Abstract
Background
Lower socioeconomic status (SES) is strongly linked to health outcomes, though the mechanisms are poorly understood. Little is known about the role of the immune system in creating and sustaining health disparities. Here we test whether SES is related to cell-mediated immunity, as measured by the host’s ability to keep persistent cytomegalovirus (CMV) antibody levels in a quiescent state.
Methods
Censored regression models are used to test the cross-sectional relationship between education, income, race/ethnicity, and antibody response to CMV, using a nationally representative sample of 9721 respondents aged 25 years and older surveyed in the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994).
Results
Among CMV seropositive respondents, those with less education, income, and non-white race/ethnicity had significantly higher levels of antibodies to CMV at all ages. On average, each additional year of age was associated with CMV antibody levels that were 0.03 (95% CI 0.03–0.04) units higher, while each additional year of education was associated with antibody levels that were 0.05 (95% CI 0.02–0.09) units lower. A doubling of family income was associated with antibody levels that were 0.25 (95% CI 0.11–0.39) units lower, the equivalent of 8 fewer years of age-related CMV antibody response. These relationships remained strong after controlling for baseline health conditions, smoking status, and BMI.
Conclusions
This study reports for the first time a significant association between SES and an indirect marker of cell-mediated immunity in a nationally representative U.S. sample. SES differences in immune control over CMV may have fundamental implications for health disparities over the life course.
Keywords: cytomegalovirus, socioeconomic status, immunity, aging, NHANES III
I. Introduction
Health disparities by socioeconomic status (SES) in the U.S. are well established for a wide range of health outcomes.1 The U.S. Department of Health and Human Services declared as one of the two major goals of the Healthy People 2010 initiative “to eliminate health disparities among different segments of the population.” The pathophysiological mechanisms linking lower SES to the earlier onset of disease are not well understood. In particular, very little is known about the role of the immune system in creating and sustaining health disparities. Recent studies have shown that cytomegalovirus (CMV) may be a factor in immunosenescence.2–4 Indeed, CMV has been considered a more important predictor of mortality than many of the well-known traditional immune function markers such as CD4:CD8 ratio, T-cell proliferation, and B cell counts in older age populations.5 CMV has also been implicated in cardiovascular disease and more recently linked to cognitive and physical decline.6–10 While primary infection with CMV is often asymptomatic and occurs at a very young age, the virus remains persistent in the infected host’s cells for life, with containment of the virus becoming an immune system priority.11, 12 Studies have identified positive age-related gradients in antibody response to CMV which are accompanied by higher CMV DNA shedding.12 Since impaired cellular immune response can result in increased CMV viral replication, these studies suggest that an increase in CMV antibody titer is a good marker of compromised cell mediated immunity observed in aging.12, 13
Previously, data from the Third National Health and Nutrition Examination Survey (NHANES) III have described large disparities in CMV prevalence in the U.S. by education, income, and race/ethnicity over a range of ages.14, 15 These disparities in seroprevalence emerge as early as age six years, converging over age 65 years as more than 90% of the population is exposed. Socioeconomic disparities in CMV seroprevalence provide important information about the link between socioeconomic determinants and exposure to CMV, but do not provide any indication of the variability in immune response to CMV among those who are seropositive. The social patterning of immune response among seropositive individuals has not been fully explored. Recent work identified an association between lower education and increased immune response to CMV and Herpes Simplex Virus Type 1 (HSV-1) in a community-based sample of Latinos ages 65 years and over.16 Nevertheless, it is unclear whether similar gradients are found in the U.S. population across a broader range of age categories and race/ethnicities. If socioeconomic gradients in CMV antibody levels are sustained across the life course, it may well represent an important immunological pathway linking socioeconomic status to adult immune function and overall health. Therefore, we analyzed NHANES III data for subjects aged 25 years and older to assess social patterning in immune response to CMV.
2. Methods
2.1 Study Population
Participants were part of the National Health and Nutrition Examination Survey (NHANES) III conducted by the National Center for Health Statistics from 1988 to 1994. The sample was a complex, stratified, multistage probability cluster of the U.S. population including interview, clinical examination, and laboratory tests. Persons <5 or >60 years of age, non-Hispanic black persons, and Mexican Americans were sampled at a higher frequency than other groups to obtain adequate sample sizes for subgroup analysis. The complete methodology and response rates of NHANES III have been published previously.17 In order to focus on adult socioeconomic status and antibody response to existing infection, our analysis includes individuals 25 years and older who are seropositive to CMV (n=10,882). Those classified as CMV seropositive represent 80.6% of respondents aged 25 years and older. As reported elsewhere, correlates of CMV seropositivity in the U.S. include lower education and income, greater age, and non-Hispanic black and Mexican American ethnicity.14, 15 Of the 10,882 subjects, 1161 CMV seropositive individuals (11.9%) were missing data on one or more covariates, primarily income, leaving a final sample of 9721. Individuals with missing income were slightly more likely to be women with less education, but did not significantly differ in CMV antibody levels.
2.2 Measures
Cytomegalovirus antibody levels were tested using frozen stored sera specimens from NHANES III to estimate the seroprevalence of CMV infection (CMV IgG) in participants aged 6 years and above and released in 2006. CMV specific IgG was measured by an Enzyme Linked Immunoassay (ELISA) (Quest International, Inc., Miami, FL). IgG optical density and ELISA index values were subsequently released in 2007 to the investigators by special request to the National Center for Health Statistics. As the original aim of the CMV testing was to identify seropositivity rather than continuous antibody levels, 31.75% of the IgG values for the full sample (and 48% of the values for the sample 25 years and over) were given a top-coded value of the maximum detectable threshold, which was set for each test batch. ELISA Index values between 0.90 and 1.05 (1.13% of the sample) were considered indeterminate with regard to seropositivity by the laboratory (Quest International, Inc., Miami, FL). We conservatively classified these individuals as seronegative to ensure that our analysis focused on antibody response among seropositive individuals and did not reflect underlying differences in seropositivity.
Education was measured as the respondent’s years of completed education. A linear specification of years of education provided the best model fit. Family income was self-reported pre-tax family income for the 12 months prior to the survey including wages, salaries, income from self-employment, veteran’s benefits, interest dividends, rental income, and public assistance. Income data were recorded in 28 income categories (none; less than $1000; $1000 to $20,000 in $1000 increments; $20,000 to $50,000 in $5000 increments; and greater than $50,000). We coded family income as the midpoint of each of the reported categories (using $65,000 for the incomes above $50,000) and adjusted for inflation between the two NHANES III phases using the Consumer Price Index. Income was log-transformed due to the skewness of the distribution. Race/ethnicity was grouped in four categories (non-Hispanic white, non-Hispanic black, Mexican-American, and other). A modified Charlson index was used to control for the potential impact of current chronic health conditions on immune response. The Charlson index employs a weighting system for each condition category based on the adjusted risk of one-year mortality.18 A higher score reflects a more severe burden of co-morbidity. Weights were assigned for having myocardial infarction, cerebrovascular/ peripheral vascular disease, chronic pulmonary disease, connective tissue/autoimmune disease, ulcer, liver disease, diabetes, moderate to severe renal disease, and any tumor. Points for each condition were summed to create the final score as previously described.18 To assess the potential effects of any immunerelated conditions or current infections not captured in our Charlson index, we created two medication variables to represent whether an individual was taking, near the time of the blood sample, any of the major classes of drugs that are: (i) treatments for concurrent infection (i.e. antibiotics, cold medicines, antivirals, antiretrovirals, etc.) or (ii) treatments for autoimmune disorders (i.e. corticosteroids, immunologic agents, immunomodulators, antiarthritics). Other control variables included sex, age in years, categorical marital status (l=married or cohabitating; 2=widowed, separated, or divorced; 3=never married), census region of residence, urban or rural residence, household size (persons in the dwelling), an indicator for current, former, or never smoking status, and body mass index (BMI).
2.3 Statistical Analysis
The association between education, income, and CMV antibodies was tested using censored normal regressions to account for the right-censoring of CMV values. This model is a generalization of the tobit model that allows censored values to vary from observation to observation, rather than a constant upper or lower censoring point.19 In our case, since different CMV testing batches were arbitrarily top-coded at different values, this model was the most appropriate. All observations were assigned the CMV value reported, and an additional indicator variable was created equal to 0 if the observation was not right-censored and equal to 1 if the observation was right-censored. All analyses were conducted using STATA 10.0 (STATA™, College Station, TX) survey commands to account for the complex survey design, and the sample completing the laboratory components was weighted to reflect the non-institutionalized population of the United States. The command “cnreg” was used in STATA for the right-censored models. Significant interactions between income and gender and between education and gender were identified, leading to subsequent stratification by gender. Other covariates included in preliminary models included age, race-ethnicity, Charlson index, census region of residence, urban or rural residence, marital status, prescription medication use, smoking status, or obesity. Of these, only age, race/ethnicity, and the Charlson index were associated with CMV antibody levels in fully adjusted models. The final model thus regresses CMV antibody levels on education, income, age, race/ethnicity, and the Charlson index, stratified by gender.
3. Results
Sample descriptives for the 9,797 participants are shown in Table 1. There was a higher mean CMV antibody level among females compared to males and lower levels of former and current smoking among females versus males. There were only slight differences in other variables between genders.
Table 1.
Sample Descriptive Statistics (Weighted) NHANES III, 1988–1994
(Adults 25 and older who are CMV seropositive)
Percentage or Mean and (Linearized Standard Error)
| MEN (N=4453) | WOMEN (N=5344) | |
|---|---|---|
| Mean or % | ||
| CMV Index Value* | 7.46 (0.11) | 8.77 (0.09)** |
| Non-Hispanic White | 81.60% | 81.23% |
| Non-Hispanic Black | 12.70% | 14.08% |
| Mexican-American | 5.70% | 4.70% |
| Years of Education | 11.87 (.10) | 11.81 (.10) |
| Family Income | 36324 (896) | 33632 (802)** |
| Age | 49.1 (0.54) | 50.5 (0.64) |
| Houshold Size (Persons in Dwelling) | 3.06 (0.05) | 2.89 (0.04) |
| Charlson Index | 4.78 (0.03) | 4.86 (0.02) |
| BMI | 26.8 (0.14) | 27 (0.18) |
| Never Smoker | 32.32% | 53.52% ** |
| Former Smoker | 37.15% | 21.29% ** |
| Current Smoker | 30.53% | 25.19%** |
Since CMV values are right-censored, this mean is the estimated expected value from the censored normal regression
gender difference significant at p=.05
CMV antibody levels, education and income: full sample
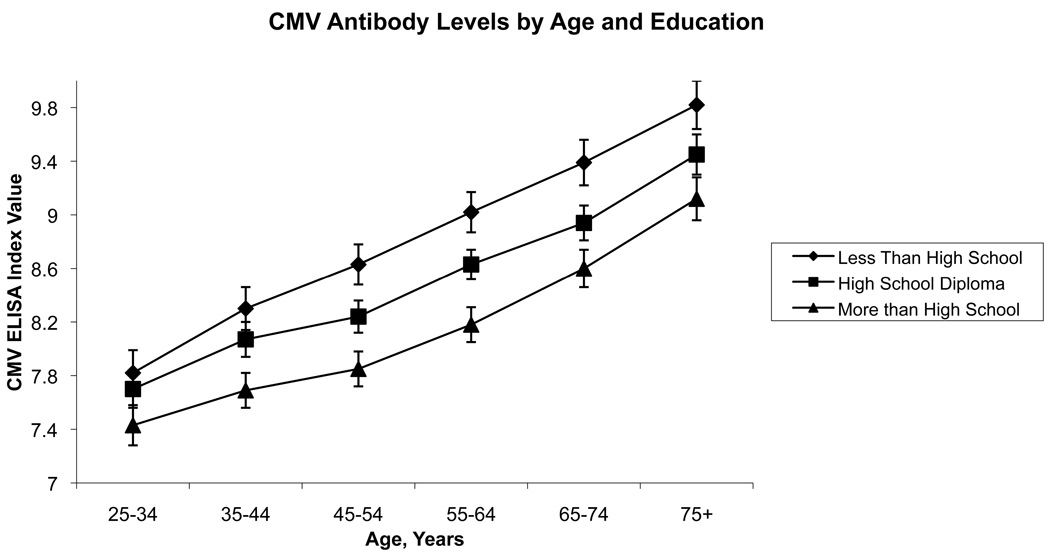
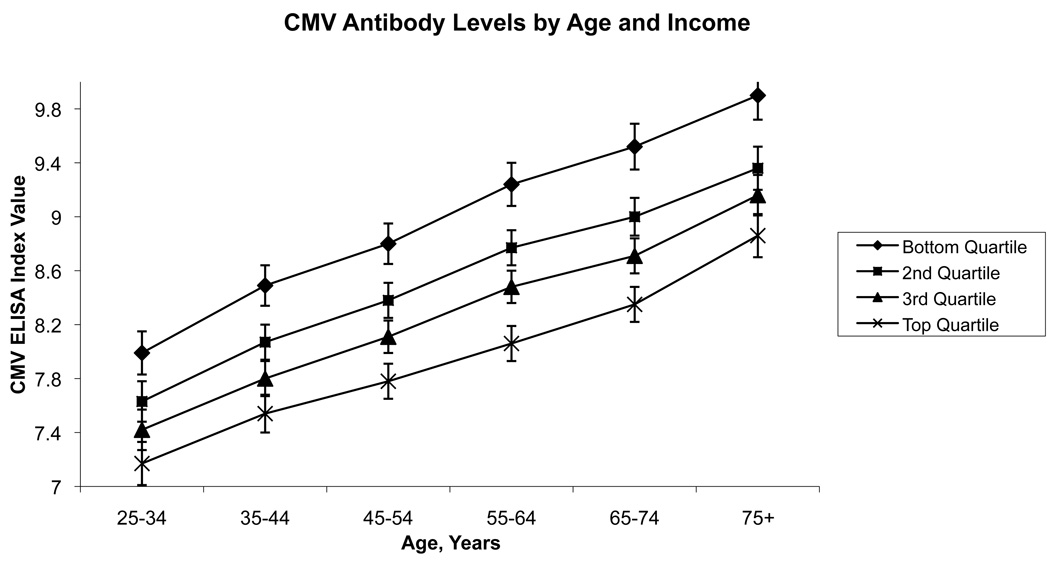
Figure 1 and Figure 2 illustrate the adjusted relationship between categorical education, income and CMV values across age based on predicted values. CMV antibody levels increased substantially with increasing age in this cross-section, while within a given age group, levels were higher for those with less education and less income. Increasing age was associated with higher CMV antibody levels at the rate of 0.03 ELISA units per year after age 25, consistent with a decreased ability of the immune system to maintain control over the virus with age (Table 2).
Figure 1.
Data from the Third National Health and Nutrition Examination Survey, 1988–1994, showing predicted values and standard errors of antibody response to cytomegalovirus (CMV) by age and level of education. Results were adjusted for race/ethnicity, income, and sex.
Figure 2.
Data from the Third National Health and Nutrition Examination Survey, 1988–1994, showing predicted values and standard errors of antibody response to cytomegalovirus (CMV) by age and quartile of income. Results were adjusted for race/ethnicity, education, and sex.
Table 2.
Censored Regression Models for CMV ELISA Index Values among seropositive respondents ages 25 and older, NHANES III 1988–1994
| Full Sample | Women | Men | |
|---|---|---|---|
| (N=9765) | (N=5336) | (N=4429) | |
| Slope (95% Confidence Interval) | |||
| Age in Years | 0.03 (0.03 – 0.04) | 0.03 (.02 – .04) | 0.04 (0.02 – 0.05) |
| Years of Education | −0.05 (−0.09 – −0.02) | −0.08 (−0.13 – 0.03) | −0.03 (−0.07 – 0.02) |
| Log (Family Income) | −0.25 (−0.39 – −0.11) | −0.12 (−0.27 – 0.04) | −0.44 (−0.65 – −0.22) |
| Non-Hispanic Black | 0.56 (0.34 – 0.78) | 0.78 (0.45 – 1.10) | 0.31 (−0.03 – 0.65) |
| Mexican-American | 0.34 (−0.10 – 0.78) | 0.91 (0.12 – 1.70) | −0.22 (−0.88 – 0.44) |
| Female | 1.12 (0.83 – 1.42) | ||
| Charlson Index | 0.10 (0.00 – 0.21) | 0.08 (−0.10 – 0.26) | 0.12 (−0.01 – 0.26) |
| Constant | 8.44 (7.13 – 9.75) | 8.69 (6.86 – 10.53) | 9.78 (7.70 –11.86) |
β Coefficients
95% Confidence Intervals in parentheses
Both education and family income were inversely associated with CMV antibody levels. Each additional year of education was associated with a 0.05 reduction in the CMV ELISA index value. Thus, holding all else constant, an additional 5 years of education was associated with roughly the same reduction in CMV values as would be seen in a respondent 8 years younger. Non-Hispanic blacks, on average, had significantly higher CMV levels compared to non-Hispanic whites, and females had higher levels on average than males, holding all else constant. In fully adjusted models, having more chronic conditions as measured by the Charlson Index was also associated with higher CMV levels.
CMV antibody levels, education and income: by sex
The associations of education and income with CMV varied by sex (Table 2). For women, years of education were more strongly associated with CMV levels than for men, for whom the association with income was stronger. For women, each additional year of education was associated with a 0.08 reduction in the CMV index, so that an additional 5 years of education was associated with a reduction in CMV values similar to that seen in women approximately 13 years younger, holding all else constant. For men, the association with income appeared to dominate that of education, such that a doubling of family income was associated with a 0.44 lower index value of CMV, holding all else constant. For men, this 0.44 reduction in the index value is equivalent to the reduction associated with being 11 years younger.
The effects of race/ethnicity appeared stronger for females compared to males. In fully adjusted models, non-Hispanic black women had a CMV index value that was 0.78 points higher than non-Hispanic white women, and Mexican-American women had an index 0.91 points higher than non-Hispanic white women, though the standard error was much larger for Mexican-Americans. The magnitude of these differences is large with respect to the 0.03 unit/year association observed for an increase in one year of age. For men, non-Hispanic blacks had CMV values 0.31 units higher on average compared to non-Hispanic whites, with no significant differences observed for Mexican-American men compared to white men, holding all else constant. In fully adjusted models, the Charlson Index was associated with higher levels of CMV for the full sample and for men alone, but not for women. Adjusting for current conditions and co-morbidities did not account for the relationship between SES, race/ethnicity, and CMV antibody response.
CMV antibody levels, education, and income-by age
To test the hypothesis that SES differences in antibody response would emerge only in older age groups at greater risk of immune decline, we interacted both education and income with continuous age as well as dichotomous age above and below age 65 years. None of these interactions were significant, suggesting that the association between education, income, and CMV antibody response does not depend on age.
4. Discussion
To our knowledge, this is the first study to report a significant association between socioeconomic status and an indirect marker of cell-mediated immunity in a nationally representative sample of the U.S population. Our study confirms recent findings of an association between level of education and CMV antibody response in a community-based study of elderly Latinos. The findings suggest that impaired cell-mediated immunity may be one way that SES is related to health outcomes in the U.S. population.
CMV has been called the “driving force” behind age-associated alterations to the T-cell immune system, and the cost of constant CMV immunological vigilance throughout life may be high.11 This theory is based on evidence that aging populations experience increased CMV specific CD8+ T-cell proliferation, reducing the availability of CD8+ T-cell carrying receptors that are specific for other pathogens or foreign antigens that the immune system may encounter. This large clonal expansion of CMV-specific CD8 T cells found in aging populations limits the capacity of the immune system to mount an efficient immune response.20 The reduction in the number of naïve cells able to combat new antigens21 resulting from CMV-specific clonal expansion can directly hinder immune response in medically important ways, as observed in poorer immune responses to influenza vaccination among those with higher CMV IgG antibody levels.22 These effects may be particularly relevant for older adults, for whom poor vaccine response is related to higher rates of clinical illness such as influenza infection and pneumonia.23
Recent work has shown that persistent CMV infection in the elderly is an important component of a set of immunological parameters designated the “immunological risk phenotype”.21 This set of immunological markers, including persistent CMV, high CD8 cells, low CD4 cell percentages, and poor T-cell proliferation, are predictive of mortality among healthy elderly individuals.2, 11 In addition to these effects, high levels of CMV IgG antibody have been found to be strongly correlated with increases in serum cytokines such as tumor necrosis factor (TNF-α) and interleukin (IL)-6 in older people.22 This inflammatory pathway may explain the observed links between CMV and cardiovascular disease, frailty, cognitive outcomes, and Alzheimer’s disease.6–10 Unfortunately, cytokines were not assayed in NHANES III to test their potential relationship to CMV antibody levels in our sample. Testing of C-reactive protein (CRP) data in NHANES III preceded high-sensitivity assay techniques, resulting in 60% of the CRP values in our sample falling below the detectable threshold, limiting its usefulness in our analyses. It is possible that immune response to latent infection may explain some of the socioeconomic gradients in cardiovascular disease and other inflammatoryassociated conditions that have thus far remained unexplained.
Given the evidence for the role of CMV in diseases of aging, the strong patterning of CMV antibody levels by income, education, and race/ethnicity in NHANES III may be a substantively important pathophysiological mechanism linking SES to health outcomes. Differences in CMV antibody levels were not accounted for by existing chronic conditions or by health behaviors such as smoking and obesity that might influence immune function. The observed differences were also not explained by factors that are significantly associated with CMV seropositivity, such as the number of persons in the household or region of residence.14
While it is clear that CMV antibody levels are higher in lower SES respondents, firm conclusions about the causes of these differences cannot be drawn from the current data. Several studies have used herpesviruses, including CMV, as a marker of subclinical immune response to psychosocial stressors.24 Increases in herpesvirus antibody titers have been linked to a wide array of stressors, including caregiving for a family member with Alzheimer’s disease,25 involvement in a poor quality marriage,26 anticipation of space flight by astronauts,27 traumatic life events,13 academic stress in medical students and military cadets,28 as well as psychological traits of loneliness and anxiety.29 Thus, it is possible that our results reflect the impact of psychological stress associated with low SES on down-regulation of the cellular immune response. Further studies should examine whether stress is an important factor in the relationship between SES and immune response to CMV in the U.S. population.
Alternative factors associated with CMV reactivation include major clinical conditions or treatments such as organ transplant and immunodeficiency.30, 31 These factors are not likely to explain the differences found in this study since these conditions are usually correlated with a high level of reactivation to the point of clinical disease, such as CMV retinitis and other disorders.32 In addition, we controlled for the use of medications that are likely to indicate altered immune function such as antivirals, antiretrovirals, and corticosteroids. Antibody levels to CMV were assessed by ELISA and therefore may not necessarily reflect all aspects of cellular immune response to this virus. Further studies are needed to examine other markers of subclinical reactivation, such as CMV DNA shedding, that were not available in this study.
It is possible that increased levels of IgG specific antibodies may indicate recent primary infection, although this is unlikely to be common in our study population since both initial as well as re-infection with CMV generally occurs at young ages.32 There is also no association between household size and CMV antibody levels in our sample, which is considered an important surrogate marker of exposure to infection and increased likelihood of transmission.14’ 33 CMV IgM antibody, which would indicate recent infection or reinfection, was measured only in women aged 12–49 years in NHANES III. For those women 25 years and older, only 1.74% tested positive for CMV IgM, suggesting that primary infection and reinfection are not important factors impacting our results.
Higher antibody response may indicate that the duration of the infection has been longer or that an individual has been repeatedly exposed throughout life. If this is the case, then high antibody levels may be a marker for early life or cumulative socioeconomic variation in level of exposure to these infections. This hypothesis is consistent with cross-sectional evidence that at current age-specific rates of seroprevalence, individuals with less education, lower income, and non-white race are likely to spend a larger number of years infected with CMV on average by the time they reach older ages.15
It has also been suggested that past history of exposure to pathogens, such as CMV, may be an important determinant of later life chronic conditions, through encouragement of a pro- or anti-inflammatory status or through gene-environment interactions.2 Emerging work has found that socioeconomic status is related to total pathogen burden, including CMV, HSV-1, and H. pylori, in the U.S. and U.K.34, 35 The causes of increased pathogen burden and earlier acquisition of persistent pathogens in lower SES individuals are not well understood. Further studies examining the extent to which these factors modify immune response and impact chronic conditions such as cardiovascular disease are warranted.
Given that our measure of co-morbidities could be considered a consequence of both our exposure and outcome of interest, we examined the possibility that inclusion of the Charlson index was producing a spurious correlation between SES and CMV antibody levels. In models excluding the Charlson index, coefficient estimates for both income and education were larger rather than smaller, suggesting that co-morbidities are likely a mediating factor between SES and antibody levels but unlikely to have introduced an upward bias in our estimates.
There were some differences in our findings by sex. In models including both education and income, education is more strongly linked to CMV levels for women, while income is associated more strongly for men. This could reflect substantive differences in the pathways linking SES and health, whereby job-related characteristics reflected in income are more important for immune response in men. It has also been suggested that household members may not have equal access to household income, so household income may be a less accurate measure of the resources available to women.36
While the continuous CMV antibody data released for NHANES III represented the first opportunity of its kind to analyze cell-mediated control of a latent herpesvirus in the general U.S. population, there were limitations to these data. The right-censoring of a substantial fraction of our CMV observations was a limitation, since it did not allow a complete picture of the distribution of this outcome variable. Fortunately, censored regression techniques were able to incorporate the information provided in these top-coded values. As in many large population surveys, there was non-trivial item non-response, especially for income. While those missing income data were slightly more likely to be females with less education, they did not differ by CMV antibody levels. A limitation to the use of CMV IgG antibody in general is the use of qualitative ELISA assays for CMV detection, making comparisons across studies difficult. Nonetheless, within studies, elevated CMV antibodies have been linked to important health risks. In a community-sample of elderly Latinos in the U.S., CMV antibody levels in the highest quartile were associated with greater all-cause mortality risk, decreased physical functioning, and increased cognitive decline.9, 37, 38 Currently, there are little data indicating standard and recognized risk categories for antibody levels among generally healthy individuals making it difficult to specify categories of risk. For now, qualitative sample based differences, such as being in the top quartile category of antibody levels, may be the most salient indicator for health risk. This is akin to how cytokines, such as IL-6, are considered as risk factors for cardiovascular health in large-scale studies, such as the Framingham Heart Study39 and the Women’s Health Study.40 While beyond the scope of this study, further research is needed to assess whether high antibody levels to CMV in the NHANES population are a risk factor for chronic health conditions, such as cardiovascular disease.
Whether through subclinical reactivation due to stress or a longer period of immune system engagement due to earlier infection, SES differences in immune control over CMV may have fundamental implications for health. Future work should test the extent to which cell-mediated immunity, as measured by CMV antibody levels, can explain socioeconomic gradients in specific health outcomes such as cardiovascular disease and mortality. The role of CMV in immune system and inflammatory changes suggests large public health benefits associated with a CMV vaccine from the standpoint of overall population health as well as health disparities. In the absence of a vaccine, future work should identify the causes of socioeconomic disparities in both CMV seroprevalence and immunity in the U.S. population in order to lessen the disproportionate health burden associated with this persistent viral infection.
References
- 1.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health. No easy solution. JAMA. 1993;269(24):3140–3145. [PubMed] [Google Scholar]
- 2.Pawelec G, Koch S, Franceschi C, Wikby A. Human immunosenescence: does it have an infectious component? Ann N Y Acad Sci. 2006;1067:56–65. doi: 10.1196/annals.1354.009. [DOI] [PubMed] [Google Scholar]
- 3.Pourgheysari B, Khan N, Best D, et al. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81(14):7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch S, Larbi A, Ozcelik D, et al. Cytomegalovirus infection: a driving force in human T cell immunosenescence. Ann N Y Acad Sci. 2007;1114:23–35. doi: 10.1196/annals.1396.043. [DOI] [PubMed] [Google Scholar]
- 5.Wikby A, Ferguson F, Forsey R, et al. An immune risk phenotype, cognitive impairment, and survival in very late life: impact of allostatic load in Swedish octogenarian and nonagenarian humans. J Gerontol A Biol Sci Med Sci. 2005;60(5):556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 6.Liu R, Moroi M, Yamamoto M, et al. Presence and severity of Chlamydia pneumoniae and Cytomegalovirus infection in coronary plaques are associated with acute coronary syndromes. Int Heart J. 2006;47(4):511–519. doi: 10.1536/ihj.47.511. [DOI] [PubMed] [Google Scholar]
- 7.Sorlie PD, Nieto FJ, Adam E, et al. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch Intern Med. 2000;160(13):2027–2032. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- 8.Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging. 2004;25(5):619–627. doi: 10.1016/j.neurobiolaging.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Aiello AE, Haan M, Blythe L, et al. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54(7):1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmaltz HN, Fried LP, Xue QL, et al. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53(5):747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 11.Koch S, Solana R, Dela Rosa O, Pawelec G. Human cytomegalovirus infection and T cell immunosenescence: a mini review. Mech Ageing Dev. 2006;127(6):538–543. doi: 10.1016/j.mad.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Stowe RP, Kozlova EV, Yetman DL, et al. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42(6):563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDade TW, Stallings JF, Angold A, et al. Epstein-Barr virus antibodies in whole blood spots: a minimally invasive method for assessing an aspect of cell-mediated immunity. Psychosom Med. 2000;62(4):560–567. doi: 10.1097/00006842-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Staras SA, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 15.Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137(1):58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowd JB, Haan MN, Blythe L, Moore K, Aiello AE. Socioeconomic gradients in immune response to latent infection. Am J Epidemiol. 2008;167(1):112–120. doi: 10.1093/aje/kwm247. [DOI] [PubMed] [Google Scholar]
- 17.Services USDoHaH, ed. 1994. National Center for Health Statistics: Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.StataCorp, Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 20.Trzonkowski P, Mysliwska J, Godlewska B, et al. Immune consequences of the spontaneous pro-inflammatory status in depressed elderly patients. Brain Behav Immun. 2004;18(2):135–148. doi: 10.1016/S0889-1591(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 21.Pawelec G, Akbar A, Caruso C, et al. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 22.Trzonkowski P, Mysliwska J, Szmit E, et al. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21(25–26):3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- 23.Gravenstein S, Drinka P, Duthie EH, et al. Efficacy of an influenza hemagglutinindiphtheria toxoid conjugate vaccine in elderly nursing home subjects during an influenza outbreak. J Am Geriatr Soc. 1994;42(3):245–251. doi: 10.1111/j.1532-5415.1994.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 24.Glaser R. Stress-associated immune dysregulation and its importance for human health: a personal history of psychoneuroimmunology. Brain Behav Immun. 2005;19(1):3–11. doi: 10.1016/j.bbi.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Glaser R, Kiecolt-Glaser JK. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type 1. Ann Behav Med. 1997;19(2):78–82. doi: 10.1007/BF02883323. [DOI] [PubMed] [Google Scholar]
- 26.Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55(4):364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000;182(6):1761–1764. doi: 10.1086/317624. [DOI] [PubMed] [Google Scholar]
- 28.Glaser R, Friedman SB, Smyth J, et al. The differential impact of training stress and final examination stress on herpesvirus latency at the United States Military Academy at West Point. Brain Behav Immun. 1999;13(3):240–251. doi: 10.1006/brbi.1999.0566. [DOI] [PubMed] [Google Scholar]
- 29.Esterling BA, Antoni MH, Kumar M, Schneiderman N. Defensiveness, trait anxiety, and Epstein-Barr viral capsid antigen antibody titers in healthy college students. Health Psychol. 1993;12(2):132–139. doi: 10.1037//0278-6133.12.2.132. [DOI] [PubMed] [Google Scholar]
- 30.Nahmias AJ, Lee FK, Beckman-Nahmias S. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl. 1990;69:19–36. [PubMed] [Google Scholar]
- 31.Soderberg-Naucler C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259(3):219–246. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 32.de Jong MD, Galasso GJ, Gazzard B, et al. Summary of the II International Symposium on Cytomegalovirus. Antiviral Res. 1998;39(3):141–162. doi: 10.1016/s0166-3542(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 33.Fowler KB, Pass RF. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics. 2006;118(2):e286–e292. doi: 10.1542/peds.2005-1142. [DOI] [PubMed] [Google Scholar]
- 34.Steptoe A, Shamaei-Tousi A, Gylfe A, et al. Socioeconomic status, pathogen burden and cardiovascular disease risk. Heart. 2007;93(12):1567–1570. doi: 10.1136/hrt.2006.113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. Journal of Gerontology: Medical Sciences. doi: 10.1093/gerona/gln012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daly MC, Duncan GJ, McDonough P, Williams DR. Optimal indicators of socioeconomic status for health research. Am J Public Health. 2002;92(7):1151–1157. doi: 10.2105/ajph.92.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63(6):610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aiello AE, Dowd JB, Simanek A, Roberts E, Haan MN. Predicting mortality from a novel biomarker of immune function. Poster presented at 41st Society for Epidemiological Reseach Annual Meeting; Chicago, IL. 2008. [Google Scholar]
- 39.Thakore AH, Guo CY, Larson MG, et al. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study) Am J Cardiol. 2007;99(11):1598–1602. doi: 10.1016/j.amjcard.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 40.Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension. 2007;49(2):304–310. doi: 10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]