Abstract
Background and purpose:
Intermedin (IMD) is a newly identified member of the calcitonin family of peptides that shares structural and functional homology with adrenomedullin (AM). In vivo cardiovascular effects of AM have been described, but relatively little is known of the in vivo actions of IMD. The purpose of this study was to compare the regional haemodynamic effects of IMD with those of AM in conscious rats, and investigate possible underlying mechanisms.
Experimental approach:
Measurements of blood pressure, heart rate and renal, mesenteric and hindquarters haemodynamics were made in conscious, chronically-instrumented rats.
Key results:
IMD caused tachycardia and vasodilatation in all three vascular beds, associated with modest hypotension. At an equimolar dose (1 nmol·kg−1), most of the cardiovascular effects of IMD were greater than those of AM. The AM receptor antagonist, AM22–52, was equally effective in attenuating the renal and mesenteric vasodilator effects of IMD (1 nmol·kg−1) and AM (3 nmol·kg−1), but inhibition of NO synthase was more effective at reducing the vasodilator effects of IMD than AM. Vascular KATP channel blockade with U-37883A did not inhibit the vasodilator effects of either peptide.
Conclusions and implications:
In vivo, the regional haemodynamic profile of IMD resembles that of AM, and some of the vasodilator effects of IMD are mediated by AM receptors and NO, but not by KATP channels. The cardiovascular effects of AM have been implicated in various pathological conditions, but whether or not endogenous IMD fulfils a similar role remains to be determined.
Keywords: intermedin, adrenomedullin, KATP channels, nitric oxide, haemodynamics
Introduction
A new member of the calcitonin peptide superfamily [which includes calcitonin, calcitonin gene-related peptide (CGRP), adrenomedullin (AM) and amylin], was described by Roh et al. (2004) and named intermedin (IMD), since high levels of expression were seen in the intermediate lobe of the pituitary. An independent group had previously isolated cDNAs encoding five AM-like peptides in pufferfish (Ogoshi et al., 2003) and in 2004, described cDNA encoding a new member of the AM family in mammals that they named AM2 (Takei et al., 2004). It is now generally understood that IMD and AM2 are the same peptide, herein referred to as IMD.
The receptor system involved in mediating the actions of CGRP and AM comprises a calcitonin receptor (CL) together with one of three receptor activity modifying proteins (RAMP1, 2 or 3) (McLatchie et al., 1998; receptor nomenclature follows Alexander et al., 2008). The CL/RAMP1 complex forms a CGRP receptor which is blocked by the CGRP fragment, CGRP8–37, whereas CL/RAMP2 and 3 complexes form AM1 and AM2 receptors, respectively, the former being blocked more effectively than the latter by the AM fragment, AM22–52 (Hay et al., 2003). Although the sequence homology between IMD and AM or CGRP is not strong (∼30% and 20%, respectively), there is evidence to suggest that many of the actions of IMD are also mediated by CL/RAMP complexes. Thus, IMD can act non-selectively as an agonist at all three CL/RAMP complexes (Roh et al., 2004), but with a pharmacological profile different from that of either CGRP or AM (Bell and McDermott, 2008). Recently, there has been some evidence published which suggests that IMD might also act at a distinct class of receptor (Owji et al., 2008).
It is clear that CGRP and AM can have important cardiovascular actions in vitro and in vivo (reviewed by Brain and Grant, 2004), and there is now accumulating evidence showing some similar cardiovascular actions of IMD (reviewed by Bell and McDermott, 2008). Thus, in vitro, vasorelaxant effects of IMD have been reported (Kobayashi et al., 2004; Pan et al., 2005; Chauhan et al., 2007; Kandilci et al., 2008), and, in vivo, hypotensive effects have been shown in anaesthetized (Fujisawa et al., 2004; Takei et al., 2004; Pan et al., 2005; Ren et al., 2006) and conscious (Roh et al., 2004; Abdelrahman and Pang, 2006; Charles et al., 2006; Fujisawa et al., 2006; 2007;) animals. It is generally assumed that IMD-induced hypotension is secondary to vasodilatation, but, although total peripheral resistance has been shown to fall in sheep following IMD administration (Charles et al., 2006), to our knowledge only one study has reported regional haemodynamic effects of IMD (Fujisawa et al., 2007), and those measurements were restricted to one point in time during IMD infusion. Thus, the first aim of the present study was to describe the regional haemodynamic profile for IMD in conscious rats and compare it with that of AM. Furthermore, to determine the extent to which AM receptors were involved in the vasodilator actions of the peptides, the effects of the AM receptor antagonist, AM22–52, on responses to AM and IMD were assessed.
There is some evidence that AM can cause vasodilatation in some vascular beds via the production of NO and/or by activation of KATP channels (Gardiner et al., 1995; Lang et al., 1997; Sabates et al., 1997; Goto et al., 2000; Terata et al., 2000). In vitro studies have shown a role for NO in the vasorelaxant effects of IMD (Kandilci et al., 2006; Chauhan et al., 2007), possibly due to up-regulation of NO synthesis (Yang et al., 2006), but, in vivo, the depressor response to IMD is reportedly unaffected by NO synthase inhibition (Taylor et al., 2005; Abdelrahman and Pang, 2007), although the accompanying regional vascular effects have not yet been evaluated. Therefore, our second aim was to compare the effects of NO synthase inhibition [with NG nitro L arginine methyl ester (L-NAME)] on the regional haemodynamic effects of AM and IMD. In addition, although KATP channels are reportedly not involved in the vasorelaxant effects of IMD in vitro (Kandilci et al., 2006; Chauhan et al., 2007; Kandilci et al., 2008), the effects of vascular KATP channel inhibition on cardiovascular responses to IMD in vivo have not been studied to date. Hence, our third aim was to compare the effects of vascular KATP channel inhibition with U-37883A (Meisheri et al., 1993) on the regional haemodynamic effects of AM and IMD. Finally, in the light of some unexpected effects of U-37883A on responses to AM but not to IMD, which could possibly be explained by an influence of the renin-angiotensin system, we compared the effects of angiotensin receptor antagonism with losartan on responses to AM and IMD.
Methods
Animals and surgery
All animal care and procedures were approved by the University of Nottingham Ethical Review Committee and were performed under Home Office Project and Personal Licence authority. Male Sprague-Dawley rats (350–400 g) were purchased from Charles River (Margate, Kent, UK) and housed in the Biomedical Services Unit, University of Nottingham for at least 10 days after delivery, with free access to standard rat chow (Teklad Global 18% protein rodent diet, Bicester, Oxon, UK) and water. Room temperatures were maintained at 21 ± 2°C, and lights were on from 06.00 h to 18.00 h.
All surgery was performed under general anaesthesia (fentanyl and medetomidine, 300 µg·kg−1 of each i.p.). Anaesthetic reversal and the provision of analgesia was achieved using atipamezole and buprenorphine (1 mg·kg−1 and 0.03 mg·kg−1, respectively, s.c.). At the first surgical stage, miniaturized pulsed Doppler flow probes were sutured around the left renal and superior mesenteric arteries, and around the distal abdominal aorta (to monitor hindquarters flow). The wires from the probes were secured at the nape of the neck and animals were returned to their home cages with free access to food and water. At least 10 days later, and following satisfactory inspection by the Named Veterinary Surgeon, the animals were anaesthetized (as above) and catheters were implanted in the distal abdominal aorta (via the ventral caudal artery) for monitoring arterial blood pressure and heart rate, and in the right jugular vein for the administration of substances. At this stage, the animals were fitted with custom-designed harnesses with a counter-balanced spring attached, to protect the catheters and allow the animals freedom of movement in their home cage with access to food and water ad libitum. The arterial catheters were connected to fluid-filled swivels for overnight infusion of heparinized saline (15 U·mL–1, 0.4 mL·h−1) to maintain catheter patency.
Data acquisition and analysis
Experiments began 24 h after catheterization. Cardiovascular variables [heart rate, arterial blood pressure, renal, mesenteric and hindquarters Doppler shifts (flow) ] were recorded continuously throughout each experimental day, using a customized, computer-based system [Haemodynamics Data Acquisition System (HDAS), University of Limburg, Maastricht, The Netherlands] connected to the transducer amplifier (Gould model 13-4615-50) and the Doppler flowmeter [Crystal Biotech VF-1 mainframe (pulse repetition frequency 125 kHz) fitted with high velocity (HVPD-20) modules]. Data were sampled by HDAS every 2 ms, averaged each cardiac cycle and stored to disc every 5 s, and analysed off-line (Datview, University of Maastricht, The Netherlands).
Data are expressed as mean ± SEM. As not all data were normally distributed, within-group analyses were carried out by a non-parametric equivalent of anova allowing for multiple comparisons (Friedman's test) (Theodorsson-Norheim, 1987), and between-group analyses were performed on the baseline values and the integrated responses to the peptides (areas under or over curves) using Wilcoxon's test or Mann–Whitney U-test as appropriate. P≤ 0.05 was taken as significant.
Experimental protocols
Experiment 1: Comparison of the regional haemodynamic effects of IMD and AM
After a period of baseline recording, rats were given an equimolar dose (1 nmol·kg−1 i.v. in 0.1 mL sterile saline) of either IMD (n= 8) or AM (n= 9) (Experiment 1a). As that dose of AM was found to have relatively modest and poorly reproducible effects (see Results), an additional experiment (Experiment 1b) was run in which the effects of IMD (1 nmol·kg−1; n= 11) were compared with those of a higher dose of AM (3 nmol·kg−1, n= 10). All further experiments used IMD at a dose of 1 nmol·kg−1 and AM at a dose of 3 nmol·kg−1.
Experiment 2: Effects of repeated doses of IMD and AM
As some of the protocols required repeat dosing with AM and IMD (see below), it was necessary to assess the reproducibility of responses under those conditions. A group of rats (n= 8) was randomized to receive AM (3 nmol·kg−1) on day 1 and 3 and IMD (1 nmol·kg−1) on day 2 and 4, or IMD on day 1 and 3, and AM on day 2 and 4, with animals only receiving one treatment each day.
Experiment 3: Involvement of AM22–52-sensitive receptors
Rats were given a 6 min i.v. infusion of saline (0.4 mL·h−1) or AM22–52 (500 nmol·kg−1·h−1) followed by IMD (1 nmol·kg−1) or AM (3 nmol·kg−1) between 5 and 10 min later. The dose of AM22–52 has previously been shown to attenuate the regional vasodilatations to AM (1 nmol·kg−1) in vivo (Gardiner et al., 1999).
Experiment 4: Involvement of NO
Because NO synthase inhibition with L-NAME causes vasoconstriction and hypertension, the control condition in this experiment involved co-infusion of angiotensin II (AII) (200 ng·kg−1·h−1) and arginine vasopressin (AVP) (20 ng·kg−1·h−1), which we have shown previously to match the regional haemodynamic effects of L-NAME (3 mg·kg−1·h−1) infusion (Gardiner et al., 2005). Animals (n= 8) were given AM (3 nmol·kg−1) or IMD (1 nmol·kg−1) on day 1 and the other peptide on day 2, 90 min after the start of the AII + AVP infusion. This protocol was repeated on days 3 and 4 with L-NAME infused instead of AII + AVP. L-NAME was always given on days 3 or 4 because of its extremely long duration of action.
Experiment 5: Involvement of KATP channels
Rats (n= 9) were given AM (3 nmol·kg−1) or IMD (1 nmol·kg−1) 30 min after administration of U-37883A (5 mg·kg−1; Meisheri et al., 1993; Champion et al., 2002) or vehicle (1 mL sterile saline infused over 30 min). The experiments ran over 4 days with each animal receiving only one treatment on each experimental day in random order. Effectiveness of U-37883A at blocking KATP channels was assessed using the KATP channel opener, levcromakalim (10 µg·kg−1·min−1 for 3 min). Whilst U-37883A was unable to completely antagonize the effects of levcromakalim, it attenuated responses to levcromakalim given 30 min after the U-37883A infusion. Thus, U-37883A reduced the levcromakalim-mediated fall in blood pressure by 75% and attenuated the renal, mesenteric and hindquarters vasodilatations by 52, 33 and 88% respectively.
Experiment 6: Effects of angiotensin (AT1) receptor antagonism
As some of the results obtained in earlier experiments could be explained by differential activation of the renin-angiotensin system (see Experiment 5 Results), the effects of the angiotensin II (AT1) receptor antagonist, losartan, on the regional haemodynamic responses to AM and IMD were measured.
A group of animals (n= 9) was given either AM (3 nmol·kg−1) or IMD (1 nmol·kg−1) on day 1 and the other peptide on day 2, 60 min after administration of losartan vehicle (0.1 mL sterile saline). On days 3 and 4, peptide administration was repeated 60 min after administration of losartan (10 mg·kg−1). This dose of losartan causes complete inhibition of the cardiovascular effects of AII (50 ng) for at least 9 h (Batin et al., 1991). Losartan was always given on the final experimental days because of its long duration of action.
Materials
Adrenomedullin (rat), intermedin (1–53), angiotensin II and arginine vasopressin were from Bachem (St Helens, UK). Adrenomedullin 22–52 was either from Peptide Institute Inc. (Scientific Marketing Associates, Barnet, UK) or Bachem (St Helens, UK). L-NAME was from Sigma (Poole, Dorset, UK), losartan potassium was from Sequoia Research Products (Oxford, UK), U-37883A was from Biomol (Plymouth Meeting, PA, USA). All drugs were dissolved in sterile saline; stock solutions of peptides were made up in sterile water for injection, and diluted in sterile saline. Injection volumes were 0.1 mL and infusion rates were 0.4 mL·h−1, except in the case of U37883A that was infused at a rate of 2 mL·h−1 for 30 min.
Fentanyl citrate was from Janssen-Cilag (High-Wycombe, UK); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were from Pfizer (Sandwich, Kent, UK); buprenorphine (Vetergesic) was from Alstoe Animal Health (York, UK)
Results
Experiment 1: Comparison of the regional haemodynamic effects of IMD and AM
Resting cardiovascular variables and integrated (0–30 min) responses to IMD and AM are shown in Table 1. In the first experiment (Experiment 1a), administration of IMD (1 nmol·kg−1) caused a fall in blood pressure associated with tachycardia and hyperaemic vasodilatation in all three vascular beds, whereas the equimolar dose of AM had no significant effect on blood pressure and caused smaller increases in renal and mesenteric vascular conductances and a more short-lived tachycardia [P < 0.05 for integrated (0–30 min) changes] (Figure 1A; Table 1). In the second experiment (Experiment 1b), the higher dose of AM (3 nmol·kg−1) caused significantly [P < 0.05 for integrated (0–30 min) changes] greater hypotension, tachycardia and renal vasodilatation compared to IMD at a dose of 1 nmol·kg−1, but the integrated mesenteric and hindquarters vasodilator effects were not different (Figure 1B; Table 1). In the light of these results and because the effects of AM (1 nmol·kg−1) were not highly reproducible (data not shown), all subsequent experiments used 1 nmol·kg−1 IMD and 3 nmol·kg−1 AM.
Table 1.
Resting cardiovascular variables and integrated responses to IMD and AM (Experiment 1)
|
Experiment 1a |
Experiment 1b |
|||
|---|---|---|---|---|
| IMD1 nmol·kg−1 | AM1 nmol·kg−1 | IMD1 nmol·kg−1 | AM3 nmol·kg−1 | |
| Resting cardiovascular variables | ||||
| Heart rate (beats·min−1) | 340 ± 8 | 320 ± 7a | 339 ± 9 | 344 ± 9 |
| Blood pressure (mmHg) | 112 ± 4 | 107 ± 3 | 108 ± 2 | 108 ± 3 |
| Renal VC (units) | 90 ± 10 | 84 ± 5 | 76 ± 9 | 98 ± 9 |
| Mesenteric VC (units) | 71 ± 5 | 77 ± 9 | 66 ± 4 | 76 ± 9 |
| Hindquarters VC (units) | 37 ± 4 | 37 ± 3 | 49 ± 4 | 48 ± 6 |
| Integrated (0–30 min) responses | ||||
| Heart rate (beats) | +1030 ± 132 | +706 ± 91a | +1136 ± 159 | +2697 ± 168a |
| Blood pressure (mmHg min) | −195 ± 52 | −45 ± 13a | −180 ± 44 | −357 ± 48a |
| Renal DS (% min) | +638 ± 142 | +460 ± 58 | +514 ± 81 | +932 ± 126a |
| Renal VC (% min) | +828 ± 140 | +467 ± 69a | +706 ± 114 | +1443 ± 190a |
| Mesenteric DS (% min) | +705 ± 97 | +278 ± 75a | +828 ± 90 | +682 ± 121 |
| Mesenteric VC (% min) | +943 ± 101 | +307 ± 86a | +1049 ± 124 | +1142 ± 134 |
| Hindquarters DS (% min) | +361 ± 87 | +417 ± 64 | +352 ± 78 | +532 ± 88 |
| Hindquarters VC (% min) | +567 ± 91 | +450 ± 63 | +528 ± 121 | +954 ± 135 |
In the first experiment (a) IMD (1 nmol·kg−1, n= 8) or AM (1 nmol·kg−1, n= 9) were given. In the second experiment (b) IMD (1 nmol·kg−1, n= 11) or AM (3 nmol·kg−1, n= 10) were given. Values are mean ± SEM, n= 8–11 per group. Units for VC are (kHz·mmHg−1) 103.
P < 0.05 versus corresponding IMD group (Mann–Whitney U-test).
AM, adrenomedullin; DS, Doppler shift; IMD, intermedin; VC, vascular conductance.
Figure 1.
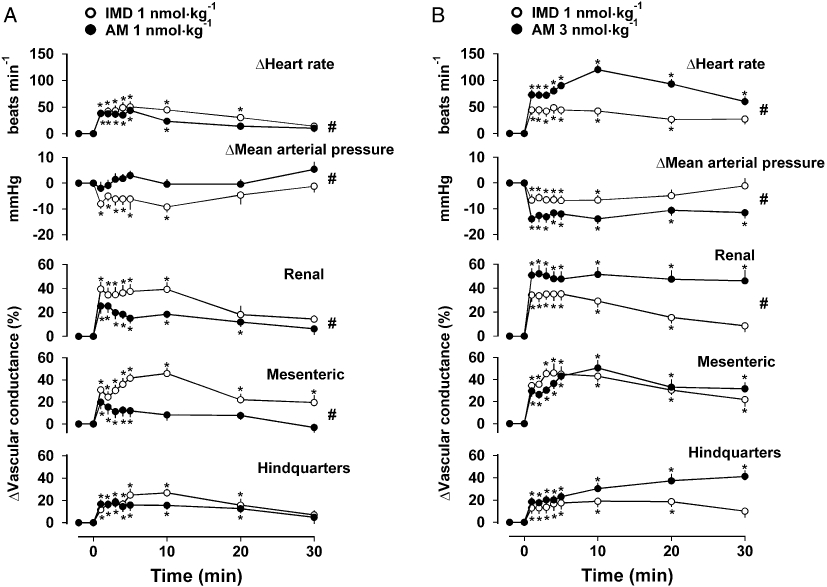
Cardiovascular responses to IMD [1 nmol·kg−1, n= 8 (A) or 11 (B)] and AM [1 nmol·kg−1, n= 9 (A) or 3 nmol·kg−1, n= 10 (B)] in conscious rats. Values are mean and vertical bars represent SEM. *P < 0.05 versus baseline (Friedman's test), #P < 0.05 for integrated (0–30 min) changes (Mann–Whitney U-test). AM, adrenomedullin; IMD, intermedin.
Experiment 2: Effects of repeated doses of IMD and AM
Resting cardiovascular variables and the integrated responses to IMD or AM on the two occasions were not different (Table 2).
Table 2.
Resting cardiovascular variables and integrated responses to repeated doses of IMD and AM (Experiment 2)
| IMD (1)1 nmol·kg−1 | IMD (2)1 nmol·kg−1 | AM (1)3 nmol·kg−1 | AM(2)3 nmol·kg−1 | |
|---|---|---|---|---|
| Resting cardiovascular variables | ||||
| Heart rate (beats·min−1) | 349 ± 13 | 354 ± 15 | 374 ± 18 | 377 ± 21 |
| Blood pressure (mmHg) | 111 ± 3 | 108 ± 4 | 110 ± 4 | 117 ± 4 |
| Renal VC (units) | 99 ± 9 | 89 ± 8 | 93 ± 7 | 93 ± 7 |
| Mesenteric VC (units) | 61 ± 5 | 64 ± 6 | 71 ± 8 | 65 ± 7 |
| Hindquarters VC (units) | 49 ± 5 | 48 ± 4 | 49 ± 4 | 46 ± 3 |
| Integrated (0–30 min) responses | ||||
| Heart rate (beats) | +1425 ± 284 | +951 ± 252 | +2315 ± 242 | +2368 ± 431 |
| Blood pressure (mmHg min) | −184 ± 52 | −218 ± 58 | −453 ± 92 | −436 ± 82 |
| Renal VC (% min) | +643 ± 73 | +868 ± 130 | +1328 ± 290 | +1257 ± 221 |
| Mesenteric VC (% min) | +854 ± 106 | +854 ± 117 | +1185 ± 240 | +939 ± 175 |
| Hindquarters VC (% min) | +454 ± 123 | +415 ± 92 | +947 ± 164 | +908 ± 191 |
Values are mean ± SEM, n= 8. Units for VC are (kHz·mmHg−1) 103.
AM, adrenomedullin; IMD, intermedin; VC, vascular conductance.
Experiment 3: Involvement of AM22–52-sensitive receptors
Administration of AM22–52 caused a small increase in mesenteric vascular conductance as reported previously (Gardiner et al., 1999), but the effect was not significant, hence resting cardiovascular variables immediately before administration of AM or IMD following treatment with saline or AM22–52 were not different, with the exception of renal vascular conductance that was higher prior to IMD in the animals treated with AM22–52 (Table 3); this was not an effect of AM22–52 inasmuch as the renal vascular conductance was higher in this group prior to AM22–52 administration.
Table 3.
Resting cardiovascular variables prior to administration of IMD or AM in animals pre-treated with saline or AM22–52 (Experiment 3)
|
Saline |
AM22–52 |
Saline |
AM22–52 |
|
|---|---|---|---|---|
| IMD (1 nmol·kg−1) | AM (3 nmol·kg−1) | |||
| Heart rate (beats·min−1) | 347 ± 10 | 338 ± 9 | 382 ± 14 | 391 ± 8 |
| Blood pressure (mmHg) | 113 ± 7 | 114 ± 3 | 111 ± 4 | 107 ± 5 |
| Renal VC (units) | 62 ± 8 | 92 ± 9a | 94 ± 8 | 107 ± 5 |
| Mesenteric VC (units) | 75 ± 11 | 82 ± 9 | 69 ± 11 | 95 ± 14 |
| Hindquarters VC (units) | 43 ± 8 | 36 ± 3 | 47 ± 4 | 46 ± 4 |
Values are mean ± SEM, n= 6–8 per group. Units for VC are (kHz·mmHg−1) 103.
P < 0.05 versus corresponding saline group (Mann–Whitney U-test).
AM, adrenomedullin; IMD, intermedin; VC, vascular conductance.
In saline-treated animals IMD (1 nmol·kg−1) caused tachycardia, hypotension and increases in renal, mesenteric and hindquarters vascular conductances, as described above. Following treatment with AM22–52, IMD-mediated tachycardia, hypotension and hindquarters vasodilatation were unaffected [P > 0.05 for integrated (0–30 min) responses] but the renal and mesenteric vasodilatations were significantly attenuated [P < 0.05 for integrated (0–30 min) responses] (Figure 2A). In saline-treated animals, AM (3 nmol·kg−1) caused a tachycardia, hypotension and increases in renal, mesenteric and hindquarters vascular conductances as described above. Following treatment with AM22–52, AM-mediated tachycardia, hypotension and hindquarters vasodilatation were not different from control, but there was a significant attenuation of the increase in renal vascular conductance [P < 0.05 for integrated (0–30 min) change], and the marked AM-mediated increase in mesenteric vascular conductance seen in control conditions was abolished (Figure 2B).
Figure 2.
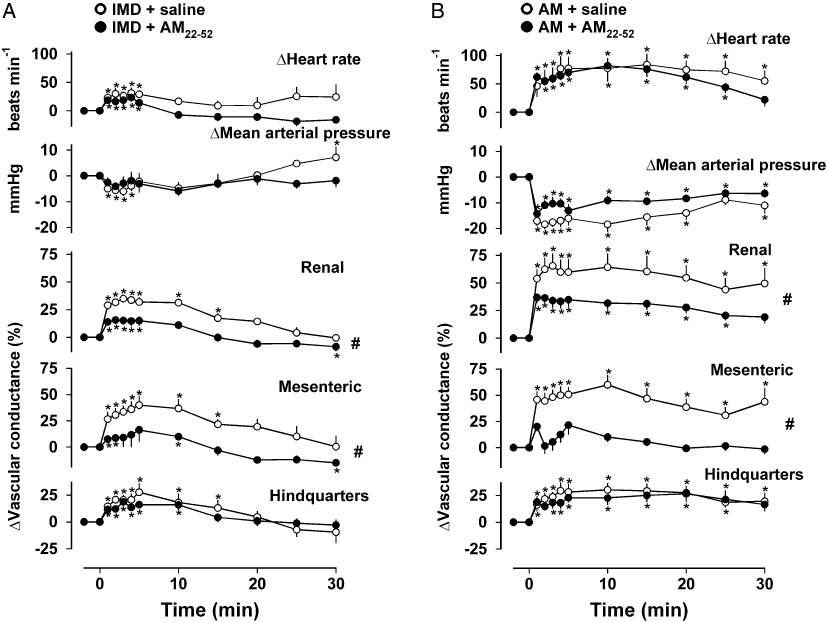
Cardiovascular responses to (A) IMD (1 nmol·kg−1) or (B) AM (3 nmol·kg−1) following administration of saline (0.4 mL·h−1) or AM22–52 (500 nmol·kg−1·h−1). Values are mean and vertical bars represent SEM, n= 6–8 per group. *P < 0.05 versus baseline (Friedman's test), #P < 0.05 for integrated (0–30 min) changes (Mann–Whitney U-test). AM, adrenomedullin; IMD, intermedin.
Experiment 4: Involvement of nitric oxide
Prior to administration of AM or IMD, resting cardiovascular variables were matched in the control (AII + AVP infused) condition and in the presence of L-NAME (Table 4).
Table 4.
Resting cardiovascular variables prior to administration of IMD or AM in animals pre-treated with co-infusion of AII+AVP or L-NAME (Experiment 4)
|
AII+AVP |
L-NAME |
AII+AVP |
L-NAME |
|
|---|---|---|---|---|
| IMD (1 nmol·kg−1) | AM (3 nmol·kg−1) | |||
| Heart rate (beats·min−1) | 296 ± 12 | 287 ± 9 | 307 ± 13 | 282 ± 9 |
| Blood pressure (mmHg) | 133 ± 5 | 138 ± 6 | 135 ± 5 | 146 ± 8 |
| Renal VC (units) | 57 ± 9 | 57 ± 9 | 56 ± 7 | 53 ± 12 |
| Mesenteric VC (units) | 36 ± 5 | 31 ± 5 | 31 ± 3 | 28 ± 5 |
| Hindquarters VC (units) | 35 ± 5 | 34 ± 2 | 30 ± 3 | 30 ± 2 |
Values are mean ± SEM, n= 9. Units for VC are (kHz·mmHg−1) 103.
AII, angiotensin II; AM, adrenomedullin; IMD, intermedin; VC, vascular conductance.
In animals infused with AII + AVP, IMD caused tachycardia, hypotension and marked renal, mesenteric and hindquarters vasodilatations (Figure 3A). In the presence of L-NAME, the integrated (0–30 min) IMD-induced tachycardia and hypotension were not different from the changes seen in the presence of AII + AVP, whereas the renal vasodilator response was significantly reduced, and there was some attenuation of the IMD-mediated mesenteric vasodilatation, although this failed to reach statistical significance [P > 0.05 for integrated (0–30 min) change]. In addition, IMD-mediated hindquarters vasodilatation was abolished (Friedman's test) although the integrated (0–30 min) changes were not different between the L-NAME and AII + AVP infused conditions (Figure 3A).
Figure 3.
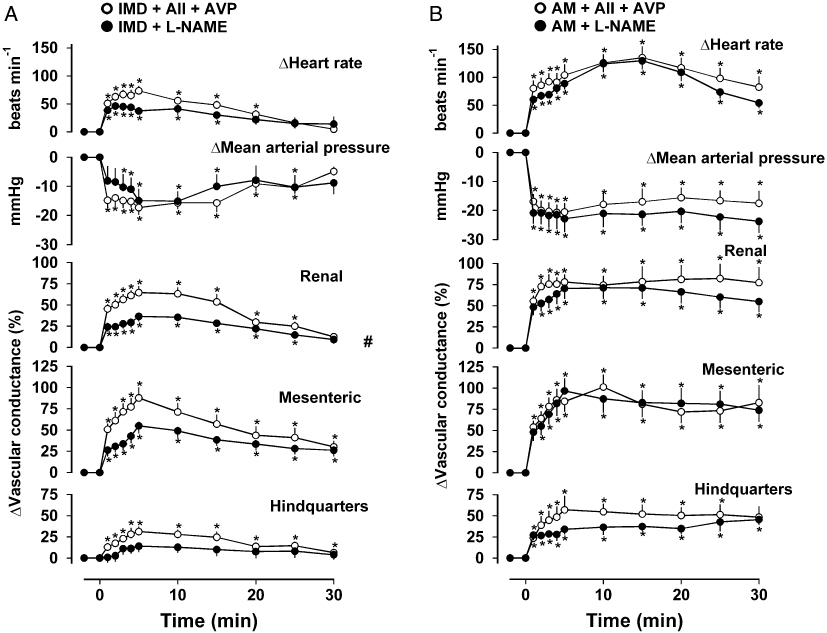
Cardiovascular responses to (A) IMD (1 nmol·kg−1) or (B) AM (3 nmol·kg−1) in the presence of angiotensin II (AII, 200 ng·kg−1·h−1) + AVP (20 ng·kg−1·h−1) or L-NAME (3 mg·kg−1·h−1). Values are mean and vertical bars represent SEM, n= 8 *P < 0.05 versus baseline (Friedman's test), #P < 0.05 for integrated (0–30 min) changes (Wilcoxon's test). AM, adrenomedullin; AVP, arginine vasopressin; IMD, intermedin.
Administration of AM during AII + AVP co-infusion caused a marked tachycardia and fall in blood pressure accompanied by large increases in renal, mesenteric and hindquarters vascular conductances (Figure 3B). These responses to AM were unaffected by L-NAME [P > 0.05 for the integrated (0–30 min) changes for each variable].
Experiment 5: Involvement of KATP channels
Resting cardiovascular variables are shown in Table 5. Administration of U-37883A on both occasions caused a significant (P < 0.05) rise in blood pressure and fall in heart rate; these changes were accompanied by falls in vascular conductance (P < 0.05 for mesenteric vascular conductance prior to IMD and hindquarters conductance prior to AM).
Table 5.
Resting cardiovascular variables prior to administration of IMD or AM in animals pre-treated with saline or U-37883A (Experiment 5)
|
Saline |
U-37883A |
Saline |
U-37883A |
|
|---|---|---|---|---|
| IMD (1 nmol·kg−1) | AM (3 nmol·kg−1) | |||
| Heart rate (beats·min−1) | 353 ± 12 | 315 ± 10a | 359 ± 14 | 294 ± 8a |
| Blood pressure (mmHg) | 113 ± 3 | 129 ± 3a | 115 ± 3 | 127 ± 2a |
| Renal VC (units) | 86 ± 9 | 76 ± 8 | 81 ± 9 | 73 ± 7 |
| Mesenteric VC (units) | 70 ± 4 | 57 ± 5a | 68 ± 4 | 60 ± 6 |
| Hindquarters VC (units) | 45 ± 4 | 37 ± 3 | 50 ± 4 | 35 ± 2a |
Values are mean ± SEM, n= 9. Units for VC are (kHz·mmHg−1) 103.
P < 0.05 versus corresponding saline group (Wilcoxon's test).
AM, adrenomedullin; IMD, intermedin; VC, vascular conductance.
The control response to IMD comprised tachycardia, hypotension and renal, mesenteric and hindquarters vasodilatations, as described above, and these responses were unaffected by pre-treatment with U-37883A [P > 0.05 for the integrated (0–30 min) changes] (Figure 4A). Administration of AM under control conditions caused tachycardia, hypotension and increases in renal, mesenteric and hindquarters vascular conductances, as described above. Following treatment with U-37883A, there was no change in AM-mediated tachycardia, hypotension and hindquarters vasodilatation. However, contrary to expectation, pre-treatment with U-37883A led to significant augmentation of AM-mediated renal and mesenteric vasodilatations when compared to controls [P < 0.05 for integrated (0–30 min) changes] (Figure 4B).
Figure 4.
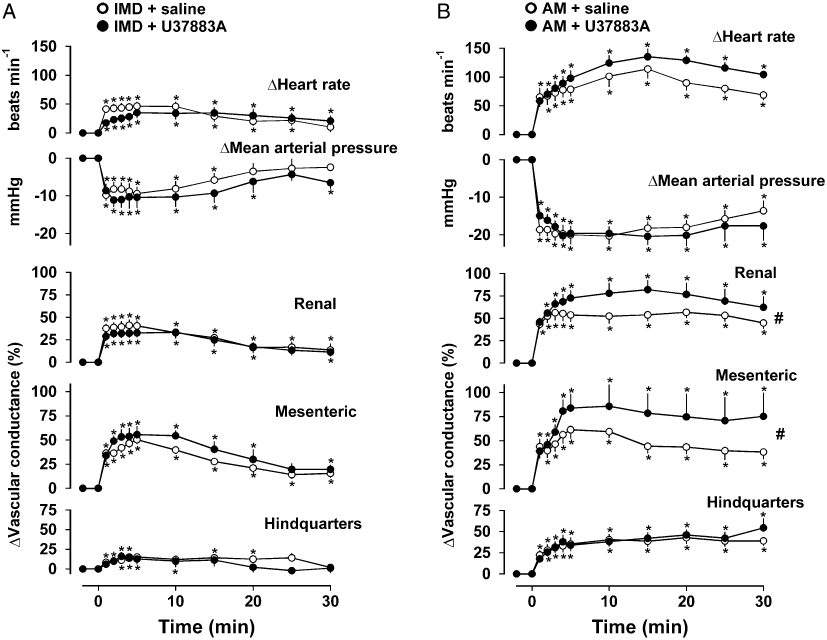
Cardiovascular responses to (A) IMD (1 nmol·kg−1) or (B) AM (3 nmol·kg−1) following administration of saline (1 mL infused over 30 min) or U-37883A (5 mg·kg−1). Values are mean and vertical bars represent SEM, n= 9 *P < 0.05 versus baseline (Friedman's test), #P < 0.05 for integrated (0–30 min) changes (Wilcoxon's test). AM, adrenomedullin; IMD, intermedin.
Experiment 6: Effects of angiotensin (AT1) receptor antagonism
Pre-treatment with losartan had no substantial effects on resting haemodynamics although there was a slight reduction in blood pressure and increase in hindquarters vascular conductance (P < 0.05 prior to administration of IMD only) (Table 6).
Table 6.
Resting cardiovascular variables prior to administration of IMD or AM in animals pre-treated with saline or losartan (Experiment 6)
|
Saline |
losartan |
Saline |
losartan |
|
|---|---|---|---|---|
| IMD (1 nmol·kg−1) | AM (3 nmol·kg−1) | |||
| Heart rate (beats·min−1) | 336 ± 9 | 355 ± 7 | 355 ± 13 | 369 ± 17 |
| Blood pressure (mmHg) | 111 ± 2 | 98 ± 4a | 111 ± 2 | 104 ± 4 |
| Renal VC (units) | 90 ± 10 | 105 ± 12 | 93 ± 12 | 81 ± 14 |
| Mesenteric VC (units) | 74 ± 4 | 89 ± 9 | 77 ± 7 | 83 ± 7 |
| Hindquarters VC (units) | 37 ± 4 | 50 ± 5a | 42 ± 5 | 47 ± 5 |
Values are mean ± SEM, n= 9. Units for VC are (kHz·mmHg−1) 103.
P < 0.05 versus corresponding saline group (Wilcoxon's test).
AM, adrenomedullin; IMD, intermedin; VC, vascular conductance.
In the absence of losartan, cardiovascular responses to IMD and AM were as described above although on this occasion, the IMD-induced fall in blood pressure was not significant (Friedman's test). Following pre-treatment with losartan, there was no change in the regional haemodynamic responses to IMD (Figure 5A), whereas AM-mediated hypotension and renal and mesenteric vasodilatations were significantly augmented [P < 0.05 for integrated (0–30 min) changes] (Figure 5B).
Figure 5.
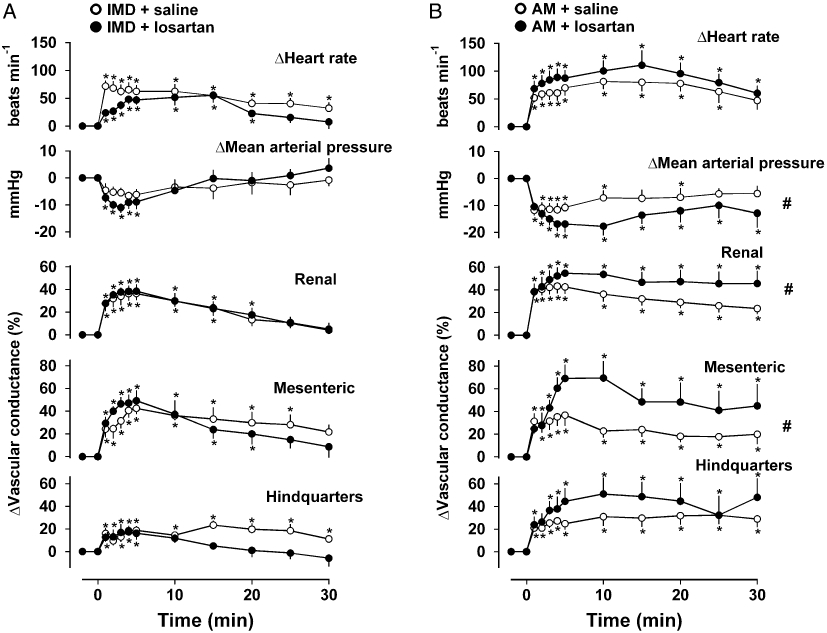
Cardiovascular responses to (A) IMD (1 nmol·kg−1) or (B) AM (3 nmol·kg−1) following administration of saline (0.1 mL) or losartan (10 mg·kg−1). Values are mean and vertical bars represent SEM, n= 9 *P < 0.05 versus baseline (Friedman's test), #P < 0.05 for integrated (0–30 min) changes (Wilcoxon's test). AM, adrenomedullin; IMD, intermedin.
Discussion
IMD (also known as AM2) is a newly identified member of the calcitonin family of peptides which shares some structural, sequence, and functional homology with AM (Roh et al., 2004; Takei et al., 2004). AM is a potent vasoactive peptide which causes relaxation of vascular smooth muscle in vitro, and vasodilatation and hypotension in vivo. The vasorelaxant effects of AM occur through increases in CL/RAMP receptor complex-mediated cAMP production, and may, to some extent, involve release of NO and activation of KATP channels (for review see Brain and Grant, 2004).
The aims of the present studies were to measure the regional haemodynamic effects of IMD and AM in conscious animals, and to compare possible mechanisms involved in the effects observed. The main findings were that, like AM, IMD causes widespread vasodilatation with a clear role for AM22–52-sensitive receptors in the renal and mesenteric, but not the hindquarters vasodilator responses, and no evidence for an involvement of KATP channels in the vasodilator responses. However, there was a significant role for NO in the renal vasodilator effects of IMD but not those of AM. Furthermore, there was an unexpected enhancement of the renal and mesenteric vasodilator actions of AM in the presence of a KATP channel blocker which could be explained by an inhibitory effect of the latter on AM-mediated renin release (see below).
To our knowledge, only one study prior to this one has described the regional haemodynamic effects of IMD (Fujisawa et al., 2007). Although, because of the nature of the measurements (i.e. radiolabelled microspheres), that study covered more vascular beds than studied here, and was restricted to one point in time during an IMD infusion. Our experiments delineated the temporal profile of response to bolus injections and showed that IMD, like AM, caused widespread vasodilatation with a rank order of potency of mesenteric ≥ renal > hindquarters. In contrast, the previous study found renal vasodilatation to be more prominent than mesenteric vasodilatation, the latter not being accompanied by an increase in flow (Fujisawa et al., 2007). Differences between the experiments which could explain the disparate findings include the strain of rat, the form of peptide (see below), and perhaps more importantly, the mode of peptide administration (i.e. bolus administration vs. continuous infusion).
In the first experiments we compared equimolar doses of IMD and AM and found the former to be more potent than the latter. Other studies comparing the potency of these peptides have produced variable results. Thus, Roh et al. (2004) described the blood pressure lowering effects of IMD and AM as similar, although the effect of IMD appeared less marked, consistent with the results of Taylor et al. (2005). In contrast, Takei et al. (2004) and Ren et al. (2006) showed more potent hypotensive effects of IMD than AM. Fujisawa et al. (2006) found similar initial hypotensive effects of AM and IMD but the effects of AM were more long lasting. It is possible that some of these apparent differences could be due to the form of the peptides used. In the original description (Roh et al., 2004), the mature peptides identified were the 47 amino acid prepro-IMD101–147(IMD1–47, also known as IMD long) and the 40 amino acid prepro-IMD108–147 (IMD8–47, also known as IMD short). However, subsequently (Yang et al., 2005), another fragment was identified, that is prepro-IMD95–147 (IMD1–53). Most of the studies described above used IMD1–47 (where specified), whereas our studies used IMD1–53 since that was the only commercially available form at the time our work was initiated. However, Ren et al. (2006) compared the hypotensive effects of IMD1–47 with those of IMD1–53 and AM, and found the rank order to be IMD1–47 > IMD1–53= AM. In contrast, we did not find the hypotensive action of IMD1–53 to be equipotent to that of AM although we compared 1 nmol·kg−1 doses of the peptides, whereas the previous study compared 3 nmol·kg−1 doses (Ren et al., 2006). In the light of the results of these first experiments, for the mechanistic studies, we chose to compare a 1 nmol·kg−1 dose of IMD with 3 nmol·kg−1 AM. These doses delivered into a plasma volume of ∼0.4 mL·kg−1 would give a theoretical peak plasma concentration of about 25 or 75 pmol·mL−1 (not allowing for binding or clearance), which are clearly higher than the normal circulating levels of IMD or AM (∼35 and 20 fmol·mL−1, respectively) (Bell and McDermott, 2008). Although the initial mesenteric and hindquarters vasodilator effects of the chosen doses of IMD and AM were matched, the renal vasodilator and hypotensive actions were greater for AM. This unavoidable mismatching is clearly a limitation to the study.
Mechanisms of IMD-mediated vasodilatation have yet to be fully elucidated but are thought to involve the non-selective activation of CL/RAMP complexes and possibly also activation of an unidentified IMD receptor (Roh et al., 2004; Takei et al., 2004; Owji et al., 2008). In isolated cell preparations, IMD appeared to show greater activity at CL/RAMP1 and CL/RAMP3 complexes than at CL/RAMP2, perhaps consistent with a more effective antagonism of the hypotensive effects with CGRP8–37 (which acts at CL/RAMP1) than AM22–52 (which acts at CL/RAMP2) (Roh et al., 2004). Furthermore, in vitro, coronary vasodilator responses to IMD have been shown to be inhibited by CGRP8–37 but not by AM22–52 (Kobayashi et al., 2004). However, here we found evidence of AM22–52-sensitive vasodilator effects of IMD in the renal and mesenteric vascular beds, but not in the hindquarters. This pattern is consistent with that observed for AM following pre-treatment with AM22–52., and suggests that IMD signals, at least in part, through AM receptors in those regions. Although AM22–52 attenuated renal and mesenteric vasodilatations, the effect was not sufficient to inhibit the accompanying fall in blood pressure, possibly because the expected increase in cardiac output was less, due to the smaller fall in afterload and/or a change in the effects on preload (Abdelrahman and Pang, 2006). Thus, the previous studies showing minimal effects of AM22–52 on the hypotensive effects of IMD (Roh et al., 2004) are not inconsistent with ours, although our interpretation is different. Interestingly, in pilot studies, we found no effect of CGRP8–37 on the regional vasodilator response to IMD (data not shown), in line with our earlier findings with AM (Gardiner et al., 1995). The lack of effect of AM22–52 on the hindquarters vasodilator response to AM is consistent with results of others in the cat hindlimb where AM-induced vasodilatation was resistant to blockade with either AM22–52 or CGRP8–37 (Champion et al., 1997).
The study of the CL/RAMP receptor system is complicated by a lack of selective antagonists, and although AM22–52 is acknowledged to be weak and lacking in specificity, it is the only available tool. Nonetheless, our findings clearly show that both AM- and IMD-mediated renal and mesenteric vasodilatations were attenuated by AM22–52, suggesting AM1 and/or AM2 receptors are activated by both peptides in a regionally selective manner.
In vitro studies have shown up-regulation of NO synthesis by IMD (Yang et al., 2006) and some degree of attenuation of IMD-induced relaxation by NO synthase inhibition (Kandilci et al., 2006; Chauhan et al., 2007), but in vivo, the depressor response to IMD is reportedly unaffected by NO synthase inhibition (Taylor et al., 2005; Abdelrahman and Pang, 2007), although the accompanying regional vascular effects have not previously been evaluated. We therefore assessed the effects of NO synthase inhibition with L-NAME on regional haemodynamic responses to IMD and AM, under conditions where controls were made for the baseline cardiovascular effects of L-NAME. Those experiments showed a tendency for all vasodilator responses to IMD to be reduced by L-NAME, although this was only significant in the renal vascular bed. While it might be expected that the reduced vasodilatations would have been manifested as an overall reduction in the blood pressure response, it is likely that along with the overall smaller peripheral vasodilatation, there was a smaller increase in cardiac output. Our findings, therefore, agree with those previously published showing no effect of NO synthase inhibition on IMD-mediated hypotension (Taylor et al., 2005; Abdelrahman and Pang, 2007), but because of the additional measurements we made, our interpretation is different, and we can conclude that there is an involvement of NO in IMD-mediated vasodilatation, particularly in the renal vascular bed.
No significant role for NO in AM-mediated vascular responses was found in the present study which is at odds with our previous studies (Gardiner et al., 1995), where we found AM-mediated hindquarters vasodilatation to be attenuated by L-NAME in conscious rats. In the present study, AM-mediated hindquarters vasodilatation was reduced in the presence of L-NAME, but this was not significantly different from control conditions. However, our earlier study used a lower dose of AM (1 nmol·kg−1) and compared integrated responses over 15 min rather than the 30 min compared here, so it is possible that the hindquarters response to AM in the presence of L-NAME would have been significantly less in the present study if responses had been compared over 15 min (Figure 3B). Nonetheless, it appears from our results that the involvement of NO may be greater for IMD than AM, although it is possible that this apparent difference was due to the differential hypotensive effects of the peptides.
There is some evidence that the cardiovascular responses to AM are sensitive to glibenclamide and U-37883A, suggesting a role for KATP channels (Sabates et al., 1997; Goto et al., 2000), although other studies have found KATP channel inhibition to have no effect on the vascular responses to AM (Miura et al., 1995; Champion et al., 1997; Terata et al., 2000). In vitro studies have failed to show an effect of glibenclamide on the vasorelaxant response to IMD (Kandilci et al., 2006; Chauhan et al., 2007), but to our knowledge there have been no in vivo studies on the possible role for vascular KATP channels in the vasodilator effects of IMD. Blocking KATP channels with U-37883A would be expected to attenuate peptide-mediated vasodilatation in areas where those channels were involved. In the present study there was no attenuation of either AM or IMD-mediated responses by U-37883A, consistent with the suggestion that KATP channels are not involved in the vasodilator responses to either peptide. However, contrary to expectation, when AM was administered following treatment with U37883A, there was a significant augmentation of AM-mediated renal and mesenteric vasodilator responses. As administration of U-37883A caused an increase in blood pressure and vasoconstriction, it could be that the augmented vasodilatation was due to animals being in a pre-constricted state. However, this is an unlikely explanation, firstly because the augmentation was still evident when the data were analysed as absolute rather than % changes (data not shown), and secondly, because the augmentation was regionally-selective and not seen in the vascular bed that was significantly constricted by U-37883A (i.e. the hindquarters). Thirdly, IMD-mediated vasodilatation was not augmented by U-37883A suggesting that the phenomenon was unique to AM and not due to a change in baseline status.
One difference between AM and IMD may be in relation to their influence on the renin-angiotensin system. Hypotension causes reflex activation of this system, but there is evidence that AM also has a direct stimulatory effect on renin release from renal juxtaglomerular cells (Jensen et al., 1997). No such evidence exists for a similar effect of IMD although IMD has been shown to increase aldosterone levels secondary to an increase in plasma renin activity (Charles et al., 2006), but it is not yet known whether this is a direct action of IMD, or secondary to its hypotensive effect. As U-37883A has been shown to inhibit renin release (Humphrey and Ludens, 1998; Vallon et al., 1998), we hypothesized that U-37883A might be inhibiting AM-induced renin release. Thus, while it has been suggested that AM might be a functional antagonist of AII (Charles et al., 2000), it was feasible that the observed augmentation of AM-mediated renal and mesenteric vasodilatations seen following treatment with U-37883A, was due to a decrease in renin activity and the consequent diminution of the vasoconstrictor effects of AII. To test this hypothesis, a separate group of animals was pre-treated with the AT1 receptor antagonist, losartan, and subsequently given AM and IMD. Interestingly, losartan pre-treatment had no effect on the vascular responses to IMD, but significantly augmented AM-mediated hypotension and renal and mesenteric vasodilatation. These data suggest that the hypotensive and vasodilator effects of AM are attenuated by its renin-stimulatory action, and when AT1 receptors are blocked, AM-mediated vasodilatations are significantly augmented in regions where there is high sensitivity to the vasoconstrictor effects of AII. Thus, the enhanced vasodilator effects of AM in the presence of U-37883A could be due to the latter's ability to decrease renin secretion (Humphrey and Ludens, 1998; Vallon et al., 1998), offsetting AM-mediated increase in renin secretion. The results of the present study do not support a similar role of the renin-angiotensin system in IMD action, although it is possible that the modest hypotensive response to IMD in this study was insufficient to trigger reflex renin release.
In conclusion, it appears that IMD and AM are similar in causing renal and mesenteric vasodilatations which are sensitive to AM22–52, suggesting that AM1 and/or AM2 receptors are activated by both peptides in a regionally selective manner, although a fuller analysis of the receptor types must await the availability of more selective antagonists. While it appears that KATP channels do not contribute to the vasodilator action of either IMD or AM, an involvement of NO is more apparent in the vasodilator actions of IMD than AM, at least at the doses used here. Moreover, the peptides also differ in that U-37883A enhances the renal and mesenteric vasodilator responses to AM, but not those to IMD, possibly due to an involvement of the renin-angiotensin system in the haemodynamic effects of the former but not the latter. The mechanisms involved in the vasodilator effects of AM and IMD which were not sensitive to AM22–52, and not mediated by NO or KATP channels, are unknown. In cats, Champion et al. (1997) showed hindlimb vasodilator responses to AM which involved activation of cAMP, but were resistant to antagonism by AM22–52, CGRP8–37, NO synthase inhibition and KATP channel blockade. Whether or not these vasodilatations involve CL/RAMP receptor complexes that are not effectively antagonized by the available tools, or in the case of IMD, the recently described distinct class of receptor (Owji et al., 2008), remains to be determined.
Acknowledgments
This work was supported by the British Heart Foundation
Glossary
Abbreviations:
- AII
angiotensin II
- AM
adrenomedullin
- AVP
arginine vasopressin
- CGRP
calcitonin gene-related peptide
- CL
calcitonin receptor
- DS
Doppler shift
- HDAS
Haemodynamics Data Acquisition System
- IMD
intermedin
- L-NAME
NG nitro L-arginine methyl ester
- RAMP
receptor activity modifying protein
- U-37883A
N-(1-Adamantyl)-N'-cyclohexyl-4-morpholinecarboxamidine·HCl
- VC
vascular conductance
Conflict of interest
None.
References
- Abdelrahman AM, Pang CCY. Effect of intermedin/adrenomedullin-2 on venous tone in conscious rats. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:376–380. doi: 10.1007/s00210-006-0076-z. [DOI] [PubMed] [Google Scholar]
- Abdelrahman AM, Pang CC. Vasodilator mechanism of intermedin/adrenomedullin-2 in anesthetized rats. Proc West Pharmacol Soc. 2007;50:43–46. [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) 3rd edn. Br J Pharmacol. 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batin P, Gardiner SM, Compton AM, Bennett T. Differential regional haemodynamic effects of the non-peptide angiotensin II antagonist, DuP 753, in water-replete and water-deprived Brattleboro rats. Life Sci. 1991;48:733–739. doi: 10.1016/0024-3205(91)90087-r. [DOI] [PubMed] [Google Scholar]
- Bell D, McDermott BJ. Intermedin (adrenomedullin-2): a novel counter-regulatory peptide in the cardiovascular and renal systems. Br J Pharmacol. 2008;153:S247–S262. doi: 10.1038/sj.bjp.0707494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Champion HC, Santiago JA, Murphy WA, Coy DH, Kadowitz PJ. Adrenomedullin-(22-52) antagonizes vasodilator responses to CGRP but not adrenomedullin in the cat. Am J Physiol Regul Integr Comp Physiol. 1997;41:R234–R242. doi: 10.1152/ajpregu.1997.272.1.R234. [DOI] [PubMed] [Google Scholar]
- Champion HC, Bivalacqua TJ, Zadina JE, Kastion AJ, Hyman AL, Kadowitz PJ. Role of nitric oxide in mediating vasodilator responses to opioid peptides in the rat. Clin Exp Pharmacol Physiol. 2002;29:229–232. doi: 10.1046/j.1440-1681.2002.03634.x. [DOI] [PubMed] [Google Scholar]
- Charles CJ, Rademaker MT, Richards AM, Cooper GJ, Coy DH, Nicholls MG. Adrenomedullin attenuates pressor responses to angiotensin II in conscious sheep. J Cardiovasc Pharmacol. 2000;36:526–532. doi: 10.1097/00005344-200010000-00017. [DOI] [PubMed] [Google Scholar]
- Charles CJ, Rademaker MT, Richards AM. Hemodynamic, hormonal, and renal actions of adrenomedullin-2 in normal conscious sheep. Endocrinology. 2006;147:1871–1877. doi: 10.1210/en.2005-1403. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Ross GR, Yallampalli U, Yallampalli C. Adrenomedullin-2, a novel calcitonin/calcitonin-gene-related peptide family peptide, relaxes rat mesenteric artery: influence of pregnancy. Endocrinology. 2007;148:1727–1735. doi: 10.1210/en.2006-1105. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Takei Y, Miura K, Shoukouji T, et al. Renal effects of a new member of adrenomedullin family, adrenomedullin 2, in rats. Eur J Pharmacol. 2004;497:75–80. doi: 10.1016/j.ejphar.2004.06.039. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Miura K, Shokoji T, Nishiyama A, et al. Roles of adrenomedullin 2 in regulating the cardiovascular and sympathetic nervous systems. Am J Physiol Heart Circ Physiol. 2006;290:H1120–H1127. doi: 10.1152/ajpheart.00461.2005. [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Nagai Y, Miyatake A, Miura K, Nishiyama A, Kimura S, et al. Effects of adrenomedullin 2 on regional hemodynamics in conscious rats. Eur J Pharmacol. 2007;558:128–132. doi: 10.1016/j.ejphar.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, Kemp PA, March JE, Bennett T. Regional haemodynamic effects of human and rat adrenomedullin in conscious rats. Br J Pharmacol. 1995;114:584–591. doi: 10.1111/j.1476-5381.1995.tb17179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Influence of CGRP (8-37), but not adrenomedullin (22-52), on the haemodynamic responses to lipopolysaccharide in conscious rats. Br J Pharmacol. 1999;127:1611–1618. doi: 10.1038/sj.bjp.0702718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Davenport AP, Wiley KE, Bennett T. Regional hemodynamic actions of selective corticotropin-releasing factor type 2 receptor ligands in conscious rats. J Pharmacol Exp Ther. 2005;312:53–60. doi: 10.1124/jpet.104.075259. [DOI] [PubMed] [Google Scholar]
- Goto K, Fujii K, Onaka U, Abe I, Fujishima M. Effects of adrenomedullin and PAMP on membrane potential and neurotransmission. Peptides. 2000;21:257–263. doi: 10.1016/s0196-9781(99)00204-1. [DOI] [PubMed] [Google Scholar]
- Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: a comparison of effects of adrenomedullin22-52, CGRP8-37 and BIBN4096BS. Br J Pharmacol. 2003;140:477–486. doi: 10.1038/sj.bjp.0705472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey SJ, Ludens JH. K-ATP blocking diuretic PNU-37883A reduces plasma renin activity in dogs. J Cardiovasc Pharmacol. 1998;31:894–903. doi: 10.1097/00005344-199806000-00013. [DOI] [PubMed] [Google Scholar]
- Jensen BL, Krämer BK, Kurtz A. Adrenomedullin stimulates renin release and renin mRNA in mouse juxtaglomerular granular cells. Hypertension. 1997;29:1148–1155. doi: 10.1161/01.hyp.29.5.1148. [DOI] [PubMed] [Google Scholar]
- Kandilci HB, Gumusel B, Wasserman A, Witriol N, Lippton H. Intermedin/adrenomedullin-2 dilates the rat pulmonary vascular bed: dependence on CGRP receptors and nitric oxide release. Peptides. 2006;27:1390–1396. doi: 10.1016/j.peptides.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Kandilci HB, Gumusel B, Lippton H. Intermedin/adrenomedullin 2 (IMD/AM2) relaxes rat main pulmonary arterial rings via cGMP-dependent pathway: role of nitric oxide and large conductance calcium-activated potassium channels (BKCa) Peptides. 2008;29:1321–1328. doi: 10.1016/j.peptides.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Liu Y-J, Gonda T, Takei Y. Coronary vasodilatory response to a novel peptide, adrenomedullin 2. Clin Exp Pharmacol Physiol. 2004;31:S49–S59. doi: 10.1111/j.1440-1681.2004.04115.x. [DOI] [PubMed] [Google Scholar]
- Lang MG, Paterno R, Faraci FM, Heistad DD. Mechanisms of adrenomedullin-induced dilatation of cerebral arterioles. Stroke. 1997;28:181–185. doi: 10.1161/01.str.28.1.181. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Meisheri KD, Humphrey SJ, Kahn SA, Cipkus-Dubray LA, Smith MP, Jones AW. 4-morpholinecarboximidine-N-1-adamantyl-N'-cyclohexylhydrochloride (U-37883A): pharmacological characterisation of a novel antagonist of vascular ATP-sensitive K+ channel openers. J Pharmacol Exp Ther. 1993;266:655–665. [PubMed] [Google Scholar]
- Miura K, Ebara T, Okamura M, Matsuura T, Kim S, Yukimura T, et al. Attenuation of adrenomedullin-induced renal vasodilatation by NG-nitro L-arginine but not glibenclamide. Br J Pharmacol. 1995;115:917–924. doi: 10.1111/j.1476-5381.1995.tb15898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoshi M, Inoue K, Takei Y. Identification of a novel adrenomedullin gene family in teleost fish. Biochem Biophys Res Commun. 2003;311:1072–1077. doi: 10.1016/j.bbrc.2003.10.111. [DOI] [PubMed] [Google Scholar]
- Owji AA, Chabot J-G, Dumont Y, Quirion R. Adrenomedullin-2/intermedin induces cAMP accumulation in dissociated rat spinal cord cells: evidence for existence of a distinct class of sites of action. J Mol Neurosci. 2008;35:355–361. doi: 10.1007/s12031-008-9062-x. [DOI] [PubMed] [Google Scholar]
- Pan C-S, Yang J-H, Cai D-Y, Zhao J, Gerns H, Yang J, et al. Cardiovascular effects of newly discovered peptide intermedin/adrenomedullin 2. Peptides. 2005;26:1640–1646. doi: 10.1016/j.peptides.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Ren Y-S, Yang J-H, Zhang J, Pan C-S, Yang J, Zhao J, et al. Intermedin 1-53 in central nervous system elevates arterial blood pressure in rats. Peptides. 2006;27:74–79. doi: 10.1016/j.peptides.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Roh J, Chang CL, Bhalla A, Klein C, Hsu SYT. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modulating protein complexes. J Biol Chem. 2004;279:7264–7247. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- Sabates BL, Pigott JD, Choe EU, Cruz MP, Lippton HL, Hyman AL, et al. Adrenomedullin mediates coronary vasodilation through adenosine receptors and KATP channels. J Surg Res. 1997;67:163–168. doi: 10.1006/jsre.1996.4985. [DOI] [PubMed] [Google Scholar]
- Takei Y, Inoue K, Ogoshi M, Kawahara T, Bannai H, Miyano S. Identification of novel adrenomedullin in mammals: a potent cardiovascular and renal regulator. FEBS Lett. 2004;556:53–58. doi: 10.1016/s0014-5793(03)01368-1. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Bagley SL, Samson WK. Intermedin/adrenomedullin-2 acts within the central nervous system to elevate blood pressure and inhibit food and water intake. Am J Physiol Regul Integr Comp Physiol. 2005;288:R919–R927. doi: 10.1152/ajpregu.00744.2004. [DOI] [PubMed] [Google Scholar]
- Terata K, Miura H, Liu Y, Loberiza F, Gutterman DD. Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and K+ channels. Am J Physiol Heart Circ Physiol. 2000;279:H2620–H2626. doi: 10.1152/ajpheart.2000.279.6.H2620. [DOI] [PubMed] [Google Scholar]
- Theodorsson-Norheim E. Friedman-Quade tests: BASIC computer program to perform nonparametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput Biol Med. 1987;17:85–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- Vallon V, Kirschenmann D, Brenner I, Albinus M, Osswald H. Potassium diet as a determinant for the renal response to systemic potassium channel modulation in anesthetised rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:245–252. doi: 10.1007/pl00005249. [DOI] [PubMed] [Google Scholar]
- Yang J-H, Jia Y-X, Pan C-S, Zhao J, Ouyang M, Yang J, et al. Effects of intermedin 1-53 on cardiac function and ischemia/reperfusion injury in isolated rat hearts. Biochem Biophys Res Commun. 2005;327:713–719. doi: 10.1016/j.bbrc.2004.12.071. [DOI] [PubMed] [Google Scholar]
- Yang J-H, Pan C-S, Jia Y-X, Zhang J, Zhao J, Pang Y-Z, et al. Intermedin 1-53 activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Biochem Biophys Res Commun. 2006;341:567–572. doi: 10.1016/j.bbrc.2006.01.010. [DOI] [PubMed] [Google Scholar]


