Abstract
Autophagy is a major catabolic pathway by which mammalian cells degrade and recycle macromolecules and organelles. It plays a critical role in removing protein aggregates as well as damaged or excess organelles in order to maintain intracellular homeostasis and to keep the cell healthy. In the heart, autophagy occurs at low levels under normal conditions, and defects in this process cause cardiac dysfunction and heart failure. However, this pathway is rapidly upregulated under environmental stress conditions, including ATP depletion, reactive oxygen species, and mitochondrial permeability transition pore opening. Although autophagy is enhanced in various pathophysiological conditions such as during ischemia and reperfusion, the functional role of increased autophagy is not clear and is currently under intense investigation. In this review, we discuss the evidence for autophagy in the heart in response to ischemia and reperfusion, identify factors that regulate autophagy, and analyze the potential roles autophagy might play in cardiac cells.
Keywords: Apoptosis, Autophagy, Mitochondria, Myocardial ischemia
Introduction
The study of autophagy in mammalian systems and in disease states is advancing rapidly, and many investigators are entering this new and exciting field. Autophagy is a physiologic process that is involved in degradation of long-lived proteins and removing excess or damaged organelles1. In this process, a double-membrane structure called the autophagosome sequesters cytoplasmic components such as ubiquitinated protein aggregates or organelles including mitochondria, peroxisomes, and endoplasmic reticulum (ER). Autophagy occurs constitutively in the normal myocardium, and disruption of this pathway results in development of left ventricular dilation and severe contractile dysfunction in the absence of any stress2. Moreover, Danon disease is due to a defect in the autophagy-lysosomal pathway that is characterized by the development of cardiomyopathy, confirming that this pathway is essential for cellular homeostasis in the heart and that disruption of this process has adverse effects3, 4. Autophagy is also induced when there is a change in the cellular environment, and is known to be an important survival mechanism that is rapidly activated in response to starvation when recycling of fatty acids and amino acids from proteins and lipids are required for survival5. Autophagy has been shown to be substantially increased in mouse hearts after fasting6. In addition, there is evidence that autophagy is enhanced in various pathological conditions including cardiac hypertrophy, cardiomyopathy, and heart failure4, 7–14. Many studies have reported that autophagy is upregulated during myocardial ischemia and reperfusion15–21 18, 22 16, 20, 23. However, the functional role of this enhancement is not clear. In this review, we discuss the evidence of enhanced autophagy in response to myocardial ischemia and reperfusion, identify signals that activate autophagy, and analyze the potential roles that autophagy might play in cells.
The Process of Autophagy
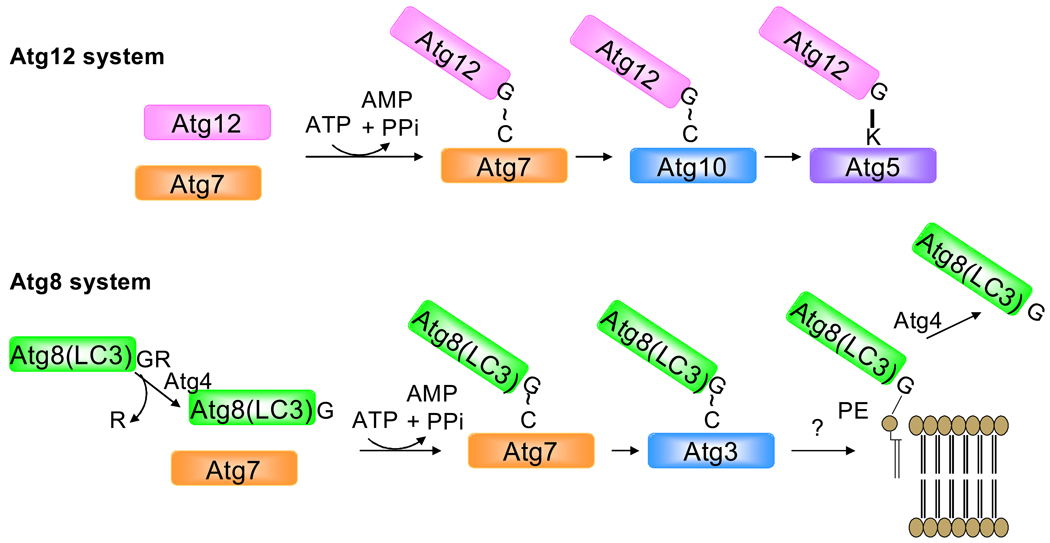
There are three main types of autophagy: macroautophagy, microautophagy and chaperone-mediated autophagy. Macroautophagy (hereafter referred to as autophagy) is the most common form of autophagy and is characterized by formation of a cup-shaped pre-autophagosomal double-membrane structure, which surrounds cytoplasmic material and closes to form the autophagosome. The outer membrane of the autophagosome then fuses with the lysosome to create a single membrane autophagolysosome, where the inner membrane of the autophagosome and its contents are digested5. This process is regulated by the autophagy (Atg) proteins. Activation of the class III PI3K/Vps34 and Beclin1(Atg6) complex causes induction of autophagy which involves formation of an isolation membrane to which the Atg proteins are recruited24, 25. Two ubiquitin-like protein conjugation pathways, Atg12-Atg5 and the microtubule associated protein light chain 3-phosphatidylethanolamine (LC3-PE) are involved in the expansion of the isolation membrane5, 26 (Figure 1). Atg12 and Atg5 are covalently conjugated when Atg12 is activated by the ubiquitin-activating E1 enzyme, Atg7 and then transferred to the E2 ubiquitin-conjugating enzyme Atg10. Atg10 is released when Atg12 is covalently conjugated to a lysine residue on Atg527. Atg12-Atg5 then binds to Atg16 and this trimeric complex then localizes to the outer membrane of the isolation membrane which is essential for recruitment of LC3 and elongation of the membrane28, 29.
Figure 1.
Ubiquitin-like conjugation systems are required for autophagosome formation. In the first case, Atg7 and Atg10 are responsible for conjugating Atg12 onto the acceptor lysine of Atg5. This is a pre-requisite for the recruitment of Atg8, which is conjugated onto phosphatidylethanolamine in the phagophore membrane in a reaction dependent upon Atg7 and Atg3.
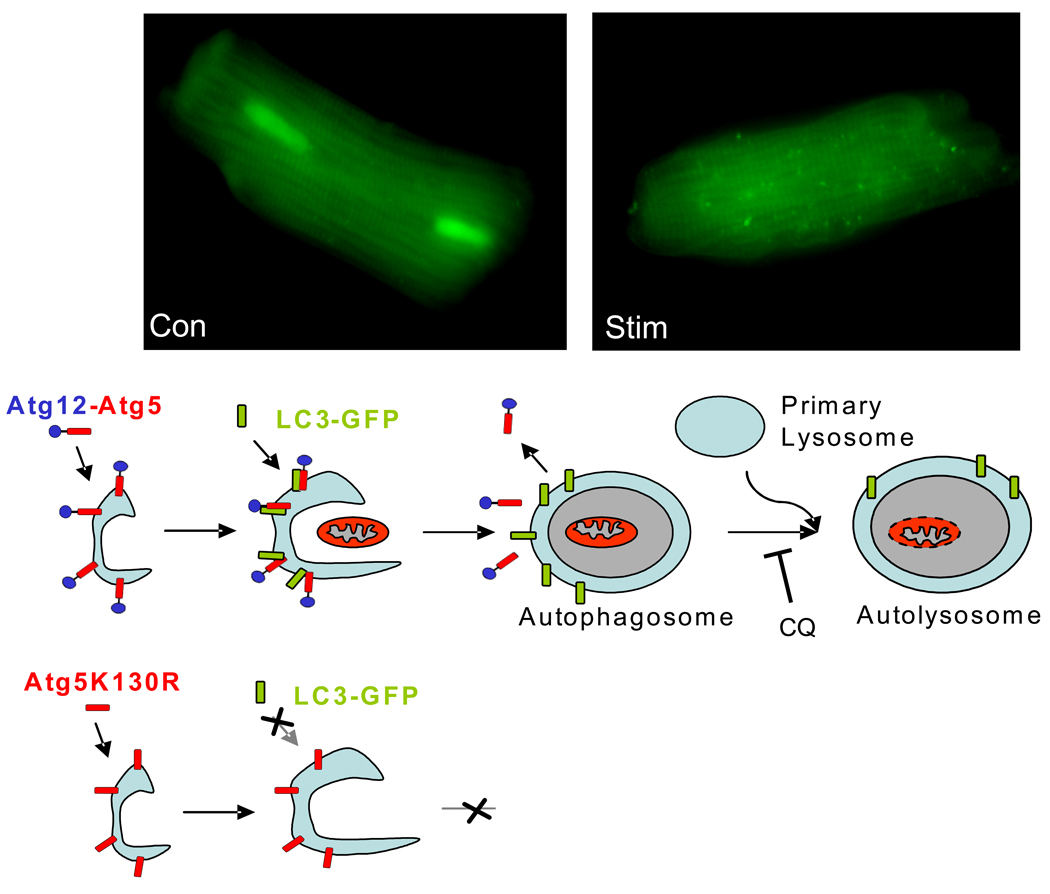
LC3 is synthesized as a larger precursor protein and is converted to LC3-I by the cysteine protease Atg4 which exposes a glycine residue at the C-terminal30. Similar to Atg12, LC3-I is activated by Atg7 and then transferred to Atg3, an E2-like enzyme, which catalyzes the covalent conjugation with PE31, 32. LC3-PE translocates to the autophagosome membrane in an Atg5-dependent manner29. LC3 fused to a fluorescent protein such as GFP or mCherry is commonly used as a marker to monitor autophagy in vivo33–35. The GFP-LC3 is diffusely distributed in the cytosol under normal conditions, but upon proteolysis of the C-terminus and lipidation, it is recruited into autophagosomes, which are evident as microscopic specks, or puncta (Figure 2). The extent to which GFP-LC3 is recruited into punctate structures correlates very well with the extent of autophagy and is now regarded as a reliable indicator of autophagic activity, with certain caveats36, 37. Atg5 is rate-limiting for autophagy and overexpression increases autophagy38, 39. Mutation of lysine130, the site for the ubiquitin-like conjugation of Atg12, causes Atg5 to function as a dominant negative (Figure 2); Atg5K130R is a useful tool to selectively block autophagy36, 40, 41.
Figure 2.
GFP-LC3 is present diffusely in the cytoplasm until it is incorporated into the developing autophagosomal membrane. The accumulation of GFP-LC3 on these structures can be visualized by fluorescence microscopy as bright fluorescent puncta. Fusion with the lysosome can be prevented with chloroquine (CQ) or Bafilomycin A1. Selective disruption of autophagy can be accomplished by using a point mutant of Atg5 in which the acceptor lysine is mutated to arginine (Atg5K130R). This prevents the incorporation of Atg12 and stalls the process before incorporation of LC3, phagophore enlargement, or target engulfment.
Autophagy in myocardial ischemia and reperfusion injury
Many studies have reported that the number of autophagosomes increases in the heart during both ischemia and reperfusion15–21 18, 22 16, 20, 23. Autophagy was initially reported to be enhanced in fetal mouse hearts in organ cultures after being subjected to hypoxia combined with glucose deprivation and subsequent reperfusion19. Later, another study found that brief ischemia (20 min) did not induce autophagy until reperfusion in Langendorff perfused rabbit hearts15. However, longer ischemia (40 min) caused an increase in the number of autophagosomes which was further increased during reperfusion, suggesting that both ischemia and reperfusion can induce autophagy. Gurusamy et al. found that 30 min of ischemia and two hours of reperfusion caused upregulation of autophagy in a Langendorff model as assessed by an increase in LC3-II and Beclin 142. Another study found enhanced autophagy and lysosomal activity in pig hearts subjected to six episodes of ischemia and reperfusion21. Enhanced autophagy has also been observed in isolated cardiac cells where exposure to hypoxia/reoxygenation or simulated I/R (sI/R) caused an increase in the number of autophagic vesicles17, 20, 42. We found that autophagic activity was inhibited during ischemia, but was re-activated during reperfusion in HL-1 myocytes16.
Signals that Induce Autophagy in Ischemia/Reperfusion
ATP levels and AMPK
Multiple signals are likely to activate autophagy during I/R. For instance, autophagy has been reported to be upregulated in response to reduced cellular content of ATP43. In cultured cardiac myocytes, glucose deprivation caused significant reduction in the levels of ATP which coincided with upregulation of autophagy18. Moreover, myocardial ischemia causes a decrease in ATP levels and an increase in the AMP/ATP ratio resulting in activation of the AMP-activated protein kinase (AMPK)44. Activated AMPK enhances uptake and oxidative metabolism of fatty acids as well as increases glucose transport and glycolysis45. Matsui et al. reported that glucose deprivation induced autophagy via activation of AMPK and inhibition of mTOR in isolated cardiac myocytes18. mTOR is a negative regulator of autophagy and functions as a sensor for cellular energy and amino acid levels and is negatively regulated by AMPK via a pathway involving the tuberous sclerosis complex (TSC1/2) and its substrate Rheb, a Ras-related small GTPase46. Moreover, induction of autophagy in response to myocardial ischemia was reduced in transgenic mice overexpressing a dominant negative AMPK18.
Hypoxia and Bnip3
The BH3-only protein Bnip3 is an important contributor to I/R injury17 and is upregulated by hypoxia47, 48. Many studies have reported that Bnip3 induces upregulation of autophagy in different cells, including cardiac cells. We found that overexpression of Bnip3 resulted in significant induction of autophagy in HL-1 myocytes17 and adult cardiac myocytes (unpubl. observation). Also, overexpression of a Bnip3 dominant negative protein reduced autophagy induced by simulated I/R in HL-1 myocytes, suggesting that Bnip3 contributes to activation of autophagy during I/R17. Autophagy is also upregulated during hypoxia. For instance, Zhang et al. found that upregulation of autophagy during hypoxia required hypoxia-dependent factor-1-expression of Bnip349, and Azad et al. reported that expression of Bnip3 siRNA or a dominant-negative form of Bnip3 reduced hypoxia-induced autophagy50. In contrast, the study by Papandreau et al. reported that hypoxia-induced activation of autophagy in cancer cell lines was not dependent on nutrient deprivation, or expression of Bnip3, but involved activation of AMPK51. Clearly, more studies are needed to define the role of Bnip3 in hypoxia-mediated autophagy, but a reasonable interpretation is that Bnip3 causes mitochondrial damage with secondary upregulation of autophagy to remove damaged organelles.
Calcium
A consequence of ischemia is elevated levels of intracellular Ca2+ due to the plasma membrane sodium calcium exchanger (NCX) operating in reverse, loading the cell with Ca2+52. It was recently demonstrated that increased cytosolic calcium is a potent inducer of autophagy. Ca2+ mobilizing agents such as vitamin D compounds, ionomycin, ATP, and thapsigargin inhibited the autophagy regulator mTOR, which resulted in massive accumulation of autophagosomes in a Beclin1- and Atg7-dependent manner53. Thus, it is very likely that elevated intracellular Ca2+ is one of the many factors that induces autophagy during ischemia.
Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)
ROS are generated in the myocardium during both ischemia and reperfusion54, 55, which can activate autophagy in cardiac myocytes. For instance, LPS treatment increased ROS and induced autophagy in neonatal cardiac myocytes56, 57. Mitochondria are major sources of ROS which can activate apoptosis, but it has recently been reported that mitochondria also induce autophagy through ROS. For instance, Chen et al. found that treatment with rotenone or TTFA to inhibit mitochondrial electron transport chain (mETC) complexes I and II, respectively, induced significant autophagy in U87 and HeLa cells58. Moreover, rotenone and TTFA treatment induced substantial ROS production in cells and the presence of a ROS scavenger decreased autophagy, suggesting that ROS generated from the mETC activates autophagy. Moreover, evadiamine, a quinozole alkaloid, was reported to induce autophagy via increased ROS production in HeLa cells59. At least one critical step in the autophagy pathway has been reported to be regulated by ROS. Atg4 is a cysteine protease that cleaves LC3 to expose a glycine residue allowing for the covalent bonding of LC3 to PE. Atg4 is also responsible for recycling of LC3 by cleaving PE from PE-conjugated LC3. Hydrogen peroxide generated during starvation was found to regulate autophagosome formation by inactivating Atg4 by oxidation of an essential cysteine residue, which led to accumulation of LC3-PE on the phagophore membrane and formation of autophagosomes60.
Nitric oxide (NO) has been reported to play an important role in protecting the heart against ischemia/reperfusion injury61, whereas excess levels of NO contribute to heart failure62. There is evidence that NO activates autophagy in cells. Treatment with the NO donor S-nitrosocysteine induced autophagy, and electron microscopy showed autophagosomes engulfing damaged mitochondria in neurons63. Moreover, Yang et al. found that nitric oxide induced autophagy in HeLa cells59. In contrast, Rabkin and Klassen evaluated changes in gene expression in neonatal cardiac myocytes treated with the NO donor SIN-1 and found that gene expression of the autophagy genes beclin 1, Atg5, and Atg12 did not change in the cells, suggesting that NO does not cause upregulation of autophagy in cardiac myocytes64. However, this study only measured changes in gene expression and did not assess whether NO induced formation of autophagosomes in cells, and as the study by Matsui et al. demonstrated, autophagy can be induced without upregulation of autophagy genes such as beclin118. Thus, further studies examining the relationship between NO and autophagy in cardiac myocytes is necessary.
mPTP opening
The mitochondrial permeability transition pore (mPTP) opening has been reported to induce autophagy in mammalian cells65–67. For instance, Arrington et al. found that overexpression of the Ca2+-activated cysteine protease Calpain 10 resulted in mitochondrial swelling and increased autophagy which was blocked by the mPTP inhibitor cyclosporine A66. We found that inhibition of the mPTP with cyclosporine A decreased the upregulation of autophagy after sI/R in HL-1 myocytes (unpubl. observation). Since reperfusion triggers opening of the mPTP68, it is possible that the mPTP serves as an upstream signal for autophagy in I/R.
ER stress and the Unfolded Protein Response (UPR)
The endoplasmic reticulum (ER) is important in protein synthesis and folding, as well as Ca2+ homeostasis. Perturbation of the ER environment induces ER stress and lead to the accumulation of unfolded or misfolded proteins which activates the UPR. The UPR is a protective and compensatory mechanism which activates multiple functions to avoid damage to the cell. It rapidly decreases protein synthesis, induces expression of ER chaperone proteins, and increases the degradation of misfolded proteins69. The UPR is activated in mouse hearts subjected to ischemia/reperfusion70, 71, as well as in surviving cardiac myocytes in the border zone of the infarct in a mouse model of in vivo myocardial infarction70, 72. Moreover, transgenic mice overexpressing the monocyte chemoattractant protein-1 (MCP-1) in the heart develop ischemic heart disease and increase the expression of many ER stress response genes73. Several studies have also shown that simulating ischemia or ischemia/reperfusion in cultured neonatal rat or adult mouse ventricular myocytes activates the UPR70, 71, 74, 75. Recently, ER stress has been linked to induction of autophagy76–78. For instance, Tannous et al. reported that pressure overload promoted upregulation of the UPR regulator and ER chaperon BiP, which correlated with upregulation of autophagy in mouse hearts79. Ogata et al. found that treatment with ER stressors tunicamycin and thapsigargin caused upregulation of autophagy in neuroblastoma cells, and IRE1, a major sensor of ER stress, was required for induction of autophagy where thapsigargin or tunicamycin failed to induce autophagy in IRE1 deficient mouse embryonic fibroblasts (MEFs)78. We showed that release of ER calcium is required for induction of autophagy80. Li et al. reported that GRP78/BiP was required for stress-induced autophagy where knockdown of GRP78 expression by siRNA in cells prevented induction of autophagy by ER stress as well as by nutrition deprivation81. These studies suggest that the UPR signaling pathways are required for induction of autophagy in response to ER stress.
Functional Role of Autophagy in Myocardial Ischemia and Reperfusion
The functional role of autophagy in the heart is currently under intense investigation and studies have characterized this process both in vitro and in vivo. Interestingly, upregulation of autophagy has been reported to both contribute as well as be the cause of cell death in the heart. Many studies have reported that enhanced autophagy contributes to cell death during I/R. For instance, Aki et al. found that glucose deprivation caused a significant increase in autophagy and cell death, and that inhibiting autophagy with 3-methyladenine (3-MA) or LY294002 reduced cell death, suggesting that autophagy contributes to cell death82. Another study reported that inhibiting autophagy by downregulation of Beclin 1 or treatment with 3-MA reduced cell death in isolated cardiac myocytes after simulated to sI/R20. Interestingly, Matsui et al. found that autophagy was protective during ischemia, but that it switched to a detrimental role during reperfusion. They reported that hearts from beclin 1 (beclin 1+/−) heterozygous mice exhibited reduced levels of autophagy during reperfusion compared to wild type litter mates which correlated with decreased apoptosis and reduced infarct size18. Increased levels of autophagy have also been reported to contribute to cell death in pressure overload-induced heart failure14. Zhu et al. found that mice with Beclin 1+/− had reduced levels of autophagy which coincided with diminished cardiac remodeling induced by pressure overload compared to wild type, whereas transgenic mice with cardiac specific overexpression of Beclin 1 had increased autophagy and enhanced pathological remodeling in response to stress.
In contrast, many studies have reported that autophagy is a beneficial response to I/R. One of the first studies of autophagy in the heart reported that increased levels of autophagy correlated with functional recovery and salvage of the myocardium after I/R, but impairment of the autophagosome-lysosome pathway during extended ischemia, likely due to depletion of ATP, was associated with irreversible injury and contractile dysfunction15. Similarly, Yan et al. reported that cardiac myocytes with enhanced autophagy were negative for apoptosis, whereas apoptotic cells were negative for autophagy in a porcine model of chronic myocardial ischemia and hibernating myocardium, suggesting that autophagy served to protect cells against apoptosis in this model21. Authophagy has also been shown to protect in cell culture models that mimic I/R injury. For instance, Matsui et al. reported that inhibition of autophagy enhanced glucose deprivation-mediated cell death18. We found that enhancing autophagy protected against sI/R mediated cell death in HL-1 myocytes, whereas inhibition of autophagy enhanced cell death16. These studies suggest that upregulation of autophagy promotes survival during ischemia/reperfusion.
Ischemic preconditioning is the well-recognized phenomenon in which a brief episode of ischemia followed by reperfusion confers protection against a subsequent more severe ischemic insult. Recently, Gurusamy et al. reported that preconditioning enhanced autophagy and that inhibition of autophagy abolished the cardioprotective effects of preconditioning42. Many agents that have been shown to be cardioprotective also induce autophagy. For instance, rapamycin, a powerful inducer of autophagy, was shown to reduce infarct size in a Langendorff model83. Statins, which have been shown to be beneficial in the post-MI setting regardless of effects on cholesterol levels, are powerful inducers of autophagy; however, pravastatin, which showed less benefit in the PROVE IT-TIMI 22 trial, failed to induce autophagy84, 85. Exercise is another intervention that has been shown to confer cardioprotection against I/R injury86, 87 as well as to induce autophagy88.
Many other studies have also found that autophagy protects against various cellular stressors. For instance, LPS treatment of neonatal myocytes induced production of ROS and autophagy, but did not induce cell death57. However, when autophagy was inhibited with 3-MA in LPS-treated cells, there was significant cell death. This suggests that increased oxidative stress by LPS leads to upregulation of autophagy by the cells to protect itself from damage by these reactive oxygen species. Chen et al. found that autophagy provided protection against cell death in response to mild oxidative stress, but excessive oxidative stress inhibited the autophagic process, resulting in irreversible damage to the cell and activation of apoptosis58.
Thus, these studies suggest that autophagy has a dual role in the heart depending on the stimulus. It has been speculated that too much autophagy can cause cell death by excessive degradation of essential proteins and organelles. Clearly, further work will be necessary to establish the role of autophagy in cardioprotection and cell death.
Potential Mechanisms of Cardioprotection by Autophagy in I/R
ATP Maintenance
The primary function of autophagy is catabolic. Within the autophagolysosome, proteins and membranes are degraded into amino acids which can feed into the tricarboxylic acid (TCA) cycle to generate ATP. Fatty acids generated from breakdown of organelle membrane lipids which can be used as fuel by mitochondria in the context of nutrient limitations. Matsui et al. found that glucose deprivation significantly reduced the cellular ATP content which correlated with upregulation of autophagy in cardiac myocytes18. Moreover, inhibition of autophagy with 3-MA further reduced the levels of ATP and increased cell death in response to glucose deprivation18. This suggests that activation of autophagy during ischemia promotes survival by maintaining ATP production.
Mitophagy
Mitochondrial integrity is essential to cellular survival. Damaged mitochondria may generate excessive reactive oxygen species (ROS), and if uncoupled, could consume ATP. Therefore their elimination by autophagy is an efficient cytoprotective response. Furthermore, damaged or unstable mitochondria may release cytochrome c, AIF, and other apoptosis-promoting factors which would promote damage to neighboring mitochondria. Autophagy is the only process by which the cell can degrade organelles, and transmission electron microscopy studies have shown that many autophagosomes formed during I/R contain mitochondria17, 42. Moreover, ultrastructural analysis of hearts lacking Atg5 showed aggregation of mitochondria and disorganized sarcomeres. Mitochondria also play a major role in inducing autophagy in the cell; autophagy is upregulated when mitochondria fail to maintain ATP levels43, or when mitochondria are damaged89. HL-1 cells subjected to simulated ischemia/reperfusion (sI/R) exhibit widespread mitochondrial fragmentation that begins during ischemia and precedes the development of autophagy16. A number of investigators have suggested that mitochondrial fragmentation is a prerequisite for efficient mitochondrial autophagy (mitophagy) (reviewed in90, 91). Mitochondrial fission is known to precede autophagy, and some have suggested that fission is a prerequisite for autophagic removal of mitochondria. Indeed, it has been suggested that in aging, mitochondrial fission is impaired, resulting in the accumulation of large, senescent mitochondria91. As we have recently published92, mitochondrial fission occurs during ischemia, well before a detectable increase in autophagy. Furthermore, inhibition of mitochondrial fission in response to simulated ischemia prevents upregulation of autophagy.
Protein Clearance
The autophagy-lysosomal pathway is important in degradation of long-lived proteins, whereas the ubiquitin-proteasome system (UPS) regulates turnover of short-lived proteins93. It has been reported that the function of the proteasome is inhibited in by ischemia/reperfusion injury which is likely due to the substantial increase in the number of substrates that the proteasome degrades as well as decreased activity of the 20S and/or 26S proteasome94, 95. There is evidence that these two degradation systems are functionally coupled. During certain conditions, accumulation of ubiquitinated proteins might exceed proteasomal degradation resulting in a buildup of ubiquitinated proteins and formation of protein aggregates. These large protein complexes are poor substrates for the proteasome96, but can be degraded by the autophagosome-lysosome pathway. Deletion of atg5 or atg7 in the brain disrupts autophagy and results in the accumulation of polyubiquitinated proteins in neurons97, 98, and deletion of atg5 in the heart increases the levels polyubiquitinated proteins as well as the activity of the proteasome2. Peripheral myelin protein 22 (PMP22) is a short-lived molecule that forms aggresomes in response to mutations or inhibition of the proteasome, and removal of the PMP22 aggregates is mediated by autophagy. The accumulation of the aggregates was suppressed by induction of autophagy, and enhancement of autophagy during proteasome inhibition prevented protein aggregate formation and correlated with a reduction in accumulated proteasome substrates. Conversely, simultaneous inhibition of autophagy and the proteasome increased the formation of aggregates99. Moreover, it has been reported that inhibition of the proteasome triggered activation of autophagy and that suppression of autophagy caused accumulation of ubiquitinated proteins in isolated cardiac myocytes, suggesting that autophagy is an important mechanism in clearing protein aggregates in cardiac myocytes79. Activation of autophagy with rapamycin has been shown to increase clearance of aggregate-prone proteins and decreased accumulation of protein aggregates in vitro and in vivo 100–102. Recently, Tannous et al. reported that accumulation of ubiquitinated proteins in the heart in response to pressure overload which was not due to diminished proteasome activity, suggesting that the accumulation of ubiquitinated proteins exceeded the capacity of the proteasome79. This suggests that both proteasomal and autophagic clearance pathways are activated in response to pressure-overload stress. It is not clear exactly how autophagy attenuates ER stress, but it is likely that the protective effect of autophagy is at the level of protein clearance, which would reduce ER stress caused by these proteins.
Autophagy and Bcl-2 Family Proteins
The Bcl-2 family proteins are important regulators of apoptosis in the cardiovascular system. Transgenic mice with cardiac specific overexpression of anti-apoptotic Bcl-2 are protected against I/R injury103–105, whereas mice deficient for pro-apoptotic Bax have reduced I/R injury106. Recently, the Bcl-2 family proteins have been linked to autophagy. Structural and functional analysis showed that Beclin1 is a novel BH3-only protein and that anti-apoptotic Bcl-2 and Bcl-XL can bind to the BH3 domain and inhibit Beclin1107, 108. A BH3 mutant of Beclin1 which has reduced affinity for Bcl-XL/Bcl-2 was a more potent inducer of autophagy than wild type Beclin1, and mutants of Bcl-2 and Bcl-XL that were unable to interact with Beclin1 failed to inhibit autophagy6, 108. Pattingre et al. found that overexpression of Bcl-2 in the heart reduced starvation-induced autophagy compared to wild type6. Based on the literature, it is possible that cardiac specific overexpression of Bcl-2 protects against I/R injury by blocking apoptosis and by preventing activation of autophagy by inhibiting Beclin1. This is supported by the fact that Beclin1+/− hearts have reduced autophagy and infarct size after I/R18. In cells where autophagy serves as a mechanism of cell death, activation of both apoptosis and autophagy may be a way for the cell to fully ensure cell death. Also, Rashmi et al. found that the BH3-only protein Bik induced cell death via the autophagic pathway in MEFs lacking Bcl-2 but not in wild type MEFs109. If the role of Bcl-2 is to maintain Beclin1 inactive, then these cells may be more sensitive to activation of autophagy leading which may lead to excessive induction of autophagy and cell death. Several of the pro-apoptotic BH3-only members have been reported to activate autophagy. For example, the BH3-only protein Bad or the BH3-mimetic ABT737 directly activated autophagy by disrupting the interaction between Bcl-2/Bcl-XL and Beclin1108, 110. As discussed above, Bnip3 causes rapid upregulation of autophagy in various cells17, 49, 50, 111, 112, possibly by competing with Beclin1 for binding to Bcl-249, and by inhibiting mTOR by directly interacting with Rheb113. To date, Bnip3 is the only BH3-only protein that has been reported to induce autophagy in cardiac cells, but based on data in other cell types, it is likely that other BH3-only proteins will induce autophagy in the heart.
A newly identified BH3-only protein, ApoLipoprotein L1 (ApoL1), was also found to induce cell death via autophagy. Overexpression of ApoL1 in cells led to cell death which was reduced by inhibiting autophagy with 3-MA or wortmannin, and autophagy deficient cells were resistant to ApoL1-mediated cell death114. In contrast, upregulation of autophagy in response to Bnip3 in HL-1 cardiac myocytes was shown to serve as a protective response against Bnip3-mediated injury17, 22. The Bnip3 homolog Nix (Bnip3L), which has been implicated in cardiac hypertrophy and development of cardiomyopathy115, was recently reported to play an essential role in mitophagy in erythrocytes. Nix-dependent loss of mitochondrial membrane potential was important for targeting the mitochondria into autophagosomes for clearance during erythroid maturation, and interference with this function impaired erythroid maturation116. It is clear that different BH3-only proteins have different effects on cells depending on the stimulus and the cell type.
Conclusions
Enhanced levels of autophagy in cardiac myocytes are observed in many cardiovascular diseases, but the functional role of autophagy in these settings is unclear. Increasing evidence from in vitro and in vivo studies suggest that basal levels of autophagy are important for maintaining cellular homeostasis and for protecting cells against excess or dysfunctional organelles. However, it appears that enhancing autophagy can also promote survival in response to milder stress, such as brief hypoxia and lower levels of oxidative stress, possibly by removing damaged and harmful organelles as well as recycling of macromolecules to maintain energy levels and protein synthesis. In contrast, it is possible that severe stress, such as prolonged hypoxia and subsequent reperfusion, results in excessive and/or long-term upregulation of autophagy which causes cell death by promoting cell death by excessive self digestion of essential organelles and proteins. Substantial loss of cardiac myocytes after I/R can lead to contractile dysfunction and heart failure. Thus, manipulation of autophagy may represent a potential future therapeutic target to treat or prevent development of heart disease. However, there are still many unanswered questions about its role(s) in the heart and it is possible that different pathways trigger death-associated and survival-associated autophagy. Therefore, it is important to elucidate the molecular mechanism of how and under what conditions autophagy contributes to survival or cell death in the heart.
Acknowledgments
Sources of Funding
This manuscript was supported by a Scientist Development Award from the AHA, and NIH grant HL087023 to ÅBG, and NIH grants HL071091, HL060590, HL085577, and AG025168 to RAG.
Footnotes
Disclosures: None.
References
- 1.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka Y, Guhde G, Suter A, Eskelinen EL, Hartmann D, Lullmann-Rauch R, Janssen PM, Blanz J, von Figura K, Saftig P. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature. 2000;406:902–906. doi: 10.1038/35022595. [DOI] [PubMed] [Google Scholar]
- 4.Nishino I, Fu J, Tanji K, Yamada T, Shimojo S, Koori T, Mora M, Riggs JE, Oh SJ, Koga Y, Sue CM, Yamamoto A, Murakami N, Shanske S, Byrne E, Bonilla E, Nonaka I, DiMauro S, Hirano M. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease) Nature. 2000;406:906–910. doi: 10.1038/35022604. [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev.Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 6.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S, Sawada K, Shimomura H, Kawamura K, James TN. On the nature of cell death during remodeling of hypertrophied human myocardium. J Mol Cell Cardiol. 2000;32:161–175. doi: 10.1006/jmcc.1999.1064. [DOI] [PubMed] [Google Scholar]
- 8.Knaapen MW, Davies MJ, De Bie M, Haven AJ, Martinet W, Kockx MM. Apoptotic versus autophagic cell death in heart failure. Cardiovasc Res. 2001;51:304–312. doi: 10.1016/s0008-6363(01)00290-5. [DOI] [PubMed] [Google Scholar]
- 9.Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, Schaper J. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 10.Dammrich J, Pfeifer U. Cardiac hypertrophy in rats after supravalvular aortic constriction. II. Inhibition of cellular autophagy in hypertrophying cardiomyocytes. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;43:287–307. doi: 10.1007/BF02932962. [DOI] [PubMed] [Google Scholar]
- 11.Miyata S, Takemura G, Kawase Y, Li Y, Okada H, Maruyama R, Ushikoshi H, Esaki M, Kanamori H, Li L, Misao Y, Tezuka A, Toyo-Oka T, Minatoguchi S, Fujiwara T, Fujiwara H. Autophagic cardiomyocyte death in cardiomyopathic hamsters and its prevention by granulocyte colony-stimulating factor. Am J Pathol. 2006;168:386–397. doi: 10.2353/ajpath.2006.050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeifer U, Fohr J, Wilhelm W, Dammrich J. Short-term inhibition of cardiac cellular autophagy by isoproterenol. J Mol Cell Cardiol. 1987;19:1179–1184. doi: 10.1016/s0022-2828(87)80528-x. [DOI] [PubMed] [Google Scholar]
- 13.Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn.Circ.J. 2001;65:965–968. doi: 10.1253/jcj.65.965. [DOI] [PubMed] [Google Scholar]
- 14.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decker RS, Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. I. Ultrastructural and cytochemical changes. Am J Pathol. 1980;98:425–444. [PMC free article] [PubMed] [Google Scholar]
- 16.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–29787. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 17.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 18.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 19.Sybers HD, Ingwall J, DeLuca M. Autophagy in cardiac myocytes. Recent Adv Stud Cardiac Struct Metab. 1976;12:453–463. [PubMed] [Google Scholar]
- 20.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 21.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci U S A. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a Protective Response to Bnip3-Mediated Apoptotic Signaling in the Heart. Autophagy. 2006;2:307–309. doi: 10.4161/auto.2947. [DOI] [PubMed] [Google Scholar]
- 23.Dosenko VE, Nagibin VS, Tumanovska LV, Moibenko AA. Protective effect of autophagy in anoxia-reoxygenation of isolated cardiomyocyte? Autophagy. 2006;2:305–306. doi: 10.4161/auto.2946. [DOI] [PubMed] [Google Scholar]
- 24.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 26.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 27.Shintani T, Mizushima N, Ogawa Y, Matsuura A, Noda T, Ohsumi Y. Apg10p, a novel protein-conjugating enzyme essential for autophagy in yeast. Embo J. 1999;18:5234–5241. doi: 10.1093/emboj/18.19.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- 29.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000;151:263–276. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlumpberger M, Schaeffeler E, Straub M, Bredschneider M, Wolf DH, Thumm M. AUT1, a gene essential for autophagocytosis in the yeast Saccharomyces cerevisiae. J Bacteriol. 1997;179:1068–1076. doi: 10.1128/jb.179.4.1068-1076.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Huang WP, Klionsky DJ. Membrane recruitment of Aut7p in the autophagy and cytoplasm to vacuole targeting pathways requires Aut1p, Aut2p, and the autophagy conjugation complex. J Cell Biol. 2001;152:51–64. doi: 10.1083/jcb.152.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679–1688. doi: 10.1242/jcs.00381. [DOI] [PubMed] [Google Scholar]
- 35.Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, Gottlieb RA. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4:322–329. doi: 10.4161/auto.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2006;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 37.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 39.George MD, Baba M, Scott SV, Mizushima N, Garrison BS, Ohsumi Y, Klionsky DJ. Apg5p functions in the sequestration step in the cytoplasm-to-vacuole targeting and macroautophagy pathways. Mol Biol Cell. 2000;11:969–982. doi: 10.1091/mbc.11.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamacher-Brady A, Brady NR, Gottlieb RA, Gustafsson AB. Autophagy as a protective response to Bnip3-mediated apoptotic signaling in the heart. Autophagy. 2006;2(4):307–309. doi: 10.4161/auto.2947. [DOI] [PubMed] [Google Scholar]
- 41.Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the HL-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. FEBS Journal. 2007;274:3184–3197. doi: 10.1111/j.1742-4658.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- 42.Gurusamy N, Lekli I, Gorbunov N, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by adaptation to ischemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 44.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 45.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 46.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–231. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–10903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Azad MB, Chen Y, Henson ES, Cizeau J, McMillan-Ward E, Israels SJ, Gibson SB. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy. 2008;4:195–204. doi: 10.4161/auto.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.84. [DOI] [PubMed] [Google Scholar]
- 52.Karmazyn M, Moffat MP. Role of Na+/H+ exchange in cardiac physiology and pathophysiology: mediation of myocardial reperfusion injury by the pH paradox. Cardiovasc Res. 1993;27:915–924. doi: 10.1093/cvr/27.6.915. [DOI] [PubMed] [Google Scholar]
- 53.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 55.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 56.Khadour FH, Panas D, Ferdinandy P, Schulze C, Csont T, Lalu MM, Wildhirt SM, Schulz R. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1108–H1115. doi: 10.1152/ajpheart.00549.2001. [DOI] [PubMed] [Google Scholar]
- 57.Hickson-Bick DL, Jones C, Buja LM. Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death. J Mol Cell Cardiol. 2008;44:411–418. doi: 10.1016/j.yjmcc.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Wu LJ, Tashino S, Onodera S, Ikejima T. Reactive oxygen species and nitric oxide regulate mitochondria-dependent apoptosis and autophagy in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic Res. 2008;42:492–504. doi: 10.1080/10715760802112791. [DOI] [PubMed] [Google Scholar]
- 60.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. Embo J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Loyer X, Heymes C, Samuel JL. Constitutive nitric oxide synthases in the heart from hypertrophy to failure. Clin Exp Pharmacol Physiol. 2008;35:483–488. doi: 10.1111/j.1440-1681.2008.04901.x. [DOI] [PubMed] [Google Scholar]
- 63.Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. Embo J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rabkin SW, Klassen SS. Nitric oxide differentially regulates the gene expression of caspase genes but not some autophagic genes. Nitric Oxide. 2007;16:339–347. doi: 10.1016/j.niox.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- 66.Arrington DD, Van Vleet TR, Schnellmann RG. Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol. 2006;291:C1159–C1171. doi: 10.1152/ajpcell.00207.2006. [DOI] [PubMed] [Google Scholar]
- 67.Teckman JH, An JK, Blomenkamp K, Schmidt B, Perlmutter D. Mitochondrial autophagy and injury in the liver in alpha 1-antitrypsin deficiency. Am J Physiol Gastrointest Liver Physiol. 2004;286:G851–G862. doi: 10.1152/ajpgi.00175.2003. [DOI] [PubMed] [Google Scholar]
- 68.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 69.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 71.Qi X, Vallentin A, Churchill E, Mochly-Rosen D. deltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart. J Mol Cell Cardiol. 2007;43:420–428. doi: 10.1016/j.yjmcc.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Severino A, Campioni M, Straino S, Salloum FN, Schmidt N, Herbrand U, Frede S, Toietta G, Di Rocco G, Bussani R, Silvestri F, Piro M, Liuzzo G, Biasucci LM, Mellone P, Feroce F, Capogrossi M, Baldi F, Fandrey J, Ehrmann M, Crea F, Abbate A, Baldi A. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll Cardiol. 2007;50:1029–1037. doi: 10.1016/j.jacc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol. 2006;291:H1411–H1420. doi: 10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szegezdi E, Duffy A, O'Mahoney ME, Logue SE, Mylotte LA, O'Brien T, Samali A. ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;349:1406–1411. doi: 10.1016/j.bbrc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 75.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic reticulum stress triggers autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 78.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tannous P, Zhu H, Nemchenko A, Berry JM, Johnstone JL, Shelton JM, Miller FJ, Jr, Rothermel BA, Hill JA. Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation. 2008;117:3070–3078. doi: 10.1161/CIRCULATIONAHA.107.763870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. Febs J. 2007;274:3184–3197. doi: 10.1111/j.1742-4658.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- 81.Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aki T, Yamaguchi K, Fujimiya T, Mizukami Y. Phosphoinositide 3-kinase accelerates autophagic cell death during glucose deprivation in the rat cardiomyocyte-derived cell line H9c2. Oncogene. 2003;22:8529–8535. doi: 10.1038/sj.onc.1207197. [DOI] [PubMed] [Google Scholar]
- 83.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol. 2006;41:256–264. doi: 10.1016/j.yjmcc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 84.Araki M, Motojima K. Hydrophobic statins induce autophagy in cultured human rhabdomyosarcoma cells. Biochem Biophys Res Commun. 2008;367:462–467. doi: 10.1016/j.bbrc.2007.12.166. [DOI] [PubMed] [Google Scholar]
- 85.Lotfi A, Schweiger MJ, Giugliano GR, Murphy SA, Cannon CP. High-dose atorvastatin does not negatively influence clinical outcomes among clopidogrel treated acute coronary syndrome patients--a Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) analysis. Am Heart J. 2008;155:954–958. doi: 10.1016/j.ahj.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 86.Ascensao A, Ferreira R, Magalhaes J. Exercise-induced cardioprotection -- biochemical, morphological and functional evidence in whole tissue and isolated mitochondria. International Journal of Cardiology. 2007;117:16–30. doi: 10.1016/j.ijcard.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 87.Powers SK, Quindry JC, Kavazis AN. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radical Biology and Medicine. 2008;44:193–201. doi: 10.1016/j.freeradbiomed.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 88.Salminen A, Vihko V. Autophagic response to strenuous exercise in mouse skeletal muscle fibers. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:97–106. doi: 10.1007/BF02889856. [DOI] [PubMed] [Google Scholar]
- 89.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Experimental Gerontology. 2003;38:863–876. doi: 10.1016/s0531-5565(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 91.Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: Accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 92.Brady NR, Hamacher-Brady A, Gottlieb RA. Proapoptotic BCL-2 family members and mitochondrial dysfunction during ischemia/reperfusion injury, a study employing cardiac HL-1 cells and GFP biosensors. Biochim Biophys Acta. 2006;1757:667–678. doi: 10.1016/j.bbabio.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 94.Willis MS, Patterson C. Into the heart: the emerging role of the ubiquitin-proteasome system. J Mol Cell Cardiol. 2006;41:567–579. doi: 10.1016/j.yjmcc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 95.Powell SR, Wang P, Katzeff H, Shringarpure R, Teoh C, Khaliulin I, Das DK, Davies KJ, Schwalb H. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: essential role of the proteasome. Antioxid Redox Signal. 2005;7:538–546. doi: 10.1089/ars.2005.7.538. [DOI] [PubMed] [Google Scholar]
- 96.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 97.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 98.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 99.Fortun J, Verrier JD, Go JC, Madorsky I, Dunn WA, Notterpek L. The formation of peripheral myelin protein 22 aggregates is hindered by the enhancement of autophagy and expression of cytoplasmic chaperones. Neurobiol Dis. 2007;25:252–265. doi: 10.1016/j.nbd.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet. 2002;11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- 101.Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 102.Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, Schmitt I, Wullner U, Evert BO, O'Kane CJ, Rubinsztein DC. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- 103.Brocheriou V, Hagege AA, Oubenaissa A, Lambert M, Mallet VO, Duriez M, Wassef M, Kahn A, Menasche P, Gilgenkrantz H. Cardiac functional improvement by a human Bcl-2 transgene in a mouse model of ischemia/reperfusion injury. J Gene Med. 2000;2:326–333. doi: 10.1002/1521-2254(200009/10)2:5<326::AID-JGM133>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 104.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 105.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 106.Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 107.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 108.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. Embo J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rashmi R, Pillai SG, Vijayalingam S, Ryerse J, Chinnadurai G. BH3-only protein BIK induces caspase-independent cell death with autophagic features in Bcl-2 null cells. Oncogene. 2008;27:1366–1375. doi: 10.1038/sj.onc.1210783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–376. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 111.Kanzawa T, Zhang L, Xiao L, Germano IM, Kondo Y, Kondo S. Arsenic trioxide induces autophagic cell death in malignant glioma cells by upregulation of mitochondrial cell death protein BNIP3. Oncogene. 2005;24:980–991. doi: 10.1038/sj.onc.1208095. [DOI] [PubMed] [Google Scholar]
- 112.Tracy K, Dibling BC, Spike BT, Knabb JR, Schumacker P, Macleod KF. BNIP3 is an RB/E2F target gene required for hypoxia-induced autophagy. Mol Cell Biol. 2007;27:6229–6242. doi: 10.1128/MCB.02246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Y, Wang Y, Kim E, Beemiller P, Wang CY, Swanson J, You M, Guan KL. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. J Biol Chem. 2007;282:35803–35813. doi: 10.1074/jbc.M705231200. [DOI] [PubMed] [Google Scholar]
- 114.Wan G, Zhaorigetu S, Liu Z, Kaini R, Jiang Z, Hu CA. Apolipoprotein l1, a novel BH3-only lipid binding protein, induces autophagic cell death. J Biol Chem. 2008;283:21540–21549. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yussman MG, Toyokawa T, Odley A, Lynch RA, Wu G, Colbert MC, Aronow BJ, Lorenz JN, Dorn GW., 2nd Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 116.Sandoval H, Thiagarajan P, Dasgupta SK, Schumacher A, Prchal JT, Chen M, Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]