Abstract
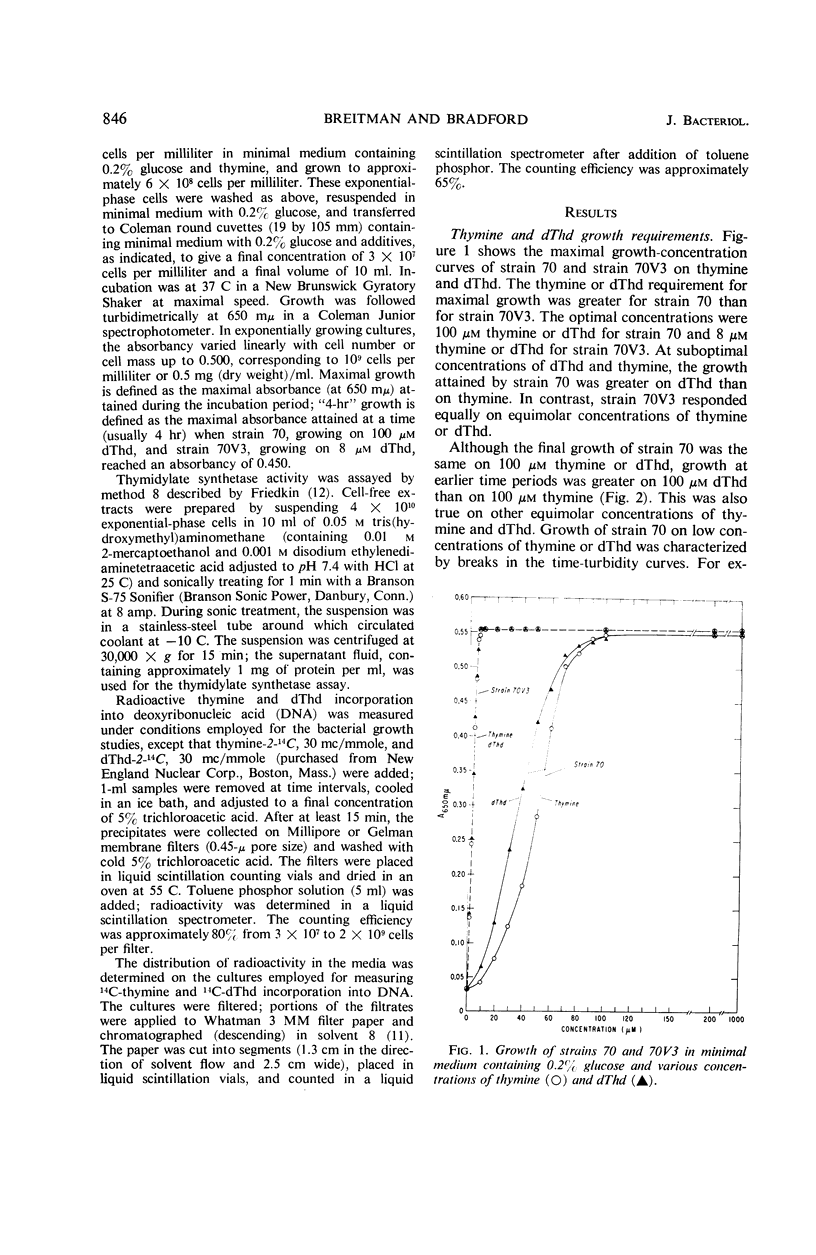
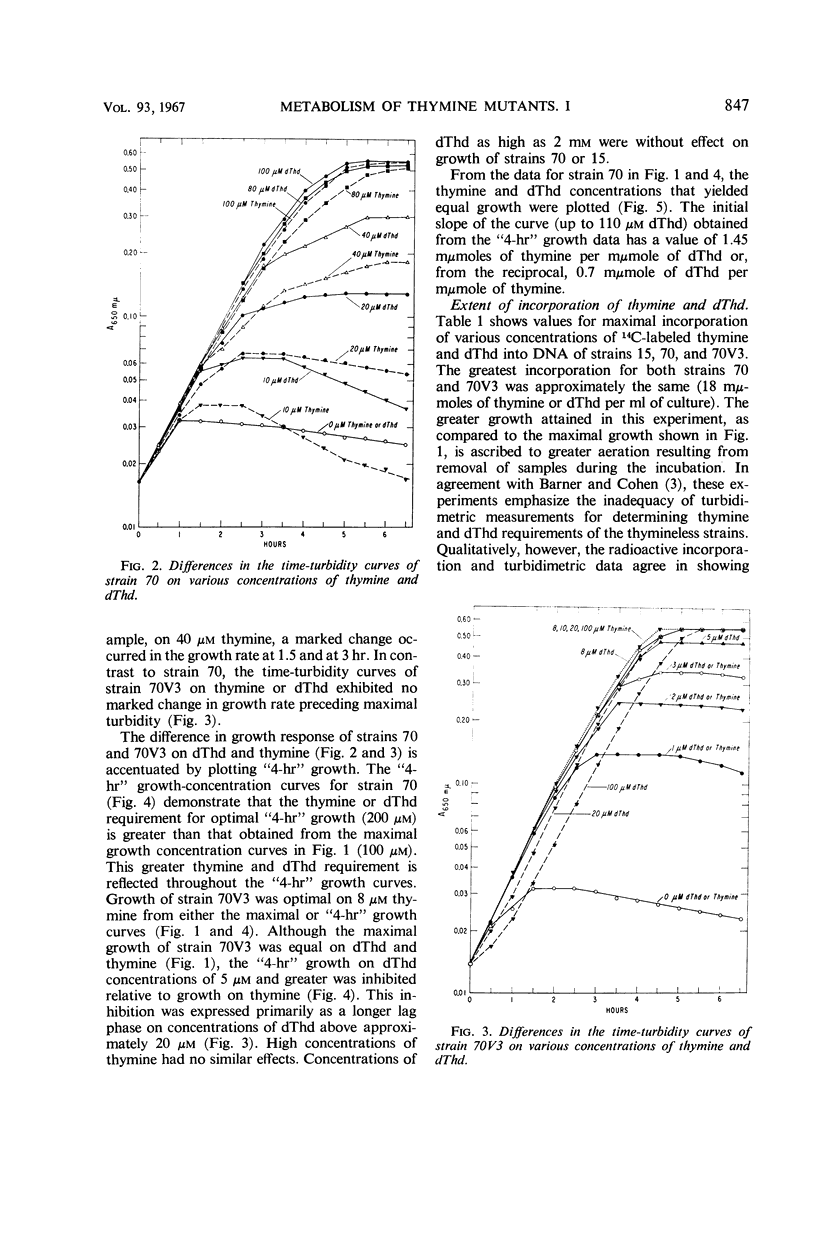
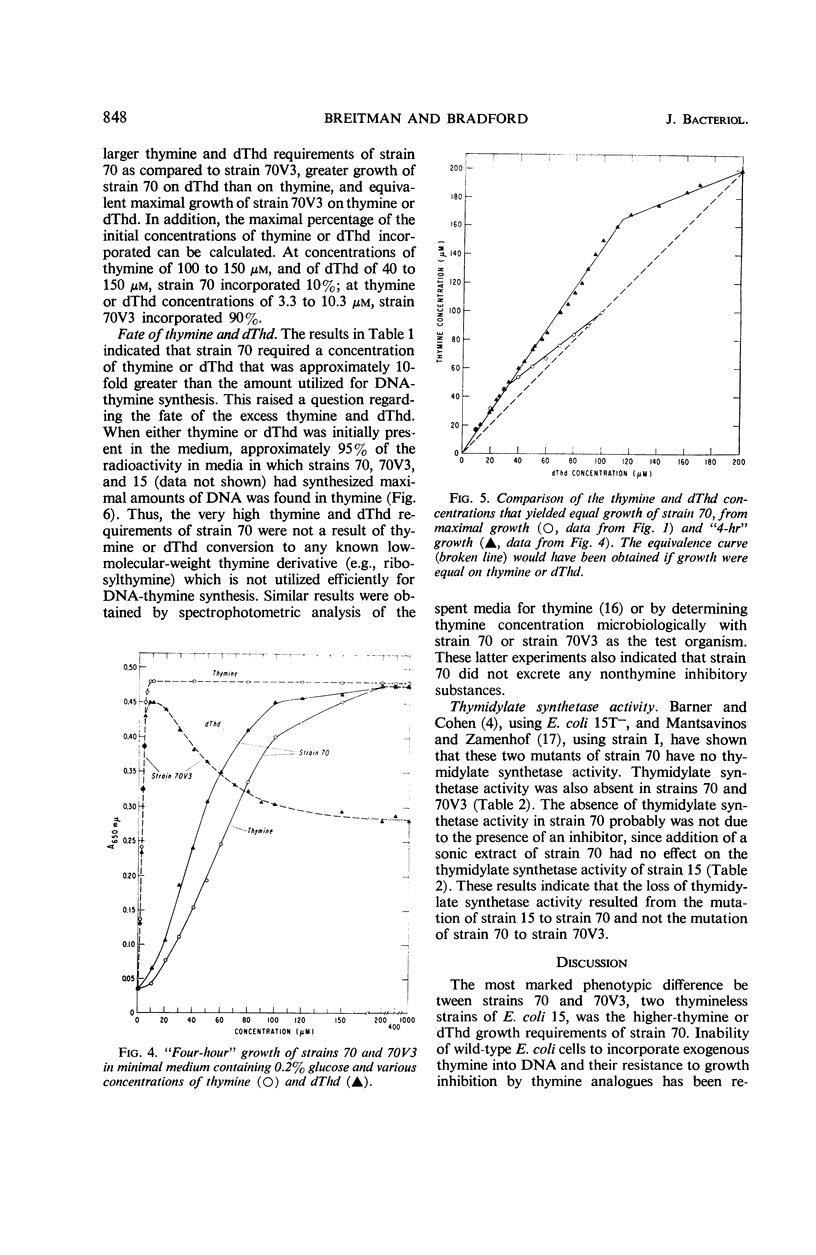
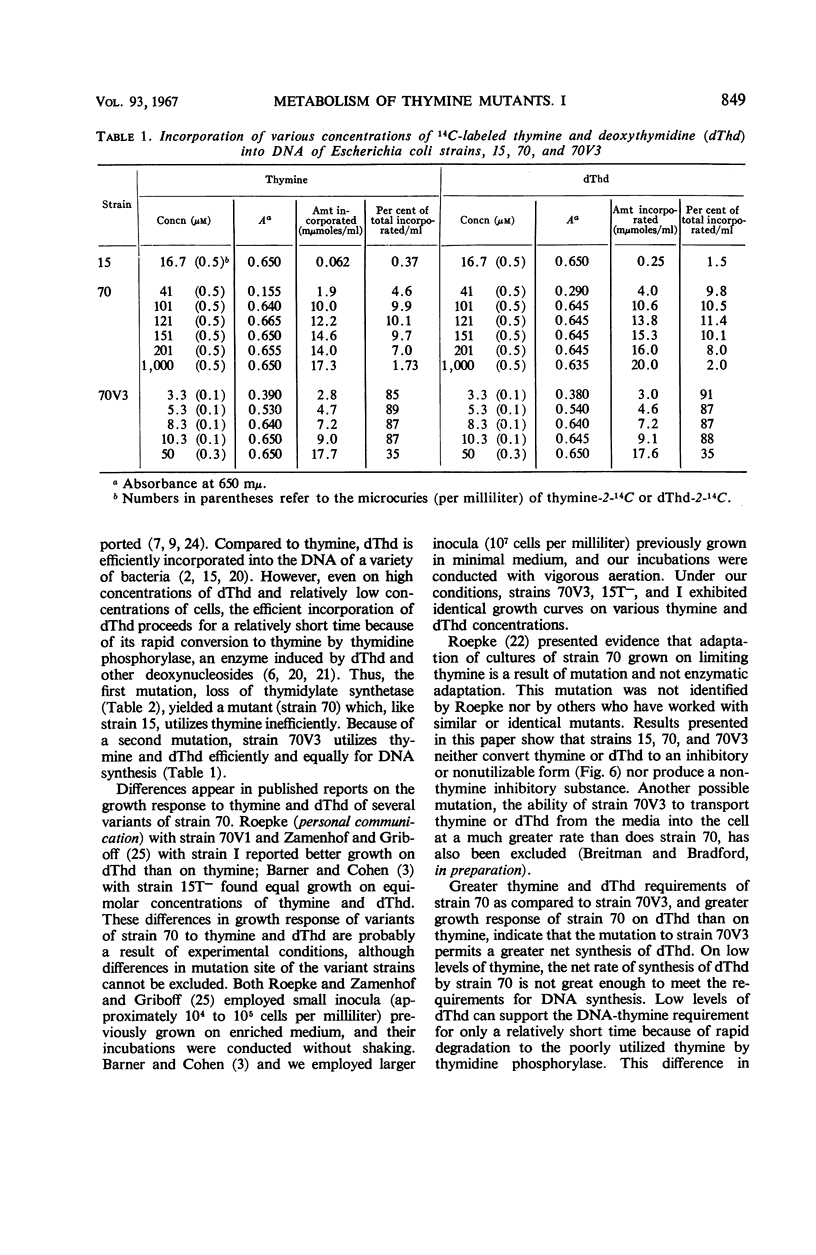
A study of optimal thymine and deoxythymidine (dThd) growth requirements of the thymineless mutants of Escherichia coli 15, E. coli 70–462 (strain 70), and a variant, E. coli 70V3–462 (strain 70V3), showed that for maximal turbidity (growth) strain 70 required 10-fold greater concentrations of thymine or dThd than did strain 70V3. On suboptimal concentrations of thymine or dThd, growth of strain 70 was greater on dThd than on thymine. In contrast, maximal growth of strain 70V3 was the same on equimolar concentrations of thymine and dThd. Growth rate of strain 70V3 was the same on equimolar concentrations of thymine and dThd up to 4 μm; at concentrations of 5 μm and greater, the “4-hr” growth was lower on dThd than on corresponding concentrations of thymine. Cultures of both thymineless mutants synthesized equal maximal amounts of DNA. Whereas strain 70V3 incorporated a maximum of 90% of the thymine or dThd in the media, strain 70 incorporated a maximum of only 10%. This poor utilization by strain 70 was neither a result of thymine or dThd conversion to a low-molecular-weight thymine derivative nor the production of a nonthymine inhibitory substance. Since strains 70 and 70V3 exhibited no thymidylate synthetase activity, the first mutation (strain 15 to strain 70) resulted in the loss of this activity. The second mutation (strain 70 to strain 70V3) probably brought about the loss of an enzyme(s) that catabolizes deoxyribose phosphate, permitting a greater net synthesis of dThd from thymine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPEN E. L., MANDEL H. G. A rapid assay method for tritium in bacterial cells. Biochim Biophys Acta. 1960 Sep 23;43:317–321. doi: 10.1016/0006-3002(60)90442-x. [DOI] [PubMed] [Google Scholar]
- Alikhanian S. I., Iljina T. S., Kaliaeva E. S., Kameneva S. V., Sukhodolec V. V. Mutants of Escherichia coli K12 lacking thymine. Nature. 1965 May 22;206(4986):848–849. doi: 10.1038/206848a0. [DOI] [PubMed] [Google Scholar]
- BARNER H. D., COHEN S. S. The induction of thymine synthesis by T2 infection of a thymine requiring mutant of Escherichia coli. J Bacteriol. 1954 Jul;68(1):80–88. doi: 10.1128/jb.68.1.80-88.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNER H. D., COHEN S. S. Virus-induced acquisition of metabolic function. IV. Thymidylate synthetase in thymine-requiring Escherichia coli infected by T2 and T5 bacteriophages. J Biol Chem. 1959 Nov;234:2987–2991. [PubMed] [Google Scholar]
- COHEN S. S., BARNER H. D. Studies on the induction of thymine deficiency and on the effects of thymine and thymidine analogues in Escherichia coli. J Bacteriol. 1956 May;71(5):588–597. doi: 10.1128/jb.71.5.588-597.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V. Thymine metabolism in strains of Escherichia coli. Biochim Biophys Acta. 1958 Nov;30(2):428–429. doi: 10.1016/0006-3002(58)90071-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN D. B., SMITH J. D., ZAMENHOF S., GRIBOFF G. Incorporation of halogenated pyrimidines into the deoxyribonucleic acids of Bacterium coli and its bacteriophages. Nature. 1954 Aug 14;174(4424):305–307. [PubMed] [Google Scholar]
- FINK K., CLINE R. E., HENDERSON R. B., FINK R. M. Metabolism of thymine (methyl-C14 or -2-C14) by rat liver in vitro. J Biol Chem. 1956 Jul;221(1):425–433. [PubMed] [Google Scholar]
- FRIEDKIN M., ROBERTS D. The enzymatic synthesis of nucleosides. I. Thymidine phosphorylase in mammalian tissue. J Biol Chem. 1954 Mar;207(1):245–256. [PubMed] [Google Scholar]
- HASH J. H. Determination of tritium in whole cells and cellular fractions of Bacillus megaterium using liquid scintillation techniques. Anal Biochem. 1962 Sep;4:257–267. doi: 10.1016/0003-2697(62)90009-x. [DOI] [PubMed] [Google Scholar]
- Harrison A. P., Jr Thymine incorporation and metabolism by various classes of thymine-less bacteria. J Gen Microbiol. 1965 Dec;41(3):321–333. doi: 10.1099/00221287-41-3-321. [DOI] [PubMed] [Google Scholar]
- MANTSAVINOS R., ZAMENHOF S. Pathways for the biosynthesis of thymidylic acid in bacterial mutants. J Biol Chem. 1961 Mar;236:876–882. [PubMed] [Google Scholar]
- OKADA T., YANAGISAWA K., RYAN F. J. A method for securing thymineless mutants from strains of E. coli. Z Vererbungsl. 1961;92:403–412. doi: 10.1007/BF00890061. [DOI] [PubMed] [Google Scholar]
- RACHMELER M., GERHART J., ROSNER J. Limited thymidine uptake in Escherichia coli due to an inducible thymidine phosphorylase. Biochim Biophys Acta. 1961 Apr 29;49:222–225. doi: 10.1016/0006-3002(61)90888-5. [DOI] [PubMed] [Google Scholar]
- RAZZELL W. E., CASSHYAP P. SUBSTRATE SPECIFICITY AND INDUCTION OF THYMIDINE PHOSPHORYLASE IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1789–1793. [PubMed] [Google Scholar]
- Roepke R. R., Mercer F. E. Lethal and Sublethal Effects of X-Rays on Escherichia coli as Related to the Yield of Biochemical Mutants. J Bacteriol. 1947 Dec;54(6):731–743. doi: 10.1128/jb.54.6.731-743.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke R. R. Relation between different thymineless mutants derived from Escherichia coli. J Bacteriol. 1967 Mar;93(3):1188–1189. doi: 10.1128/jb.93.3.1188-1189.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMENHOF S., DE GIOVANNI R., RICH K. Escherichia coli containing unnatural pyrimidines in its deoxyribonucleic acid. J Bacteriol. 1956 Jan;71(1):60–69. doi: 10.1128/jb.71.1.60-69.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMENHOF S., REINER B., DE GIOVANNI R., RICH K. Introduction of unnatural pyrimidines into deoxyribonucleic acid of Escherichia coli. J Biol Chem. 1956 Mar;219(1):165–173. [PubMed] [Google Scholar]


