Abstract
In all vertebrate brains, there is a period of widespread embryonic neurogenesis followed by specific regional neurogenesis that continues into adult stages. The Wnt signaling pathway, which is essential for numerous developmental processes, has also been suggested to be involved in neurogenesis. To help investigate the exact roles of canonical Wnt signaling in neurogenesis, here we examine the identity of Wnt-responsive cells in the zebrafish hypothalamus. This tissue is a useful diencephalic neurogenesis model containing evolutionarily conserved populations of neurons. We first performed in situ hybridization to show the expression patterns of Tcf family members and a canonical Wnt signaling reporter in the 50 hpf embryonic hypothalamus and larval/adult hypothalamus. We then used immunohistochemistry to identify the cell types of Wnt-responsive and Lef1-positive cells in both 50 hpf embryonic and adult hypothalamus. Our results indicate that Wnt-responsive cells in the hypothalamus are likely to be both mitotic progenitors and postmitotic precursors at embryonic stages, but only precursors at the adult stage. These data suggest that canonical Wnt signaling may be functionally required for maintenance of neural progenitor and precursor pools in the embryo, and for ongoing neurogenesis in the adult zebrafish.
Introduction and Review
The Wnt signaling pathway regulates early patterning, morphogenesis, and cellular function throughout the animal kingdom, with evolutionary conservation between vertebrates and invertebrates. The canonical Wnt pathway involves a signaling cascade that ultimately results in the stabilization of β-catenin, which is translocated into the nucleus, where it binds to Lef/Tcf high mobility group (HMG) transcription factors and activates transcription.1 Previous reviews have summarized how Wnt signaling generally regulates embryonic development, and the roles of Wnt signaling in neural development.2 Here we will emphasize the roles of Wnt signaling in vertebrate brain neurogenesis.
Although the roles of Wnt signaling in early embryonic patterning are relatively well understood, the function of this pathway in neurogenesis is less clear. Neurogenesis at the cellular level consists of three distinct processes: (1) proliferation and cell cycle exit, (2) proneural specification, and (3) neuronal (and glial) differentiation. Identification of canonical Wnt pathway targets supports roles in all of these processes. For example, Cyclin D1, Cdx4, Neurogenin1, and Sox3 are all identified direct transcriptional targets.3–6 The first two genes encode regulators of cell proliferation, while the second two encode regulators of proneural specification. In addition, canonical Wnt signaling has been suggested to regulate multiple neuronal/glial differentiation factors.3,7 However, it is not clear whether Wnt signaling plays consistently positive or negative roles in any of these processes.
Conflicting roles for Wnt signaling in neurogenesis
Most published data support the idea that the Wnt signaling pathway promotes neural progenitor proliferation. At the ligand level, Wnt1 acts as a mitogen and an apoptosis inhibitor in the developing CNS. Ectopic Wnt1 can induce overproliferation of caudal midbrain via expansion of the progenitor pool, which may be attributed to the shorter cell cycle length and decreased cell cycle exit.8–11 Wnt3a, Wnt7a, Wnt7b, and Wnt10b also stimulate the proliferation of neural progenitors. In mouse embryos lacking Wnt3a, a total loss of hippocampus and reduction of caudomedial cerebral cortex are observed, as the hypothesized result of progenitor proliferation defects,12,13 and zebrafish embryos lacking Wnt3a, Wnt1, and Wnt10b undergo extensive apoptosis in the midbrain and cerebellum.14 Similarly, in Lef1 null mutants, LRP6 mutants, and D6-Cre lines with conditional inactivation of β-catenin or activation of Dkk1 (a secreted Wnt inhibitor) in the mouse cerebral cortex, the hippocampal fields are significantly reduced in size.7,15–18 Specifically, neurogenesis in the dentate gyrus is decreased in both the premigratory and migratory progenitor pools, and LRP6 null mutants with a single Lef1 null allele exhibit more severe defects, as do embryos expressing a Lef1-lacZ fusion gene, which encodes a dominant-negative protein that blocks all Lef/Tcf function.15,17 Finally, β-catenin has been shown to be essential for maintenance and proliferation of neural progenitors, as it can promote proliferation of Mash1+progenitor cells in the subventricular zone, and the overexpression of β-catenin can induce a larger forebrain with increased neuronal production.19–21 Together, these data suggest that Wnt signaling promotes neurogenesis primarily by increasing the size of the progenitor pool.
Other studies suggest that Wnt activity must be down-regulated for progenitors to differentiate. Ectopic expression of a β-catenin/Lef1 fusion protein delays the expression onset of neural markers and subsequent neurogenesis, and conditional ablation of β-catenin can also accelerate expression of some neural markers.22 Targeted inhibition of β-catenin signaling during embryonic development causes cortical progenitor cells to prematurely differentiate into neurons and migrate to the cortex.23 However, these findings are contradicted by other studies showing that Wnt activity directly leads to the specification of neural progenitors. In Xenopus, ectopic expression of mouse Wnt8, X-Wnt8, β-catenin, or dominant-negative glycogen synthase kinase 3 (GSK3) induces the expression of neural marker NCAM.24 In zebrafish, the canonical Wnt signaling pathway via Wnt8b and Lef1 has been suggested to be involved in posterior hypothalamic neural specification without significant effects on proliferation and apoptosis.4 In addition, Wnt3 is expressed in the hippocampal neurogenic niche, and overexpression of Wnt3 increases neurogenesis.25 These opposite roles of Wnt signaling during neurogenesis have been attributed to differences in context between the relevant cell populations.
Most in vitro experiments on cultured embryonic stem cells (ESCs) support the idea that Wnt signaling contributes to the maintenance of embryonic stemness while inhibiting neural differentiation. Forced expression of Wnt1, Wnt5a, or Wnt6 inhibits neural differentiation from ESCs.26,27 Manipulations that can inhibit the canonical Wnt signaling pathway, such as Sfrp2 treatment and Dkk1 induction, can stimulate neural differentiation from ESCs.26,28 Manipulations that can activate the canonical Wnt signaling pathway, such as lithium chloride treatment, adenomatous polyposis coli inactivation, and expression of a dominant active form of β-catenin, all inhibit neural differentiation.26,29 In addition, 6-bromoindirubin-3-oxime, a specific pharmacological inhibitor of GSK3, maintains the pluripotent state of ESCs indicated by the expression of ESC markers like Oct3/4, Rex1, and Nanog.30 By contrast, most in vitro experiments using neural progenitor/precursor cell culture support the idea that Wnt signaling contributes to both the proliferation of neural progenitor cells and further neural differentiation. Wnt3, Wnt3a, and Wnt5b have been suggested to be transiently required for proliferation and further differentiation into neuronal (Map2+) and astrocyte lineages in neonatal or adult neural progenitor cultures.31–33
It is possible that the Wnt signaling pathway plays different roles in nonneuralized stem cells and neural progenitors or precursors. Before stem cells or their progeny undergo neural specification, Wnt signaling could inhibit neurogenesis, while Wnt signaling could promote further differentiation in neural progenitors or precursors. In both situations, Wnt signaling seems to promote proliferation. The conflicting results obtained from in vivo experiments could be attributed to different composition of the tested tissues, as it is expected that these tissues usually contain both nonneural and neural progenitors. The relative proportion of each progenitor subtype might depend on how mature those tissues are.
The Wnt signaling pathway plays roles in neuronal and glial differentiation
Several studies have also suggested a role for the Wnt pathway in neuronal subtype differentiation. It has been shown that Wnt signaling is important for dopaminergic and GABAergic neuronal development at different contexts. Wnt1 acts to specify the midbrain-dopaminergic (mDA) precursors in mouse embryos. Loss of Wnt1 causes loss of mDA neurons and ectopic production of 5-HT serotoninergic neurons in the ventral midbrain.34,35 Wnt1 and Wnt3a can promote the proliferation of Nurr1+mDA precursors, while Wnt5a functions in the transition from Nurr1+mDA precursors to tyrosine hydroxylase (TH)–expressing mDA neurons.36,37 However, in zebrafish embryos, dopaminergic precursor number is restricted by the canonical Wnt signaling pathway (Wnt8a, Fz8a, and Lef1) in the diencephalon, which may again reflect a context-dependent difference.38 Three components of the canonical Wnt signaling pathway—GSK3β, β-catenin, and Lef1—are also involved in GAD67 regulation,39 suggesting their roles in GABAergic neuronal differentiation. The Wnt pathway also has specific effects on glial subtype differentiation. In the mouse cortex with D6-Cre–driven conditional inactivation of β-catenin, premature disassembly of the radial glial scaffold and increased numbers of astrocytes are found at newborn stages.16 Activation of Wnt signaling in vitro is also able to increase the number of GFAP-positive astrocytes but suppresses the number of oligodendroglial lineage cells labeled by PDGFR or O4.32
Mechanisms underlying the differential response to Wnt signals
Several potential mechanisms could explain how neural progenitors and precursors respond differently to Wnt signaling. One possibility is that crosstalk between Wnt and other signaling pathways such as Fgf, Shh, and RA can result in different outputs. Overexpression of β-catenin in the presence of FGF2 helps to maintain neural progenitor cells in a proliferative state, while overexpression of β-catenin in the absence of FGF2 enhances neuronal differentiation.40 Similarly, Wnt7a and Wnt7b have been shown to differentially regulate proliferation or maturation of neural precursors depending on the context of FGF2 and Shh signaling.3,13,41 Further, Wnt and FGF signaling may act simultaneously on the promoters of downstream targets such as Sox2 and can together contribute to the specification of dorsal telencephalic character.42,43 Retinoic acid (RA) is another signal that can change the response of cells to Wnt signals. Wnt1 induces neuronal differentiation in the absence of RA but inhibits neural differentiation in response to RA treatment.26,44 In addition, the Notch intracellular domain can function as a coactivator of Lef1,45,46 and the BMP signaling pathway can interact with Wnt signaling to regulate neural tube proliferation and patterning.29,47,48 Recently, epigenetic research also shows that chromatin modification status can affect the selective promoter occupancy by Tcfs.49 Taken together, these findings suggest that Wnt responses are very context dependent.
Multiple Tcf family members may contribute to the different responses to Wnt signaling
Another level of cell-intrinsic differences is determined by the expression of diverse Tcf/Lef family members. There are four closely related Tcf family members identified in human and mouse50—Lef1, Tcf7 (Tcf1), Tcf7l1 (Tcf3), and Tcf7l2 (Tcf4). One of these factors (Tcf7l1) is duplicated in zebrafish, leading to five proteins in total. All Tcf/Lef proteins have a highly similar HMG box that allows specific DNA binding. Biochemical assays using biotinylated oligonucleotides and the HMG domain of Tcf4 have calculated a binding affinity matrix with CCTTTGATG as the highest affinity sequence.51 Although all Tcf family members contain similar domains, alternative splicing and promoter usage may produce dominant-negative isoforms, contributing to functional diversity.52 For example, Lef1 is normally a β-catenin–dependent transcriptional activator, but a truncated form of Lef1 lacking the β-catenin binding domain can be produced in colon cancer and lymphocyte development.53–55 In addition, the cysteinerich domain of Lef1 can be alternatively spliced in response to TGF-β signaling.56
Among the four Tcfs, Tcf7 and Lef1 are highly functionally redundant. Both factors show redundancy in paraxial mesoderm and ectoderm morphogenesis as well as limb development.57–59 However, more careful investigation suggests that the two factors are not completely interchangeable and have distinct responses to Wnt signaling.58,60 Partial functional redundancy also exists between Tcf7 and Tcf7l2,61 which both have dual functions as repressors and activators.62–64 Tcf7 and Tcf7l2 also share similar C-termini that can cooperate with β-catenin and p300 to form a specialized transcription factor complex for the Cdx1 promoter, unlike Lef1.65,66 However, each factor also plays some distinct roles; for example, Tcf7l2 has been suggested to be specifically essential for intestinal development and cancer.67–69
By contrast, Tcf7l1 appears to function complimentarily to other Lef/Tcf factors, acting primarily as a repressor. Tcf7l1 is expressed broadly in the early embryo and contributes to tissue patterning together with other Tcfs.58,60,70–72 Although Tcf7l1 and Lef1 are usually expressed adjacently, they perform distinct functions. In hair follicles, Lef1 promotes the differentiation of hair-producing progenitors, whereas Tcf7l1 may maintain bulge stem cells. The two factors also cooperate in early embryonic ectoderm differentiation and medial pallium development in the telencephalon.15,60,73 It is likely that the combination of the activator and repressor forms of Tcfs determines the cellular response. Recently, with the development of ChIP-sequencing techniques, Tcf7l1 has been identified as a key factor together with Oct4, Sox2, and Nanog, in contributing to the core regulatory circuitry within ESCs.74–78
The zebrafish hypothalamus is a good model to investigate Tcf functions
We are focusing on Wnt-dependent neurogenesis in the zebrafish, using Tcfs as a means to investigate the canonical Wnt signaling pathway. This approach has several advantages. First, the relatively small number of Tcfs compared with 21 identified vertebrate Wnt ligands allows loss-of-function analyses. In addition, these factors act cell autonomously as opposed to secreted Wnt ligands, allowing us to analyze the phenotypes of single cells from a defined population. Finally, Tcf-mediated transcription is the final step of the canonical Wnt signaling pathway, allowing us to analyze a single output and use biochemical tools. In zebrafish, the expression and function of multiple Tcf factors have been examined in detail. In the CNS, canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis.4,79 tcf7 Is specifically expressed in the early dorsal retina and forebrain, but does not alone appear to have a required role in embryonic development. Together with lef1, tcf7 is required for fin and ectoderm development.59,80 tcf7l2 is alternatively spliced in zebrafish and exhibits transient expression in the embryonic telencephalon and midbrain,81 but little is known about its function. tcf7l1a and tcf7l1b share similar expression patterns and contribute redundantly to early A-P brain patterning.82,83
In the mouse, most studies of neurogenesis are performed in the hippocampus, for which there are no comparable structures in the zebrafish embryo. Further, in zebrafish most Tcf family members are absent from the telencephalon after early embryogenesis. We have chosen the hypothalamus as a model to investigate the role of Tcfs in neurogenesis. Previous publications and preliminary work have suggested the expression of transgenic β-catenin–dependent reporters and canonical Wnt components in the embryonic and adult hypothalamus.4,84,85 In addition, both the embryonic and adult hypothalamus contain a diversity of neurons, glial cells, and proliferating cells.86,87 Despite these facts, the hypothalamus has been poorly exploited as a model for neurogenesis. It is an evolutionarily conserved endocrine and autonomic organ in vertebrates, and it has recently been suggested that both the vertebrate hypothalamus and the insect pars intercerebralis trace back to a simple brain with neurosecretory cells that existed in common bilaterian ancestors.88,89 The anatomical and functional conservation of the hypothalamus between diverse vertebrate species makes it an attractive system for uncovering mechanisms of neurogenesis in model organisms. While the role of Wnt signaling in early patterning of the embryonic hypothalamus has been well studied in zebrafish,90 the function of this pathway in later neurogenesis is poorly understood. Here we examine the identity of TCF-expressing and Wnt-responsive cells in the embryonic, larval, and adult hypothalamus, as an initial step leading to further functional studies.
Materials and Methods
Zebrafish
Embryos were obtained from natural spawning of wild-type (AB*), Tg(TOP:dGFP)w25, and Tg(1.4dlx5a-dlx6a:GFP)ot1 zebrafish lines.91,92 Adult brains were dissected from anesthetized adult fish fixed in 4% paraformaldehyde for 2 days.
In situ hybridization and immunohistochemistry
Probe synthesis and in situ hybridization were performed as described elsewhere.93 The following RNA probes were used: lef1,79 tcf7,80 tcf7l1a/b,82 tcf7l2,81 gfp,91 and axin2 (made in our laboratory).
Antibodies and their working dilution ratios are listed below:
Rabbit-anti-GFP (Molecular Probes [Carlsbad, CA], A11122, 1:500)
Mouse-anti-GFP (Molecular Probes, A11120, 1:250)
Affinity-purified rabbit anti-Lef1 (Open Biosystems [Huntsville, AL], 1:500)
Rabbit-anti-GABA (Sigma [St. Louis, MO], A2052, 1:500)
Rabbit-anti-5HT (ImmunoStar [Hudson, WI], Part 20080, 1:1000)
Mouse-anti-TH (ImmunoStar, Part 22941, 1:500)
Mouse-anti-Hu (Molecular Probes, A21271, 1:500)
Mouse-anti-PCNA (Sigma, P8825, 1:1000)
Mouse-anti-GFAP (zrf-1; Zebrafish International Resource Center [Eugene, OR], 1:1000)
TO-PRO-3 iodide (Molecular Probes, T3605, 1:1500)
For whole-mount immunostaining, embryos were fixed with 4% paraformaldehyde for 3 h at room temperature, and incubated with primary and secondary antibodies at 4°C overnight. For whole-mount photography after all staining methods, yolks and eyes of embryos were dissected.
Sectioning and microscopy
Cryosections were cut at a thickness of 12 μm for embryos and 25 μm for adults. Plastic sections were cut at a thickness of 10 μm. Fluorescent images of whole-mount embryos and cryosections were taken using an Olympus FV1000 confocal microscope and a fluorescent dissecting microscope. Bright-field images were obtained using a conventional compound microscope.
Results
The embryonic hypothalamus contains Wnt-responsive cells
Although Tcfs and the Wnt reporter TOP:dGFP have been shown to be expressed in the brain during early zebrafish embryogenesis,4,79–82 expression after 40 hpf has not been characterized. To assess the precise expression patterns of these markers in the late embryonic hypothalamus, we analyzed embryos at 50 hpf. We chose this stage because the hypothalamus is anatomically distinct and contains dividing progenitors as well as postmitotic precursors and multiple differentiated cell types.
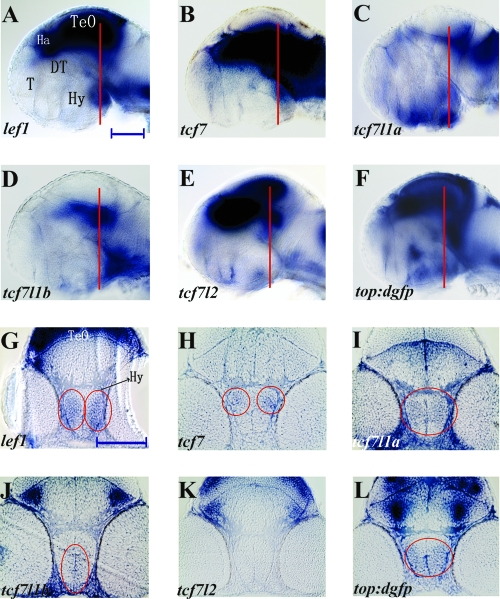
At 50 hpf, lef1 is expressed strongly in the tectum opticum (TeO), habenula (Ha), and the posterior hypothalamus (Fig. 1A). In the hypothalamus, lef1 expression can be observed around the presumptive posterior recess of the diencephalic ventricle (Fig. 1A). tcf7 Is also expressed in the posterior hypothalamus, around the presumptive posterior recess of the diencephalic ventricle (Fig. 1B). Cross sections through the posterior hypothalamus show that lef1 and tcf7 are expressed most strongly in the marginal regions, where postmitotic precursors and neurons reside (Fig. 1G, H). At 50 hpf, tcf7l1a and tcf7l1b are both expressed at low levels throughout the posterior hypothalamus (Fig. 1C, D). Cross sections through the posterior hypothalamus show that tcf7l1a is expressed more broadly, whereas tcf7l1b is expressed primarily in the medial region adjacent to the ventricle, where neural progenitors/stem cells reside (Fig. 1I, J). By contrast, tcf7l2 expression is almost absent from the entire ventral diencephalon (Fig. 1E, K). At 50 hpf, the only expression of tcf7l2 in the hypothalamus is found right above the future hypophysis (Fig. 1E), consistent with the finding that Tcf7l2 negatively regulates pituitary growth in early mouse embryos.94 Expression of the canonical Wnt reporter top:dgfp,91 as detected by in situ hybridization for gfp mRNA, is found throughout the hypothalamus at 50 hpf (Fig. 1F, L), suggesting the presence of canonical Wnt activity in a large region. Considering the requirement of tcf gene function for transcription of the gfp reporter, broad expression of gfp may reflect the combined expression of multiple Lef/Tcf factors earlier in development.
FIG. 1.
Expression of tcf and top:dgfp mRNA in the 50 hpf embryonic zebrafish hypothalamus. (A–F) Lateral views of whole-mount brains at 50 hpf. (G–L) Ten-micron plastic cross sections of the posterior hypothalamus. Sectioned regions are indicated in panels (A–F). Circled regions in (G–L) indicate domains of specific gene expression. top:dgfp and all tcf genes except tcf7l2 are expressed in the posterior hypothalamus. T, telencephalon; Ha, habenula; TeO, tectum opticum; DT, dorsal thalamus (thalamus); Hy, hypothalamus. Scale bars: 100 μm.
Tcf expression and canonical Wnt signaling activity persist in the adult hypothalamus
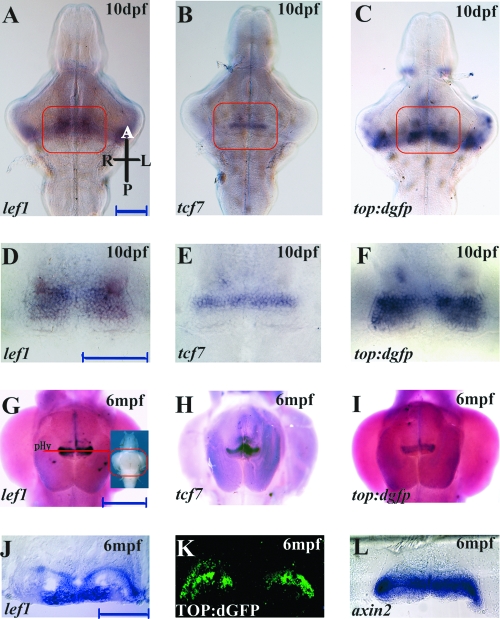
To determine whether Wnt-responsive cells exist in the hypothalamus at later stages, we examined brains from larvae at 10 days postfertilization and 6-month-old adults. At larval stages, specific expression of tcf7l1a/b and tcf7l2 was not observed in the posterior hypothalamus (data not shown). By contrast, lef1, tcf7, and top:dgfp were all expressed in the caudal zone of the periventricular hypothalamus (Fig. 2A–C). The expression pattern of top:dgfp appeared to overlap with the combined domains of lef1 and tcf7, with higher intensity in the zone closest to the posterior recess of the diencephalic ventricle (Fig. 2D–F). We found that the expression of lef1, tcf7, and top:dgfp also persists in the adult brain, specifically in the caudal zone of the periventricular hypothalamus (Fig. 2G–I). Cross sections through this region of the adult brain indicated that lef1, top:dgfp, and axin2 (a candidate Wnt target95) are strongly expressed in the posterior recess of the diencephalic ventricle (Fig. 2J–L), where potential adult stem cells reside as marked by labeling with PCNA and BrdU.86 These data suggest that canonical Wnt signaling may be involved in adult hypothalamic neurogenesis.
FIG. 2.
Expression of tcf genes and top:dgfp in the larval and adult zebrafish hypothalamus. (A–C) Ventral view of 10 dpf larval brains; orientation is indicated in panel (A). (D–F) Higher power images of the fields indicated in (A–C). top:dgfp Expression encompasses the combined domains of lef1 and tcf7. (G–I) Ventral views of 6mpf adult brains. (J) Cross section through adult hypothalamus at the level indicated in panel (G). (K) GFP antibody staining on an adult TOP:dGFP hypothalamus cross section at the same level as panel (J). (L) axin2 expression on an adult hypothalamus cross section at the same level as panel (J). lef1 and GFP/axin2 occupy different regions of the periventricular zone. pHy, periventricular hypothalamus. Scale bars: (A, D) 100 μm; (G) 500 μm; (J) 200 μm.
Characterization of Wnt-responsive and Lef1-positive cell types within the embryonic hypothalamus
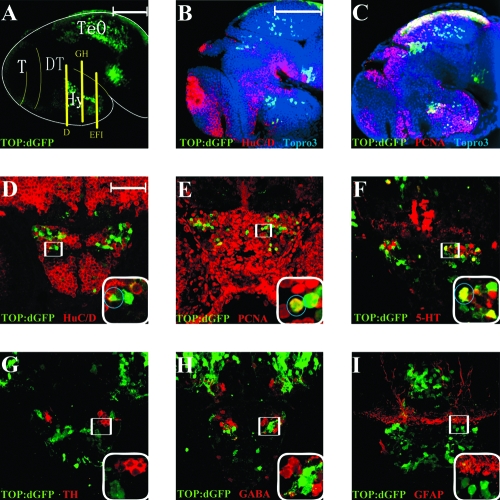
To further investigate the potential role of canonical Wnt signaling in hypothalamic neurogenesis, we determined which cell types expressed TOP:dGFP and Lef1 protein in 50 hpf embryos. We found that in the posterior hypothalamus, TOP:dGFP spanned the proximal to distal extent of this region (Fig. 3A), in which we observed a gradient of neuronal differentiation. The neuronal marker HuC/D was primarily restricted to cells more proximal to the thalamus (Fig. 3B), while the proliferation marker PCNA was mainly expressed in distal cells (Fig. 3C). Cross-section analysis revealed that TOP:dGFP-positive cells were either negative or weakly positive for HuC/D (circle, Fig. 3D). Some of these cells also co-expressed PCNA (circle, Fig. 3E), suggesting that the Wnt-responsive population spans the period of cell cycle exit and early differentiation. We found that some TOP:dGFP-positive cells expressed serotonin (5-HT, circle, Fig. 3F), but did not express TH, GABA, or the glial marker GFAP (Fig. 3G–I). These data indicate that Wnt-responsive cells may adopt particular neuronal fates characteristic of their position in the hypothalamus. Taken together, TOP:dGFP-positive cells in the 50 hpf hypothalamus are likely to be neural progenitors, precursors, and specific subtypes of neurons.
FIG. 3.
Immunohistochemical identification of Wnt-responsive cells in the 50 hpf embryonic hypothalamus. (A) Lateral view of TOP:dGFP whole-mount brain stained with GFP antibody, observed in a 100-μm confocal projection. (B, C) Triple labeling for TOP:dGFP, HuC/D or PCNA, and Topro3, observed in a single confocal slice. (D–I) Twelve-micron cryosections immunostained for the markers listed in each panel. Positions of cross sections are indicated in panel (A). Boxed region is shown at higher magnification in lower right corner, and circled cells are double labeled. GFP staining partially overlaps with HuC/D, PCNA, and 5-HT, but not with TH, GABA, or GFAP. T, telencephalon; TeO, tectum opticum; DT, dorsal thalamus (thalamus); Hy, hypothalamus. Scale bars: (A, B) 100 μm; (D) 50 μm.
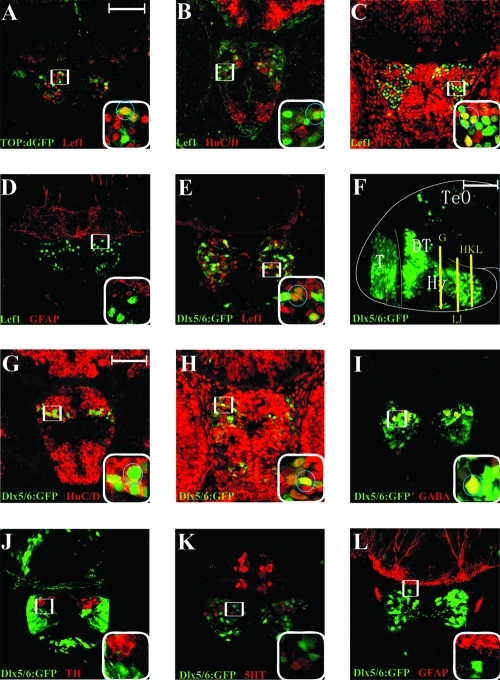
Surprisingly, we found that Lef1 and TOP:dGFP expression overlapped only occasionally (circle, Fig. 4A). Similar to the Wnt-responsive cells described above, some Lef1-positive cells were weakly positive for HuC/D and others were positive for PCNA (circles, Fig. 4B, C). In addition, none of the Lef1-positive cells expressed GFAP (Fig. 4D). Due to difficulties with antibody specificity, we were unable to directly analyze the neuronal subtypes of Lef1-positive cells. However, we took advantage of a transgenic fish line that expresses GFP driven by a dlx5/6 enhancer.92 These two genes have been reported to label GABAergic progenitors and precursors in mouse.96,97 We found that many of the dlx5/6:gfp-positive cells in the posterior hypothalamus co-expressed Lef1 (circle, Fig. 4E). The transgene labels a large number of cells within the posterior hypothalamus, spanning both postmitotic and progenitor regions (Fig. 4F–H, circled cells are double labeled). Immunohistochemical analysis revealed that as in mouse, GFP-positive cells were primarily GABAergic (circle, Fig. 4I), and we did not detect overlap with TH, 5-HT, or GFAP (Fig. 4J–L). This indirect analysis suggests that Lef1-positive cells in the 50 hpf hypothalamus are likely to be neural progenitors, GABAergic precursors, and immature neurons.
FIG. 4.
Immunohistochemical identification of Lef1-expressing cells in the 50 hpf embryonic hypothalamus. (A–E) Twelve-micron cryosections immunostained for the markers listed in each panel. Positions of cross sections for panels (A) and (C–E) are at the same level as Figure 3E, and panel (B) is at the same level as Figure 3G. Boxed region is shown at higher magnification in lower right corner, and circled cells are double labeled. Lef1 staining partially overlaps with TOP:dGFP, HuC/D, PCNA, and dlx5/6:gfp, but not with GFAP. (F) Lateral view of dlx5/6:gfp whole-mount brain, observed with a 100-μm confocal projection. (G–L) Twelve-micron cryosections immunostained for the markers listed in each panel. Positions of cross sections are indicated in panel (F). Boxed region is shown at higher magnification in lower right corner, and circled cells are double labeled. GFP staining partially overlaps with HuC/D, PCNA, and GABA, but not with TH, 5-HT, or GFAP. T, telencephalon; TeO, tectum opticum; DT, dorsal thalamus (thalamus); Hy, hypothalamus. Scale bars: (A, G) 50 μm; (F) 100 μm.
Characterization of Wnt-responsive cells within the adult hypothalamus
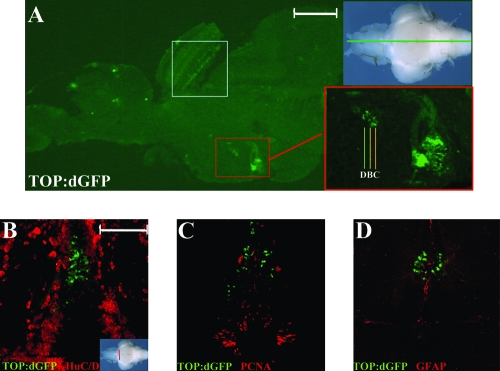
We found that cells with strongest expression of TOP:dGFP in the adult hypothalamus are mainly located adjacent to the diencephalic ventricle (Fig. 5A). We further analyzed these cells in cross sections through the diencephalic ventricle, and found that TOP:dGFP-positive cells did not express HuC/D, PCNA, or GFAP, but instead were located between the HuC/D and PCNA/GFAP-positive cell populations (Fig. 5B–D). Because HuC/D labels postmitotic neurons, and PCNA labels proliferating cells, while GFAP has also been reported to be expressed in adult astrocyte-like neural stem cells,98 our data suggest that Wnt-responsive cells in the adult hypothalamus occupy a developmental state between proliferating progenitors/stem cells and postmitotic neurons.
FIG. 5.
Immunohistochemical identification of Wnt-responsive and Lef1-expressing cells in the adult hypothalamus. (A) GFP antibody staining on a sagittal section through the midline of a TOP:dGFP adult brain. Specific expression of GFP is observed in the optic tectum (white box) and the periventricular hypothalamus (red box). (B–D) Twenty-five-micron hypothalamic cross sections immunostained for the markers listed in each panel. GFP-positive cells do not express HuC/D, PCNA, or GFAP. Scale bars: (A) 300 μm; (B) 100 μm.
Discussion
Wnt-responsive cells in the hypothalamus are likely to be neuronal precursors
Based on our data, Wnt-responsive and Lef1-expressing cells in the 50 hpf embryonic hypothalamus are most likely to be a subset of mitotic progenitors and postmitotic precursors, although Wnt reporter expression may persist as cells become immature neurons due to the rapid rate of differentiation. Wnt-responsive cells in the adult are most likely to be postmitotic precursors, residing between mitotic progenitors and neurons. It is also possible that these cells could be quiescent (PCNA-negative) neural stem cells, as it is known that the cell cycle inhibitor p21 promotes degradation of PCNA during an extended G1 phase.99 However, their position and lack of GFAP expression makes this possibility unlikely. A core question remains as to what role the canonical Wnt signaling pathway plays in the process of precursor maintenance and specification.
Our data also suggest that the regulation of canonical Wnt signaling inside the hypothalamus may be modified by cellintrinsic states. The expression patterns of TOP:dGFP and Lef1 are not fully overlapping, and in the posterior hypothalamus, where Lef1 is strongly expressed at 50 hpf, the intensity of TOP:dGFP is not particularly strong. This suggests that other Tcfs such as Tcf7 and Tcf7l1a/b, which are expressed in this region as well, may compete to keep canonical Wnt activity at an intermediate level. Further, GFP-positive cells express 5-HT, while Lef1-positive cells appear to become GABAergic, indicating that these represent two divergent lineages. Cells that have stopped responding to Wnt signals may retain Lef1 protein for some time, as it is known that Lef1 expression itself is upregulated by the Wnt pathway through autoregulation.54,100 In addition, alternative lef1 transcripts encoding dominant-repressor forms of the protein have been reported in other contexts, and these isoforms could be recognized by our antibody and mRNA probe. Further, other Wnt pathway modulators could also influence the transcriptional output.
Wnt signaling and neural stem cells
Canonical Wnt signaling has been suggested to be generally responsible for the expansion of neural stem cells or progenitor pools in previous publications. However, in the adult brain we found strong TOP:dGFP expression only in the optic tectum and hypothalamus, while at least 10 distinct regions within the adult zebrafish brain have been identified as proliferation zones with neural progenitors.86,101 It is therefore unlikely that the canonical Wnt signaling pathway is a generally required factor for all neural stem cell maintenance. Instead, the more restricted expression pattern we observe suggests that Wnt-responsive cells may be limited to particular progenitor or precursor populations, and those lineages may be as evolutionarily conserved as Wnt signaling itself. It has been suggested that postnatal neural stem cells are likely to be fate restricted,102,103 which further supports the idea that Wnt signaling is required for specific neurogenesis processes.
Intriguingly, Tcf7l1 has been reported to function as a transcriptional repressor in the maintenance of ESCs,77 which may suggest that inhibition of Wnt transcriptional targets is a key condition for stem cell/progenitor maintenance. As most previous studies have focused on the ultimate phenotypes following Wnt pathway manipulation in the brain, it is not clear whether particular transcriptional targets are repressed or activated in these instances. It is therefore important to understand the mechanism of Tcf function in detail when examining the more general role of Wnt signaling in vertebrate neurogenesis.
Adult neurogenesis and the hypothalamus
Adult neurogenesis has become an attractive target for potential therapeutic strategies in neural degenerative diseases and injury, and the Wnt signaling pathway has also been suggested to have therapeutic potential for Alzheimer's disease and Parkinson's disease.35,104 The generation of new neurons in the adult mammalian brain has long been thought to be restricted primarily to two regions: the subventricular zone of the lateral ventricle, which generates olfactory bulb GABAergic interneurons via the rostral migratory stream, and the subgranular zone of the dentate gyrus, which generates hippocampal granular neurons, both in the telencephalon. Recently, investigation has revealed the diencephalic third ventricle as another proliferating region in mammalian forebrain, contributing to the adult hypothalamic neurogenesis.105,106 The hypothalamus has also been confirmed as a site of adult neurogenesis in the teleost,86,87,107 highlighting this part of the brain as a good model to investigate adult neurogenesis in both mammalian and nonmammalian vertebrates.
Acknowledgments
We thank Marc Ekker for sharing the dlx5/6:gfp transgenic line, and Jan Kaslin for sharing unpublished data.
Disclosure Statement
No competing financial interests exist.
References
- 1.Seidensticker MJ. Behrens J. Biochemical interactions in the wnt pathway. Biochim Biophys Acta. 2000;1495:168–182. doi: 10.1016/s0167-4889(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 2.Grigoryan T. Wend P. Klaus A. Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirabayashi Y. Itoh Y. Tabata H. Nakajima K. Akiyama T. Masuyama N, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131:2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- 4.Lee JE. Wu SF. Goering LM. Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006;133:4451–4461. doi: 10.1242/dev.02613. [DOI] [PubMed] [Google Scholar]
- 5.Pilon N. Oh K. Sylvestre JR. Bouchard N. Savory J. Lohnes D. Cdx4 is a direct target of the canonical Wnt pathway. Dev Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Shtutman M. Zhurinsky J. Simcha I. Albanese C. D'Amico M. Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou CJ. Zhao C. Pleasure SJ. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson ME. Krumlauf R. McMahon AP. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- 9.Ikeya M. Lee SMK. Johnson JE. McMahon AP. Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- 10.Panhuysen M. Vogt Weisenhorn DM. Blanquet V. Brodski C. Heinzmann U. Beisker W, et al. Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Mol Cell Neurosci. 2004;26:101–111. doi: 10.1016/j.mcn.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Serbedzija GN. Dickinson M. McMahon AP. Cell death in the CNS of the Wnt-1 mutant mouse. J Neurobiol. 1996;31:275–282. doi: 10.1002/(SICI)1097-4695(199611)31:3<275::AID-NEU1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 12.Lee SM. Tole S. Grove E. McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 13.Viti J. Gulacsi A. Lillien L. Wnt regulation of progenitor maturation in the cortex depends on Shh or fibroblast growth factor 2. J Neurosci. 2003;23:5919–5927. doi: 10.1523/JNEUROSCI.23-13-05919.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckles GR. Thorpe CJ. Ramel MC. Lekven AC. Combinatorial Wnt control of zebrafish midbrain-hindbrain boundary formation. Mech Dev. 2004;121:437–447. doi: 10.1016/j.mod.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 15.Galceran J. Miyashita-Lin EM. Devaney E. Rubenstein JL. Grosschedl R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127:469–482. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- 16.Machon O. van den Bout CJ. Backman M. Kemler R. Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- 17.Solberg N. Machon O. Krauss S. Effect of canonical Wnt inhibition in the neurogenic cortex, hippocampus, and premigratory dentate gyrus progenitor pool. Dev Dyn. 2008;237:1799–1811. doi: 10.1002/dvdy.21586. [DOI] [PubMed] [Google Scholar]
- 18.Zhou CJ. Borello U. Rubenstein JL. Pleasure SJ. Neuronal production and precursor proliferation defects in the neo-cortex of mice with loss of function in the canonical Wnt signaling pathway. Neuroscience. 2006;142:1119–1131. doi: 10.1016/j.neuroscience.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Adachi K. Mirzadeh Z. Sakaguchi M. Yamashita T. Nikolcheva T. Gotoh Y, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 20.Chenn A. Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 21.Zechner D. Fujita Y. Hulsken J. Muller T. Walther I. Taketo MM, et al. Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 22.Machon O. Backman M. Machonova O. Kozmik Z. Vacik T. Andersen L, et al. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311:223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 23.Woodhead GJ. Mutch CA. Olson EC. Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci. 2006;26:12620–12630. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker JC. Beddington RS. Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lie DC. Colamarino SA. Song HJ. Desire L. Mira H. Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 26.Aubert J. Dunstan H. Chambers I. Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 2002;20:1240–1245. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- 27.Hao J. Li TG. Qi X. Zhao DF. Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Verani R. Cappuccio I. Spinsanti P. Gradini R. Caruso A. Magnotti MC, et al. Expression of the Wnt inhibitor Dick-kopf-1 is required for the induction of neural markers in mouse embryonic stem cells differentiating in response to retinoic acid. J Neurochem. 2007;100:242–250. doi: 10.1111/j.1471-4159.2006.04207.x. [DOI] [PubMed] [Google Scholar]
- 29.Haegele L. Ingold B. Naumann H. Tabatabai G. Ledermann B. Brandner S. Wnt signalling inhibits neural differentiation of embryonic stem cells by controlling bone morphogenetic protein expression. Mol Cell Neurosci. 2003;24:696–708. doi: 10.1016/s1044-7431(03)00232-x. [DOI] [PubMed] [Google Scholar]
- 30.Sato N. Meijer L. Skaltsounis L. Greengard P. Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch C. Campano LM. Wohrle S. Hecht A. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp Cell Res. 2007;313:572–587. doi: 10.1016/j.yexcr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Muroyama Y. Kondoh H. Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun. 2004;313:915–921. doi: 10.1016/j.bbrc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Yu JM. Kim JH. Song GS. Jung JS. Increase in proliferation and differentiation of neural progenitor cells isolated from postnatal and adult mice brain by Wnt-3a and Wnt-5a. Mol Cell Biochem. 2006;288:17–28. doi: 10.1007/s11010-005-9113-3. [DOI] [PubMed] [Google Scholar]
- 34.Prakash N. Brodski C. Naserke T. Puelles E. Gogoi R. Hall A, et al. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development. 2006;133:89–98. doi: 10.1242/dev.02181. [DOI] [PubMed] [Google Scholar]
- 35.Prakash N. Wurst W. Genetic networks controlling the development of midbrain dopaminergic neurons. J Physiol. 2006;575(Pt 2):403–410. doi: 10.1113/jphysiol.2006.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castelo-Branco G. Rawal N. Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J Cell Sci. 2004;117(Pt 24):5731–5737. doi: 10.1242/jcs.01505. [DOI] [PubMed] [Google Scholar]
- 37.Castelo-Branco G. Wagner J. Rodriguez FJ. Kele J. Sousa K. Rawal N, et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russek-Blum N. Gutnick A. Nabel-Rosen H. Blechman J. Staudt N. Dorsky RI, et al. Dopaminergic neuronal cluster size is determined during early forebrain patterning. Development. 2008;135:3401–3413. doi: 10.1242/dev.024232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benes FM. Lim B. Matzilevich D. Walsh JP. Subburaju S. Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Israsena N. Hu M. Fu W. Kan L. Kessler JA. The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol. 2004;268:220–231. doi: 10.1016/j.ydbio.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 41.Hirabayashi Y. Gotoh Y. Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci Res. 2005;51:331–336. doi: 10.1016/j.neures.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Gunhaga L. Marklund M. Sjodal M. Hsieh JC. Jessell TM. Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- 43.Takemoto T. Uchikawa M. Kamachi Y. Kondoh H. Convergence of Wnt and FGF signals in the genesis of posterior neural plate through activation of the Sox2 enhancer N-1. Development. 2006;133:297–306. doi: 10.1242/dev.02196. [DOI] [PubMed] [Google Scholar]
- 44.Tang K. Yang J. Gao X. Wang C. Liu L. Kitani H, et al. Wnt-1 promotes neuronal differentiation and inhibits gliogenesis in P19 cells. Biochem Biophys Res Commun. 2002;293:167–173. doi: 10.1016/S0006-291X(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 45.de Strooper B. Annaert W. Where Notch and Wnt signaling meet. The presenilin hub. J Cell Biol. 2001;152:F17–F20. doi: 10.1083/jcb.152.4.f17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ross DA. Kadesch T. The notch intracellular domain can function as a coactivator for LEF-1. Mol Cell Biol. 2001;21:7537–7544. doi: 10.1128/MCB.21.22.7537-7544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chesnutt C. Burrus LW. Brown AM. Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Wilson SI. Rydstrom A. Trimborn T. Willert K. Nusse R. Jessell TM, et al. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- 49.Wohrle S. Wallmen B. Hecht A. Differential control of Wnt target genes involves epigenetic mechanisms and selective promoter occupancy by T-cell factors. Mol Cell Biol. 2007;27:8164–8177. doi: 10.1128/MCB.00555-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurlstone A. Clevers H. T-cell factors: turn-ons and turnoffs. EMBO J. 2002;21:2303–2311. doi: 10.1093/emboj/21.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hallikas O. Palin K. Sinjushina N. Rautiainen R. Partanen J. Ukkonen E, et al. Genome-wide prediction of mammalian enhancers based on analysis of transcription-factor binding affinity. Cell. 2006;124:47–59. doi: 10.1016/j.cell.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 52.van de Wetering M. Castrop J. Korinek V. Clevers H. Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol Cell Biol. 1996;16:745–752. doi: 10.1128/mcb.16.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Billin AN. Thirlwell H. Ayer DE. Beta-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol Cell Biol. 2000;20:6882–6890. doi: 10.1128/mcb.20.18.6882-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hovanes K. Li TW. Waterman ML. The human LEF-1 gene contains a promoter preferentially active in lymphocytes and encodes multiple isoforms derived from alternative splicing. Nucleic Acids Res. 2000;28:1994–2003. doi: 10.1093/nar/28.9.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li TW. Ting JH. Yokoyama NN. Bernstein A. van de Wetering M. Waterman ML. Wnt activation and alternative promoter repression of LEF1 in colon cancer. Mol Cell Biol. 2006;26:5284–5299. doi: 10.1128/MCB.00105-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cordray P. Satterwhite DJ. TGF-beta induces novel Lef-1 splice variants through a Smad-independent signaling pathway. Dev Dyn. 2005;232:969–978. doi: 10.1002/dvdy.20275. [DOI] [PubMed] [Google Scholar]
- 57.Galceran J. Farinas I. Depew MJ. Clevers H. Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1(−/−)Tcf1(−/−) mice. Genes Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu F. van den Broek O. Destree O. Hoppler S. Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/beta-catenin signalling in mesoderm development. Development. 2005;132:5375–5385. doi: 10.1242/dev.02152. [DOI] [PubMed] [Google Scholar]
- 59.Nagayoshi S. Hayashi E. Abe G. Osato N. Asakawa K. Urasaki A, et al. Insertional mutagenesis by the Tol2 transposon-mediated enhancer trap approach generated mutations in two developmental genes: tcf7 and synembryn-like. Development. 2008;135:159–169. doi: 10.1242/dev.009050. [DOI] [PubMed] [Google Scholar]
- 60.Heeg-Truesdell E. LaBonne C. Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Dev Biol. 2006;298:71–86. doi: 10.1016/j.ydbio.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 61.Gregorieff A. Grosschedl R. Clevers H. Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. EMBO J. 2004;23:1825–1833. doi: 10.1038/sj.emboj.7600191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arce L. Yokoyama NN. Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- 63.Pukrop T. Gradl D. Henningfeld KA. Knochel W. Wedlich D. Kuhl M. Identification of two regulatory elements within the high mobility group box transcription factor XTCF-4. J Biol Chem. 2001;276:8968–8978. doi: 10.1074/jbc.M007533200. [DOI] [PubMed] [Google Scholar]
- 64.Shulewitz M. Soloviev I. Wu T. Koeppen H. Polakis P. Sakanaka C. Repressor roles for TCF-4 and Sfrp1 in Wnt signaling in breast cancer. Oncogene. 2006;25:4361–4369. doi: 10.1038/sj.onc.1209470. [DOI] [PubMed] [Google Scholar]
- 65.Atcha FA. Syed A. Wu B. Hoverter NP. Yokoyama NN. Ting JH, et al. A unique DNA binding domain converts T-cell factors into strong Wnt effectors. Mol Cell Biol. 2007;27:8352–8363. doi: 10.1128/MCB.02132-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hecht A. Stemmler MP. Identification of a promoter-specific transcriptional activation domain at the C terminus of the Wnt effector protein T-cell factor 4. J Biol Chem. 2003;278:3776–3785. doi: 10.1074/jbc.M210081200. [DOI] [PubMed] [Google Scholar]
- 67.Korinek V. Barker N. Moerer P. van Donselaar E. Huls G. Peters PJ, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 68.Nateri AS. Spencer-Dene B. Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- 69.van de Wetering M. Sancho E. Verweij C. de Lau W. Oving I. Hurlstone A, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 70.Bonner J. Gribble SL. Veien ES. Nikolaus OB. Weidinger G. Dorsky RI. Proliferation and patterning are mediated independently in the dorsal spinal cord downstream of canonical Wnt signaling. Dev Biol. 2008;313:398–407. doi: 10.1016/j.ydbio.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Molenaar M. Roose J. Peterson J. Venanzi S. Clevers H. Destree O. Differential expression of the HMG box transcription factors XTcf-3 and XLef-1 during early Xenopus development. Mech Dev. 1998;75:151–154. doi: 10.1016/s0925-4773(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 72.Roel G. Hamilton FS. Gent Y. Bain AA. Destree O. Hoppler S. Lef-1 and Tcf-3 transcription factors mediate tissue-specific Wnt signaling during Xenopus development. Curr Biol. 2002;12:1941–1945. doi: 10.1016/s0960-9822(02)01280-0. [DOI] [PubMed] [Google Scholar]
- 73.Merrill BJ. Gat U. DasGupta R. Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cole MF. Johnstone SE. Newman JJ. Kagey MH. Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marson A. Levine SS. Cole MF. Frampton GM. Brambrink T. Johnstone S, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134:521–533. doi: 10.1016/j.cell.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira L. Yi F. Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol. 2006;26:7479–7491. doi: 10.1128/MCB.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tam WL. Lim CY. Han J. Zhang J. Ang YS. Ng HH, et al. T-cell factor 3 regulates embryonic stem cell pluripotency and self-renewal by the transcriptional control of multiple lineage pathways. Stem Cells. 2008;26:2019–2031. doi: 10.1634/stemcells.2007-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi F. Pereira L. Merrill BJ. Tcf3 functions as a steady-state limiter of transcriptional programs of mouse embryonic stem cell self-renewal. Stem Cells. 2008;26:1951–1960. doi: 10.1634/stemcells.2008-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dorsky RI. Snyder A. Cretekos CJ. Grunwald DJ. Geisler R. Haffter P, et al. Maternal and embryonic expression of zebrafish lef1. Mech Dev. 1999;86:147–150. doi: 10.1016/s0925-4773(99)00101-x. [DOI] [PubMed] [Google Scholar]
- 80.Veien ES. Grierson MJ. Saund RS. Dorsky RI. Expression pattern of zebrafish tcf7 suggests unexplored domains of Wnt/beta-catenin activity. Dev Dyn. 2005;233:233–239. doi: 10.1002/dvdy.20330. [DOI] [PubMed] [Google Scholar]
- 81.Young R. Reyes A. Allende M. Expression and splice variant analysis of the zebrafish tcf4 transcription factor. Mech Dev. 2002;117:269–273. doi: 10.1016/s0925-4773(02)00180-6. [DOI] [PubMed] [Google Scholar]
- 82.Dorsky RI. Itoh M. Moon RT. Chitnis A. Two tcf3 genes cooperate to pattern the zebrafish brain. Development. 2003;130:1937–1947. doi: 10.1242/dev.00402. [DOI] [PubMed] [Google Scholar]
- 83.Kim CH. Oda T. Itoh M. Jiang D. Artinger KB. Chandrasekharappa SC, et al. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu YA. Gu X. Liu J. Yang Y. Yan Y. Zhao C. Expression pattern of Wnt inhibitor factor 1(Wif1) during the development in mouse CNS. Gene Expr Patterns. 2008;8:515–522. doi: 10.1016/j.gep.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 85.Potok MA. Cha KB. Hunt A. Brinkmeier ML. Leitges M. Kispert A, et al. WNT signaling affects gene expression in the ventral diencephalon and pituitary gland growth. Dev Dyn. 2008;237:1006–1020. doi: 10.1002/dvdy.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chapouton P. Jagasia R. Bally-Cuif L. Adult neurogenesis in non-mammalian vertebrates. Bioessays. 2007;29:745–757. doi: 10.1002/bies.20615. [DOI] [PubMed] [Google Scholar]
- 87.Kaslin J. Ganz J. Brand M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:101–122. doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Velasco B. Erclik T. Shy D. Sclafani J. Lipshitz H. McInnes R, et al. Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev Biol. 2007;302:309–323. doi: 10.1016/j.ydbio.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 89.Tessmar-Raible K. Raible F. Christodoulou F. Guy K. Rembold M. Hausen H, et al. Conserved sensory-neurosecretory cell types in annelid and fish forebrain: insights into hypothalamus evolution. Cell. 2007;129:1389–1400. doi: 10.1016/j.cell.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 90.Kapsimali M. Caneparo L. Houart C. Wilson SW. Inhibition of Wnt/Axin/beta-catenin pathway activity promotes ventral CNS midline tissue to adopt hypothalamic rather than floorplate identity. Development. 2004;131:5923–5933. doi: 10.1242/dev.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dorsky RI. Sheldahl LC. Moon RT. A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish Development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- 92.Ghanem N. Jarinova O. Amores A. Long Q. Hatch G. Park BK, et al. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res. 2003;13:533–543. doi: 10.1101/gr.716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oxtoby E. Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brinkmeier ML. Potok MA. Davis SW. Camper SA. TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol. 2007;311:396–407. doi: 10.1016/j.ydbio.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jho EH. Zhang T. Domon C. Joo CK. Freund JN. Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eisenstat DD. Liu JK. Mione M. Zhong W. Yu G. Anderson SA, et al. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J Comp Neurol. 1999;414:217–237. doi: 10.1002/(sici)1096-9861(19991115)414:2<217::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 97.Petryniak MA. Potter GB. Rowitch DH. Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Imura T. Nakano I. Kornblum HI. Sofroniew MV. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–293. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- 99.Kippin TE. Martens DJ. van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filali M. Cheng N. Abbott D. Leontiev V. Engelhardt JF. Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J Biol Chem. 2002;277:33398–33410. doi: 10.1074/jbc.M107977200. [DOI] [PubMed] [Google Scholar]
- 101.Grandel H. Kaslin J. Ganz J. Wenzel I. Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol. 2006;295:263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 102.Adolf B. Chapouton P. Lam CS. Topp S. Tannhauser B. Strahle U, et al. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol. 2006;295:278–293. doi: 10.1016/j.ydbio.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 103.Merkle FT. Mirzadeh Z. Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 104.Caricasole A. Copani A. Caruso A. Caraci F. Iacovelli L. Sortino MA, et al. The Wnt pathway, cell-cycle activation and beta-amyloid: novel therapeutic strategies in Alzheimer's disease? Trends Pharmacol Sci. 2003;24:233–238. doi: 10.1016/s0165-6147(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 105.Kokoeva MV. Yin H. Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 106.Xu Y. Tamamaki N. Noda T. Kimura K. Itokazu Y. Matsumoto N, et al. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 107.Zikopoulos B. Kentouri M. Dermon CR. Proliferation zones in the adult brain of a sequential hermaphrodite teleost species (Sparus aurata) Brain Behav Evol. 2000;56:310–322. doi: 10.1159/000047215. [DOI] [PubMed] [Google Scholar]