Abstract
Purpose
To gain a better understanding of the roles of interleukins (ILs) in subconjunctival fibrosis, we investigated their expression in transforming growth factor-β1 (TGF-β1)-stimulated Tenon’s fibroblasts and examined their association with the transdifferentiation of fibroblasts to myofibroblasts.
Methods
After primary culture, fibroblasts derived from human Tenon’s capsule were exposed to TGF-β1. The expression of α-smooth muscle actin (α-SMA) protein was assessed by western immunoblots and immunofluorescence. The mRNA levels of various ILs were also evaluated by multiplex reverse transcription (RT)-PCR. Using the small interfering RNAs (siRNAs) specific for IL-6 and IL-11 and the promoter deletion assay, the contributions of IL-6 and IL-11 to TGF-β1-induced induction of α-SMA were determined.
Results
In human Tenon’s fibroblasts, TGF-β1 stimulated the expression of α-SMA protein determined by western blot analysis and also increased the mRNA levels of IL-6 and IL-11 determined by multiplex RT-PCR. On the western immunoblots and immunofluorescence, the increased expression of α-SMA was attenuated only by the siRNAs specific for IL-6 but not by the siRNAs specific for IL-11. When the activator protein-1 binding sites of the IL-6 promoter region were deleted, the stimulation effects of TGF-β1 decreased.
Conclusions
Our data show that autocrine IL-6 may participate in the TGF-β1-induced transdifferentiation of human Tenon’s fibroblasts to myofibroblasts, which is known to be an essential step for subconjunctival fibrosis.
Introduction
Subconjunctival fibrosis is an essential wound-healing process of the ocular surface, but if excessive it can result in ocular morbidity, as seen in patients with oculocutaneous disorders, such as ocular cicatricial pemphigoid, and patients who have undergone glaucoma-filtering surgery [1-6]. Even though transforming growth factor-β (TGF-β) is known to play a crucial role in this fibrosis [7-9], detailed mechanisms of how it functions have not yet been elucidated. Several recently published research papers that demonstrated antifibrotic effects of anti-TGF-β molecules have re-stimulated interest in TGF-β-mediated fibrosis [10-14].
In the present study, we were interested in investigating the relationship between inflammation and fibrosis in human Tenon’s fibroblasts. In lung and heart, certain types of inflammation recruit and stimulate fibroblasts in a TGF-β-dependent manner [15-18]. These activated fibroblasts then transdifferentiate to myofibroblasts that produce extracellular matrix (ECM); these contractile cells ultimately cause extensive fibrosis. In this study we investigated which of the proinflammatory cytokines of the interleukin (IL) family are stimulated by TGF-β1, and we monitored changes in α-smooth muscle actin (α-SMA), a phenotypic hallmark of myofobroblasts [19], to investigate the effect of the TGF-β1-stimulated ILs on the transdifferentiation of fibroblasts to myofibroblasts. The effects of blocking these ILs with small interfering RNA (siRNA) were also investigated.
Methods
Cell culture
After obtaining approval from the Institutional Review Board of our institution, 6 human Tenon’s capsule specimens were excised during strabismus surgeries in compliance with the provisions of the Declaration of Helsinki. A total of six participants who have no other disease except strabismus and no previous ocular surgery/trauma history were included. Written informed consent was obtained before operative excision. Briefly, 5x5-mm sections of Tenon’s capsule were collected, minced, and placed in a 35-mm culture dish containing Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen Co., Carlsbad, CA) supplemented with 10% fetal calf serum (Invitrogen), and 50 U/ml penicillin and 50 μg/ml streptomycin (Invitrogen). Cells were allowed to migrate from the explanted tissue and were then incubated at 37 °C and 5% CO2. Cells between the third and fifth passages were used for this study. Cultures were allowed to reach about 80% confluence. Depending on the experiments, fibroblasts were treated with various concentrations of TGF-β1 (R&D System, Minneapolis, MN) after 24 h of serum starvation in serum-free DMEM.
Western immunoblot analysis
Whole cellular proteins were isolated from primary cultured fibroblasts of human Tenon’s capsules, as described previously [20]. Briefly, total cell lysates were obtained by using lysis buffer (25 mM HEPES [pH 7.5], 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.05% Triton X-100, 0.5 mM dithiothreitol [DTT], and 0.4 mM phenylmethylsulfonyl fluoride [PMSF]; Sigma-Aldrich, Co., St. Louis, MO), 2 μg/ml leupeptin (Sigma-Aldrich), and 2 μg/ml aprotinin (Sigma-Aldrich). Equal amounts of protein (20 μg) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were probed with primary antibodies against human α-SMA (diluted 1:500; Dako Corporation, Carpinteria, CA) and β-actin (diluted 1:5,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Immunoreactive bands were detected with horseradish peroxidase-conjugated secondary antibodies (diluted 1:5,000; Invitrogen) and visualized by enhanced chemiluminescence detection reagents on autoradiograph films.
Multiplex reverse transcription-PCR
Total RNA was extracted from fibroblasts and converted into complementary (c)DNA by a first-strand synthesis system (SuperScript III; Invitrogen). Subsequently, the cDNA was used as a template for multiplex reverse transcription (RT)-PCR assays (MegaXpression; Seegene, Inc., Seoul, Korea) [21,22]. The following IL gene segments were amplified: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12A, IL-12B, IL-13, IL-15, IL-16, IL-18, and IL-1 receptor antagonist (IL-1RN). In addition, gene segments of β-actin, β2-microblobulin (β2M), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), succinate dehydrogenase complex subunit A (SDHA), and ribosomal protein L13a (RPL13a) were amplified as housekeeping genes.
RNA interference assay
siRNA molecules targeting IL-6 and IL-11 mRNA were purchased from Ambion, Inc. (Austin, TX). The RNA duplex against IL-6 had the sequence 5'-GGA CAU GAC AAC UCA UCU CTT-3' (sense) and 5'-GAG AUG AGU UGU CAU GUC CTG-3' (antisense); and the RNA duplex against IL-11 had the sequence 5'-GCA ACA UGG UGC AUC UGU GTT-3' (sense) and 5'-CAC AGA UGC ACC AUG UUG CTT-3' (antisense). siRNAs were delivered into cells according to the manufacturer’s instructions. Briefly, the diluted transfection reagent (siPORT Amine; Ambion) was mixed with the diluted siRNA to allow the formation of transfection complexes. This mixture with a final RNA concentration of 20 nM was then dispensed onto the cells and incubated for 24 h.
Immunofluorescence analysis
To carry out immunostaining, fibroblasts were fixed in 4% paraformaldehyde for 5 min and permeabilized in 0.2% Triton X-100 for 5 min at room temperature. Fibroblasts were then incubated with the primary antibody against human α-SMA (diluted 1:50) overnight at 4 °C and probed with a fluoroscein isothiocyanate-conjugated secondary antibody (diluted 1:100) for 2 h at room temperature in the dark. Subsequently, they were washed and viewed by immunofluorescence microscopy (IX71; Olympus, Tokyo, Japan).
Cell transfection and luciferase assay
Three recombinant plasmids, in which different fragments of the activator protein-1 (AP-1)-binding site of the human IL-6 promoter were deleted (p1168hu.IL6P-luc+, wild type; p1168h.IL6m3AP1-luc+, 3'-terminal deletion; and p1168h.IL6m5AP1-luc+, 5'-terminal deletion), were used as reporter constructs. These plasmids were obtained from the Belgian Coordinated Collections of Microorganism (BCCM/LMBP) Plasmid Collection (Zwijnaarde, Belgium) and have been described previously [23,24].
Cells were transfected with an equal amount of reporter plasmid using Lipofectamine 2000 (Invitrogen). The luciferase activities were determined using a luciferase assay system (Promega, Co., Madison, WI), following the manufacturer’s instruction. Briefly, the 96-well plate containing 20 μl of cell lysate per well was placed into the luminometer with injector. The injector added 100 μl of luciferase assay reagent per well, then the well was read immediately. The plate was advanced to the next well for a repeat of the inject-then-read process. The typical delay time was 2 s and the typical read time was 10 s.
Data analysis
Densitometric data are expressed as means ± standard deviation (SD). Data were compared with Kruskal-Wallis one-way analysis of variance and Mann-Whitney U test using the SPSS program for Windows, version 12.0.1 (SPSS Inc., Chicago, IL). A p value less than 0.05 was considered statistically significant.
Results
Transforming growth factor-β1 induces transdifferentiation of fibroblasts to myofibroblasts
To evaluate the effect of TGF-β1 on the expression of α-SMA, we measured α-SMA protein levels in human Tenon’s fibroblasts stimulated with different concentrations of TGF-β1 for different durations. Exposure of fibroblasts to TGF-β1 resulted in a dose- and time-dependent increase in expression of α-SMA (Figure 1). TGF-β1 treatment (10 ng/ml) for 72 h resulted in a significant increase (p<0.001) in α-SMA expression. Because the presence of α-SMA is a phenotypic hallmark of myofobroblasts, this finding indicates that TGF-β1 induced the transdifferentiation of fibroblasts to myofibroblasts.
Figure 1.
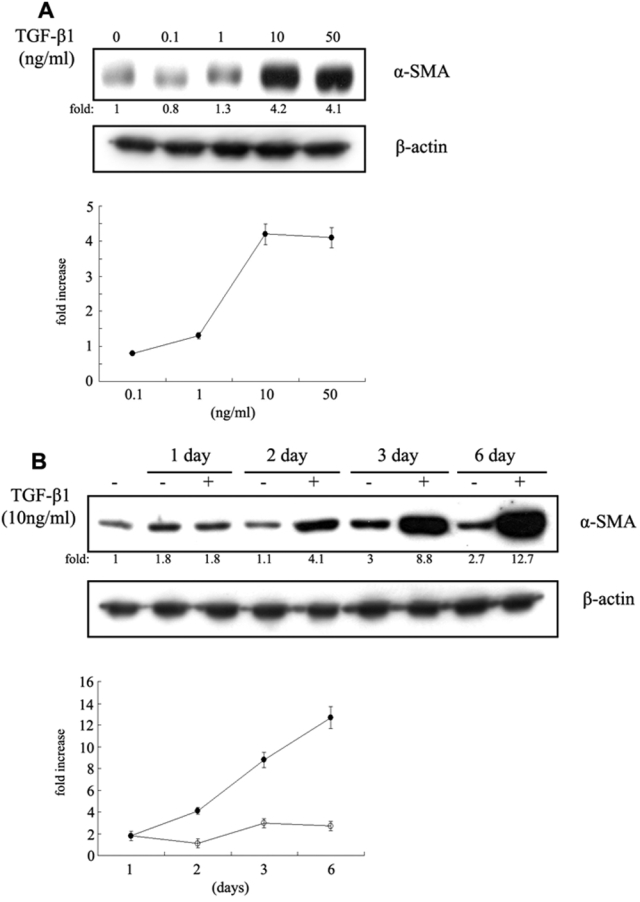
Representative western blot bands for α-smooth muscle actin. A: Dose-response effects: fibroblasts were exposed to various concentrations of transforming growth factor (TGF)-β1 for 72 h. B: Time-response effects: fibroblasts were incubated with or without 10 ng/ml of TGF-β1 for up to 6 days. β-actin was used as an internal control. Densitimetric data represent the mean±SD of results from three independent experiments.
Upregulation of IL-6 and IL-11 by transforming growth factor-β1 treatment
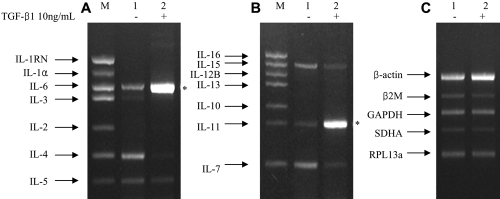
To assess the effect of TGF-β1 on the transcription of ILs, the IL mRNA levels in TGF-β1-treated human Tenon’s fibroblasts were evaluated using multiplex RT-PCR. After fibroblasts were incubated with 10 ng/ml TGF-β1 for 72 h, multiplex RT-PCR was performed. For the first set of genes (IL-1RN, IL-1α, IL-2, IL-3, IL-4, IL-5, and IL-6), only IL-6 mRNA was increased significantly (p<0.001; Figure 2A); for the second set of genes (IL-7, IL-10, IL-11, IL-12B, IL-13, IL-15, and IL-16), only IL-11 mRNA was increased significantly (p<0.001; Figure 2B). The levels of IL-1β, IL-8, IL-9, IL-12A, and IL-18 were not changed (data not shown) and the expression of β-actin, β2M, GAPDH, SDHA, and RPL13a was not influenced by TGF-β1 (Figure 2C). These data demonstrate that TGF-β1 induces the transcription of IL-6 and IL-11 in human Tenon’s fibroblasts.
Figure 2.
Multiplex reverse transcription PCR. Multiplex reverse transcription PCR sets for interleukins (ILs; A, B) and housekeeping genes (C). For each set of data, lane 1 represents the control group with no transforming growth factor (TGF)-β1 treatment and lane 2 represents the study group with 10 ng/ml TGF-β1 treatment for 72 h. Lane M contains positive markers. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-1RN, interleukin-1 receptor antagonist; RPL13a; SDHA, succinate dehydrogenase complex subunit A; β2M, β2-microblobulin. Two asterisks refer to a significant increase (p<0.001) in expression of IL-6 and IL-11 in the presence of TGF-β1.
Transforming growth factor-β1-induced expression of α-smooth muscle actin is reduced by IL-6-specific small interfering RNAs
To more directly address the possible involvement of IL-6 and IL-11 in TGF-β1-induced myofibroblast transdifferentiation, we evaluated the effects of IL-6 and IL-11 knockdown using specific siRNAs. Since the accumulation of ECM proteins is an indicator of myofibroblast transdifferentiation, we studied the effect of IL-6- and IL-11-specific siRNAs on TGF-β1-induced expression of the ECM component α-SMA. Treatment with IL-6-specific siRNAs strongly inhibited the TGF-β1-induced α-SMA expression (Figure 3A), whereas IL-11-specific siRNAs had little effect (Figure 3B).
Figure 3.
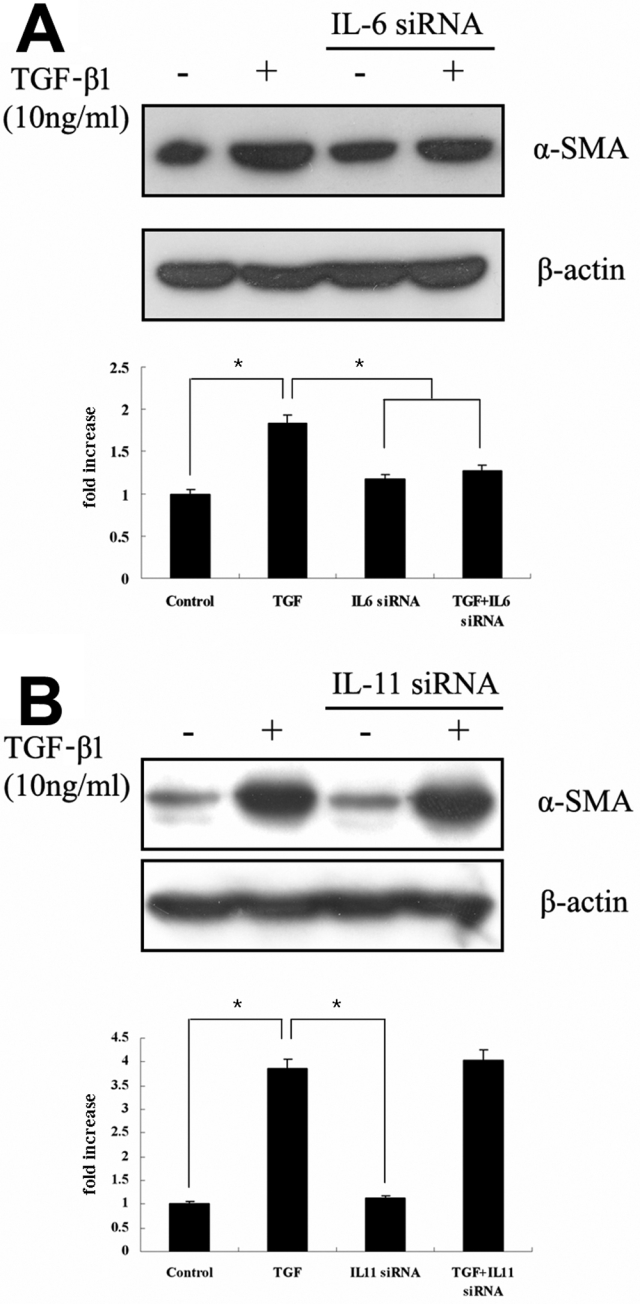
RNA interference assay for IL-6 and IL-11. After administering small interfering RNAs targeting IL-6 (A) and IL-11 (B), fibroblasts were exposed to 10 ng/ml transforming growth factor (TGF)-β1 for 72 h. Then, the expression of α-smooth muscle actin (α-SMA) was evaluated using western blots. Densitimetric data represent the mean±SD of results from three independent experiments. The asterisks refer to a significant difference compared to TGF-β treatment only (p<0.001).
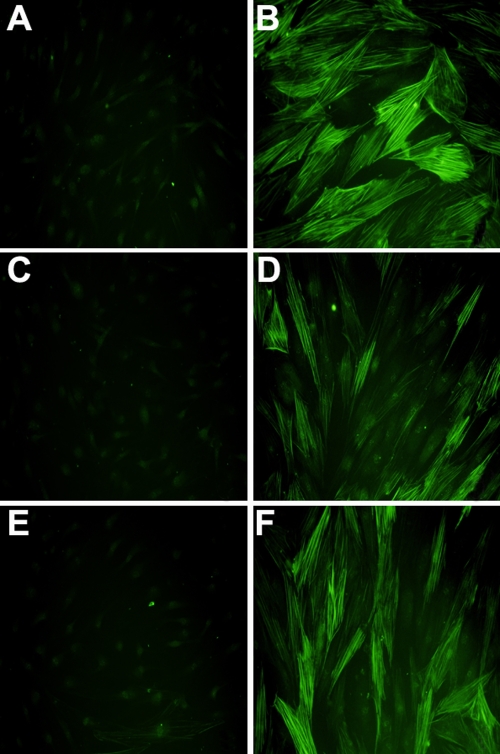
To confirm this finding, we assessed the effects of IL-6- and IL-11-specific siRNAs on TGF-β1-induced α-SMA upregulation by immunofluorescent staining and obtained similar results with western immunoblot analysis (Figure 4). Compared to untreated control fibroblasts, TGF-β1-stimulated fibroblasts showed a strong cytoplasmic α-SMA signal with numerous intracellular stress fibers (Figure 4B). Fibroblasts transfected with IL-6-specific siRNAs showed a significant reduction in the effect of TGF-β1 on α-SMA expression (Figure 4D); but cytoplasmic staining for α-SMA was little diminished in the presence of IL-11-specific siRNAs (Figure 4F). These data demonstrate that knockdown of IL-6 reduces the ability of TGF-β1 to induce α-SMA expression.
Figure 4.
Immunofluorescent analysis of the expression of α-smooth muscle actin. A: No treatment control, B: 10 ng/ml transforming growth factor (TGF)-β1 for 72 h, C: IL-6-specific small interfering (si)RNA, D: IL-6-specific siRNA plus 10 ng/ml TGF-β1 for 72 h, E: IL-11-specific siRNA, and F: IL-11-specific siRNA plus 10 ng/ml TGF-β1 for 72 h. The secondary antibody was conjugated with fluoroscein isothiocyanate, resulting in green fluorescence. Total magnification was 400×.
Activator protein-1 activity at the IL-6 promoter in the presence of transforming growth factor-β1
To determine whether TGF-β1-mediated myofibroblast transdifferentiation might be related to AP-1-binding site-dependent effects on IL-6 transcription, we used wild-type and mutant IL-6 promoter-reporter constructs to analyze IL-6 promoter activity (Figure 5). TGF-β1 increased the promoter activity of the wild-type-reported plasmid about 2.5-fold above the no-treatment control (p<0.001). However when the 3' or 5' end of the AP-1-binding site of the IL-6 promoter was deleted, the increased promoter activity by TGF-β1 was significantly attenuated (p<0.001). Our results indicate that the effect of TGF-β1 on IL-6 expression is dependent on the AP-1-binding site of the IL-6 promoter region and suggest that TGF-β1-induced myofibroblast transdifferentiation is dependent, at least in part, on TGF-β1-induced IL-6 expression.
Figure 5.
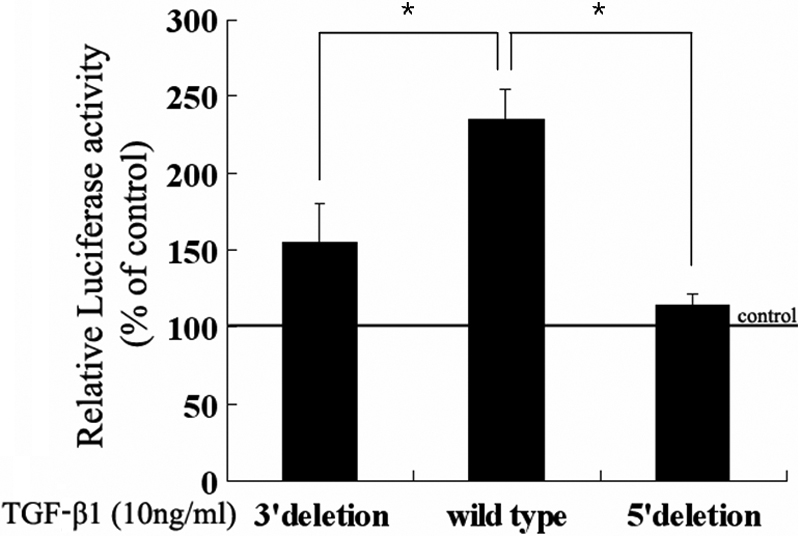
Promoter deletion assay for interleukin-6 (IL-6). Three recombinant plasmids, in which different fragments of the activator protein-1 (AP-1)-binding site on the human IL-6 promoter were deleted, were used. After transfection with each plasmid, fibroblasts were incubated with 10 ng/ml transforming growth factor (TGF)-β1 for 72 h. The luciferase activity was then determined and expressed as the percent of the no-TGF-β1 treatment control. Data represent the mean±SD of results from three independent experiments. The asterisks refer to a significant difference (p<0.001) compared to the wild type.
Discussion
Transdifferentiation of fibroblasts to myofibroblasts is at the core of the fibrotic process. Although the fibrosis caused by these activated fibroblasts is essential for natural wound healing, it can also result in excessive scarring, which is one of the most common causes of surgical failure after various ocular surgeries, including glaucoma-filtering procedures [1-6]. Although adjunctive antimetabolites can suppress the proliferation of fibroblasts and reduce subconjunctival fibrosis, the use of these compounds may result in serious complications; thus a new treatment strategy to prevent excessive fibrosis is required.
In various organs, including the lung and heart, inflammation is known to result in the development of fibrosis; TGF-β seems to be at the center of this process [15-18]. At the ocular surface, TGF-β plays a key role as a potent fibrogenic cytokine [25-29]. It is thought to stimulate the chemotaxis and transdifferentiation of fibroblasts, resulting in the overproduction of collagen, fibronectin, and other ECM components. Furthermore, TGF-β reduces the degradation of synthesized ECM through suppression of the activity of matrix metalloproteases and the activation of protease inhibitors.
IL-6 is essentially a chemoattractant and stimulator of lymphocytes [30-33]. It also functions as a pleiotropic mediator in the acute-phase response and stimulates the differentiation and proliferation of various target cells [33]. IL-6 exerts its effects through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway [34]. Because the IL-6 receptor does not have intrinsic tyrosine kinase activity, when IL-6 binds to the extracellular ligand-binding domain of its receptor, members of the JAK family are activated and phosphorylate the tyrosine residues of the IL-6 receptor, which then act as docking sites for the SH2 domains of STATs. IL-6-activated T lymphocytes then secrete TGF-β and trigger the fibrotic cascade.
Consistent with a previous report that IL-6 participates directly in the transdifferentiation of Tenon’s capsule fibroblasts to myofibroblasts [35], in the present study we verified the presence of autocrine IL-6 from activated Tenon’s capsule fibroblasts.
We found that the transcription of IL-6 and IL-11 was increased by TGF-β1 stimulation in human Tenon’s fibroblasts, and that this was accompanied by the upregulated expression of α-SMA. When IL-6-specific siRNAs were used, the TGF-β1-stimulated increase in expression of α-SMA was blocked, but this did not occur when IL-11-specific siRNAs were used. Our results indicate that autocrine IL-6 produced as a result of TGF-β-stimulation of human Tenon’s fibroblasts stimulates the transdifferentiation of these fibroblasts to myofibroblasts, which is thought to be essential for subconjunctival fibrosis. Modulation of autocrine IL-6 production might be useful as a novel therapeutic strategy for controlling postoperative subconjunctival fibrosis.
Acknowledgments
This work was supported by a Faculty Research Grant from Yonsei University College of Medicine (6-2008-0156).
References
- 1.Skuta GL, Parrish RK., 2nd Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987;32:149–70. doi: 10.1016/0039-6257(87)90091-9. [DOI] [PubMed] [Google Scholar]
- 2.Dutt JE, Ledoux D, Baer H, Foster CS. Collagen abnormalities in conjunctiva of patients with cicatricial pemphigoid. Cornea. 1996;15:606–11. [PubMed] [Google Scholar]
- 3.Razzaque MS, Foster CS, Ahmed AR. Role of collagen-binding heat shock protein 47 and transforming growth factor-beta1 in conjunctival scarring in ocular cicatricial pemphigoid. Invest Ophthalmol Vis Sci. 2003;44:1616–21. doi: 10.1167/iovs.02-0644. [DOI] [PubMed] [Google Scholar]
- 4.Esson DW, Neelakantan A, Iyer SA, Blalock TD, Balasubramanian L, Grotendorst GR, Schultz GS, Sherwood MB. Expression of connective tissue growth factor after glaucoma filtration surgery in a rabbit model. Invest Ophthalmol Vis Sci. 2004;45:485–91. doi: 10.1167/iovs.03-0485. [DOI] [PubMed] [Google Scholar]
- 5.Andreev K, Zenkel M, Kruse F, Jünemann A, Schlötzer-Schrehardt U. Expression of bone morphogenetic proteins (BMPs), their receptors, and activins in normal and scarred conjunctiva: role of BMP-6 and activin-A in conjunctival scarring? Exp Eye Res. 2006;83:1162–70. doi: 10.1016/j.exer.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Adan ES, Matsumoto Y, Dogru M, Fukagawa K, Takano Y, Tsubota K, Fujishima H. Conjunctival in vivo confocal scanning laser microscopy in patients with atopic keratoconjunctivitis. Mol Vis. 2007;13:1379–89. [PubMed] [Google Scholar]
- 7.Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT. TGF-beta1, -beta2, and -beta3 in vitro: biphasic effects on Tenon's fibroblast contraction, proliferation, and migration. Invest Ophthalmol Vis Sci. 2000;41:756–63. [PubMed] [Google Scholar]
- 8.Meyer-ter-Vehn T, Sieprath S, Katzenberger B, Gebhardt S, Grehn F, Schlunck G. Contractility as a prerequisite for TGF-beta-induced myofibroblast transdifferentiation in human Tenon fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:4895–904. doi: 10.1167/iovs.06-0118. [DOI] [PubMed] [Google Scholar]
- 9.Meyer-Ter-Vehn T, Grehn F, Schlunck G. Localization of TGF-beta type II receptor and ED-A fibronectin in normal conjunctiva and failed filtering blebs. Mol Vis. 2008;14:136–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Siriwardena D, Khaw PT, King AJ, Donaldson ML, Overton BM, Migdal C, Cordeiro MF. Human antitransforming growth factor beta(2) monoclonal antibody--a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology. 2002;109:427–31. doi: 10.1016/s0161-6420(01)00997-6. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H, Siddiqui SS, Shen X, Malik AB, Pulido JS, Kumar NM, Yue BY. RNA interference targeting transforming growth factor-beta type II receptor suppresses ocular inflammation and fibrosis. Mol Vis. 2004;10:703–11. [PubMed] [Google Scholar]
- 12.Gomes dos Santos AL, Bochot A, Doyle A, Tsapis N, Siepmann J, Siepmann F, Schmaler J, Besnard M, Behar-Cohen F, Fattal E. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J Control Release. 2006;112:369–81. doi: 10.1016/j.jconrel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 13.CAT-152 0102 Trabeculectomy Study Group. Khaw P, Grehn F, Holló G, Overton B, Wilson R, Vogel R, Smith Z. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 2007;114:1822–30. doi: 10.1016/j.ophtha.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 14.CAT-152 Trabeculectomy Study Group. Grehn F, Holló G, Khaw P, Overton B, Wilson R, Vogel R, Smith Z. Factors affecting the outcome of trabeculectomy: an analysis based on combined data from two phase III studies of an antibody to transforming growth factor beta2, CAT-152. Ophthalmology. 2007;114:1831–8. doi: 10.1016/j.ophtha.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Krein PM, Winston BW. Roles for insulin-like growth factor I and transforming growth factor-beta in fibrotic lung disease. Chest. 2002;122:289S–93S. doi: 10.1378/chest.122.6_suppl.289s. [DOI] [PubMed] [Google Scholar]
- 16.Yun SH, Shin JO, Lim BK, Kim KL, Gil CO, Kim DK, Jeon ES. Change in the cells that express connective tissue growth factor in acute Coxsackievirus-induced myocardial fibrosis in mouse. Virus Res. 2007;126:62–8. doi: 10.1016/j.virusres.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, Assoian RK, Puré E. Fibroblast migration is mediated by CD44-dependent TGF beta activation. J Cell Sci. 2008;121:1393–402. doi: 10.1242/jcs.021683. [DOI] [PubMed] [Google Scholar]
- 18.Nonaka M, Pawankar R, Fukumoto A, Yagi T. Heterogeneous response of nasal and lung fibroblasts to transforming growth factor-beta 1. Clin Exp Allergy. 2008;38:812–21. doi: 10.1111/j.1365-2222.2008.02959.x. [DOI] [PubMed] [Google Scholar]
- 19.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–41. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung SA, Lee HK, Yoon JS, Kim SJ, Kim CY, Song H, Hwang KC, Lee JB, Lee JH. Upregulation of TGF-beta-induced tissue transglutaminase expression by PI3K-Akt pathway activation in human subconjunctival fibroblasts. Invest Ophthalmol Vis Sci. 2007;48:1952–8. doi: 10.1167/iovs.06-1164. [DOI] [PubMed] [Google Scholar]
- 21.Chun JY, Kim KJ, Hwang IT, Kim YJ, Lee DH, Lee IK, Kim JK. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 2007;35:e40. doi: 10.1093/nar/gkm051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JK, Lee HJ, Lee YJ, Chun JY, Lee IK, Lim YS, Suh DJ, Ko SY, Kim MH, Oh HB. Direct detection of lamivudine-resistant hepatitis B virus mutants by a multiplex PCR using dual-priming oligonucleotide primers. J Virol Methods. 2008;149:76–84. doi: 10.1016/j.jviromet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Plaisance S, Vanden Berghe W, Boone E, Fiers W, Haegeman G. Recombination signal sequence binding protein Jkappa is constitutively bound to the NF-kappaB site of the interleukin-6 promoter and acts as a negative regulatory factor. Mol Cell Biol. 1997;17:3733–43. doi: 10.1128/mcb.17.7.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–90. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 25.Strissel KJ, Rinehart WB, Fini ME. A corneal epithelial inhibitor of stromal cell collagenase synthesis identified as TGF-beta 2. Invest Ophthalmol Vis Sci. 1995;36:151–62. [PubMed] [Google Scholar]
- 26.Parkin BT, Smith VA, Easty DL. The control of matrix metalloproteinase-2 expression in normal and keratoconic corneal keratocyte cultures. Eur J Ophthalmol. 2000;10:276–85. doi: 10.1177/112067210001000402. [DOI] [PubMed] [Google Scholar]
- 27.Daniels JT, Schultz GS, Blalock TD, Garrett Q, Grotendorst GR, Dean NM, Khaw PT. Mediation of transforming growth factor-beta(1)-stimulated matrix contraction by fibroblasts: a role for connective tissue growth factor in contractile scarring. Am J Pathol. 2003;163:2043–52. doi: 10.1016/s0002-9440(10)63562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein AM, Twining SS, Warejcka DJ, Tall E, Masur SK. Urokinase receptor cleavage: a crucial step in fibroblast-to-myofibroblast differentiation. Mol Biol Cell. 2007;18:2716–27. doi: 10.1091/mbc.E06-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ming JE, Cernetti C, Steinman RM, Granelli-Piperno A. Interleukin 6 is the principal cytolytic T lymphocyte differentiation factor for thymocytes in human leukocyte conditioned medium. J Mol Cell Immunol. 1989;4:203–11. [PubMed] [Google Scholar]
- 31.Bacon K, Gearing A, Camp R. Induction of in vitro human lymphocyte migration by interleukin 3, interleukin 4, and interleukin 6. Cytokine. 1990;2:100–5. doi: 10.1016/1043-4666(90)90003-c. [DOI] [PubMed] [Google Scholar]
- 32.Rieckmann P, Tuscano JM, Kehrl JH. Tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) in B-lymphocyte function. Methods. 1997;11:128–32. doi: 10.1006/meth.1996.0396. [DOI] [PubMed] [Google Scholar]
- 33.Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000;202:151–67. doi: 10.1016/S0171-2985(00)80061-3. [DOI] [PubMed] [Google Scholar]
- 34.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–3. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 35.Koh SW, Coll TJ, Rose L, Matsumoto Y, Higginbotham EJ. Antiglaucoma eye drop pulses--increased interleukin-6 secretion by Tenon's capsule fibroblast cultures. J Glaucoma. 2004;13:200–9. doi: 10.1097/00061198-200406000-00005. [DOI] [PubMed] [Google Scholar]