Abstract
Aims: The present experiments examined sex differences in ethanol intake and in the influence of a social context on aversive properties of ethanol in adolescent and adult Sprague-Dawley rats. Methods: Experiment 1 examined ethanol intake, with animals receiving daily 2-h access to ethanol and water for 8 days. Experiment 2 assessed the aversive effects of ethanol using a conditioned taste aversion (CTA) paradigm, with animals placed either alone or with a same-sex, same-age peer during the ethanol intoxication phase of conditioning. Results: Ethanol intake varied with both age and sex, although the sex differences emerging at each age were opposite in nature. Adolescent males consumed more ethanol relative to their body weights than adolescent females and adults of both sexes, whereas adult females generally consumed more than adult males. The CTA test revealed no sex differences in aversive effects of ethanol in adults, whereas adolescent males were less sensitive to the aversive properties of ethanol than adolescent females when intoxication occurred in the presence of a peer. Ethanol-induced CTA was evident in adults at lower doses than in adolescents. Conclusions: These results suggest that age differences in ethanol intake in males and sex differences in intake during adolescence may be associated in part with the relative insensitivity of the male adolescents to ethanol's aversive properties, especially when intoxication occurred in a social context. However, the elevated ethanol intake observed in adult females relative to their male counterparts appears to be unrelated to the aversive properties of ethanol.
INTRODUCTION
Much of the research examining alcohol effects has focused on males. Yet, epidemiological and clinical data have shown notable sex differences in alcohol use and propensity for abuse and dependence. For instance, women have shorter intervals between the onset of drinking and the emergence of problem drinking than men (Greenfield, 2002). Women also differ from men in their sensitivity to a number of acute and chronic consequences of ethanol (e.g. NIAAA, 2004; Fillmore and Weafer, 2004). Sex differences in drinking behaviors often become more pronounced during adolescence and may be associated, in part, with puberty-related increases in gonadal hormones (see Witt (2007)).
In studies examining ethanol intake in rodents, mature females generally exhibit higher ethanol intake than their male counterparts (Cailhol and Mormede, 2001; Lê et al., 2001; Chester et al., 2006). This sex difference has been reported using 24-h intake (Lancaster et al., 1996; Doremus et al., 2005), limited access to ethanol in two-bottle choice situations (Lê et al., 2001; Chester et al., 2006) and operant self-administration (Blanchard et al., 1993; Blanchard and Glick, 1995) and is evident in outbred rats (e.g. Lancaster et al., 1996; Doremus et al., 2005), as well as in rats and mice selected for high or low alcohol intake (e.g. Lê et al., 2001; Chester et al., 2006).
Sex differences in ethanol intake may begin to emerge during adolescence (Truxell et al., 2007), a developmental period characterized by elevated ethanol consumption in both humans and laboratory rodents, with adolescent rats, for instance, exhibiting 2- to 3-fold higher ethanol intake than their adult counterparts (Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007). The majority of studies examining the impact of both age and sex on ethanol intake have utilized home cage continuous access models where ethanol intake is sufficiently dispersed throughout the 24-h period (e.g. Brunell and Spear, 2005), making it difficult to determine when or if pharmacologically relevant blood ethanol levels are reached. Given that little is known about sex differences and their ontogeny under limited access conditions that promote pharmacologically relevant blood ethanol concentrations (BECs), the aim of Experiment 1 was to assess ethanol consumption in male and female rats during adolescence and adulthood using a limited access paradigm.
One potential contributor to high ethanol intake may be insensitivity to adverse effects of ethanol that serve as cues to terminate drinking. Indeed, a negative correlation has been observed between ethanol-induced conditioned taste aversion (CTA) and ethanol intake in laboratory mice and rats (see Green and Grahame (2008) for review). These findings suggest possible age- and sex-related differences in sensitivity to ethanol-induced CTA, given that adolescents demonstrate elevated levels of ethanol intake relative to adults, whereas adult females consume more ethanol than adult males. The main objective of Experiment 2, therefore, was to investigate whether responsiveness to the aversive properties of ethanol in a CTA paradigm differed as a function of age and sex. Given that the adverse effects of ethanol may be attenuated in the context of social interactions (Gauvin et al., 1994), and that there are age and sex differences in the rewarding effects of a same-sex social peer (Douglas et al., 2004), Experiment 2 also assessed whether exposure to ethanol in the presence of a same-sex peer would modify responsiveness to its aversive properties.
MATERIALS AND METHODS
Subjects
Subjects were Sprague-Dawley male and female rats bred in our colony at Binghamton University. A total of 32 male and female rats were used in Experiment 1, whereas in Experiment 2, 160 adolescent and 128 adult rats served as experimental subjects and 80 adolescents and 64 adults were used as partners.
All animals were maintained in a temperature-controlled vivarium on a 14:10 h light:dark cycle (lights on at 0700 h), with ad libitum (ad lib) access to water and food (Purina rat chow, Lowell, MA). Animals were housed with their littermates prior to testing. To eliminate the possible confounding of litter with treatment effects, no more than one subject from a given litter was assigned to a particular experimental group (Holson and Pearce, 1992; Zorilla, 1997). Animals were assigned randomly to the testing conditions, and the order of testing was counterbalanced across the experiments. At all times, animals were treated in accordance with guidelines for animal care established by the National Institutes of Health (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996), using protocols approved by Binghamton University Institutional Animal Care and Use Committee. Given that females of different age groups were included in this study, vaginal smears were not used to determine the estrous cycle in the adult females.
Procedures: Experiment 1
To examine the limited access ethanol intake of adolescent and adult male and female subjects, a 2 (age) × 2 (sex) factorial design was used, with eight animals placed into each experimental group.
On either postnatal day (P) 26 or 68–69, animals were re-housed with a same-age, same-sex non-littermate and allowed to acclimate for 2 days before the start of ethanol intake testing. On P28 for adolescents and P70–71 for adults, animals were water deprived for 24 h prior to the first 2-h limited access session. Ethanol intakes were assessed daily and continued for 8 days. Fifteen minutes prior to each session, animals in each housing pair were separated from each other in their home cage by a mesh divider in order to assess intake of each animal individually. During the limited access sessions, animals were given access to two bottles: one containing water and the other containing ethanol sweetened with 0.1% saccharin at concentrations of 6% ethanol (Days 1–4) and 10% ethanol (Days 5–8). Although research in our laboratory has revealed that non-nutritive sweetener does not impact ethanol consumption in adolescent or adult male rats (Vetter et al., 2007), we chose to sweeten the ethanol solution presented to animals during the 2-h access session with saccharin for comparability to previous ethanol intake studies conducted in our laboratory in male and female adolescents and adults (see Brunell and Spear (2005) and Doremus et al. (2005)). The use of sweetened ethanol also more closely models drinking in human adolescents who self-report preferential consumption of sweetened alcoholic drinks (Wechsler et al., 2000). The position of the two bottles was alternated daily to avoid development of side preferences. Fifteen minutes after the end of the 2-h access session, the divider was removed from the home cage, and shortly thereafter animals were given supplementary water.
Body weights of animals were monitored daily with the goal of maintaining each animal's weight trajectory at ∼85–90% of that of non-deprived animals of the corresponding age and sex (ad lib weights) through slight water deprivation. To achieve this goal, animals were given supplementary water post-session and the amount of water provided each day was adjusted to maintain these weight trajectories. Access to supplementary water was not limited, although once all of the supplementary water provided was consumed, animals did not receive access to fluids again until the next day's 2-h access session. Average ad lib body weights and supplementary water amounts were determined based on data from an earlier study examining normal body weight gain and trajectories of food and water consumption in free-feeding adolescent and adult male and female rats (Vetter and Spear, 2007). Body weights assessed on Day 1 and Day 8 are presented in Table 1, along with average ad lib weights for animals of the same age and sex. The body weights of the adolescent and adult male and female subjects were ∼84–89% of average ad lib weights and did not differ significantly across age or sex.
Table 1.
Mean ± standard error of the mean of the body weights (g) of non-deprived animals (determined from Vetter and Spear (2007)) and actual body weights (g) of water-restricted animals from Experiment 1 are shown by age and sex on the first (Day 1) and last day (Day 8) of ethanol intake testing.
| Day 1 | Day 8 | ||||||
|---|---|---|---|---|---|---|---|
| Percent of ad | Percent of ad | ||||||
| Age | Sex | Ad lib weight | Actual weight | lib weight | Ad lib weight | Actual weight | lib weight |
| Adolescent | Male | 118.9 ± 2.4 | 101.2 ± 2.6 | 84.7 ± 2.3 | 171.3 ± 3.6 | 144.2 ± 2.7 | 84.1 ± 1.6 |
| Female | 97.5 ± 2.5 | 84.4 ± 3.1 | 86.4 ± 3.1 | 149.4 ± 5.0 | 127.3 ± 3.5 | 86.7 ± 2.4 | |
| Adult | Male | 390.4 ± 5.5 | 351.3 ± 7.1 | 89.9 ± 1.8 | 415.6 ± 5.8 | 369.5 ± 8.1 | 88.8 ± 1.9 |
| Female | 245.8 ± 4.0 | 214.1 ± 6.6 | 87.0 ± 2.7 | 257.4 ± 4.3 | 227.7 ± 8.0 | 88.2 ± 3.0 | |
Blood samples from the tail were collected immediately following the 2-h access session on the last day of the 4 days of access to 6% ethanol in heparinized collection tubes and stored at −80°C until the time of assay of BECs. On the last day of the intake period (i.e. after 4 days of access to 10% ethanol), all animals were killed via decapitation immediately following testing, with trunk blood collected in heparinized tubes, frozen and stored at −80°C for later analysis of BECs. BECs were determined by means of head-space gas chromatography, using a Hewlett Packard (HP) 5890 series II Gas Chromatograph and a HP 7694E Headspace Sampler (see Varlinskaya and Spear (2006) for details).
Procedures: Experiment 2
This experiment was designed to examine sex and age differences in the influence of a social context on aversive properties of ethanol through assessment of CTAs. Given pronounced age-related differences in sensitivity to the aversive properties of ethanol observed in pilot studies in our laboratory using the CTA paradigm (Varlinskaya and Spear, 2006), the dose range of ethanol chosen to be paired with the saccharin CS was different in adolescents (0, 0.5, 1.0, 1.5 and 2.0 g/kg) and adults (0, 0.5, 1.0 and 1.5 g/kg), with experimental animals of both ages placed either into a non-social or social context following ethanol injection. Therefore, for adolescents, the design of Experiment 2 was a 2 (sex) × 5 (ethanol dose) × 2 (context) factorial, whereas for adults, the design was a 2(sex) × 4 (ethanol dose) × 2 (context) factorial, with eight animals placed into each experimental group at each age.
On Day 1 of the experimental protocol (P28 for adolescents and P70 for adults), animals were placed into individual cages with ad libitum access to food and water and on Day 2 were given 50% of the volume of water they ingested during the previous 24-h period. On Day 3 (P30 or P72), animals were given 30-min access to a single bottle containing a 0.1% saccharin solution, with intake being recorded. Following this 30-min period, each animal was injected intraperitoneally (i.p.) with either isotonic saline or ethanol (12.6% ethanol solution in isotonic saline, v/v) at doses of 0, 0.5, 1.0, 1.5 or 2.0 g/kg for adolescents, whereas adults were injected with 0, 0.5, 1.0 and 1.5 g/kg ethanol. Immediately following injection, animals under the non-social condition were left alone in their cages, whereas animals under the social condition were paired in their home cages with an unfamiliar non-manipulated partner of the same sex and age for 24 h. All animals were given ad libitum access to water during this 24-h post-conditioning period. On Day 4, partners were removed in order to avoid any social interactions between water-deprived animals. Experimental subjects were given 50% of the volume of water they ingested on Day 1. On Day 5 (P32 or P74), experimental animals were given access to one bottle containing a 0.1% saccharin solution for a 60-min test period in their home cages. Actual body weights for experimental subjects following the first (Day 3) and second (Day 5) water deprivation are presented in Table 2, along with average ad lib weights of animals of the same age and sex. Animals in all experimental conditions gained ∼95–96% of their expected weights, percentages that did not differ across age or sex.
Table 2.
Body weights of water restricted male and female adolescent and adult rats from experiment 2 compared to non-deprived rats
| Day 3 (conditioning) | Day 5 (testing) | ||||||
|---|---|---|---|---|---|---|---|
| Percent of ad | Percent of ad | ||||||
| Age | Sex | Ad lib weight | Actual weight | lib weight | Ad lib weight | Actual weight | lib weight |
| Adolescent | Male | 119.5 ± 1.3 | 114.9 ± 1.3 | 95.9 ± 1.3 | 132.6 ± 1.6 | 126.3 ± 1.3 | 95.3 ± 1.1 |
| Female | 99.7 ± 1.2 | 96.1 ± 1.4 | 96.3 ± 1.1 | 110.4 ± 1.5 | 105.9 ± 1.2 | 95.9 ± 1.2 | |
| Adult | Male | 398.7 ± 3.2 | 385.3 ± 7.1 | 96.6 ± 1.2 | 409.6 ± 1.3 | 393.5 ± 1.4 | 95.8 ± 1.2 |
| Female | 252.8 ± 2.3 | 244.4 ± 2.2 | 96.5 ± 1.0 | 256.8 ± 1.2 | 245.8 ± 1.2 | 95.7 ± 0.9 | |
Mean ± standard error of the mean of the body weights of non-deprived (ad lib) animals (determined from Vetter and Spear (2007)) and actual body weight (g) animals from Experiment 2 are shown by age and sex on the conditioning (Day 3) and testing day (Day 5) following 50% water deprivation.
Data analyses
Ethanol intake, preference and BEC data were first analyzed in Experiment 1 using omnibus repeated measures analyses of variance (ANOVAs). In order to further examine sex-related differences at each age, in instances where an age effect was revealed in the overall ANOVA, data were analyzed separately by age. All data were subjected to post hoc contrasts with Fisher's LSD tests to determine the locus of significant main effects and interactions. Due to the different ethanol doses examined in adolescents and adults in Experiment 2, saccharin intake (mL/kg) during conditioning and testing was analyzed separately for each age using ANOVA procedures. CTA was defined as a significant decrease in saccharin intake in ethanol-exposed animals relative to the corresponding saline-injected control group within each age/sex/social condition. Prior to these ANOVAs, Levene's tests were used to examine homogeneity of variance within each data set, with no data sets violating this assumption.
RESULTS
Experiment 1
Ethanol intake (g/kg)
The overall omnibus ANOVA of the ethanol intake data revealed a significant age × sex interaction [F(1, 28) = 18.84, P < 0.01], with adolescent males drinking more than adolescent females and more than adults of both sexes, and adult females drinking more than adult males. To further explore effects at each age, data were analyzed separately by age.
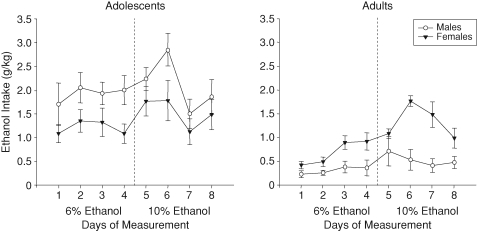
As presented in Fig. 1, the analysis of adolescent ethanol intake revealed a main effect of sex, with adolescent males consuming more ethanol than adolescent females [F(1, 14) = 6.38, P < 0.05]. Adolescent intake increased across days to peak on Day 6 (i.e. P33), followed by a decline [main effect of the day, F(7, 89) = 2.93, P < 0.01]. In the analysis of ethanol intake among adults, a day × sex interaction emerged [F(7, 89) = 3.2, P < 0.01]. Intake in adult females peaked to reach levels significantly elevated above male intake on Days 6 and 7, whereas intake of adult males remained relatively low and stable across days.
Fig. 1.
The mean intake of ethanol (g/kg) by adolescent and adult male and female rats across the 8 days of measurement. The vertical dashed line represents the increase in the ethanol concentration (from 6% to 10%) provided to animals during the 2-h limited access. Bars represent standard errors, as in all other graphs in these experiments.
Preference ratio
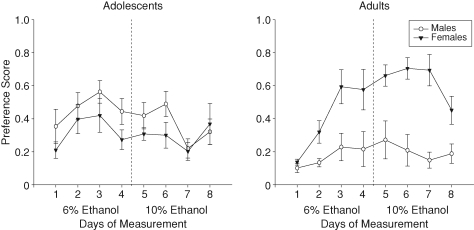
Preference scores were calculated via the formula: (mL ethanol solution intake)/(mL ethanol solution intake + mL water intake), with values >0.5 reflecting a preference for the ethanol solution and scores <0.5 reflecting a water preference. In the overall analysis of preference, an age × sex interaction [F(1, 28) = 23.86, P < 0.01] emerged (see Fig. 2). Adult females showed significantly higher preference scores than adult males and adolescent females, whereas adolescent males showed significantly higher preference scores than adult males. When preference data were analyzed separately by age, only a main effect of day emerged in the adolescents [F(7, 98) = 2.89, P < 0.01], with preference scores peaking on Day 3 and declining thereafter. In adults, a day × sex interaction was revealed [F(7, 98) = 2.85, P < 0.01], with adult female preference increasing from Day 1 to Day 3 to remain significantly elevated over that of adult males from Days 3–8, whereas adult male preference remained relatively low and stable across days.
Fig. 2.
Preference scores for the ethanol solution relative to water in adolescent and adult rats across the 8 days of measurement. Scores >0.5 reflect a preference for the ethanol solution, whereas scores < 0.5 reflect a preference for water. The vertical dashed line represents the increase in the ethanol concentration (from 6% to 10%) provided to animals during the 2-h limited access.
Blood ethanol concentrations
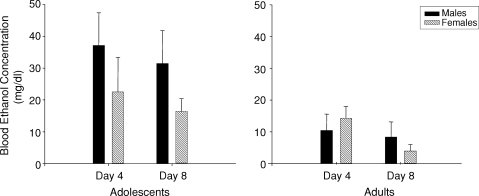
Blood samples were collected on Day 4 (fourth day of access to 6% ethanol) and Day 8 (fourth day of access to 10% ethanol). The repeated measures analysis of BEC revealed a significant main effect of age [F(1, 28) = 11.77, P < 0.01], with adolescents showing 2- to 3-fold higher BECs than adults (Fig. 3). In the adolescent analysis, there was a trend for elevated BECs among adolescent males compared to adolescent females, but this trend did not reach significance. In the adults, there was a significant day effect [F(1, 14) = 5.59, P < 0.05], with adults exhibiting greater BECs on Day 4 than Day 8; although this effect appears to be driven by the adult females, the day × sex interaction did not reach significance.
Fig. 3.
Blood ethanol concentrations (mg/dL) at the end of the 2-h ethanol access sessions on Days 4 and 8 in adolescent and adult male and female rats.
Experiment 2
Conditioning
In adolescent animals, saccharin intake during conditioning differed as a function of sex [F(1, 140) = 5.74, P < 0.05], with adolescent males consuming significantly more saccharin on the conditioning day (50.7 ± 1.7 mL/kg) than their female counterparts (44.6 ± 1.8 mL/kg). Sex-related differences in saccharin intake during conditioning were also seen in adult animals [F(1, 112) = 4.58, P < 0.05]; however, in contrast to adolescents, adult females consumed more saccharin (19.1 ± 1.1 mL/kg) than adult males (16.1 ± 0.8 mL/kg). At both ages, animals within each sex randomly assigned to the different ethanol/social conditions did not differ in terms of their initial intake of the saccharin CS.
Testing
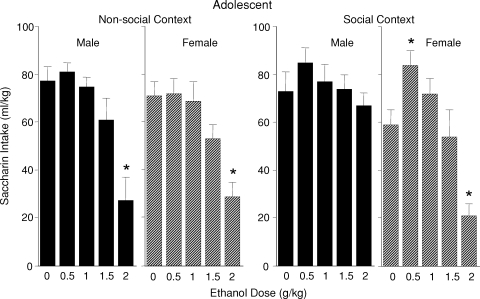
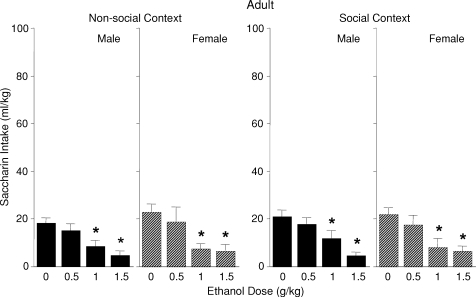
The ANOVA analysis of saccharin intake in adolescent animals revealed a significant sex × ethanol dose × context interaction [F(1, 140) = 2.50, P < 0.05] (see Fig. 4). Adolescent males and females housed alone during the intoxication period (i.e. non-social context) showed ethanol-induced CTA only at a does of 2.0 g/kg. Female adolescents who experienced intoxication in the presence of a peer likewise showed a significant reduction of saccharin intake at 2.0 g/kg relative to corresponding saline controls, whereas the presence of the peer blocked expression of CTA in adolescent males. Adolescent females who received 0.5 g/kg ethanol in the social context ingested more saccharin at test than their corresponding saline controls. In contrast, as seen in Fig. 5, adults of both sexes showed a CTA following 1.0 and 1.5 g/kg ethanol regardless of the social context [a significant main effect of ethanol dose, F(1, 112) = 20.13, P < 0.0001].
Fig. 4.
Saccharin consumption (mL/kg) of adolescent male and female rats on the CTA test day that were either isolated (non-social context) or placed with a social partner during the intoxication period (social context).
Fig. 5.
Saccharin consumption (mL/kg) of adult male and female rats on the CTA test day that were either isolated (non-social context) or placed with a social partner (social context) during the intoxication period.
DISCUSSION
Age and sex differences in ethanol intake were evident using the 2-h limited access paradigm, with sex differences in ethanol intake emerging at both ages, albeit opposite in nature. Adult females generally consumed more ethanol relative to their body weights than adult males, with adult female intake increasing over days to a level significantly elevated above that of adult males. In contrast, adolescent males consumed more ethanol per kilogram of body weight than their female counterparts and adults of both sexes. No sex-related differences were seen in adult animals when tested in the CTA paradigm, whereas adolescent males were less sensitive to the aversive properties of ethanol than their female counterparts following exposure to ethanol in the presence of a peer. Age differences were also observed, with ethanol-induced CTA evident at lower doses in adult animals than in adolescents.
The elevated intake of adult females relative to adult males observed in the present study is consistent with other intake data in adult rodents (Cailhol and Mormede, 2001; Lê et al., 2001; Doremus et al., 2005; Chester et al., 2006). Similarly, when intake was expressed as preference scores, sex-related differences were also observed, with adult females showing greater preference scores than that of adult males, an effect previously reported in our laboratory (Doremus et al., 2005). Interestingly, in our study, female ethanol consumption was highest when the solution presented contained 10% ethanol. It is possible that the increase in the ethanol concentration from 6% to 10% could play a role in this increase in ethanol intake over days in the adult females. There is limited evidence that sex differences in ethanol intake may be more robust at a concentration of 10% than at 6% or 8% concentrations of ethanol solution, although this effect was not statistically compared (Cailhol and Mormede, 2001). Although estrous cyclicity was not assessed in adult females in this experiment, the variability among adult females was not greater than that of any other group, suggesting that the phase of estrous cycle may not exert a strong influence on overall ethanol consumption. Other studies have found that the total ethanol intake was unaffected by the stage of estrous cycle (Roberts et al., 1998; Ford et al., 2002), although the microstructure of ethanol drinking does vary across estrous phase (Ford et al., 2002).
In marked contrast to the greater intake of ethanol in females than males during adulthood, adolescent males consumed significantly more ethanol than adolescent females. The findings are mixed for those few studies that have examined sex differences in ethanol consumption during adolescence, with some reporting greater intake among females (Doremus et al., 2005; Truxell et al., 2007) and others reporting no difference between the sexes (Lancaster et al., 1996). Specific test parameters such as tube type, ethanol concentration or housing conditions may explain this variation, given the notable influence of different environmental and procedural variables on the intake of adolescent and adult animals (Doremus et al., 2005).
In addition to the sex differences in ethanol intake evident during adolescence and adulthood, age differences were also evident. Adolescent male ethanol consumption was ∼3-fold higher than that of adult males and 2-fold greater than adult female intake, age differences reminiscent of those previously obtained in our laboratory (Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007), whereas there were no significant differences observed in intake between adolescent and adult females. Similarly, when intake was expressed as preference scores, male but not female adolescents showed preference scores elevated above those of their adult counterparts. While ethanol intake promoted by this 2-h access model used in the present study did not produce BECs in the ‘binge’ range as defined by NIAAA (i.e. ≥80 mg/dL, NIAAA, 2004), average BECs among adolescents were in the moderate (20–80 mg/dL) consumption range (Eckardt et al., 1998). Thus, when tested during adolescence, this limited access model of ethanol consumption promotes sex differences in ethanol intake that are of functional relevance, and reminiscent of sex differences in human consumption during mid-to-late adolescence, with young men drinking more per occasion and more often than young women (McPherson, 2004).
It is unlikely that the consumption patterns observed in adolescent and adult males and females in Experiment 1 were due solely to the addition of saccharin to the ethanol solution. For instance, a study conducted in our laboratory found that adolescent males drank the same amount (g/kg) of an ethanol solution whether it was sweetened with saccharin or unsweetened, an effect that was also observed in adult males (Vetter et al., 2007). Moreover, sex differences in ethanol consumption in adult rodents are evident even when the ethanol solution is unsweetened (Cailhol and Mormede, 2001; Chester et al., 2006; Lê et al., 2001).
BECs reached among adolescents self-administering ethanol were notably greater among adolescent males than those of adults. Given the higher ethanol intake of adolescent males than adolescent females, it was not surprising that a trend for higher BECs in males emerged in the analysis of the adolescent data, although this trend did not reach statistical significance. Among adults, despite higher ethanol intakes in females than males, BECs did not differ as a function of sex. This may be related in part to blood samples not being collected on days where the most notable sex differences in intake were observed. It is also possible that the lack of sex differences in BECs could be associated with different temporal patterns of ethanol consumption between males and females over the 2-h period. That is, it is possible that adult females consumed more ethanol than males early in the 2-h session, leading to BECs that peaked earlier and could have declined somewhat by the time that the blood sample was taken. Another potential explanation for the lack of sex differences in BECs could be due to differences in ethanol elimination or absorption. While some studies did not obtain significant sex differences in the rate of ethanol elimination (Silveri and Spear, 2000), others have reported slightly faster ethanol clearance rates in females than males (Collins et al., 1975; Crippens et al., 1999).
The results of Experiment 2 suggest that sex-related differences in ethanol intake evident in adulthood were not associated with responsiveness to the aversive properties of ethanol. That is, no differences in sensitivity to ethanol-induced CTA were evident in adult animals, with adult males and females, regardless of the social context, substantially decreasing their intake of saccharin following conditioning with 1.0 and 1.5 g/kg ethanol. A similar lack of sex differences in the aversive properties of ethanol have been reported in adult Wistar Kyoto and Wistar Kyoto hyperactive rats, although male spontaneously hypertensive rats have been reported to be more sensitive than females to the aversive properties of ethanol (Cailhol and Mormede, 2002).
Insensitivity to the aversive properties of ethanol may be associated with age-related differences in ethanol intake among males. Doses of ethanol effective at inducing CTA in adults had no effect in adolescents, with adolescents of both sexes who were socially isolated during the intoxication period and adolescent females in the social context condition demonstrating ethanol-induced CTA only at 2.0 g/kg, and socially exposed adolescent males not even showing CTA at this dose. These results add to the list of the consequences of ethanol for which adolescents appear to be less sensitive than adults. That is, the attenuated sensitivity of adolescents to ethanol-induced anxiolysis (Varlinskaya and Spear, 2002), social inhibition (Varlinskaya and Spear, 2002, 2004), motor impairment (Hollstedt et al., 1980; Silveri and Spear, 2001; White et al., 2002) and sedation (Little et al., 1996; Moy et al., 1998; Silveri and Spear, 1998; Draski et al., 2001) is now extended to include ethanol-induced CTA. Such aversive effects of ethanol may normally serve as cues to limit intake, and hence the attenuated sensitivity of adolescents to these cues may represent a permissive factor contributing to the relatively high levels of ethanol consumption during adolescence.
The results of these experiments extend earlier findings exploring the role of social context in attenuating the aversive effects of ethanol (Gauvin et al., 1994) and in influencing ethanol intake (Doremus et al., 2005). In Experiment 1, while adolescent animals were separated during the 2-h access to ethanol, they were reunited while still exhibiting pharmacologically relevant BECs (as confirmed by BEC data obtained on Day 4), allowing for possible learning about the social context during intoxication. This may be especially important in male adolescents who consumed more ethanol during the 2-h access session than adolescent females or adults. Similarly, Experiment 2 suggests that exposure to a social context during intoxication may decrease sensitivity to the aversive effects of ethanol as index by CTA, an effect observed only in adolescent males. In contrast, adolescent females who received 0.5 g/kg ethanol in a social context ingested more saccharin at test than saline controls, suggesting the intriguing possibility that the presence of a social partner following exposure to a low dose of ethanol during conditioning may have induced a conditioned taste preference for saccharin.
Taken together, these experiments suggest the possibility that elevated ethanol intake in adolescent males relative to adults as well as adolescent females may be associated, in part, with an attenuated sensitivity of adolescent males to the aversive effects of ethanol after exposure to social cues. In contrast, the elevated intake observed in adult females relative to adult males appears to be unrelated to sex differences in ethanol's aversive properties.
Acknowledgments
The research presented in this article was supported by National Institute of Alcohol Abuse and Alcoholism Grants R01 AA07135501 and R01 AA016887 to Linda P. Spear and Grant R01 AA12453 to Elena I. Varlinskaya.
References
- Blanchard BA, Glick SD. Sex differences in mesolimbic dopamine responses to ethanol and relationship to ethanol intake in rats. Recent Dev Alcohol. 1995;12:231–41. doi: 10.1007/0-306-47138-8_15. [DOI] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, et al. Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res. 1993;17:968–73. doi: 10.1111/j.1530-0277.1993.tb05650.x. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–53. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–9. [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Conditioned taste aversion and alcohol drinking: strain and gender differences. J Stud Alcohol. 2002;63:91–9. [PubMed] [Google Scholar]
- Chester JA, de Paula Barrenha G, DeMaria A, et al. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- Collins AC, Yeager TN, Lebsack ME, et al. Variations in alcohol metabolism: influence of sex and age. Pharmacol Biochem Behav. 1975;3:973–8. doi: 10.1016/0091-3057(75)90004-0. [DOI] [PubMed] [Google Scholar]
- Crippens D, White ML, George MA, et al. Gender differences in blood levels, but not brain levels, of ethanol in rats. Alcohol Clin Exp Res. 1999;23:414–20. [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, et al. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev Psychobiol. 2004;45:153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Draski LJ, Bice PJ, Deitrich RA. Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacol Biochem Behav. 2001;70:387–96. doi: 10.1016/s0091-3057(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Alcohol impairment of behavior in men and women. Addiction. 2004;99:1237–46. doi: 10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in comsumption patterns. Alcohol Clin Exp Res. 2002;26:635–43. [PubMed] [Google Scholar]
- Gauvin DV, Briscoe RJ, Goulden KL, et al. Aversive attributes of ethanol can be attenuated by dyadic social interaction in the rat. Alcohol. 1994;11:247–51. doi: 10.1016/0741-8329(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: Is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF. Women and alcohol use disorders. Harv Rev Psychiatry. 2002;10:76–85. doi: 10.1080/10673220216212. [DOI] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–8. [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, et al. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–9. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Lê AD, Israel Y, Juzytsch W, et al. Genetic selection for high and low alcohol consumption in a limited-access paradigm. Alcohol Clin Exp Res. 2001;25:1613–20. doi: 10.1111/j.1530-0277.2001.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, et al. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–51. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- McPherson M, Casswell S, Pledger M. Gender convergence in alcohol consumption and related problems: issues and outcomes from comparisons of New Zealand survey data. Addiction. 2004;99:738–48. doi: 10.1111/j.1360-0443.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, et al. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–92. [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- An important women's health issue. Alcohol Alert. 2004;62 [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. NIAAA Council approves definition of binge drinking. 2004 NIAAA Newsletter No. 3, Winter. [Google Scholar]
- Roberts AJ, Smith AD, Weiss F, et al. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22:156–1569. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–8. [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–65. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Falkowitz S, Spear LP. Adolescent-associated insensitivity to ethanol-induced taste aversions. 2006. Paper presented at the Society for Neuroscience, Atlanta, GA.
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Ann N Y Acad Sci. 2004;1021:459–61. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–44. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Spear LP. Age-associated trajectories of c consumption and body weight gain in pair- and isolate- housed adolescent and adult sprague-dawley rats. 2007. Poster presented at the International Society for Developmental Psychobiology conference, San Diego, CA.
- Wechsler H, Kuo M, Lee H, et al. Environmental correlates of underage alcohol use and related problems of college students. Am J Prev Med. 2000;19:24–9. doi: 10.1016/s0749-3797(00)00163-x. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, et al. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73:673–7. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–50. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]