSION
Among patients selected to undergo surgical bypass for infrainguinal disease, this parsimonious risk stratification model reliably identified a category of CLI patients with a >50% chance of death or major amputation at 1 year. Calculation of a “PIII risk score” may be useful for surgical decision making and for clinical trial designs in the CLI population.
INTRODUCTION
Critical limb ischemia (CLI), the most advanced form of peripheral arterial disease (PAD), is associated with a high risk of cardiovascular events that include major limb loss, myocardial infarction, stroke, and death.1–4 The estimated rate of all-cause mortality in patients with CLI has been reported to be as high as 20% within 6 months of diagnosis and surpasses 50% at 5 years post diagnosis.5, 6 These rates exceed those seen in patients with symptomatic coronary artery disease (CAD)7, 8 and reflect the global atherosclerotic burden that accompanies a diagnosis of CLI.
Open surgical bypass using autogenous vein has traditionally served as the gold standard limb revascularization strategy for patients with CLI and infrainguinal disease. However, over the last decade, the introduction of endovascular treatment methods has begun to challenge this concept.5, 9 Much of the impetus driving this paradigm shift has stemmed from patients and physicians seeking reduced procedural risk, albeit, with potential trade-offs of inferior durability and greater cost. As a result, precise risk stratification for patients who present with CLI has become increasingly important in order to improve clinical decision making and to determine the most appropriate therapy for individual patients.
This study sought to address this topic by utilizing the Project of Ex-Vivo graft Engineering via Transfection III (PREVENT III) database. PREVENT III was a prospective, randomized, double-blinded, multicenter trial designed to examine the efficacy of a novel pharmacologic agent (edifoligide) in preventing autogenous vein graft failure in 1404 patients who underwent infrainguinal vein bypass exclusively for the treatment of CLI.10 This trial incorporated mandated duplex surveillance, independent adjudication of endpoints by a Clinical Events Committee, and external contract research organization (CRO) monitoring of all study data per industry standards. The objective of the current investigation was to utilize this unique database to develop and then validate a precise and easy-to-use prognostic risk index for amputation free survival (AFS) in a population of CLI patients selected to undergo surgical revascularization.
PATIENTS AND METHODS
Data Sources
I. The PREVENT III cohort
Details of the PREVENT III (PIII) trial design have been described elsewhere11 and only relevant features are briefly reviewed here. Edifoligide is a short double-stranded DNA molecule that inhibits cell cycle gene expression and was hypothesized to reduce neointimal hyperplasia. However, in the primary PIII analysis, the treatment of vein grafts with edifoligide was found to confer no benefit on the pre-specified primary and secondary endpoints.10
The study cohort consisted of 1404 patients with CLI drawn from 83 community and university hospitals located across Canada and the United States. All participating institutions underwent independent review of the study and received approval from their respective institutional review boards. Enrollment initiated in November, 2001 and was completed in October, 2003. Detailed characteristics of the study population may be found in the primary trial report.10 The inclusion criteria specified patients at least 18 years of age who underwent IB with autogenous vein for CLI (defined as gangrene, nonhealing ischemic ulcer, or ischemic rest pain with hemodynamic corroboration). Exclusion criteria included claudication as an indication for IB surgery, or use of a non-autogenous conduit. Due to the nature of study drug treatment in PIII (ex-vivo application to the vein), in-situ vein reconstructions were also excluded.
II. External validation cohort
This cohort was accumulated from three diverse hospitals (two academic—Brigham and Women’s Hospital and University of South Florida, one community-based—Sarasota Memorial Hospital) in the United States. All patients (n=716) in the external validation cohort had undergone infrainguinal vein bypass surgery for CLI at one of the three sites, and each subject had a minimum of 1 year follow-up. Datasets from each of these three institutions were comprised of consecutive, non-selected cohorts spanning the inclusive period of 2001–2005. Each of these institutions were participating sites in PIII (contributing a total of 126 patients), and although practice patterns are not assumed to be uniform amongst the individual surgeons, all three groups employ similar approaches including postoperative duplex surveillance and prophylactic reintervention to maintain graft patency, consistent with the PIII study protocol.
Covariates examined
Demographic variables as well as a detailed vascular exam (including an ankle-brachial index (ABI) measurement) were collected prior to surgery as part of a comprehensive history and physical exam. Age was treated as a dichotomous variable using 75 years of age as the cutoff threshold. Patients were defined as having advanced coronary artery disease (CAD) based on documentation of a prior myocardial infarction or surgical or percutaneous revascularization. Medication usage corresponds to prescription at discharge from the index operation; in PIII, the decision to prescribe any concomitant medication before surgery was not protocol-driven and was left to the discretion of the operating surgeon. Protocol-specific technical variables related to the surgery were recorded at the time of bypass by the operating surgeon. These variables included, but were not limited to conduit type and bypass configuration (site of proximal anastomosis, site of distal anastomosis).
Outcomes
The study subjects were followed for 1 year from the time of surgery with postoperative visits at 1, 3, 6, 9, and 12 months post surgery. Outcome events in PIII were tracked by investigators and their study staff at participating centers using specifically designed case report forms, supported by source documentation. All data were audited by a CRO before being entered into the trial database. The primary endpoint, AFS, was a composite endpoint defined as freedom from ipsilateral amputation proximal to the ankle and freedom from all-cause mortality. A total of 44 patients (3.2%) either withdrew or were lost to follow-up in PREVENT III.
Prediction rule development
Two-thirds (N=953) of the PREVENT III cohort were randomly assigned to a derivation set (PIII derivation set) and one-third (N=451) were assigned to the validation set (PIII validation set). The baseline demographic and clinical features of the derivation and validation sets were compared using the t test for continuous variables and the x2 test for categorical variables.
A univariate screen to identify potential significant predictors of AFS was conducted in the PIII derivation set. Kaplan-Meier survival estimates were determined for each covariate and Log rank p-values were generated to compare group differences. Cox proportional hazard models were used to obtain hazard ratios (HR) and 95% confidence intervals. Associations that yielded a p-value < 0.20 on univariate screen (as well as gender), were then included in a multivariable forward stepwise selection model (significance criteria 0.25 for entry, 0.05 for removal).
An integer, or whole number, risk score for AFS at 1 year was constructed by assigning weighted points to each statistically significant independent predictor yielded from the multivariable analysis. These weighted points were calculated by dividing the β-coefficient of the predictor by 0.25 and then rounding off to the nearest integer value. The integer risk score range was then stratified into 3 distinct risk categories based on a qualitative assessment of the Kaplan-Meier survival estimates for each integer score value: low risk, medium risk, high risk.
Internal and external validation
The prediction rule for 1 year AFS was applied to the PIII validation set and the resulting AFS rates were compared to those obtained from the PIII derivation set. Subsequently, the prediction rule was applied to the external multicenter cohort.
All tests were considered statistically significant at an alpha level of 0.05 (p=0.05, two-tailed). All analyses were performed using SAS version 9.1 (Cary, North Carolina).
RESULTS
The PIII cohort consisted of 1404 patients of whom 953 patients were randomly assigned to the PIII derivation set and 451 patients were randomly assigned to the PIII validation set. Baseline patient demographics, medication usage, co-morbid medical conditions, and surgical characteristics were equivalent between the derivation and validation sets (Table 1). The estimated 1 year AFS in the PIII derivation set was 76.3% and in the PIII validation set was 72.5%. With regards to the absolute number of events in the entire PIII cohort, 354 patients either underwent major amputation or died during the 1 year of follow-up; more specifically, there were 162 amputation events and 228 death events (two events in a single patient were not counted twice).
Table 1.
Patient characteristics in the PREVENT III derivation set and the PREVENT III validation set.
| Characteristics | PIII Derivation Set n=953 (%) |
PIII Validation Set n=451 (%) |
|---|---|---|
| DEMOGRAPHICS | ||
| Female | 348 (36.5) | 159 (35.3) |
| Age > 75 | 307 (32.2) | 153 (33.9) |
| African American | 173 (18.2) | 76 (16.9) |
| MEDICATIONS | ||
| Statin | 431 (45.2) | 205 (45.5) |
| Antiplatelet | 759 (79.6) | 362 (80.3) |
| Beta-blocker | 554 (58.1) | 281 (62.3) |
| RISK FACTORS | ||
| Tissue loss (ulcer or gangrene) | 701 (73.6) | 345 (76.5) |
| History of advanced CAD | 393 (41.2) | 193 (42.8) |
| Previous ipsilateral bypass | 138 (14.5) | 58 (12.9) |
| Smoking (ever) | 709 (74.6) | 324 (72.0) |
| Diabetes | 610 (64.0) | 290 (64.3) |
| Hypertension | 774 (81.2) | 372 (82.5) |
| High cholesterol | 445 (46.7) | 189 (41.9) |
| Dialysis-dependent renal failure | 105 (11.0) | 65 (14.4) |
| Ankle brachial index <0.3 | 457 (73.2) | 216 (74.5) |
| Baseline Hematocrit ≤ 30 % | 127 (13.5) | 57 (12.9) |
| Baseline Lymphocyte < 1500 cells/ml3 | 345 (43.1) | 153 (40.1) |
| Weight >75 kg | 502 (53.5) | 256 (57.5) |
| SURGICAL CHARACTERISTICS | ||
| Proximal anastomosis site | ||
| CFA | 464 (48.7) | 223 (49.5) |
| SFA | 234 (24.6) | 113 (25.1) |
| Popliteal | 165 (17.3) | 75 (16.6) |
| Distal anastomosis site | ||
| Popliteal | 320 (34.5) | 137 (30.9) |
| Tibial | 505 (54.4) | 243 (54.9) |
| Pedal | 103 (11.1) | 63 (14.2) |
| Single segment GSV | 781 (82.0) | 350 (77.6) |
CAD, coronary artery disease; CFA, common femoral artery; SFA, superficial femoral artery; GSV, greater saphenous vein.
Univariate screen
In the univariate analysis (Table 2), all covariates suspected to have a potential effect on the outcome AFS were tested, given the following two requirements: 1. The variable had to be obtainable preoperatively (in order to be useful for a clinician prior to deciding on a treatment strategy). 2. The variable had to have been collected during the PREVENT III trial. Statistically significant predictors of AFS included age greater than or equal to 75 years (p=.0009), tissue loss at time of presentation (p<.0001), diabetes (p=.003), history of tobacco use (p=.0002), history of advanced CAD (p=.008), CKD class 3 (p=0.04) and CKD class 4 (p<.0001), dialysis (p<.0001), hematocrit ≤ 30% (p=.004), and lymphocyte count < 1500 (p=.0001). As mentioned above, in order to remain broadly inclusive during covariate evaluation, any predictor with a univariate p-value of < 0.2 was also included in the multivariable model; these factors included elevated cholesterol (p=.05), distal anastomosis to a tibial vessel (p=.05), and weight greater than 75 kg (p=.08).
Table 2.
Univariate amputation-free survival rates and hazard ratios in the PREVENT 5III derivation set.
| Covariate | Amputation-free survival |
HR (95% CI) | p-value | |
|---|---|---|---|---|
| Female | Yes | 75.3 | 1.08 (0.82–1.41) | 0.6 |
| No | 76.7 | 1.0 (ref) | ||
| Age ≥ 75 years | Yes | 69.7 | 1.57 (1.20–2.04) | 0.0009 |
| No | 79.4 | 1.0 (ref) | ||
| African American | Yes | 73.8 | 1.19 (0.86–1.64) | 0.304 |
| No | 76.8 | 1.0 (ref) | ||
| Statin | Yes | 77.7 | 0.86 (0.66–1.21) | 0.27 |
| No | 74.9 | 1.0 (ref) | ||
| Beta-blocker | Yes | 76.8 | 0.93 (0.72–1.22) | 0.61 |
| No | 75.4 | 1.0 (ref) | ||
| Anti-platelet | Yes | 76.5 | 0.93 (0.68–1.28) | 0.66 |
| No | 74.9 | 1.0 (ref) | ||
| CLI criterion | Tissue loss | 72.1 | 2.50 (1.72–3.66) | <0.0001 |
| Rest pain | 87.5 | 1.0 (ref) | ||
| Diabetes | Yes | 73.2 | 1.57 (1.17–3.00) | 0.003 |
| No | 81.7 | 1.0 (ref) | ||
| Insulin controlled diabetes | Yes | 72 | 1.11 (0.82–1.51) | 0.51 |
| No | 74.6 | 1.0 (ref) | ||
| Hypertension | Yes | 76 | 1.08 (0.77–1.52) | 0.21 |
| No | 77.3 | 1.0 (ref) | ||
| Elevated cholesterol | Yes | 73.8 | 0.78 (0.59–1.00) | 0.05 |
| No | 78.9 | 1.0 (ref) | ||
| Smoking (ever) | Yes | 79.1 | 0.59 (0.45–0.78) | 0.0002 |
| No | 67.8 | 1.0 (ref) | ||
| History of advanced CAD | Yes | 71.9 | 1.42 (1.10–1.84) | 0.008 |
| No | 79.2 | 1.0 (ref) | ||
| CKD class | GFR ≥ 60 (ref.) | 81.7 | 1.0 (ref) | |
| GFR 30–60 | 77.8 | 1.29 (0.93–1.78) | 0.13 | |
| GFR 15–30 | 70.5 | 1.84 (1.03–3.29) | 0.04 | |
| GFR < 15 | 54.7 | 3.03 (2.19–4.20) | <.0001 | |
| Dialysis | Yes | 49.3 | 3.09 (2.27–4.20) | <0.0001 |
| No | 79.5 | 1.0 (ref) | ||
| Proximal anastomosis site | CFA | 77.4 | 1.0 (ref) | |
| SFA | 75.9 | 1.11 (0.81–1.54) | 0.52 | |
| Popliteal | 71.9 | 1.31 (0.92–1.85) | 0.13 | |
| Distal anastomosis site | Popliteal | 80 | 1.0 (ref) | |
| Tibeal | 74 | 1.36 (1.00–1.83) | 0.05 | |
| Pedal | 77.3 | 1.24 (0.78–1.99) | 0.36 | |
| Prior ipsilateral bypass | Yes | 79.4 | 0.82 (0.55–1.21) | 0.31 |
| No | 75.6 | 1.0 (ref) | ||
| ABI (median) | >4.2 | 79 | 1.0 (ref) | |
| <4.2 | 74.9 | 1.22 (0.88–1.69) | 0.24 | |
| ABI | >0.3 | 80.1 | 1.0 (ref) | |
| <0.3 | 75.8 | 1.24 (0.84–1.83) | 0.28 | |
| SS-GSV | Yes | 76 | 1.0 (ref) | |
| No | 77.1 | 1.06 (0.75–1.50) | 0.74 | |
| Hematocrit < 30 | Yes | 66.4 | 1.65 (1.18–2.31) | 0.004 |
| No | 77.7 | 1.0 (ref) | ||
| Lymphocyte < 1500 | Yes | 69.6 | 1.75 (1.31–2.33) | 0.0001 |
| No | 81.5 | 1.0 (ref) | ||
| Weight >75 kg | Yes | 78.5 | 0.79 (0.61–1.03) | 0.08 |
| No | 73.7 | 1.0 (ref) |
Independent determinants of AFS and discrimination into 3 strata of risk
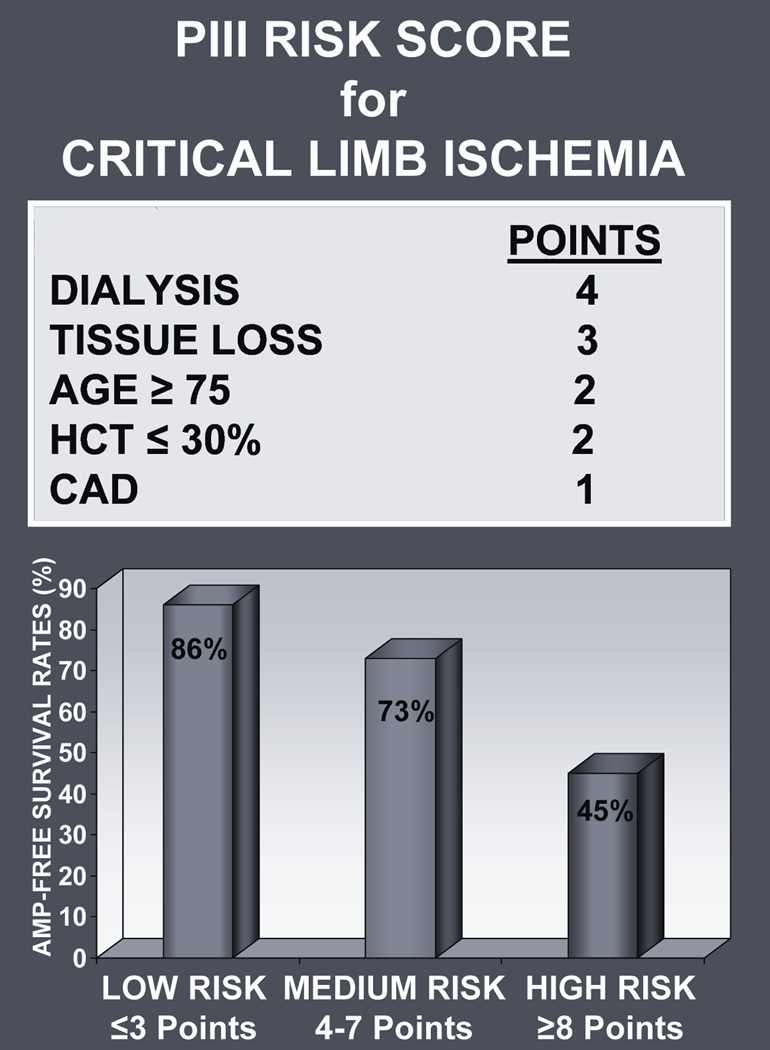
The multivariable Cox proportional hazards model identified 5 statistically significant predictors of AFS (Table 3): dialysis, tissue loss, age ≥ 75 years, hematocrit ≤ 30, and a history of advanced CAD. According to the magnitude of the HRs, the strongest predictor for death or major amputation within 1 year of surgery was dialysis-dependency (HR 2.81, CI 1.97–3.99). Tissue loss as an indication for revascularization was associated with a HR of 2.22, CI 1.43–3.44. Age ≥ 75 years and hematocrit ≤ 30% each had a greater than 1.5 fold risk for loss of AFS (HR 1.64, CI 1.21–2.22 and HR 1.61, CI 1.11–2.34, respectively) while a history of advanced CAD was associated with a HR of 1.41, CI 1.05–1.88.
Table 3.
Multivariable model for the prediction of amputation-free survival with an integer risk score assigned to each covariate (PIII derivation set).
| COVARIATES | β coefficient | Integer score | HR (95% CI) | P-Value |
|---|---|---|---|---|
| Dialysis | 1.03 | 4 | 2.81 (1.97, 3.99) | <0.0001 |
| CLI criterion | 0.80 | 3 | 2.22 (1.43, 3.44) | 0.0004 |
| Age ≥ 75 years | 0.50 | 2 | 1.64 (1.21–2.22) | 0.001 |
| Hematocrit < 30 | 0.48 | 2 | 1.61 (1.11, 2.34) | 0.012 |
| History of advanced CAD | 0.34 | 1 | 1.41 (1.05, 1.88) | 0.021 |
The integer score assigned to each covariate was used to calculate each individual patient’s risk score for 1 year AFS. The scores ranged from 0 to 12 (median 4, interquartile range 3–5). As shown in Table 4, a clear gradient of increased risk ranging from 93.1% AFS to 33.0% AFS was noted to correlate with the magnitude of the risk score. Based on the Kaplan-Meier estimated AFS rates in the PIII derivation set, three risk categories were assigned: low risk (score ≤ 3), medium risk (score 4–7), and high risk (score ≥ 8). The 1 year AFS rates associated with each risk category were significantly different (p-value <.0001 for each comparison): AFS in the low risk group (44.4% of cohort) was 85.9%, AFS in the medium risk group (46.7% of cohort) was 73.0%, and AFS in high risk group (8.8% of cohort) was 44.6% (Table 5).
Table 4.
Estimated 1 year amputation-free survival stratified according to the calculated risk score (PIII derivation set).
| Risk Score | Amputation-Free Survival (%) |
|---|---|
| 1 | 93.1 |
| 2 | 89.7 |
| 3 | 86.2 |
| 4 | 81.1 |
| 5 | 76.1 |
| 6 | 74.5 |
| 7 | 71.7 |
| 8 | 63 |
| 9 | 51 |
| 10 | 44 |
| 11 | 27.3 |
| 12 | 33 |
Table 5.
Estimated one year amputation-free survival and the associated hazard ratios based on stratification into low, medium, and high risk groups (PIII derivation set).
| Risk Categories | Integer Score | Amputation-Free Survival |
HR | p-value |
|---|---|---|---|---|
| Low | ≤3 | 85.9 | 1.0 (ref) | — |
| Medium | 4–7 | 73.0 | 2.11 (1.54–2.89) | <.0001 |
| High | ≥8 | 44.6 | 5.50 (3.73–8.10) | <.0001 |
Validation
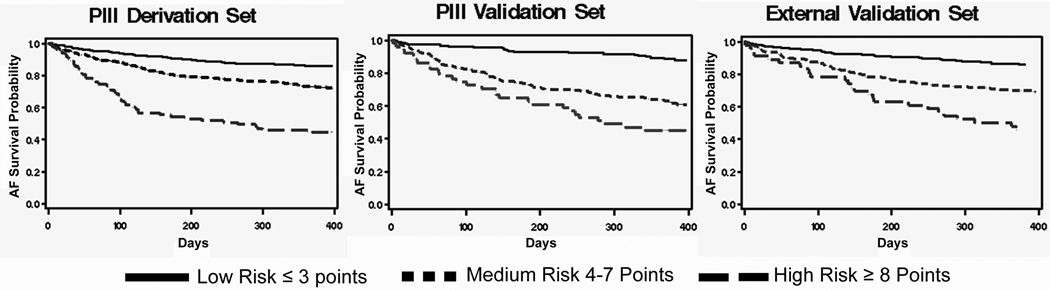
The integer scoring system derived above was applied to each patient in the PIII validation set and to each patient in the external validation set. After stratification by risk category, the AFS rate was extremely similar between the PIII derivation set, the PIII validation set, and the external validation set (Figure 1). The stratified Kaplan-Meier curves displayed in Figure 2 demonstrate that all 3 cohorts had comparable AFS within each of the three risk categories.
Figure 1.
Figure 2.
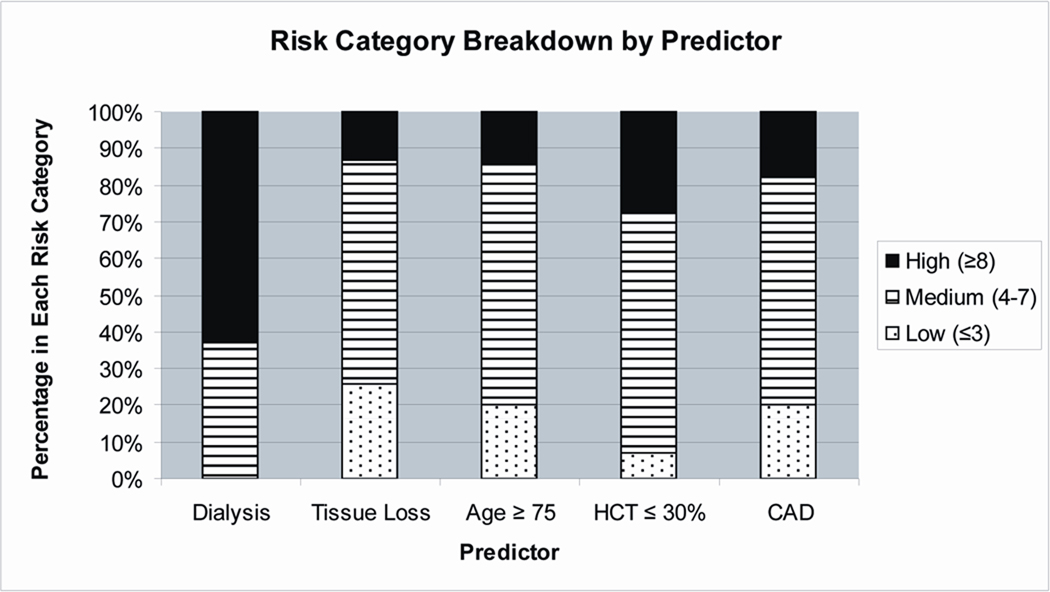
Composition of each risk group (Figure 3)
Figure 3.
The entire PIII cohort was analyzed to determine the breakdown of patients in each risk group according to each independent predictor. High risk classification was assigned to 63% of the patients on dialysis, 13% of the patients with tissue loss, 14% of the patients with age ≥ 75 years, 27% of the patients with a HCT ≤ 30%, and 18% of the patients with CAD.
DISCUSSION
Among patients selected to undergo surgical bypass, this parsimonious risk stratification model reliably identified a subgroup of high risk patients who experienced a > 50% incidence of either death or major amputation within 1 year. All of the information necessary (dialysis, tissue loss, advanced age, low hematocrit, and advanced CAD) to calculate the “PIII risk score” can be easily obtained at the time of initial presentation. We believe that the PIII risk score (Figure 4) serves as a useful clinical tool for surgical decision making, enabling physicians to generate a pre-procedure risk estimate that addresses an individual patient’s likelihood of success or failure after surgical revascularization; future prospective validation studies are necessary to support this claim.
Figure 4.
The decision to model AFS as the critical endpoint of interest was based on our belief that in order to deem a revascularization procedure a successful and worthwhile endeavor, at minimum, there should be a reasonable probability that the patient will remain alive with an intact index limb at one year. If this goal cannot be achieved with a given therapy such as surgical bypass, then alternative therapies including conservative care, endovascular options, or primary amputation should be strongly considered. The PIII risk score may aid in making these, often difficult, treatment decisions.
The five independent predictors of AFS identified in this study have all been shown in prior reports to be associated with survival and/or limb salvage in patients undergoing infrainguinal revascularization:
Dialysis-dependent renal failure as a predictor of AFS is especially relevant given the remarkably high reported coprevalence of PAD in the end stage renal disease population which has been estimated at 24%.12 This factor was clearly the single most dominant determinant of AFS in this cohort of patients with an associated hazard ratio of 2.81, CI 1.97–3.99. Owens and colleagues demonstrated a similar pronounced effect on AFS in a heterogeneous population of patients with claudication and CLI who underwent first-time surgical bypass (adjusted hazard ratio in their analysis 3.19).13
Tissue loss, defined as a non-healing ulcer or gangrene, is reported as the primary manifestation in 74% to 82% of patients with CLI.5, 10, 14 Previous studies have identified tissue loss as a significant predictor of amputation.15, 16
Advanced patient age is a common concern for vascular physicians as increasing numbers of elderly patients are presenting with PAD.17 Brosi and colleagues demonstrated a greater than 5 fold increase in 1 year mortality when octogenerians were treated with surgical bypass for CLI as compared to non-octogenerians.14
Anemia, to our knowledge, has not been shown to play an independent role in either amputation or death after bypass surgery. However, prior reports have demonstrated an association between anemia and incisional complications18 as well as with early loss of bypass graft patency;19 these types of complications have been demonstrated to ultimately lead to increased limb loss and mortality in patients with CLI.15 In addition, anemia has been shown using the National Veterans Administration Surgical Quality Improvement Program (NSQIP) dataset to confer increased rates of perioperative infection and mortality in noncardiac surgical patients.20
CAD, either symptomatic or asymptomatic, has been estimated to be present in greater than 50% of patients with CLI.21, 22 It has been well established that the large systemic burden of atherosclerotic disease inherent to CLI subjects patients, not only to the immediate risk of morbidity and mortality secondary to the affected limb, but also to an increased risk of cardiac death.1, 2, 4
The absence of diabetes from the final model is deserving of special comment. In several series of patients with advanced PAD, patients with diabetes have been noted to have reduced survival22 and limb salvage16. Indeed, that was the case in the univariate analysis of this study. However, when dialysis and tissue loss were incorporated into the stepwise regression model, the association between diabetes and AFS became attenuated and was no longer significant. Among the population of diabetics analyzed in the entire PIII cohort, the proportion assigned to each of the three risk categories was 34%, 54%, and 12% (low risk, medium risk, and high risk, respectively).
The use of statin therapy is also conspicuously absent from the final model. In fact, statin use did not demonstrate an associated with AFS on univariate analysis (p=0.27). This finding may seem at odds with previous publications linking statins to improved outcomes in patients with peripheral arterial disease.23–26 However, it is important to remember that these other studies did not model AFS as the study endpoint. While statins may have an important protective role for mortality and major cardiovascular events (stroke, myocardial infarction), these data did not demonstrate an association with AFS.
The PIII risk score may be useful to refine patient stratification for future study designs evaluating new treatment modalities for CLI. It is our opinion that the results of novel treatments in low risk patients (44% of this cohort, estimated AFS 86%) should be held to a high standard with durability outcomes comparable to those obtained with surgical bypass. Conversely, in high risk patients (9% of this cohort), where AFS at 1 year has been shown to be less than 50%, inferior durability may be an acceptable trade-off in exchange for lower periprocedural risk.
Several limitations inherent to this study design merit consideration. All patients included in this study underwent surgical bypass and, by necessity, had to have been deemed to be acceptable surgical candidates. Furthermore, all of the patients from whom the prediction rule was derived were enrolled in a randomized controlled trial. Due to this upfront selection bias, the PIII risk score may lack generalizability to all patients presenting with CLI; there is likely to be a group of greater risk patients who were not available for evaluation because they were not offered surgical bypass in the first place. Future prospective validation studies will be necessary to address this important issue. Therefore, this risk index should be currently considered only within the context of patients with CLI that are considered as potential candidates for an open bypass. We are unable to comment on how this model would perform in patients undergoing endovascular interventions because none of the CLI patients from whom it was derived or validated underwent endovascular methods.
Quality of life (QOL) and functional outcomes are of clear importance and only recently have such measures (and the use of appropriately validated instruments to assess them) been incorporated into the analysis of interventions for CLI.27–29 QOL results have been reported from PIII,30 but were not available from the external datasets. We therefore did not include QOL as an endpoint for modeling in this study.
Although all of the data analyzed in this study were prospectively collected, they were reviewed retrospectively. As a result, in the design of the present study, we were limited to those variables that were collected during the original trial and unable to assess any additional factors that had not been recorded. Among the notable covariates not available for analysis were inflammatory markers. Over the last decade, a wide body of literature has developed pointing to the role that inflammation plays in atherosclerosis31, highlighted by the establishment of c-reactive protein as a key prognostic indicator.32–34 Unfortunately, we are unable to comment on the potential role that inflammatory markers may play in determining outcomes in patients with CLI. Similarly, functional/ambulatory status at the time of presentation is another factor that often weighs heavily into the decision-making process when evaluating treatment strategies for patients with CLI; these factors were not available for this analysis.
Finally, with regards to our methodology, we used Kaplan-Meier and Cox regression survival analysis in order to include all patients and to account for any censoring. As a result, for the purpose of validation, we were unable to create receiver operator curves, with an associated c-statistic, as is often done with logistic regression-based prediction rules. Validation in this study is therefore subjective (Figure 1) and does not have an associated p-value. Nonetheless, the different risk strata are clearly comparable between the PIII derivation set, PIII validation set, and the external validation set.
CONCLUSIONS
The PIII risk score utilizes five easily obtainable binary variables—dialysis-dependency, presence of tissue loss, age ≥ 75 years, hematocrit ≤ 30%, and a history of advanced CAD—to stratify patients with CLI and surgically correctable infrainguinal disease into three distinct categories of expected amputation-free survival. Prospective evaluation of this risk score will clarify its utility for clinical decision making and in clinical trial design.
Supplementary Material
Footnotes
Presented at the 2008 Vascular Annual Meeting, San Diego, CA
REFERENCES
- 1.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 2.McKenna M, Wolfson S, Kuller L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis. 1991;87:119–128. doi: 10.1016/0021-9150(91)90014-t. [DOI] [PubMed] [Google Scholar]
- 3.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 4.Howell MA, Colgan MP, Seeger RW, Ramsey DE, Sumner DS. Relationship of severity of lower limb peripheral vascular disease to mortality and morbidity: a six-year follow-up study. J Vasc Surg. 1989;9:691–696. doi: 10.1067/mva.1989.vs0090691. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 5.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 6.Stoyioglou A, Jaff MR. Medical treatment of peripheral arterial disease: a comprehensive review. J Vasc Interv Radiol. 2004;15:1197–1207. doi: 10.1097/01.RVI.0000137978.15352.C6. [DOI] [PubMed] [Google Scholar]
- 7.Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. Jama. 2007;297:1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 8.Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord. 2005;5:14. doi: 10.1186/1471-2261-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeRubertis BG, Faries PL, McKinsey JF, et al. Shifting paradigms in the treatment of lower extremity vascular disease: a report of 1000 percutaneous interventions. Ann Surg. 2007;246:415–422. doi: 10.1097/SLA.0b013e31814699a2. discussion 22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 51. [DOI] [PubMed] [Google Scholar]
- 11.Conte MS, Lorenz TJ, Bandyk DF, Clowes AW, Moneta GL, Seely BL. Design and rationale of the PREVENT III clinical trial: edifoligide for the prevention of infrainguinal vein graft failure. Vasc Endovascular Surg. 2005;39:15–23. doi: 10.1177/153857440503900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004;109:320–323. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 13.Owens CD, Ho KJ, Kim S, et al. Refinement of survival prediction in patients undergoing lower extremity bypass surgery: stratification by chronic kidney disease classification. J Vasc Surg. 2007;45:944–952. doi: 10.1016/j.jvs.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Brosi P, Dick F, Do DD, Schmidli J, Baumgartner I, Diehm N. Revascularization for chronic critical lower limb ischemia in octogenarians is worthwhile. J Vasc Surg. 2007;46:1198–1207. doi: 10.1016/j.jvs.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen LL, Brahmanandam S, Bandyk DF, et al. Female gender and oral anticoagulants are associated with wound complications in lower extremity vein bypass: an analysis of 1404 operations for critical limb ischemia. J Vasc Surg. 2007;46:1191–1197. doi: 10.1016/j.jvs.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor SM, Kalbaugh CA, Blackhurst DW, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44:747–755. doi: 10.1016/j.jvs.2006.06.015. discussion 55–6. [DOI] [PubMed] [Google Scholar]
- 17.Diehm C, Schuster A, Allenberg JR, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105. doi: 10.1016/s0021-9150(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 18.Nam JH, Gahtan V, Roberts AB, Kerstein MD. Influence of incisional complications on infrainguinal vein bypass graft outcome. Ann Vasc Surg. 1999;13:77–83. doi: 10.1007/s100169900224. [DOI] [PubMed] [Google Scholar]
- 19.Singh N, Sidawy AN, DeZee KJ, Neville RF, Akbari C, Henderson W. Factors associated with early failure of infrainguinal lower extremity arterial bypass. J Vasc Surg. 2008;47:556–561. doi: 10.1016/j.jvs.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 20.Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res. 2002;102:237–244. doi: 10.1006/jsre.2001.6330. [DOI] [PubMed] [Google Scholar]
- 21.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81–157. [PubMed] [Google Scholar]
- 22.Feringa HH, Bax JJ, Hoeks S, et al. A prognostic risk index for long-term mortality in patients with peripheral arterial disease. Arch Intern Med. 2007;167:2482–2489. doi: 10.1001/archinte.167.22.2482. [DOI] [PubMed] [Google Scholar]
- 23.Schanzer A, Hevelone N, Owens CD, Beckman JA, Belkin M, Conte MS. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47:774–781. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 24.Feringa HH, Karagiannis SE, van Waning VH, et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45:936–943. doi: 10.1016/j.jvs.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 25.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 26.Ward RP, Leeper NJ, Kirkpatrick JN, Lang RM, Sorrentino MJ, Williams KA. The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery. Int J Cardiol. 2005;104:264–268. doi: 10.1016/j.ijcard.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Czajkowski SM. Health-related quality of life outcomes in clinical research: NHLBI policy and perspectives. Ann Thorac Surg. 1998;66:1486–1487. doi: 10.1016/s0003-4975(98)00837-6. [DOI] [PubMed] [Google Scholar]
- 28.Abou-Zamzam AM, Jr, Lee RW, Moneta GL, Taylor LM, Jr, Porter JM. Functional outcome after infrainguinal bypass for limb salvage. J Vasc Surg. 1997;25:287–295. doi: 10.1016/s0741-5214(97)70350-1. discussion 95–7. [DOI] [PubMed] [Google Scholar]
- 29.Thorsen H, McKenna S, Tennant A, Holstein P. Nottingham health profile scores predict the outcome and support aggressive revascularisation for critical ischaemia. Eur J Vasc Endovasc Surg. 2002;23:495–499. doi: 10.1053/ejvs.2002.1648. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen LL, Moneta GL, Conte MS, Bandyk DF, Clowes AW, Seely BL. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg. 2006;44:977–983. doi: 10.1016/j.jvs.2006.07.015. discussion 83–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 32.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 34.Owens CD, Ridker PM, Belkin M, et al. Elevated C-reactive protein levels are associated with postoperative events in patients undergoing lower extremity vein bypass surgery. J Vasc Surg. 2006 doi: 10.1016/j.jvs.2006.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.