Abstract
Background and purpose:
Several anticancer drugs with diverse chemical structures can induce differentiation of cancer cells. This study was undertaken to explore the potential contribution of caspase-3 to pharmacologically-induced differentiation of K562 cells.
Experimental approach:
We assessed differentiation by measuring the expression of glycophorin A and haemoglobin synthesis in K562 cells treated with low concentrations of doxorubicin, hydroxyurea, cytosine arabinoside, cisplatin and haemin. Caspase-3 activation, mitochondrial membrane potential dissipation and viability were assessed by FACS. GATA-1-binding activity was evaluated by EMSA.
Key results:
Treatment of K562 cells with low concentrations of the tested drugs activated caspase-3 but did not trigger detectable apoptosis. Instead, elevated levels of haemoglobin-positive and glycophorin A/caspase-3-double-positive cells were observed, suggesting involvement of caspase-3 in drug-induced differentiation. Inhibition of caspase-3 activity significantly reduced the ability of K562 cells to execute the differentiation programme. Mitochondrial membrane potential dissipation was observed, indicating involvement of the mitochondrial pathway. Binding activity of GATA-1, transcription factor responsible for differentiation and cell survival, was not diminished by increased caspase-3 activity during drug-stimulated differentiation.
Conclusions and implications:
Our results could explain how anticancer drugs, with diverse structures and modes of action, can stimulate erythroid differentiation in leukaemic cells with appropriate genetic backgrounds. Our findings imply that some similarities exist between pharmacologically-induced differentiation of erythroleukaemic cells and normal erythropoiesis, both involving caspase-3 activation at high levels of anti-apoptotic protein Bcl-XL and chaperone protein Hsp70 (heat shock protein 70). Therefore, the functions of caspase-3, unrelated to cell death, can be extended to pharmacologically-induced differentiation of some cancer cells.
Keywords: caspase-3, differentiation, K562 cells, doxorubicin, Ara-C, hydroxyurea, cisplatin, haemin
Introduction
The human K562 cell line represents a widely used in vitro model system for chronic myelogenous leukaemia (CML). Several studies have indicated that many compounds, very diverse in their chemical structure, can induce differentiation of K562 cells at low non-toxic concentrations. For example, 12-0-tetradecanoylphorbol-13-acetate (TPA) stimulates megakaryocytic development, while anthracyclines, haemin, hydroxyurea, GTP, cyclopentenyl cytosine (CPEC), cytosine arabinoside (Ara-C), cisplatin, imatinib (STI571) and butyric acid have been shown to cause erythroid differentiation of K562 cells (Morceau et al., 1996; Belhacène et al., 1998;Cortesi et al., 1999; Bianchi et al., 2000; Witt et al., 2000; Park et al., 2001; Jacquel et al., 2003; Huang et al., 2004; Woessmann et al., 2004; Czyz et al., 2005; 2008; Moosavi et al., 2007). However, it is unclear how such diverse drugs can trigger differentiation of K562 cells. Our recent study has indicated that caspase-3 activation is required for erythroid differentiation of K562 cells induced by doxorubicin (Czyz et al., 2008), whereas differentiation stimulated by STI571, an inhibitor of Bcr-Abl kinase, is not associated with caspase-3 activation (Jacquel et al., 2007; Czyz et al., 2008). To further explore caspase-3 involvement in pharmacologically-induced differentiation, we have chosen well-recognized inducers of K562 differentiation. Their distinct mechanisms of drug action were the basis for the selection of cytostatic compounds in the current study. Doxorubicin mainly intercalates into DNA and inhibits topoisomerase II (Gewirtz, 1999), cisplatin interferes with replication by interaction with DNA via intrastrand cross links with two neighbouring guanosines (Siddik, 2003), hydroxyurea inhibits ribonucleotide reductase, which catalyses the reduction of ribonucleotides to the corresponding deoxyribonucleotides required for DNA synthesis (King, 2003), and Ara-C inhibits replication by incorporating into DNA (Iwasaki et al., 1997). Only haemin, an iron-containing porphyrin, which is the prosthetic moiety for a broad range of proteins involved in oxygen delivery, is not employed in anticancer chemotherapy as a cytostatic drug, but is used in acute porphyria and in patients with thalassaemia intermedia (Nakamichi et al., 2005). In each case, differentiation of K562 cells appeared to be a common outcome when tested drugs were used at low non-toxic concentrations (Morceau et al., 1996; Cortesi et al., 1999; Bianchi et al., 2000; Park et al., 2001; Woessmann and Mivechi, 2001; Czyz et al., 2005; 2008; Di Pietro et al., 2007).
In this study, glycophorin A (GPA) expression and haemoglobin synthesis were related to changes in mitochondrial transmembrane potential (ΔΨm) and caspase-3 activity. It was presumed that induction of caspase-3 activity via the mitochondrial pathway could be a common feature of the tested drugs resulting in pharmacologically-induced erythroid differentiation of K562 cells.
Methods
Cell culture conditions, cell growth and viability assays
Human K562 cells were grown in a humidified atmosphere of 5% CO2 at 37°C in RPMI 1640, supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 50 mg·mL−1 gentamycin. For experiments, K562 cells were seeded at the density 3.5 × 104 mL−1, and 22 h later tested drugs at indicated concentrations were added. In some experiments, the caspase-3 inhibitor zDEVD-fmk at the final concentrations of 50 µM or DMSO (0.25%) were added 30 min prior to the various tested compounds. Cell growth and viability were determined by Trypan blue dye exclusion assay. In the cell growth assay, only viable cells were taken into account. For each experimental condition, comparisons were made relative to values obtained for untreated controls, and expressed as percentages of control. In the viability assay, the number of dead cells taking up Trypan blue is expressed as a percentage of the total cell number (viable and dead) in each experimental condition. Each experiment was conducted in triplicate and repeated four times. All subsequent experiments were performed at drug concentrations for which cell viability higher than 90% on day 3 was observed.
Benzidine staining
Cells were incubated with tested drugs for up to 5 days. In selected experiments, culture medium was supplemented with the caspase inhibitor zDEVD-fmk or DMSO as described above. To assess erythroid differentiation of K562 cells, the benzidine/H2O2 reaction was used. Cells were collected on days 3, 4 and 5, washed with ice-cold phosphate-buffered saline (PBS) and resuspended in 0.9% NaCl; 0.2% benzidine solution in 0.5 M acetic acid supplemented with 0.6% H2O2 was used to start the reaction. After 30 min of incubation at room temperature, 400 cells were counted to determine the percentage of blue haeme-containing cells.
Acridine orange/ethidium bromide staining
Cell death was studied morphologically by using fluorescent dyes: acridine orange (AO) and ethidium bromide (EB). Briefly, the cells were cultured for 5 days with or without the test drugs at indicated concentrations. Cells (1 × 105) were collected by centrifugation and resuspended in 30 µL of staining solution, a mixture (1:1) of EB (100 µg·mL−1) and AO (100 µg·mL−1). Then, they were examined by ultraviolet fluorescence microscopy (Olympus BX 41). In each experiment, more than 300 cells were analysed, and then percentages of early/late apoptotic or necrotic cells were calculated.
Flow cytometry
All stained cells were analysed by using the standard optics of a FACSCalibur flow cytometer and CellQuest software.
Assessment of GPA expression and caspase-3 activity
K562 cells were treated with 100 nM doxorubicin, 5 µM cisplatin, 250 nM Ara-C, 600 µM hydroxyurea or 30 µM haemin for 3, 4 and 5 days. In selected experiments, culture medium was supplemented with the caspase inhibitor zDEVD-fmk or DMSO as described above. In experiments presented in Figure 2, where only caspase-3 activity is demonstrated, propidium iodide (PI)-positive cells were excluded. To relate differentiation with caspase-3 activation, cells were double-stained with fluorescein isothiocyanate (FITC)-conjugated NucView 488 caspase-3 substrate to detect caspase-3 activity and with phycoerythrin (PE)-conjugated mouse monoclonal antibody to the human GPA antigen to detect a cell surface marker of erythroid differentiation. In these experiments, cells were washed with PBS, and 3 µL of NucView was added to 1 × 105 cells in 0.2 mL PBS. After 20 min of incubation (room temperature, darkness) cells were centrifuged, washed with PBS containing 2% FBS and resuspended in PBS containing 2% FBS. Five microlitres of PE-conjugated mouse monoclonal antibody to the human GPA antigen was added to 0.1 mL of the cell suspension. After 15 min of incubation (room temperature, darkness) cells were washed and resuspended in 0.3 mL PBS containing 2% FBS and analysed by flow cytometry. FL1 and FL2 channels were used for detection of caspase-3 activity and GPA expression, respectively.
Figure 2.
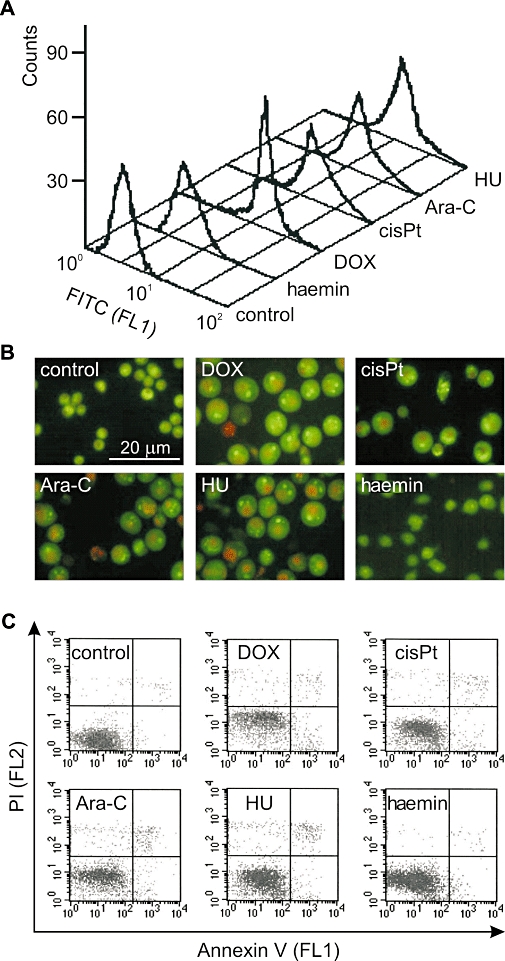
Activation of caspase-3 in K562 cells treated with DOX, cisPt, Ara-C, HU and haemin is not related to apoptosis. (A) Untreated cells or cells treated with 100 nM DOX, 5 µM cisPt, 250 nM Ara-C, 600 µM HU and 30 µM haemin for 4 days were stained with NucViewTM 488 caspase-3 substrate and analysed by flow cytometry (FL1 channel). PI-positive cells were excluded from the analysis. The histograms represent results from a single typical experiment. (B) Untreated cells or cells treated with tested drugs for 5 days were stained with acridine orange and ethidium bromide and examined by fluorescence microscopy. The numbers of apoptotic or necrotic cells were below 4% as assessed in 300 cells. Representative microscopic fields are shown. (C) Untreated K562 cells or cells treated with tested drugs for 4 days were stained with Annexin V and PI and analysed by flow cytometry (FL1 and FL2 channel respectively). Histograms represent results from a single typical experiment. Ara-C, cytosine arabinoside; cisPt, cisplatin; DOX, doxorubicin; FITC, fluorescein isothiocyanate; HU, hydroxyurea; PI, propidium iodide.
Apoptosis assessment by Annexin-V/PI staining
To assess apoptosis, staining with Annexin V and PI (Annexin-V-FLUOS Staining Kit) was used. After drug treatment for 4 days, 1 × 105 cells were washed in cold PBS and incubated for 10 min with 50 µL staining solution containing 2 µL Annexin V-FITC. Next, 50 µL staining solution containing 2 µL PI was added. Cells were analysed by flow cytometry by using FL1 and FL2 channels.
Propidium iodide staining
Propidium iodide staining was employed to assess the percentage of dead cells. After drug treatment, cells were harvested (1 × 105 cells), centrifuged and resuspended with 0.3 mL of PI (8 µg·mL−1) in PBS. After 10 min of incubation at room temperature in darkness, cells were analysed by flow cytometry (FL2 channel).
Mitochondrial transmembrane potential dissipation (ΔΨm)
To monitor changes in ΔΨm, drug-treated or untreated cells were loaded with tetramethylrhodamine ethyl ester (TMRE) at 25 nM concentration for 20 min at 37°C and analysed by flow cytometry (FL2 channel).
Electrophoretic mobility shift assay (EMSA)
Preparation of nuclear extracts from either untreated or drug-treated K562 cells was performed as described previously (Moll et al., 1995). The extracts were frozen in aliquots and stored at −80°C. Double-stranded oligonucleotide: 5′-AAT TAA GGC GGA TTT GAA TGT AG-3′ containing GATA-1-binding site (underlined) from Bcl-2-related gene (Bcl-XL) promoter was labelled by using [α-32P]-dATP and purified as described previously (Szulawska et al., 2005). Labelled oligonucleotides (100 000 cpm), 12 µg nuclear extracts, 2 µg of non-specific competitor poly[dI-dC]·poly[dI-dC] and binding buffer (Czyz and Gniazdowski, 1998) were incubated for 20 min at room temperature in a final volume of 20 µL. The reaction mixtures were analysed by electrophoresis on 5% polyacrylamide gel in 1× Tris-borate buffer. Gels were vacuum-dried and visualized by phosphorimaging (Molecular Imager). The specificity of the complex was confirmed by competition experiments by using a 100-fold excess of non-radioactive GATA-1 oligonucleotide as specific competitor or oligonucleotide with unrelated sequence: 5′-AAT TGC CTG GGA AAG TCC CCT CAA CT-3′[nuclear factor κB (NFκB)-binding site] as non-specific one. Relative band intensity was quantified by the densitometric analysis using Quantity One version 4.4.1. Software (BioRad, Hercules, CA, USA). The data from three independent experiments were expressed as means ± SD.
Statistical analysis
The data from at least three independent experiments were expressed as means ± SD. Student's one-tailed t-test was used to determine the statistical significance of differences between means. Differences were considered as significant when P < 0.05.
Drugs and materials
Doxorubicin was kindly provided by Dr Irena Oszczapowicz and Dr Malgorzata Wasowska-Lukawska from the Institute of Biotechnology and Antibiotics, Warsaw. Ara-C, hydroxyurea and haemin were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Stock solutions of each compound (doxorubicin, Ara-C and hydroxyurea in sterile water and haemin in 0.1 M NaOH) were stored at −20°C and diluted in RPMI 1640 medium immediately before use. Cisplatin (0.5 mg·mL−1 in 0.9% NaCl) was from EBEWE Arzneimittel G.m.b.H (Unterach, Austria).
Human K562 cells were a gift from Professor Jean Claude D'Halluin (INSERM 125, Lille, France); RPMI 1640 and FBS, Gibco (Scotland, UK); gentamycin, Polfa (Tarchomin, Poland); zDEVD-fmk, BD Pharmingen (CA, USA); Trypan blue dye exclusion assay, AO and benzidine solution, Sigma-Aldrich (St. Louis, MO, USA); FACSCalibur flow cytometer and CellQuest software, Becton Dickinson (San Jose, CA, USA); FITC-conjugated NucView 488 caspase-3 substrate, Biotium, Inc. (Hayward, CA, USA); PE-conjugated mouse monoclonal antibody, Caltag Laboratories (Invitrogen Corporation, USA); Annexin-V-FLUOS Staining Kit, Roche Diagnostics (Mannheim, Germany); TMRE, Molecular Probes (Invitrogen, OR, USA); Molecular Imager, BioRad.
Results
Doxorubicin, cisplatin, Ara-C, hydroxyurea inhibit proliferation and induce differentiation of K562 cells
To determine optimal concentrations for induction of differentiation while keeping a high viability level, K562 cells were treated with drugs at various concentrations for 4 days (Figure 1A). Erythroid differentiation was assessed by benzidine staining for haemoglobin synthesis. The highest percentages of benzidine-positive cells were obtained in cultures treated with 100 nM doxorubicin, 5 µM cisplatin, 250 nM Ara-C, 600 µM hydroxyurea and 30 µM haemin. At those concentrations 46%, 32%, 35%, 23% and 31% of cells were benzidine-positive respectively. Increasing the concentrations of cisplatin and hydroxyurea reduced the percentage of benzidine-positive cells, whereas higher concentrations of doxorubicin increased the number of differentiated cells but at the expense of reduced viability (not shown). As already published by us (Czyz et al., 2005) and others (Cortesi et al., 1999; Bianchi et al., 2000; Rodrigue et al., 2001; Grebenováet al., 2006; Moosavi et al., 2007), differentiation of K562 cells decreases the number of cells able to proliferate. After treatment with 100 nM doxorubicin, 5 µM cisplatin, 250 nM Ara-C or 600 µM hydroxyurea, cell growth was reduced to about 10% of control (Figure 1A).
Figure 1.
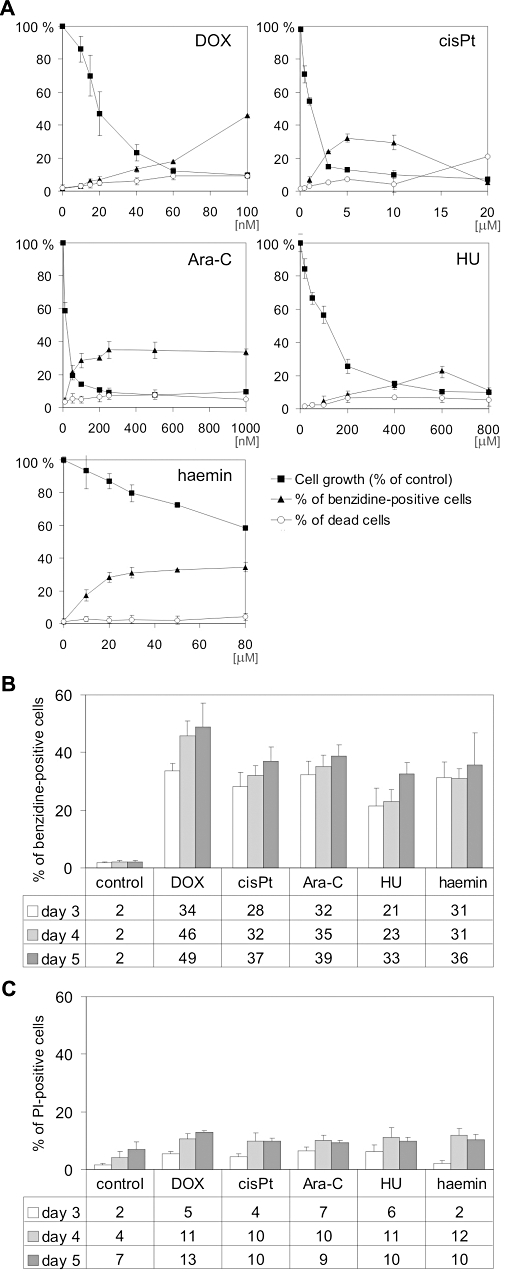
(A) Concentration-dependent effect of DOX, cisPt, Ara-C, HU and haemin on cell growth, viability and differentiation of K562 cells. Cells were cultured in the presence of various concentrations of DOX, cisPt, Ara-C, HU and haemin. After 3 days, cell growth and viability were determined. To evaluate erythroid differentiation, the percentage of haeme-containing cells was assessed by benzidine staining. Cell growth and viability were determined by Trypan blue exclusion assay. (B) Time-dependent effect of drugs on differentiation of K562 cells. Cells were cultured in the presence of selected concentrations of drugs: 100 nM DOX, 5 µM cisPt, 250 nM Ara-C, 600 µM HU and 30 µM haemin for 3, 4 and 5 days. The percentage of differentiated cells was determined by benzidine staining. (C) Time-dependent effect of drugs on viability of K562 cells. Untreated cells or cells treated for 3, 4 and 5 days with 100 nM DOX, 5 µM cisPt, 250 nM Ara-C, 600 µM HU or 30 µM haemin were stained with propidium iodide and analysed by flow cytometry. Data represent the mean ± SD of at least three independent experiments carried out in triplicate. Significant differences (P < 0.001) from the control data points were obtained for all values shown in the figure. Ara-C, cytosine arabinoside; cisPt, cisplatin; DOX, doxorubicin; HU, hydroxyurea; PI, propidium iodide.
Surprisingly, haemin used at a concentration of 30 µM reduced cell growth only to about 80% of control. This raised the question as to whether the method applied to evaluate differentiation induced by haemin was the correct one. Haemin is the Fe3+ oxidation product of haeme, and eventually might be reduced to haeme in cells. Therefore, it is possible that the benzidine test used to assess the effects of adding haemin into the culture could produce false positives. To test this hypothesis, we estimated the percentage of cells that were benzidine-positive after 2, 6 and 24 h of treatment with 30, 60 and 100 µM haemin; after the first 2 h of treatment, 7%, 15% and 20% of cells were ‘benzidine-positive’ respectively. After 6 h, the results were almost identical. Even if the differentiation programme had already been triggered, the endogenous synthesis of haeme seemed unlikely. After 24 h of treatment the percentages were raised to about 25% for all concentrations tested. In all experiments only 1% of control cells were benzidine-positive. This strongly suggested that haemin, when diffusing into cells, could become a substrate in the benzidine reaction and therefore only a number of the cells, counted as benzidine-positive, could be considered as truly differentiated cells. Haemin was included in all other experiments, but possible misleading results were taken into account.
Prolonged incubation with tested drugs (up to 5 days) increased the percentages of benzidine-positive cells (Figure 1B) without decreasing cellular viability below 90%, except for doxorubicin, as assessed by PI staining and flow cytometry (Figure 1C). Further prolongation of treatment markedly reduced viability of cells (not shown).
Activation of caspase-3 in K562 cells treated with doxorubicin, cisplatin, Ara-C, hydroxyurea and haemin is not related to apoptosis
To determine whether caspase-3 was activated in K562 cells treated with low concentrations of doxorubicin, cisplatin, Ara-C, hydroxyurea and haemin, the cleavage of a cell membrane-permeable fluorogenic, NucViewTM 488 caspase-3 substrate, was monitored by fluorescence-activated cell sorting (FACS). This method detects caspase-3 activity within individual intact cells. Non-fluorescent caspase-3 substrate enters the cell cytoplasm, where it is cleaved by caspase-3 to form a fluorescent high-affinity DNA dye able to stain the nucleus. The mean fluorescence intensity produced by the cleavage of the FITC-conjugated caspase-3 substrate increased from 4.3 in untreated cells to 19.5, 25.3, 22.8, 17.8 in cells treated with doxorubicin, Ara-C, cisplatin, hydroxyurea for 4 days respectively (Figure 2A). Because viability was high, there were no marked differences between mean values obtained for the whole population of drug-treated cells and those PI-negative (not shown). In this way, false positives due to autofluorescence of dead cells were excluded. The effects of haemin on caspase-3 activity were weak in comparison with other tested drugs; it induced a mean intensity of FITC fluorescence of 7.3. These data indicate that doxorubicin, cisplatin, Ara-C and hydroxyurea induced caspase-3 activation within 4 days and haemin was less efficient than the other drugs. To exclude the possibility that the observed caspase-3 activation resulted in apoptosis, cells were treated with drugs for 5 days and then analysed by fluorescence microscopy after AO/EB staining (Figure 2B). All tested drugs, except haemin, caused an increase in cell size (no pyknosis) and in 300 cells the percentages of apoptotic or necrotic cells were still below 4% (not shown). The induction of apoptosis in K562 cells after drug treatment was also evaluated by Annexin V/PI staining (Figure 2C). The present results and those published previously (Czyz et al., 2008) exclude the possibility that caspase-3 activation triggered by low concentrations of anticancer drugs may favour apoptosis in K562 cells.
Drug-induced differentiation of K562 cells is associated with caspase-3 activation
To relate differentiation to caspase-3 activity, double-staining for activated caspase-3 and GPA was employed in flow cytometry. PE-conjugated mouse monoclonal antibody to the human GPA, a surface marker of erythroid differentiation, was used together with the fluorogenic substrate for detecting caspase-3 activity within living cells. A marked increase in the percentages of GPA/caspase-3-double-positive cells was observed in cultures treated for 3 days with tested compounds (Figure 3A). They were raised from 1% in the control culture to about 51%, 39%, 47% and 36% in the cultures stimulated with 100 nM doxorubicin, 5 µM cisplatin, 250 nM Ara-C and 600 µM hydroxyurea respectively. The percentage of double-positive cells was not significantly increased after haemin treatment. Activation of caspase-3 was a much slower process in haemin-treated cells than observed in cells treated with other tested drugs, and a marked increase was observed on day 5. GPA expression in haemin-treated cells was still at a similar level to that observed in untreated cells and therefore no double-positive cells were visible on day 5. Most of the cells stimulated with other drugs were already double-positive, simultaneously carrying a marker for differentiation and expressing caspase-3 activity. To further confirm the involvement of caspase-3 in erythroid differentiation, an irreversible and specific caspase-3 inhibitor zDEVD-fmk was added to the cultures 30 min prior to tested drugs. The optimal concentrations of drugs were used to stimulate differentiation of K562 cells. Neither the DMSO vehicle nor the caspase inhibitor was toxic to the K562 cells or increased cell viability (not shown). In addition, they did not influence the differentiation level of K562 cells. After 4 days, zDEVD-fmk restrained caspase-3 activation in more than 50% of cells treated with cisplatin, Ara-C, hydroxyurea and haemin and in about 40% of cells treated with doxorubicin (Figure 3B). In parallel, a marked reduction in the percentages of cells with evidence of differentiation was observed. As shown in Figure 3C, zDEVD-fmk significantly reduced the level of GPA-positive cells in cultures treated with doxorubicin, cisplatin, hydroxyurea and Ara-C. The percentages of benzidine-positive cells were also significantly decreased by caspase-3 inhibitor (Figure 3E). In haemin-treated cultures, the decrease in the percentages of benzidine-positive cells or GPA-positive cells was not statistically significant (P > 0.05). After caspase-3 inhibition, the pool of double-positive cells in doxorubicin-, cisplatin- and hydroxyurea-treated cultures was decreased by more than 60%, and in Ara-C-treated cultures by more than 40% (Figure 3D). These results clearly indicate that the caspase-3 inhibitor zDEVD-fmk suppressed erythroid differentiation induced by doxorubicin, hydroxyurea, Ara-C and cisplatin. Haemin (30 µM) did not induce differentiation within 5 days, as indicated by the low level of GPA-positive cells. In addition, the caspase-3 inhibitor did not reduce the high level of benzidine-positive cells.
Figure 3.
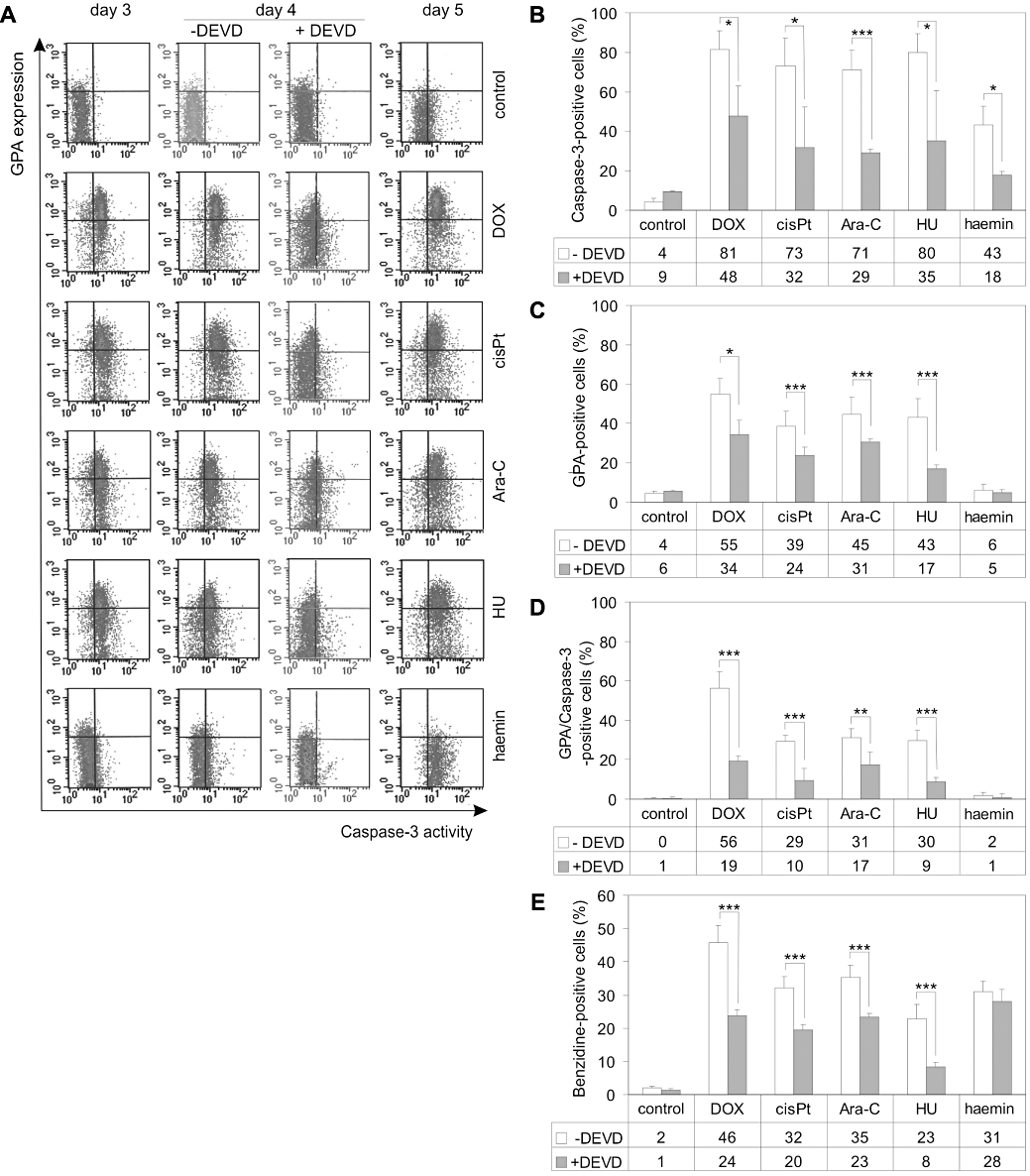
Caspase-3 is involved in the pharmacologically-induced differentiation of K562 cells. (A) Untreated cells or cells treated for 3, 4 and 5 days with 100 nM DOX, 5 µM cisPt, 250 nM Ara-C, 600 µM HU and 30 µM haemin were incubated with FITC-conjugated NucView caspase-3 substrate and PE-conjugated anti-GPA monoclonal antibodies and analysed by flow cytometry (FL1 and FL2 channels respectively). In selected experiments, the caspase-3 inhibitor zDEVD-fmk (50 µM) was added to the cultures 30 min prior to drug applications and incubated with drugs for 4 days. Density plots are from a single typical experiment. The percentage of caspase-3-positive (B), GPA-positive (C) and GPA/caspase-3-double-positive (D) cells were determined from density plots obtained in drug-treated cultures with or without zDEVD-fmk. (E) K562 cells were treated with tested drugs at selected concentrations for 4 days with or without zDEVD-fmk (50 µM). Next, differentiation was assessed by benzidine staining. Data represent the mean ± SD of at least three independent experiments carried out in triplicate. Asterisks indicate significant differences (*P < 0.05; **P < 0.01; ***P < 0.001) for the data points obtained with versus without zDEVD-fmk. Ara-C, cytosine arabinoside; cisPt, cisplatin; DOX, doxorubicin; FITC, fluorescein isothiocyanate; GPA, glycophorin A; HU, hydroxyurea; PE, phycoerythrin; PI, propidium iodide.
Doxorubicin, cisplatin, Ara-C, hydroxyurea and haemin treatment of K562 cells caused mitochondrial transmembrane potential dissipation (ΔΨm)
Caspase-3 can be activated along two major pathways: the extrinsic (death receptor-mediated) and intrinsic (mitochondria-dependent) pathways. Of these two, the mitochondrial pathway is commonly triggered during chemotherapy-induced apoptosis. To determine whether caspase-3 is activated along the mitochondrial pathway in drug-induced differentiation, changes in ΔΨm were assessed in K562 cells treated with 100 nM doxorubicin, 5 µM cisplatin, 250 nM Ara-C, 600 µM hydroxyurea and 30 µM haemin. All tested drugs caused a progressive loss of ΔΨm in K562 cells (Figure 4). Already on day 3, more than 10% of cells treated with doxorubicin, Ara-C, cisplatin and hydroxyurea exerted ΔΨm dissipation. Only in haemin-treated cells, was the ΔΨm not affected on day 3. On day 5, as much as 54% of doxorubicin-treated cells exhibited low ΔΨm. Cisplatin, Ara-C, hydroxyurea and haemin decreased ΔΨm in 50%, 48%, 45% and 26% of cells, respectively. At the same time, only about 10% of cells were PI-positive, as assessed by FACS (Figure 1C). The addition of zDEVD-fmk to the drug-treated cultures did not significantly affect the dissipation of the ΔΨm (not shown).
Figure 4.
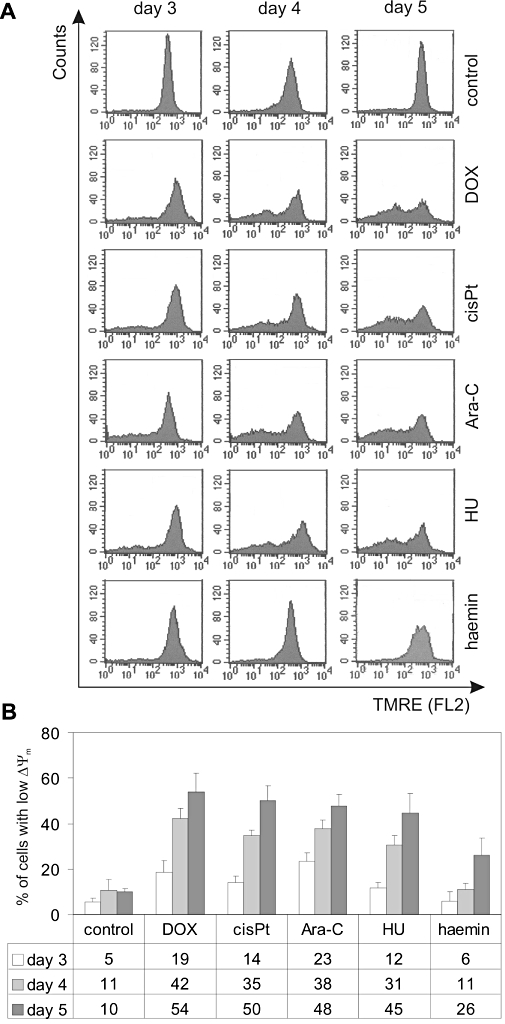
Mitochondrial transmembrane potential (ΔΨm) dissipation is observed in cells treated with DOX, cisPt, Ara-C, HU and haemin. Cells were cultured in the presence of 100 nM DOX, 5 µM cisPt, 250 nM Ara-C, 600 µM HU and 30 µM haemin for 3, 4 and 5 days at indicated concentrations. After treatment, cells were loaded with TMRE and analysed by flow cytometry (FL2 channel). (A) Histograms are shown from a single typical experiment. (B) Quantitative data are presented as means ± SD of percentages of cells with low TMRE fluorescence intensity (<2 × 102) from at least three independent experiments. Significant differences (P < 0.001) from the control data points were obtained, except for haemin on days 3 and 4 (P > 0.05). Ara-C, cytosine arabinoside; cisPt, cisplatin; DOX, doxorubicin; HU, hydroxyurea; TMRE, tetramethylrhodamine ethyl ester.
GATA-1 activity was not diminished with increased caspase-3 activity upon treatment of K562 cells with doxorubicin, hydroxyurea, cisplatin, Ara-C and haemin
GATA-1 transcription factor is essential for cell survival and maturation of erythroid progenitors. On the other hand, it is a target of caspase-3, and it is cleaved upon erythropoietin (Epo) starvation or induction of death receptor Fas (De Maria et al., 1999; Gregoli and Bondurant, 1999). Using the EMSA we investigated the levels of GATA-1-binding activity in nuclear extracts prepared from K562 cells after 3 days of treatment with doxorubicin, hydroxyurea, cisplatin, Ara-C and haemin at the indicated concentrations. EMSA, using the double-stranded oligonucleotide containing GATA-1-binding site from the Bcl-XL promoter, was performed on equal amounts of protein from nuclear extracts. The specificity of the protein–DNA complexes was confirmed by competition with ds-oligonucleotides: specific and non-specific (NFκB-binding site). The results revealed that the binding of GATA-1 to DNA was not lost but rather slightly increased on day 3, when the high activity of caspase-3 was already observed (Figure 5). Band intensities quantified by densitometric analysis were increased in cells treated with haemin by 1.34 ± 0.18-fold, cisplatin 1.44 ± 0.15-fold, Ara-C 1.58 ± 0.59-fold, doxorubicin 1.61 ± 0.44-fold and hydroxyurea 1.70 ± 0.69-fold. This suggests that even if induction of GATA-1 and activation of caspase-3 by cytostatic compounds were coincident, caspase-3 did not diminish the activity of GATA-1 by cleavage of this erythroid transcription factor. These results are in line with previously published ones (Szulawska et al., 2007) showing an enhancement of GATA-1 DNA-binding activity, and an increase in GATA-1 and γ-globin mRNA levels in doxorubicin-treated K562 cells.
Figure 5.
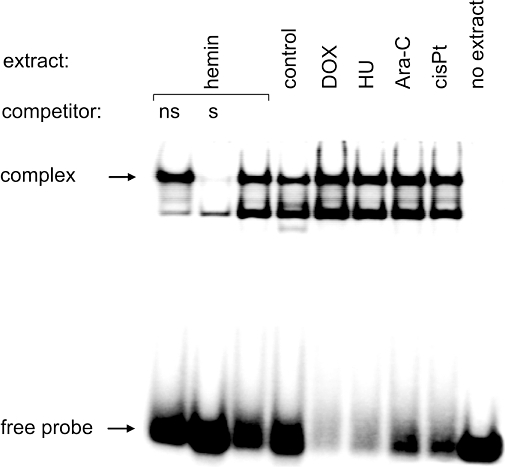
EMSA comparing the level of GATA-1-binding activity in nuclear extracts of untreated and DOX-, HU-, Ara-C-, cisPt- and haemin-treated K562 cells. EMSA was performed after incubation of 32P-labelled GATA-1 ds-oligonucleotides with nuclear extracts as described in Methods. Competition experiments showing the specificity of the protein–DNA complexes are included: lane 1 contains unrelated ds-oligonucleotide denoted as ‘ns’, and lane 2 contains specific competitor (non-radioactive GATA-1 ds-oligonucleotide) denoted as ‘s’. The position of DNA–GATA-1 complexes and the free DNA probe are indicated by arrows. Quantitative results from three independent experiments showing band intensity obtained with nuclear extracts from drug-treated versus control cells are presented in the Results section. Ara-C, cytosine arabinoside; cisPt, cisplatin; DOX, doxorubicin; EMSA, electrophoretic mobility shift assay; HU, hydroxyurea.
Discussion and conclusions
Caspases play a central role in apoptosis. In addition, they have been found to be essential for cell survival, differentiation, proliferation, NFκB activation and homeostasis in a variety of cells. Recent reports indicate that they are involved in differentiation of embryonic keratinocytes (Okuyama et al., 2004), neural stem cells (Fernando et al., 2005), lens cells (Weber and Menko, 2005; Zandy et al., 2005), monocytes into macrophages (Sordet et al., 2002), skeletal muscles (Fernando et al., 2002) and osteoblasts (Miura et al., 2004). Caspase-3 is needed to mediate differentiation of embryonic stem cells (Fujita et al., 2008). Several reviews have summarized the importance of caspases in cellular differentiation (Schwerk and Schulze-Osthoff, 2003; Launay et al., 2005; Kuranaga and Miura, 2007; Lamkanfi et al., 2007; Droin et al., 2008; Galluzzi et al., 2008). In addition, the apoptotic machinery is involved in normal erythropoiesis. A non-apoptotic role for active caspase-3 was proven to be essential for erythroid differentiation (Zermati et al., 2001; Aispuru et al., 2008). Caspase-3 is transiently activated through the mitochondrial pathway (Zermati et al., 2001), and its activity peaks at the erythroid colony-forming unit (CFU-E) stage (Carlile et al., 2004). To counterbalance the appearance of pro-apoptotic molecules, Epo promotes erythroid precursor survival by maintaining the expression of Bcl-XL (Zermati et al., 2001). Bcl-XL acts by preventing the release of pro-apoptotic molecules from mitochondria (Gregoli and Bondurant, 1999). The transcription factor GATA-1 is involved in Epo-mediated up-regulation of Bcl-XL (Gregory et al., 1999) and positively regulates expression of erythroid genes (Pevny et al., 1991). This transcription factor is a target for caspases in cells undergoing death upon Epo starvation or induction of death receptor Fas (De Maria et al., 1999; Gregoli and Bondurant, 1999), but it remains uncleaved during cell differentiation stimulated with Epo (Zermati et al., 2001; Carlile et al., 2004). The chaperone protein Hsp70 (heat shock protein 70) protects GATA-1 from caspase-3 cleavage in erythroid precursors undergoing differentiation (Ribeil et al., 2007). It was shown that the use of Hsp70-targeting siRNA in Epo-treated cultures of erythroid precursors led to GATA-1 cleavage, a decrease in haemoglobin content, down-regulation of Bcl-XL and cell death by apoptosis (Ribeil et al., 2007).
Several genetic alterations present in cancer cells introduce changes to the mechanisms responsible for cell fate. K562, an erythroleukaemic cell line derived from a patient with CML in blast crisis, bears interesting examples of such alterations. In this clearly defined genetic background, pharmacologically-induced differentiation has been studied extensively (Morceau et al., 1996; Belhacène et al., 1998; Cortesi et al., 1999; Bianchi et al., 2000; Witt et al., 2000; Park et al., 2001; Jacquel et al., 2003; Huang et al., 2004; Woessmann et al., 2004; Czyz et al., 2005; 2008; Moosavi et al., 2007; Szulawska et al., 2007; Jakubowska et al., 2008). Due to the constitutive activity of the fusion kinase Bcr-Abl, K562 cells overexpress anti-apoptotic protein Bcl-XL, independently from Epo. A high expression of Bcl-XL and a mutation in the p53 gene make K562 cells resistant to apoptosis induced by anticancer drugs.
The fact that caspase-3 is activated during Epo-induced differentiation of erythroid progenitors (Zermati et al., 2001; Droin et al., 2008) raised the question as to whether it could be also involved in pharmacologically-induced differentiation of erythroleukaemic cells. To investigate this possibility, K562 cells were used to assess the induction of caspase-3 activity simultaneously with the induction of differentiation. A distinct increase in caspase-3 activity accompanied differentiation not apoptosis. GPA, a marker of erythroid cells, which appears during erythropoiesis at the basophilic normoblast stage of development and then increases throughout the rest of erythroid differentiation, was detected on day 3 in drug-treated K562 cells (Figure 3). The GPA levels increased during the following 2 days of treatment, and the highest change was observed for doxorubicin, from 54% on day 3 to 72% on day 5. The number of haemoglobin-positive cells was also growing (Figure 1B). To further substantiate the role of caspase-3 in pharmacologically-induced differentiation, a specific inhibitor of caspase-3 zDEVD-fmk was used (Figure 3). Along with caspase-3 inhibition, percentages of GPA-positive and haemoglobinized cells were decreased. Our results attempt to provide evidence that caspase-3 is a prerequisite for drug-induced differentiation of erythroleukaemic cells. As in normal erythropoiesis, the involvement of the mitochondria in the pathway leading to the drug-induced caspase-3 activation was shown (Figure 4).
Another important point addressed in this study pertained to the role of caspase-3 in the proteolysis of molecules important for erythroid differentiation. The non-apoptotic processes controlled by caspase-3 require the cleavage of selected targets but are different from those needed for apoptosis (Launay et al., 2005). For instance, during normal erythropoiesis, GATA-1 is not cleaved by caspase-3 but it is a substrate of this protease in progenitors undergoing apoptosis upon Epo starvation (De Maria et al., 1999). Accordingly, our results indicate that GATA-1 was not a target of caspase-3 during pharmacologically-induced differentiation of K562 cells. GATA-1 can be activated in K562 cells by several drugs including butyrate (Chénais, 1998), N-acetylcysteine (Partington and Patient, 1999) and anthracyclines (Jeannesson et al., 1997); (Gillet et al., 2002). The mRNA level of GATA-1 is increased by more than twofold in the presence of doxorubicin or its morpholine derivatives as assessed by real-time PCR (Szulawska and Czyz, 2007). Also, expression of the GATA-1-dependent gene was enhanced, as shown previously for γ-globin in doxorubicin-stimulated cells (Szulawska et al., 2007). In the present study, GATA-1 activity was also slightly increased upon treatment with doxorubicin, hydroxyurea, cisplatin, Ara-C and haemin (Figure 5). This might be connected with increased stability of GATA-1 mRNA, as shown previously for doxorubicin (Szulawska et al., 2007). The influence of other drugs used in the study on GATA-1 mRNA stability were not tested and distinct mechanisms such as increased transcription rate, as observed for instance in the case of aclarubicin treatment (Jeannesson et al., 1997), should also be considered. Nevertheless, in pharmacologically-induced differentiation that is accompanied by increased caspase-3 activity, the DNA-binding activity of GATA-1 was not diminished, but rather increased (Figure 5). The chaperone protein Hsp70 should be considered as a protective molecule for GATA-1, as it exerts its anti-apoptotic function downstream of caspase-3 activation (Jäätteläet al., 1998). Indeed, K562 cells contain an elevated level of endogenous Hsp70 (Guo et al., 2005), which could protect GATA-1 from caspase-3 cleavage, similarly to normal erythroid development.
Among other anti-apoptotic events, GATA-1-dependent expression of Bcl-XL is required for the survival of erythroid cells during differentiation (Gregoli and Bondurant, 1997). Our recent findings (Czyz et al., 2008) have indicated the importance of Bcl-XL, which is also overexpressed in K562 cells (Benito et al., 1996), in keeping cells on the differentiation track after stimulation with cytostatic drugs. It was shown that doxorubicin alone induced differentiation, whereas in combination with STI571 it induced apoptosis in about 50% of K562 cells and differentiation in only a few percent of cells (Czyz et al., 2008). The inhibition of Bcr-Abl activity by STI571 could decrease the activity of Bcl-XL to a level that is not sufficient to counterbalance the massive release of pro-apoptotic molecules induced by stimulation with doxorubicin. We cannot exclude the possibility that caspase-3 activation in response to cytostatic drugs is limited, at least partially, by the lower level of Apaf-1 in K562 cells, the protein important for apoptosome formation (Fu et al., 2003) or the failure of Bax targeting to mitochondria, as was observed for K562 cells stimulated with etoposide (Jia et al., 2001).
Results obtained for haemin-treated K562 cells are controversial. Although in our experiments haemin led to the appearance of benzidine-positive cells (Figure 1), as shown previously (Baliga et al., 1993; Addya et al., 2004), we were not able to detect a substantial percentage of GPA-positive cells after treatment with 30 µM haemin for 5 days (Figure 3). In this respect, there is a contradiction between our results and those previously obtained for 30 µM haemin, where about 50% of K562 cells were CD71/GPA-positive after 4 days of treatment (Di Pietro et al., 2007). On the other hand, the possibility that benzidine positivity after haemin treatment could be due to the presence of free haemin being taken up by the cells from the medium should be taken into account (Cioe et al., 1981). Our results do not exclude the possibility that haemin is a weak inducer of differentiation, and in our experimental system a longer time would be necessary to stimulate erythroid markers. This notion is supported by the delay in the ΔΨm dissipation observed in haemin-treated K562 cells (Figure 4). Caspase-3 activity was also increased later than in cultures treated with the other drugs (Figure 3). In addition, it has been found that haemin-induced differentiation is reversible (Dean et al., 1981) and does not involve up-regulation of GATA-1 and NF-E2 transcription factors (Morceau et al., 1996).
Taken together, our data obtained for four diverse anticancer drugs: doxorubicin, hydroxyurea, cisplatin and Ara-C indicate that: (i) caspase-3 is involved in pharmacologically-induced differentiation of the CML cell line K562; (ii) as with the apoptotic response to many anticancer drugs, mitochondrial membrane potential dissipation seems to be necessary to activate caspase-3 for this non-apoptotic function; and (iii) GATA-1 is not a target for activated caspase-3 as its DNA-binding activity is increased rather than diminished. In addition, we could speculate, that the observed phenomena depend on the specific genetic background of K562 cells such as an elevated level of chaperone protein Hsp70 and a high level of anti-apoptotic protein Bcl-XL (Figure 6). Apart from its significance in the regulation of growth of cancer cells, these findings may also have some impact on our understanding of how important the genetic background of cancer cells is for the type of response to cytotoxic drugs. Future studies are needed to elucidate the specific role of caspase-3 in pharmacologically-induced differentiation, which would provide a valuable insight into the function of other pro- and anti-apoptotic molecules in the context of cell differentiation mediated by anticancer drugs. For instance, differentiation of erythroid progenitors is associated with the caspase-mediated proteolysis of several proteins otherwise involved in the apoptotic process such as poly (ADP-ribose) polymerase (PARP)1, lamin B and acinus (Droin et al., 2008). It would be of interest to evaluate whether the drug-induced caspase-3 activity is sufficient to trigger proteolysis of these substrates. It has been demonstrated, for instance, that a dose-dependent increase in cleaved caspase-3 and cleaved PARP levels occurs in K562 cells treated with atiprimod (Faderl et al., 2007). Pretreatment with the differentiating agent sodium butyrate has also been found to enhance PARP cleavage in etoposide-treated K562 cells (Kurz et al., 2001). Cleavage of these targets is thought to induce enucleation during erythropoiesis (Droin et al., 2008). However, the pharmacologically-induced differentiation of K562 cells, which results in the inhibition of cell growth and the induction of the haemoglobin synthesis, never leads to the appearance of enucleated mature erythrocytes.
Figure 6.
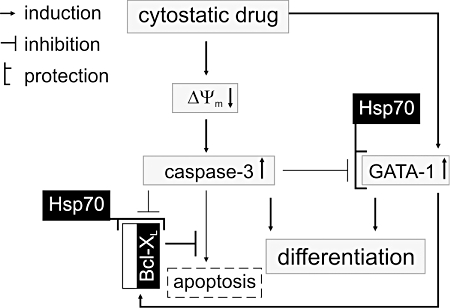
A hypothetical model of caspase-3 involvement in pharmacologically-induced erythroid differentiation of K562 cells. Cytostatic drugs trigger activity of transcription factor GATA-1 (Figure 5; Jeannesson et al., 1997; Chénais, 1998; Partington and Patient, 1999; Gillet et al., 2002; Szulawska et al., 2007). Caspase-3 activity is simultaneously induced via mitochondrial pathway (Figure 4; Czyz et al., 2008). However, an elevated level of Bcl-XL in K562 (Benito et al., 1996) can prevent the massive release of apoptotic molecules from mitochondria to the cytosol. High endogenous levels of chaperone protein Hsp70 (Guo et al., 2005) can protect GATA-1 and Bcl-XL from caspase-3-mediated proteolysis. In turn, GATA-1 can promote expression of erythroid and anti-apoptotic genes (Weiss and Orkin, 1995; Szulawska et al., 2007). A pharmacologically-induced differentiation is, therefore, similar to normal erythropoiesis, also involving the GATA-1, Bcl-XL and Hsp70 proteins (Ribeil et al., 2007), but as the Bcl-XL and Hsp70 proteins are overexpressed in K562, erythropoietin is not needed to trigger the signalling cascades leading to the differentiation of erythroleukaemic cells. Thus, both proteins Bcl-XL and Hsp70, which are expressed in K562 cells at high levels, might be important for keeping cells on track of differentiation in the presence of drug-activated caspases. The importance of Bcl-XL is also supported by the observation that inhibition of Bcr-Abl by STI571 induces apoptosis in CML cells by down-regulation of Bcl-XL (Oetzel et al., 2000). Grey boxes – changes induced by cytotoxic drugs; black boxes – proteins already present at high levels in K562 cells. ΔΨm, mitochondrial transmembrane potential; Bcl-XL, Bcl-2-related gene, long isoform; CML, chronic myelogenous leukaemia; Hsp70, heat shock protein 70.
Acknowledgments
The authors thank Dr Markus Duechler for critical reading of the manuscript. This research was supported by Grants 502-11-602 and 503-1099-2 from Medical University of Lodz.
Glossary
Abbreviations:
- ΔΨm
mitochondrial transmembrane potential
- AO
acridine orange
- Ara-C
cytosine arabinoside
- Bcl-XL
Bcl-2-related gene, long isoform
- cisPt
cisplatin
- CML
chronic myelogenous leukaemia
- DOX
doxorubicin
- EB
ethidium bromide
- EMSA
electrophoretic mobility shift assay
- Epo
erythropoietin
- FACS
fluorescence-activated cell sorting
- FITC
fluorescein isothiocyanate
- GPA
glycophorin A
- Hsp70
heat shock protein 70
- HU
hydroxyurea
- PE
phycoerythrin
- PI
propidium iodide
- TMRE
tetramethylrhodamine ethyl ester
- PARP
poly (ADP-ribose) polymerase
Conflict of interest
The authors state no conflict of interest.
References
- Addya S, Keller MA, Delgrosso K, Ponte CM, Vadigepalli R, Gonye GE, et al. Erythroid-induced commitment of K562 cells results in clusters of differentially expressed genes enriched for specific transcription regulatory elements. Physiol Genomics. 2004;19:117–130. doi: 10.1152/physiolgenomics.00028.2004. [DOI] [PubMed] [Google Scholar]
- Aispuru GR, Aguirre MV, Aquino-Esperanza JA, Lettieri CN, Juaristi JA, Brandan NC. Erythroid expansion and survival in response to acute anemia stress: the role of EPO receptor, GATA-1, Bcl-xL and caspase-3. Cell Biol Int. 2008;32:966–978. doi: 10.1016/j.cellbi.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Baliga BS, Mankad M, Shah AK, Mankad VN. Mechanism of differentiation of human erythroleukaemic cell line K562 by haemin. Cell Prolif. 1993;26:519–529. doi: 10.1111/j.1365-2184.1993.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Belhacène N, Maulon L, Guérin S, Ricci JE, Mari B, Colin Y, et al. Differential expression of the Kell blood group and CD10 antigens: two related membrane metallopeptidases during differentiation of K562 cells by phorbol ester and hemin. FASEB J. 1998;12:531–539. doi: 10.1096/fasebj.12.7.531. [DOI] [PubMed] [Google Scholar]
- Benito A, Silva M, Grillot D, Nuñez G, Fernández-Luna JL. Apoptosis induced by erythroid differentiation of human leukemia cell lines is inhibited by Bcl-XL. Blood. 1996;87:3837–3843. [PubMed] [Google Scholar]
- Bianchi N, Ongaro F, Chiarabelli C, Gualandi L, Mischiati C, Bergamini P, et al. Induction of erythroid differentiation of human K562 cells by cisplatin analogs. Biochem Pharmacol. 2000;60:31–40. doi: 10.1016/s0006-2952(00)00297-5. [DOI] [PubMed] [Google Scholar]
- Carlile GW, Smith DH, Wiedmann M. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood. 2004;103:4310–4316. doi: 10.1182/blood-2003-09-3362. [DOI] [PubMed] [Google Scholar]
- Chénais B. Requirement of GATA-1 and p45 NF-E2 expression in butyric acid-induced erythroid differentiation. Biochem Biophys Res Commun. 1998;253:883–886. doi: 10.1006/bbrc.1998.9869. [DOI] [PubMed] [Google Scholar]
- Cioe L, McNab A, Hubbell HR, Meo P, Curtis P, Rovera G. Differential expression of the globin genes in human leukaemia K562(S) cells induced to differentiate by hemin or butyric acid. Cancer Res. 1981;41:237–243. [PubMed] [Google Scholar]
- Cortesi R, Gui V, Gambari R, Nastruzzi C. In vitro effect on human leukemic K562 cells of co-administration of liposome-associated retinoids and cytosine arabinoside (Ara-C) Am J Hematol. 1999;62:33–43. doi: 10.1002/(sici)1096-8652(199909)62:1<33::aid-ajh6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Czyz M, Gniazdowski M. Actinomycin D specifically inhibits the interaction between transcription factor Sp1 and its binding site. Acta Biochim Pol. 1998;45:67–73. [PubMed] [Google Scholar]
- Czyz M, Szulawska A, Bednarek AK, Düchler M. Effects of anthracycline derivatives on human leukemia K562 cell growth and differentiation. Biochem Pharmacol. 2005;70:1431–1442. doi: 10.1016/j.bcp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Czyz M, Jakubowska J, Sztiller-Sikorska M. STI571/doxorubicin concentration-dependent switch for diverse caspase actions in CML cell line K562. Biochem Pharmacol. 2008;75:1761–1773. doi: 10.1016/j.bcp.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Dean A, Erard F, Schneider AP, Schechter AN. Induction of hemoglobin accumulation in human K562 cells by hemin is reversible. Science. 1981;212:459–461. doi: 10.1126/science.6163216. [DOI] [PubMed] [Google Scholar]
- De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, et al. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 1999;401:489–493. doi: 10.1038/46809. [DOI] [PubMed] [Google Scholar]
- Di Pietro R, di Giacomo V, Caravatta L, Sancilio S, Rana RA, Cataldi A. Cyclic nucleotide response element binding (CREB) protein activation is involved in K562 erythroleukemia cells differentiation. J Cell Biochem. 2007;100:1070–1079. doi: 10.1002/jcb.21106. [DOI] [PubMed] [Google Scholar]
- Droin N, Cathelin S, Jacquel A, Guéry L, Garrido C, Fontenay M, et al. A role for caspases in the differentiation of erythroid cells and macrophages. Biochimie. 2008;90:416–422. doi: 10.1016/j.biochi.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Faderl S, Ferrajoli A, Harris D, Van Q, Kantarjian H, Estrov Z. Atiprimod blocks phosphorylation of JAK-STAT and inhibits proliferation of acute myeloid leukemia (AML) cells. Leuk Res. 2007;31:91–95. doi: 10.1016/j.leukres.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci USA. 2002;99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando P, Brunette S, Megeney LA. Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J. 2005;19:1671–1673. doi: 10.1096/fj.04-2981fje. [DOI] [PubMed] [Google Scholar]
- Fu WN, Bertoni F, Kelsey SM, McElwaine SM, Cotter FE, Newland AC, et al. Role of DNA methylation in the suppression of Apaf-1 protein in human leukaemia. Oncogene. 2003;22:451–455. doi: 10.1038/sj.onc.1206147. [DOI] [PubMed] [Google Scholar]
- Fujita J, Crane AM, Souza MK, Dejosez M, Kyba M, Flavell RA, et al. Caspase activity mediates the differentiation of embryonic stem cells. Cell Stem Cell. 2008;2:595–601. doi: 10.1016/j.stem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Joza N, Tasdemir E, Maiuri MC, Hengartner M, Abrams JM, et al. No death without life: vital functions of apoptotic effectors. Cell Death Differ. 2008;15:1113–1123. doi: 10.1038/cdd.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Gillet R, Bobichon H, Trentesaux C. Nuclear transcription factor GATA-1 is activated during aclacinomycin-induced erythroid differentiation. Biol Cell. 2002;94:267–273. doi: 10.1016/s0248-4900(02)01201-7. [DOI] [PubMed] [Google Scholar]
- Grebenová D, Kuzelová K, Pluskalová M, Peslová G, Halada P, Hrkal Z. The proteomic study of sodium butyrate antiproliferative/cytodifferentiation effects on K562 cells. Blood Cells Mol Dis. 2006;37:210–217. doi: 10.1016/j.bcmd.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Gregoli PA, Bondurant MC. The roles of Bcl-X(L) and apopain in the control of erythropoiesis by erythropoietin. Blood. 1997;90:630–640. [PubMed] [Google Scholar]
- Gregoli PA, Bondurant MC. Function of caspases in regulating apoptosis caused by erythropoietin deprivation in erythroid progenitors. J Cell Physiol. 1999;178:133–143. doi: 10.1002/(SICI)1097-4652(199902)178:2<133::AID-JCP2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Gregory T, Yu C, Ma A, Orkin SH, Blobel GA, Weiss MJ. GATA-1 and erythropoietin cooperate to promote erythroid cell survival by regulating bcl-xL expression. Blood. 1999;94:87–96. [PubMed] [Google Scholar]
- Guo F, Sigua C, Bali P, George P, Fiskus W, Scuto A, et al. Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood. 2005;105:1246–1255. doi: 10.1182/blood-2004-05-2041. [DOI] [PubMed] [Google Scholar]
- Huang M, Wang Y, Collins M, Graves LM. CPEC induces erythroid differentiation of human myeloid leukemia K562 cells through CTP depletion and p38 MAP kinase. Leukemia. 2004;18:1857–1863. doi: 10.1038/sj.leu.2403490. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Huang P, Keating MJ, Plunkett W. Differential incorporation of ara-C, gemcitabine, and fludarabine into replicating and repairing DNA in proliferating human leukemia cells. Blood. 1997;90:270–278. [PubMed] [Google Scholar]
- Jäättelä M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquel A, Herrant M, Legros L, Belhacene N, Luciano F, Pages G, et al. Imatinib induces mitochondria-dependent apoptosis of the Bcr-Abl-positive K562 cell line and its differentiation toward the erythroid lineage. FASEB J. 2003;17:2160–2162. doi: 10.1096/fj.03-0322. [DOI] [PubMed] [Google Scholar]
- Jacquel A, Colosetti P, Grosso S, Belhacene N, Puissant A, Marchetti S, et al. Apoptosis and erythroid differentiation triggered by Bcr-Abl inhibitors in CML cell lines are fully distinguishable processes that exhibit different sensitivity to caspase inhibition. Oncogene. 2007;26:2445–2458. doi: 10.1038/sj.onc.1210034. [DOI] [PubMed] [Google Scholar]
- Jakubowska J, Wasowska-Lukawska M, Czyz M. STI571 and morpholine derivative of doxorubicin collaborate in inhibition of K562 cell proliferation by inducing differentiation and mitochondrial pathway of apoptosis. Eur J Pharmacol. 2008;596:41–49. doi: 10.1016/j.ejphar.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Jeannesson P, Lahlil R, Chenais B, Devy L, Gillet R, Aries A, et al. Anthracyclines as tumor cell differentiating agents: effects on the regulation of erythroid gene expression. Leuk Lymphoma. 1997;26:575–587. doi: 10.3109/10428199709050893. [DOI] [PubMed] [Google Scholar]
- Jia L, Patwari Y, Srinivasula SM, Newland AC, Fernandes-Alnemri T, Alnemri ES, et al. Bax translocation is crucial for the sensitivity of leukaemic cells to etoposide-induced apoptosis. Oncogene. 2001;20:4817–4826. doi: 10.1038/sj.onc.1204628. [DOI] [PubMed] [Google Scholar]
- King SB. The nitric oxide producing reactions of hydroxyurea. Curr Med Chem. 2003;10:437–452. doi: 10.2174/0929867033368213. [DOI] [PubMed] [Google Scholar]
- Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17:135–144. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Kurz EU, Wilson SE, Leader KB, Sampey BP, Allan WP, Yalowich JC, et al. The histone deacetylase inhibitor sodium butyrate induces DNA topoisomerase II alpha expression and confers hypersensitivity to etoposide in human leukemic cell lines. Mol Cancer Ther. 2001;1:121–131. [PubMed] [Google Scholar]
- Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- Launay S, Hermine O, Fontenay M, Kroemer G, Solary E, Garrido C. Vital functions for lethal caspases. Oncogene. 2005;24:5137–5148. doi: 10.1038/sj.onc.1208524. [DOI] [PubMed] [Google Scholar]
- Miura M, Chen XD, Allen MR, Bi Y, Gronthos S, Seo BM, et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–1713. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll T, Czyz M, Holzmüller H, Hofer-Warbinek R, Wagner E, Winkler H, et al. Regulation of the tissue factor promoter in endothelial cells. Binding of NF kappa B-, AP-1-, and Sp1-like transcription factors. J Biol Chem. 1995;270:3849–3857. doi: 10.1074/jbc.270.8.3849. [DOI] [PubMed] [Google Scholar]
- Moosavi MA, Yazdanparast R, Lotfi A. ERK1/2 inactivation and p38 MAPK-dependent caspase activation during guanosine 5′-triphosphate-mediated terminal erythroid differentiation of K562 cells. Int J Biochem Cell Biol. 2007;39:1685–1697. doi: 10.1016/j.biocel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Morceau F, Aries A, Lahlil R, Devy L, Jardillier JC, Jeannesson P, et al. Evidence for distinct regulation processes in the aclacinomycin- and doxorubicin-mediated differentiation of human erythroleukemic cells. Biochem Pharmacol. 1996;51:839–845. doi: 10.1016/0006-2952(95)02240-6. [DOI] [PubMed] [Google Scholar]
- Nakamichi I, Habtezion A, Zhong B, Contag CH, Butcher EC, Omary MB. Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction. J Clin Invest. 2005;115:3007–3014. doi: 10.1172/JCI24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetzel C, Jonuleit T, Götz A, van der Kuip H, Michels H, Duyster J, et al. The tyrosine kinase inhibitor CGP 57148 (ST1 571) induces apoptosis in BCR-ABL-positive cells by down-regulating BCL-X. Clin Cancer Res. 2000;6:1958–1968. [PubMed] [Google Scholar]
- Okuyama R, Nguyen BC, Talora C, Ogawa E, Tommasi di Vignano A, Lioumi M, et al. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev Cell. 2004;6:551–562. doi: 10.1016/s1534-5807(04)00098-x. [DOI] [PubMed] [Google Scholar]
- Park JI, Choi HS, Jeong JS, Han JY, Kim IH. Involvement of p38 kinase in hydroxyurea-induced differentiation of K562 cells. Cell Growth Differ. 2001;12:481–486. [PubMed] [Google Scholar]
- Partington GA, Patient RK. Phosphorylation of GATA-1 increases its DNA-binding affinity and is correlated with induction of human K562 erythroleukaemia cells. Nucleic Acids Res. 1999;27:1168–1175. doi: 10.1093/nar/27.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D'Agati V, et al. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Ribeil JA, Zermati Y, Vandekerckhove J, Cathelin S, Kersual J, Dussiot M, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445:102–105. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]
- Rodrigue CM, Arous N, Bachir D, Smith-Ravin J, Romeo PH, Galacteros F, et al. Resveratrol, a natural dietary phytoalexin, possesses similar properties to hydroxyurea towards erythroid differentiation. Br J Haematol. 2001;113:500–507. doi: 10.1046/j.1365-2141.2001.02746.x. [DOI] [PubMed] [Google Scholar]
- Schwerk C, Schulze-Osthoff K. Non-apoptotic functions of caspases in cellular proliferation and differentiation. Biochem Pharmacol. 2003;66:1453–1458. doi: 10.1016/s0006-2952(03)00497-0. [DOI] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Sordet O, Rébé C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100:4446–4453. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]
- Szulawska A, Gniazdowski M, Czyz M. Sequence specificity of formaldehyde-mediated covalent binding of anthracycline derivatives to DNA. Biochem Pharmacol. 2005;69:7–18. doi: 10.1016/j.bcp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Szulawska A, Arkusinska J, Czyz M. Accumulation of gamma-globin mRNA and induction of irreversible erythroid differentiation after treatment of CML cell line K562 with new doxorubicin derivatives. Biochem Pharmacol. 2007;73:175–184. doi: 10.1016/j.bcp.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Weber GF, Menko AS. The canonical intrinsic mitochondrial death pathway has a non-apoptotic role in signaling lens cell differentiation. J Biol Chem. 2005;280:22135–22145. doi: 10.1074/jbc.M414270200. [DOI] [PubMed] [Google Scholar]
- Weiss MJ, Orkin SH. Transcription factor GATA-1 permits survival and maturation of erythroid precursors by preventing apoptosis. Proc Natl Acad Sci USA. 1995;92:9623–9627. doi: 10.1073/pnas.92.21.9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt O, Sand K, Pekrun A. Butyrate-induced erythroid differentiation of human K562 leukemia cells involves inhibition of ERK and activation of p38 MAP kinase pathways. Blood. 2000;95:2391–2396. [PubMed] [Google Scholar]
- Woessmann W, Mivechi NF. Role of ERK activation in growth and erythroid differentiation of K562 cells. Exp Cell Res. 2001;264:193–200. doi: 10.1006/excr.2000.5124. [DOI] [PubMed] [Google Scholar]
- Woessmann W, Zwanzger D, Borkhardt A. ERK signaling pathway is differentially involved in erythroid differentiation of K562 cells depending on time and the inducing agent. Cell Biol Int. 2004;28:403–410. doi: 10.1016/j.cellbi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Zandy AJ, Lakhani S, Zheng T, Flavell RA, Bassnett S. Role of the executioner caspases during lens development. J Biol Chem. 2005;280:30263–30272. doi: 10.1074/jbc.M504007200. [DOI] [PubMed] [Google Scholar]
- Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, et al. Caspase activation is required for terminal erythroid differentiation. J Exp Med. 2001;193:247–254. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]


