Abstract
Background and purpose:
Oily extracts of Sichuan and Melegueta peppers evoke pungent sensations mediated by different alkylamides [mainly hydroxy-α-sanshool (α-SOH)] and hydroxyarylalkanones (6-shogaol and 6-paradol). We assessed how transient receptor potential ankyrin 1 (TRPA1) and TRP vanilloid 1 (TRPV1), two chemosensory ion channels, participate in these pungent sensations.
Experimental approach:
The structure–activity relationships of these molecules on TRPA1 and TRPV1 was measured by testing natural and synthetic analogues using calcium and voltage imaging on dissociated dorsal root ganglia neurons and human embryonic kidney 293 cells expressing the wild-type channels or specific cysteine mutants using glutathione trapping as a model to probe TRPA1 activation. In addition, using Trpv1 knockout mice, the compounds' aversive responses were measured in a taste brief-access test.
Key results:
For TRPA1 activation, the cis C6 double bond in the polyenic chain of α-SOH was critical, whereas no structural specificity was required for activation of TRPV1. Both 6-shogaol and 6-paradol were found to activate TRPV1 and TRPA1 channels, whereas linalool, an abundant terpene in Sichuan pepper, activated TRPA1 but not TRPV1 channels. Alkylamides and 6-shogaol act on TRPA1 by covalent bonding whereas none of these compounds activated TRPV1 through such interactions. Finally, TRPV1 mutant mice retained sensitivity to 6-shogaol but were not responsive to α-SOH.
Conclusions and implications:
The pungent nature of components of Sichuan and Melegueta peppers was mediated via interactions with TRPA1 and TRPV1 channels and may explain the aversive properties of these compounds.
Keywords: chemosensation, structure–activity, calcium imaging, covalent binding, taste preference, TRP channel, TRPV1 knockout
Introduction
Certain spices impart complex sensations including pungency, described by sharp and biting sensory effects classically produced by mustard oil, garlic or the burning sensation of capsaicin. Among these spices, Sichuan pepper (genus Zanthoxylum) imparts unique sensory qualities, characterized as tingling, numbing and burning. These pungent sensations have been attributed to several alkylamides present in the pericarp of the dried fruit (Sugai et al., 2005a). Work on alkylamide-based pungency demonstrated that these compounds were agonists of both the capsaicin-activated receptor, transient receptor potential vanilloid 1 (TRPV1) (Caterina et al., 1997; Sugai et al., 2005b) and the cinnamaldehyde-activated receptor, TRP ankyrin 1 (TRPA1) (Story et al., 2003; Jordt et al., 2004; Koo et al., 2007; receptor nomenclature follows Alexander et al., 2008). More recently, two-pore potassium channels were found to be modulated by hydroxy-α-sanshool (α-SOH) in mechanoreceptors, potentially rationalizing some of the complex tingling and numbing perceptions associated with this compound (Bautista et al., 2008). Their pungent ‘sharp’ and ‘biting’ sensations may be attributed to TRPV1 and TRPA1 stimulation. Interestingly, oily extracts from Sichuan pepper are extremely rich in terpene compounds, with linalool being the most abundant (75% by weight). Linalool has been reported as a weak agonist of the menthol receptor, TRP melastatin 8 (TRPM8) (Behrendt et al., 2004), but little is known about its activity on other TRPs or its gustatory profile.
The essential oil of Melegueta pepper (Aframomum melegueta K. Schum) contains the hydroxyarylalkanones 6-shogaol and 6-paradol in approximately equal concentrations (Tackie et al., 1975). Like capsaicin, they contain a vanilloid moiety (see Figure 1) and activate TRPV1 and thus have been reported to be pungent (Lee and Surh, 1998; Witte et al., 2002). Whether they stimulate other TRP channels to mediate their sensory effects is unknown.
Figure 1.
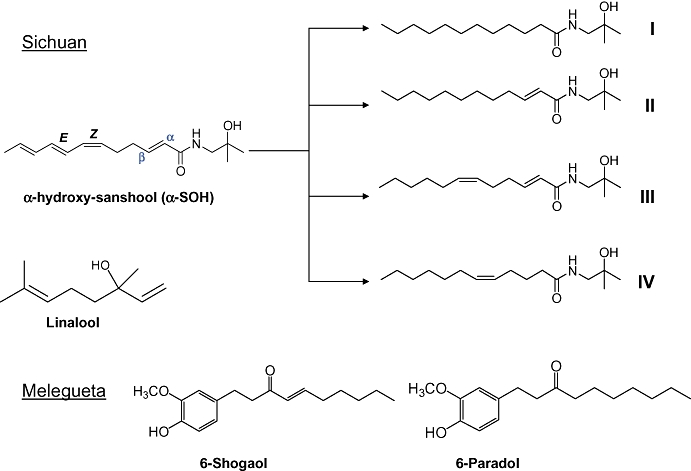
Chemical structures of compounds tested for activation of TRPV1, TRPA1 and TRPM8 channels. The natural compounds are contained in Sichuan (α-SOH and linalool) and Melegueta (6-paradol and 6-shogaol) peppers, whereas the synthetic compounds (I–IV) are analogues of α-SOH. The four synthetic analogues I–IV were tested to obtain their structure–activity relations. Linalool, is a monoterpene that markedly differs from the sanshools. The vanilloids, 6-paradol and 6-shogaol, only differ from each other in the α,β unsaturation. TRPA1, transient receptor potential ankyrin 1; TRPM8, transient receptor potential melastatin 8; TRPV1, transient receptor potential vanilloid 1.
Two studies have proposed that cinnamaldehyde and allyl-isothiocyanate activate TRPA1 through covalent binding on specific cysteine residues present in the ankyrin repeats of the channels (Hinman et al., 2006; Macpherson et al., 2007). Interestingly, the mutation of one or several reactive cysteine residues to serine leads to loss of sensitivity of TRPA1 to electrophilic agonists, but not to non-electrophilic compounds (Macpherson et al., 2007). This suggests that TRPA1 contains both a ‘traditional’ binding pocket and cysteine residues involved in covalent channel activation. The stimulation of TRPV1 receptors by capsaicin and other vanilloids is believed to occur via a non-covalent binding pocket in the transmembrane domain through π-stacking interactions between the aromatic moiety of Tyr 511 and the vanilloid ring moiety of capsaicin (Jordt and Julius, 2002). Plants of the Allium genus (onion and garlic) also stimulate TRPV1 in addition to TRPA1 (Macpherson et al., 2007) and recently they have been shown to act covalently on one intracellular cysteine residue in the N-terminal region of TRPV1 (Salazar et al., 2008). These findings raise the possibility that covalent modification of cysteine residues in the cytoplasmic terminus of the channels is the common mechanism for pungent TRPA1 and TRPV1 activation.
As many pungent compounds stimulate either TRPA1 and/or TRPV1, we evaluated the effects of the main constituents of Sichuan and Melegueta peppers and four synthetic analogues of α-SOH on both dissociated rat dorsal root ganglia (DRG) cells and on HEK293 cells expressing the human TRPA1 and TRPV1 receptors. We established that molecules present in these spices specifically stimulate TRPA1- and TRPV1-containing neurons with the exception of linalool that stimulates only TRPA1. In addition, we tested the effects of these molecules on cysteine mutants of TRPA1 and TRPV1 to address whether their mode of action on both TRPs would be similar. We found that covalent binding is critical for the stimulation of TRPA1 whereas it is not required for TRPV1. These results provide new insights into the understanding of TRPA1 and TRPV1 coding and their pharmacological responses to pungent compounds.
Methods
Technical sensory trials
Solutions of food-grade linalool (Sigma-Aldrich) diluted in Vittel® were evaluated by three volunteers. Solutions of 10 µM, 100 µM, 500 µM and 1 mM were kept in mouth for 30 s to evaluate the pungency with rinsing the mouth between each trial. Pungency of analogues (I–IV) of α-SOH was not assessed as these molecules are non-food-grade synthetic reagents and consequently we had no protocol for such compounds.
Glutathione adduct reaction
Compounds at 10 mM in water were incubated for several hours with an equimolar concentration of glutathione (GSH) to form adducts. Products of reactions were diluted 10 times in a solution of 50% MeOH and measured by electrospray ionization mass spectrometry.
Cloning and expression of human TRPV1 and TRPA1 receptors in HEK293 cells
Cloning and expression of these receptors was performed following previously published protocols (Riera et al., 2007). Briefly, cloned human TRPV1 cDNA was obtained from RZPD (Germany) and hTRPA1 cDNA from OriGene (Rockville, MD). Genes were subcloned into pcDNA5/FRT (Invitrogen, Carlsbad, CA) to generate stable cell lines using the Flp-In system (Invitrogen) after sequencing verification.
Site-directed mutagenesis on TRPA1 and TRPV1
Point mutants were generated using the Quick Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) on the hTRPA1 and the hTRPV1 clone. A triple TRPA1 cysteine mutant (C621S-C641S-C665S) and the cysteine point mutant of TRPV1 C158A were generated. For C158A, we verified that this region is conserved across humans, rats and mice. After sequence verification, mutants were transiently expressed in HEK293 cells using Lipofectamine 2000 (Invitrogen) and the respective response to various agonists was obtained using voltage imaging (see below).
Quantitative PCR analysis of cultured DRG neurons
Total RNA samples were isolated from cultured DRG neurons treated with β-NGF using the Nucleospin RNA II kit (Macherey-Nagel, Oensingen, Switzerland). Rat RNAs were reverse-transcribed into cDNA using SuperScript III (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The cDNA (equivalent to 50 ng RNA) was amplified by real time (RT)-PCR using an ABIPRISM 7900HT sequence detection system (Applied Biosystems, Foster City, CA). Taqman primers and probes were purchased from Applied Biosystems (Rn00583727_m1 for rat KCNK3, Rn00755967_m1 for rat KCNK9, Rn02345764_m1 for rat KCNK18, Rn01473803_m1 for rat TRPA1, Rn00676891_m1 for rat TRPV1). Detection of amplification relied on monitoring a reporter dye (6-FAM) linked to the 5′ end of a probe complementary to the sequence amplified by the primers. The cycling conditions were 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, and then 40 cycles of 95°C for 15 s and 60°C for 1 min. The β-actin primers were used as a positive control.
Measurement of intracellular calcium levels [Ca2+]i and membrane potential variation in HEK293 cells using a fluorescent plate reader
Cell lines stably expressing TRP channels were seeded into 96-well plates previously coated with poly-D-lysine. Cells were incubated in Hank's Balanced Salt Solution (HBSS) supplemented with 2 mM CaCl2 and 20 mM HEPES (pH 7.4), containing the cytoplasmic calcium indicator Fura-2/AM at 2 µM (Molecular Devices, Sunnyvale, CA). For membrane potential assays, cells were loaded with a voltage-sensitive dye according to protocol (Red dye, Molecular Devices) and fluorescence changes were measured after application of the test compounds (λex1= 530 nm, λem= 565 nm). Experiments were conducted at room temperature. Changes in [Ca2+]i from a homogenous cell population (approximately 100 000 cells) were measured as changes in fluorescence intensity when stimulated with agonists using a FLEXstation (Molecular Devices) (Riera et al., 2007). Cells expressing TRP channels and non-transfected controls were then challenged with the different compounds shown in Figure 1. Responses to molecules in HEK293 cells were expressed as a percentage of maximum responses evoked by 150 µM cinnamaldehyde for TRPA1 and 1 µM capsaicin for TRPV1 (these concentrations were assessed independently to be saturating under these conditions). For all experiments, calcium fluxes and voltage changes were measured as changes in fluorescence intensity, before and after the addition of agonists. The peak response was taken to be the characteristic value and was obtained by subtracting the peak value from the baseline (value before injection). A signal was considered as a response when greater than 5% over baseline. Dose–response curves were fitted using the Hill equation (GraphPad Prism Software, San Diego, CA) to obtain EC50 values and Hill coefficients. Data obtained from this study were expressed as mean ± SEM.
DRG culture and calcium imaging
Dissociated DRG neurons from neonatal (2–3 days) Sprague-Dawley rats were obtained frozen from Cambrex Bio Science (Walkersville, MD). Cells were cultured as previously described (Riera et al., 2007) and supplemented with nerve growth factor (β-NGF, Sigma-Aldrich) at a concentration of 100 ng·ml−1. Changes in [Ca2+]i were measured using ratiometric digital fluorescence imaging using Fura-2/AM. Images of individual neurons were acquired with a cooled, charge-coupled device camera (Cascade II, Photometrics, USA) mounted to an AxioObserver D1 inverted microscope. Autofluorescence was negligible and with illumination times of 100–300 ms, F340/F380 remained stable. Coverslips with attached neurons were placed in a chamber with continuous flow of supplemented HBSS. To provide a more physiological environment related to mouth physiology, chemical stimuli present in HBSS were applied at 30–33°C to the flow chamber for 5 s and cells were rinsed in supplemented HBSS between stimuli. [Ca2+]i transients are represented as an increase in the fluorescence ratio of Fura-2/AM-loaded DRG neurons (450 cells from two separate cultures were analysed). To reduce channel desensitization, we used relatively short stimulations (5 s) of one or two compounds per coverslip and then applied in some order cinnamaldehyde (TRPA1), capsaicin (TRPV1) or menthol (TRPM8) to determine TRPA1, TRPV1 and TRPM8 expressing DRGs. For each neuron, the average fluorescence ratio F340/F380 was calculated using Metafluor software (Universal Imaging Corp.).
Brief-access taste tests
Ten wild-type (WT) and 10 TRPV1 knockout (KO) mice (Jackson Laboratory; Bar Harbor, ME), age- and gender-matched, males and females, 100% C57BL/6J background were tested with three compounds. Brief-access taste tests were conducted using a gustometer (Davis MS160-Mouse gustometer; Dilog Instruments, Tallahassee, FL). The training and testing procedures were conducted as described previously (Glendinning et al., 2002; Damak et al., 2006). Mice were presented alternatively with two bottles; one containing the test compound (from ethanol stock dissolved in distilled water) and the other containing vehicle (equivalent volume of ethanol in distilled water) for 30 min testing periods. Licking starts a 5 s trial after which the shutter is closed whereupon the other bottle is presented. The number of licks per trial is recorded. Mice that did not get accustomed to the gustometer were excluded from the study. The animals were exposed every 2 days to the compounds. Initially, compound I and α-SOH were given in a crossed test at increasing concentrations. Mice were allowed to rest for a week before being challenged with increasing concentrations of 6-shogaol. The preference ratio (PR) was calculated as the mean number of licks per trial for the test compound (L1) divided by the mean number of licks per trial for tastant plus mean number of licks per trial for water (Lt). A PR of 0.5 indicates indifference, a ratio above 0.5 indicates preference and a ratio below 0.5 indicates avoidance.
Statistical analysis
The PRs for brief-access test were analysed by one-way anova for three factors (concentration, compound, genotype) using the general linear model repeated measures of the statistics package spss. A P value of less than 0.05 was considered significant.
Materials
Capsaicin, cinnamaldehyde, linalool, 2-aminoethyl diphenyl borate (2-APB), menthol, cis-6-nonenal and 2-aminoethyl methanethiosulphonate hydrobromide (MTSEA) were obtained from Sigma Aldrich (Buchs, Switzerland). 6-shogaol was purchased from Extrasynthese (Lyon, France) and 6-paradol was synthesized from 6-shogaol (see Supporting information S3). α-SOH was purified from the extract of green Sichuan pepper, Zanthoxylum spp. (Supporting information S1). Four analogues of α-SOH (I–IV, see Figure 1) were synthesized as outlined in S2. All compounds were prepared as stock solutions (1 M) in ethanol and were soluble at working concentrations in aqueous solutions. Pluronic acid was not used to increase compound solubility as it can affect responses of TRP channels (Bautista et al., 2008).
Results
Components of Sichuan and Melegueta peppers and synthetic alkylamides derived from α-SOH
Sichuan pepper oil extract contains primarily linalool, an acyclic monoterpene, as well as various alkylamides, the most abundant being α-SOH (Figure 1). To evaluate the role of double bonds and their configuration in the polyenic chain of α-SOH, we synthesized four analogues of this compound and measured their activity on DRG neurons, hTRPV1 and hTRPA1 channels. The analogues of α-SOH comprise a fully saturated α-SOH (I), a mono-α,β unsaturated analogue (II), a di-unsaturated analogue of α-SOH possessing both the α,β double bond and the cis C6 double bond (III) and mono-cis C5 unsaturated compound (IV). Melegueta pepper contains hydroxyarylalkanones: 6-paradol and 6-shogaol, which differ from each other by the unsaturation in the α,β position (Figure 1). We determined which of three TRP (V1, A1 and M8) channels are activated by these compounds, their concentration dependence and if the activation occurs via covalent or non-covalent interactions.
Responses of DRG neurons to Sichuan and Melegueta compounds
To address if any of the compounds shown in Figure 1 would stimulate dissociated DRG neurons we measured compound-induced changes in [Ca2+]i. in 450 neurons. Figure 2 shows changes in [Ca2+]i in response to specific stimuli for three representative neurons. Figure 2A shows representative responses to α-SOH. One representative neuron is shown to have responded to α-SOH, cinnamaldehyde and capsaicin, suggesting that it expresses TRPA1 and TRPV1; another responded to α-SOH and capsaicin but not to cinnamaldehyde, suggesting that it contained TRPV1 but not TRPA1. A third neuron responded to menthol and not to α-SOH or capsaicin and thus is consistent with it containing TRPM8 channels. Analogues of α-SOH (I–IV) were found to stimulate capsaicin- and cinnamaldehyde-sensitive DRGs, but they had no effect on menthol-responsive DRGs (Figure 2B and C). In none of the 450 neurons tested was pure cinnamaldehyde (TRPA1) sensing identified. Of these 450 neurons, 135 responded to capsaicin, 85 responded to cinnamaldehyde and capsaicin and 89 responded only to menthol. The remaining neurons did not respond to any of these compounds.
Figure 2.
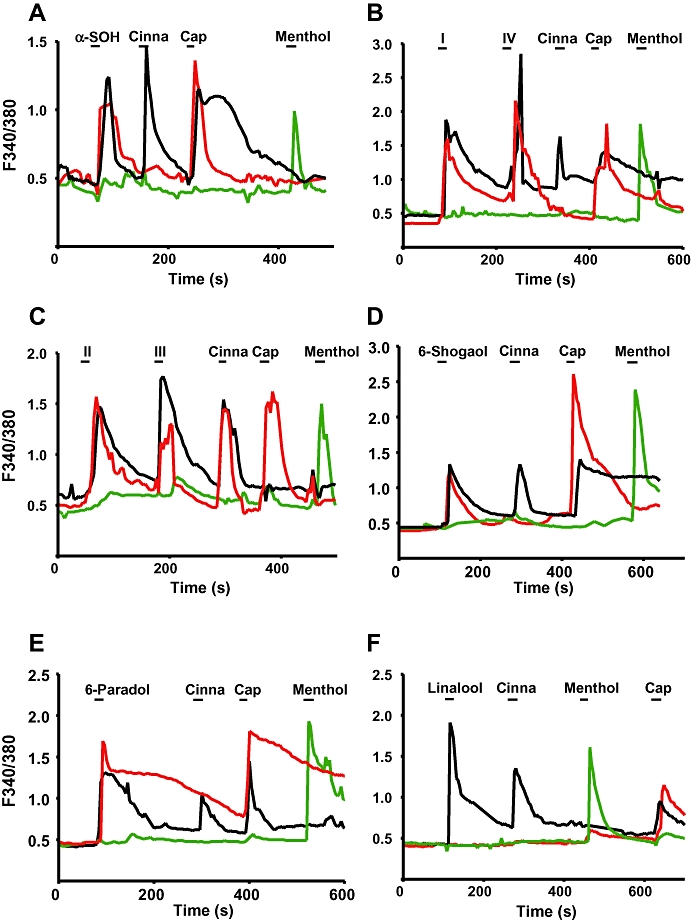
Sanshools, 6-shogaol, 6-paradol and linalool activate capsaicin- and cinnamaldehyde-sensitive dorsal root ganglia neurons. Each panel shows representative changes in [Ca2+]i of three neurons to capsaicin (Cap; TRPV1), cinnamaldehyde (Cinna; TRPA1) and menthol (TRPM8). Compounds were applied successively for 5 s, as shown by horizontal bars. Between stimulations neurons were perfused with supplemented HBSS. Maximal concentrations were used in these experiments. (A) 500 µM α-SOH, 100 µM Cinna, 1 µM Cap, 500 µM Menthol; (B) 500 µM I, 500 µM IV, 100 µM Cinna, 1 µM Cap, 500 µM Menthol; (C) 500 µM II, 500 µM III, 100 µM Cinna, 1 µM Cap, 500 µM Menthol; (D) 500 µM 6-shogaol, 100 µM Cinna, 1 µM Cap, 500 µM Menthol; (E) 500 µM 6-paradol, 100 µM Cinna, 1 µM Cap, 500 µM Menthol; (F) 500 µM linalool, 100 µM Cinna, 500 µM Menthol, 1 µM Cap. α-SOH, hydroxy-α-sanshool; TRPA1, transient receptor potential ankyrin 1; TRPM8, transient receptor potential melastatin 8; TRPV1, transient receptor potential vanilloid 1.
Both 6-shogaol and 6-paradol stimulated cinnamaldehyde- and capsaicin-sensitive DRGs, but neither compound had an effect on menthol-responding DRGs (Figure 2D and E). Neurons activated by linalool also responded both to cinnamaldehyde and capsaicin, but no changes in [Ca2+]i were evoked on purely capsaicin-sensitive DRGs, thus suggesting the involvement of TRPA1 (Figure 2F). Finally, linalool did not induce an increase in [Ca2+]i in menthol-sensitive DRGs.
Heterologously expressed TRPA1 and TRPV1 channels respond to natural and synthetic stimuli derived from Sichuan and Melegueta peppers
For all the compounds tested, only DRG neurons putatively expressing TRPA1 and TRPV1 responded. For this reason, menthol-activated TRPM8 channels were excluded from further investigation. Although the responses evoked in DRG neurons are suggestive of the channels they activate, the assignment is not definitive because many compounds activate numerous receptors and common antagonists in use for TRPA1 and TRPM8 are non-specific. Thus, to address this issue more directly, we used a heterologous system to test if these molecules would activate the cloned cinnamaldehyde-activated channel (hTRPA1) and the capsaicin-activated channel (hTRPV1).
To test the stimulus effect on TRPA1 and TRPV1 in HEK293 cells, changes in [Ca2+]i were typically monitored over time at different agonist concentrations. Although all eight compounds were tested, two representative examples are presented for increasing concentrations of compound IV and 6-paradol on TRPA1 (Figure 3A and C) and TRPV1 (Figure 3B and D). Exposure to both compounds produced robust increases in [Ca2+]i in TRPA1- and TRPV1-expressing neurons. In non-transfected cells none of the eight compounds produced significant increases in [Ca2+]i. over the concentration range tested (data not shown).
Figure 3.
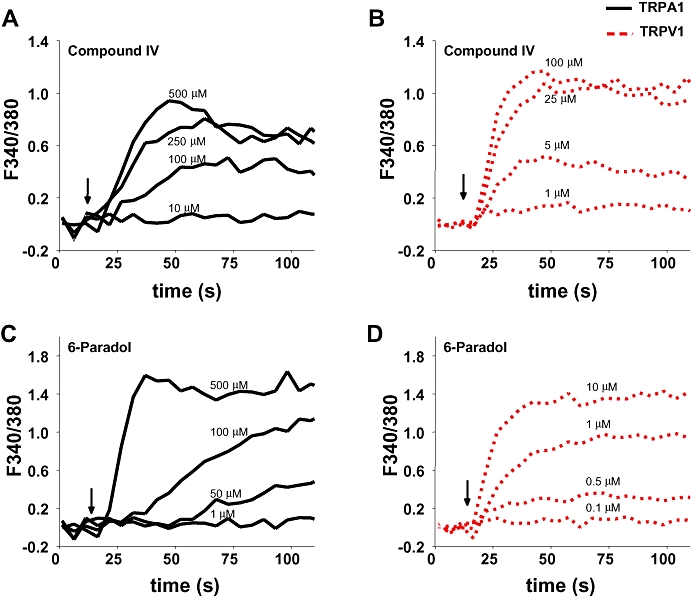
[Ca2+]i transients in HEK293 cells expressing TRPV1 and TRPA1. Typical traces obtained for TRPA1- and TRPV1-containing cells are shown for compound IV and 6-paradol. The changes in concentrations were obtained in separate experiments. TRPA1-expressing HEK293 cells respond to increased concentrations of IV (A) and 6-paradol (C). TRPV1-expressing HEK293 cells respond to increased concentrations of IV (B) and 6-paradol (D). At the end of each of these test compounds, maximal concentrations of cinnamaldehyde (150 µM, TRPA1) or capsaicin (1 µM, TRPV1) were tested (not shown). TRPA1, transient receptor potential ankyrin 1; TRPV1, transient receptor potential vanilloid 1.
Figure 4 shows the dose–response relation for each of the compounds on TRPA1 (Figure 4A and B) and TRPV1 channels (Figure 4C and D). Table 1 gives the EC50 values and the Hill coefficients of these compounds. The rankings of the EC50 values for TRPA1 are cinnamaldehyde > 6-shogaol > 6-paradol =α-SOH > IV > linalool> III > II > I. Note that the unsaturated analogue (I) did not evoke an appreciable effect and the monounsaturated α,β sanshool analogue (II) was a partial agonist whereas after cinnamaldehyde, the two vanilloids were the most potent activators. Linalool and 6-shogaol evoked about 90%, and 6-paradol 70% of the maximum cinnamaldehyde response. In contrast, α-SOH and analogues III and IV produced about 60% of the maximum response. As seen in the dose–responses curves, compound III displayed the same potency as compound IV, suggesting that the replacement of the cis C6 by a cis C5 olefin is a suitable model to study the structural arrangement of the natural molecule.
Figure 4.
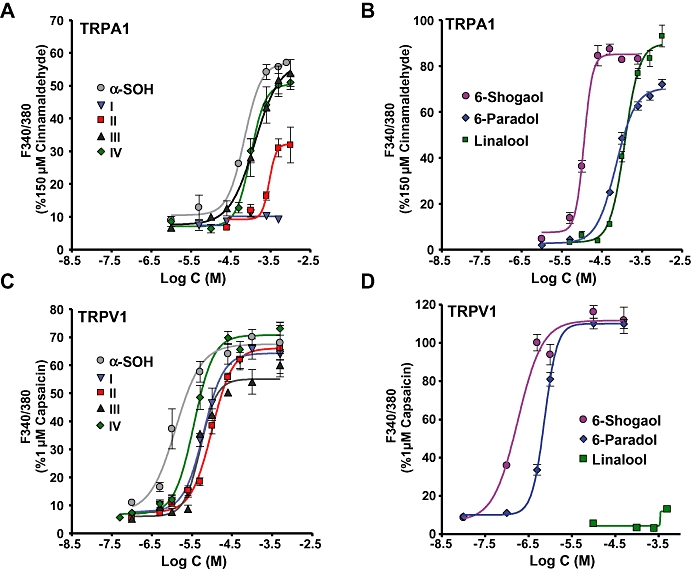
Dose–response profiles in HEK293 cells expressing TRPV1 or TRPA1. (A and B) TRPA1. (C and D) TRPV1. (A) Dose–responses for α-SOH and its analogues I–IV. (B) Dose–responses of 6-shogaol, 6-paradol and linalool. (C) Dose–responses for α-SOH and its analogues on TRPV1. (D) Dose–responses of linalool, 6-shogaol and 6-paradol. Note that linalool has no effect on TRPV1 until 1 mM. Means ± SEM (n= 4–5). Responses were normalized to maximal concentrations of 150 µM cinnamaldehyde and 1 µM capsaicin for TRPA1 and TRPV1 respectively. Data were fitted to the Hill equation. TRPA1, transient receptor potential ankyrin 1; TRPV1, transient receptor potential vanilloid 1.
Table 1.
EC50 values and Hill coefficients (nH) of selected TRPV1 and TRPA1 ligands
|
TRPA1 |
TRPV1 |
|||
|---|---|---|---|---|
| EC50 (µM) | nH | EC50 (µM) | nH | |
| Cinnamaldehyde | 6.75 | 1.8 | – | – |
| Capsaicin | – | – | 0.037 | 1.5 |
| α-SOH | 69 | 2.0 | 1.1 | 0.8 |
| I | – | – | 7.0 | 1.4 |
| II | – | – | 10.2 | 1.8 |
| III | 125 | 1.4 | 5.0 | 2.5 |
| IV | 100 | 2.3 | 3.5 | 1.8 |
| 6-Shogaol | 11.2 | 4.1 | 0.2 | 1.5 |
| 6-Paradol | 71 | 1.8 | 0.7 | 3 |
| Linalool | 117 | 2.4 | – | – |
α-SOH, hydroxy-α-sanshool; TRPA1, transient receptor potential ankyrin 1; TRPV1, transient receptor potential vanilloid 1.
Figure 4C and D show the dose–responses for the tested compounds on hTRPV1 channels relative to the maximal capsaicin response. The ranking of the EC50 values were: capsaicin > 6-shogaol > 6-paradol > α-SOH > III = IV > I > II >> linalool. Small differences in the EC50 of compounds I–IV were identified, implying that TRPV1 responses were relatively insensitive to alkyl chain unsaturation (at least to the extent of the compounds tested). We observed that the presence of the C8–C10-conjugated moiety increased α-SOH potency from compounds III and IV fivefold. Similarly, a fourfold increase in potency from 6-paradol to 6-shogaol was obtained, once again indicating the significance of the α,β unsaturation in the alkyl chain. Linalool concentrations (>1 mM) induced small changes in [Ca2+]i amounting to about 30% of the maximum capsaicin response (not shown). All compounds tested displayed an EC50 value larger than capsaicin, however, 6-shogal and 6-paradol slightly exceeded the intensity of the capsaicin [Ca2+]i increase. All sanshool responses saturated at about 70% of the capsaicin response.
Covalent binding of tested compounds to TRPA1 and TRPV1 channels
TRPA1
To determine if the tested TRPA1 ligands would react on TRPA1 cysteines as observed with cinnamaldehyde and other α,β unsaturated aldehydes (Macpherson et al., 2007), we constructed a reactive triple cysteine mutant of TRPA1 (TRPA1–3C) and measured responses using a membrane voltage-sensitive assay at maximal agonist concentrations (Figure 5).
Figure 5.
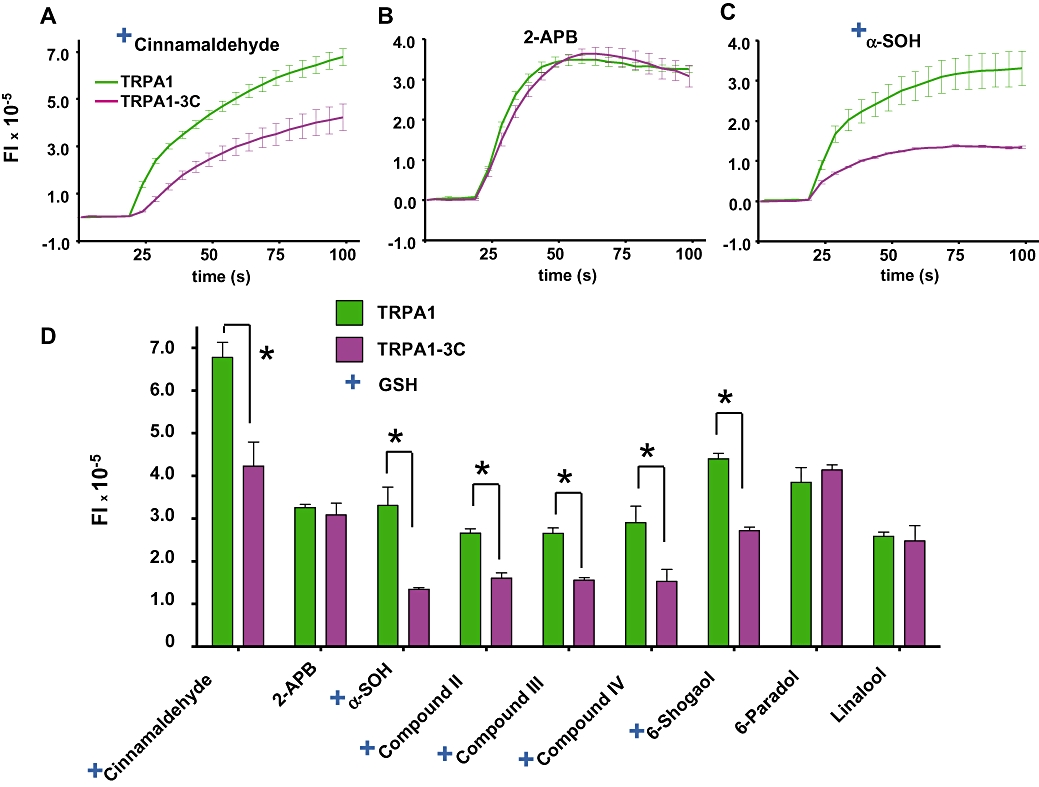
Targets of the N-terminal reactive cysteines in TRPA1. Voltage changes of HEK293 cells loaded with Red dye expressed as fluorescence intensity (FI) when stimulated with maximal concentrations of the tested compounds. Cells were transiently transfected with wild-type TRPA1 and TRPA1-3C and stimulated with (A) 150 µM cinnamaldehyde (Cinna), (B) 100 µM 2-APB. (C) 500 µM α-SOH. (D) Recapitulates peak responses of the cells to 150 µM Cinna, 100 µM 2-APB, 500 µM α-SOH, 500 µM II, 500 µM III, 500 µM IV, 100 µM 6-shogaol, 500 µM 6-paradol and 500 µM linalool. *P < 0.05 unpaired Student's t-test. + indicates the compounds that formed adducts with GSH. Means ± SEM (n= 4–5). 2-APB, 2-aminoethyl diphenyl borate; GSH, glutathione; α-SOH, hydroxy-α-sanshool; TRPA1, transient receptor potential ankyrin 1; TRPM8, transient receptor potential melastatin 8; TRPV1, transient receptor potential vanilloid 1.
As shown in the panels, with respect to the WT, the TRPA1 mutant's response to cinnamaldehyde was greatly reduced (Macpherson et al., 2007), whereas for the non-electrophile TRPA1 agonist, 2-APB (Hinman et al., 2006), the response was identical in both the WT and mutant. The response to linalool was the same in the WT and mutant, thus arguing for a non-electrophilic binding mechanism, whereas responses to α-SOH and analogues II–IV were markedly reduced in the TRPA1 triple cysteine mutant. Compound I was not tested as it was unable to produce calcium increases in hTRPA1 (Figure 4A). We also observed that the response of 6-paradol was unchanged in the mutant, although 6-shogaol was decreased by 35% under the same conditions.
To further demonstrate that the tested compounds could act covalently on TRPA1, we used GSH as a test for adduct formation (Macpherson et al., 2007). We found that cinnamaldehyde and 6-shogaol reacted covalently with the cysteine on GSH whereas 2-APB, linalool and 6-paradol did not (see Supporting information S4). We also found that α-SOH and the analogues II–IV reacted covalently with GSH. To test whether a cis unsaturated bond in the carbon backbone of the α-SOH system would be sufficient for covalent bonding, we used cis-6-nonenal, an aldehyde possessing the same cis unsaturation feature as α-SOH and found that it did not form adducts with GSH. Surprisingly, like its analogues, the fully saturated compound I formed adducts with GSH.
TRPV1
For rat TRPV1, a single reactive cysteine residue, C157A, has recently been characterized as a reactive residue for the stimulation by pungent sulphide compounds from garlic and onion (Salazar et al., 2008). Using this information we assessed for our molecules if TRPV1 reactivity was mediated by this cysteine, using the point mutant C158A. The results, shown in Figure 6, show that none of the compounds tested, with the exception of the cysteine-modifying agent MTSEA, evoked a response suggesting these ligands act via different mechanisms on TRPV1 and TRPA1 channels.
Figure 6.
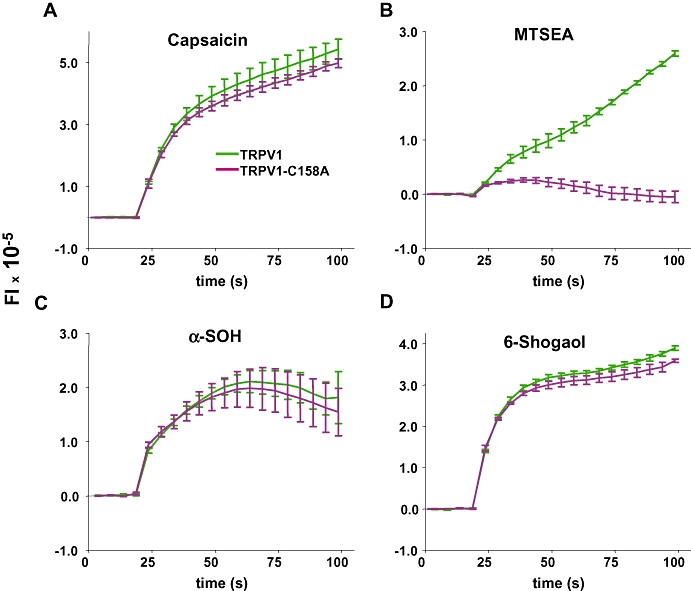
Compounds activate TRPV1 via non-covalent gating. Voltage changes of HEK293 cells loaded with Red dye expressed as a fluorescence intensity (FI) when stimulated with saturating concentrations of compounds. Cells were transiently transfected with wild-type TRPV1 and TRPV1-C158A and typical responses are shown for (A) 1 µM capsaicin (Cap), (B) 2 mM MTSEA, (C) 500 µM α-SOH. Means ± SEM (n= 4–5). MTSEA, 2-aminoethyl methanethiosulphonate hydrobromide; TRPV1, transient receptor potential vanilloid 1.
Trpv1 KO mice show diminished aversion to α-SOH and 6-shogaol
To determine whether TRPV1 KO mice would exhibit a taste aversion to α-SOH, we performed brief-access tests with both WT and KO mice when they were presented with α-SOH or vehicle (Figure 7A). The test involved determining the PR. 500 µM of α-SOH was perceived as slightly aversive by both WT and KO mice. However, 1 mM α-SOH was markedly aversive to WT animals but, in KO animals, the aversion was almost entirely eliminated (P < 0.001).
Figure 7.
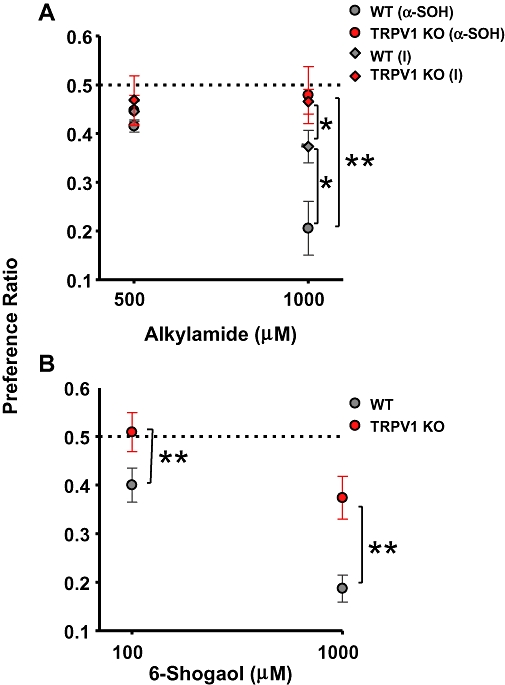
Brief-access taste preference test comparing the responses of TRPV1 KO and WT mice. Preference ratios of TRPV1 KO and WT mice for increasing concentrations of (A) α-SOH and compound I, (B) 6-shogaol. For each group data represent mean preference ratio ± SEM for 10 animals. *P < 0.05, **P < 0.001, one-way anova. KO, knockout; α-SOH, hydroxy-α-sanshool; TRPV1, transient receptor potential vanilloid 1; WT, wild type.
When tested with 1 mM of compound I, WT mice showed greater preference for it than for 1 mM α-SOH (P < 0.05). Compound I was selected because it did not activate heterologously expressed TRPA1 channels (Figure 4A). Comparison between the PR of α-SOH and compound I indicate that these compounds were not perceived as significantly different in the TRPV1 KO mice (P > 0.05).
When presented with increasing concentrations of 6-shogaol (Figure 7B), WT mice displayed an increasing aversion that was largely decreased in the KO mice (P < 0.01). For example, WT exhibited strong aversion to 1 mM 6-shogaol whereas the KO mice exhibited a significantly smaller aversive response (P < 0.01).
Discussion and conclusions
Given that sanshools and hydroxyarylalkananones produce a variety of sensations, including pungency, tingling and numbness, our goal was to determine whether and how compounds present in Zanthoxylum spp. and A. melegueta stimulate DRG neurons.
Vanilloids and sanshools stimulate TRPA1- and TRPV1-expressing DRGs
We found that sanshools and hydroxyarylalkanones induce calcium influx in neurons responding to capsaicin and cinnamaldehyde, but not to menthol (Figure 2). These results are consistent with the co-expression of TRPA1 and TRPV1 but not with TRPM8 in sensory neurons (Peier et al., 2002; Story et al., 2003). None of the compounds tested, including linalool, stimulated menthol-sensitive neurons (Figure 2F).
On many points our results confirm those of Bautista et al. (2008). We agree that sanshool responses are in capsaicin-sensitive neurons and also that they are not in TRPM8 (menthol sensitive) containing neurons. Our quantitative PCR results also show three different KCNK types of channels expressed in DRGs (Figure S6). However, our results showing that α-SOH did not activate DRGs, which did not respond to capsaicin, diverge from those of Bautista et al. (2008), who identified sanshool responses in both capsaicin-sensitive and insensitive neurons. This difference may, in part, be explained by the high expression of TRPV1 compared with KCNK channels in our rat DRG neuron preparations (Figure S6), which could be different in their mouse preparation. Also, we used frozen/thawed rat DRGs and they used primary cultures of dissected mice trigeminal ganglions and DRGs. Finally, Bautista et al. (2008) performed their imaging experiments at 22–25°C and we performed ours at 30–33°C. In this regard, KCNK channels may be less sensitive to sanshool at higher temperatures. Several studies have recently reported significant differences in the responses to TRPA1 ligands, between human and mouse as observed with caffeine (Nagatomo and Kubo, 2008) and menthol (Xiao et al., 2008). We did not, however, explore these differences. Our results diverge from those of Bautista et al. (2008) in another matter. We, as well as Koo et al. (2007), found that sanshool also activated cinnamaldehyde- and capsaicin-sensitive neurons, suggesting that sanshool activates neurons containing TRPA1 and TRPV1 channels. In contrast, Bautista et al. (2008) did not find sanshool responses in neurons that are activated by mustard oil and thus are presumably TRPA1-sensitive.
Our behavioural studies revealed that TRPV1 was essential in obtaining the aversive component of α-SOH, as TRPV1 KO animals treated 1 mM α-SOH as they did water (Figure 7A). This finding deviates from the behavioural results presented by Bautista et al. (2008) where their TRPV1/TRPA1 double KO mice remained sensitive to the aversive effect of 1 mM α-SOH. However, to assess taste preference we used a different testing paradigm from that used by Bautista et al. (2008). The brief-access test we used reflects primarily taste responses, whereas the drinking test used by Bautista et al. (2008) (3 h drinking) also includes post-ingestive effects. Taken together, the work of both studies cannot be directly compared.
The vanilloids 6-shogaol and 6-paradol stimulate TRPA1 and TRPV1 channels
Activation of TRPV1 by 6-shogaol and gingerols (Iwasaki et al., 2006) is consistent with their burning sensory profile (Govindarajan, 1982). Gingerols are highly similar to the shogaols and paradols with 6-gingerol differing from 6-paradol only by a single hydroxyl group at C6 of the alkyl chain (Figure S5). Increasing the hydrophilicity of these compounds in the transition of 6-shogaol to 6-gingerol coincides with the decreased potency on TRPV1 responses (Dedov et al., 2002).
Given its structural similarity to 6-shogaol, 6-paradol stimulation of TRPV1 was not surprising. However, that 6-paradol is less potent than 6-shogaol is likely to be a consequence of the missing α,β double bond that might weaken its binding in the capsaicin binding pocket. The large change in the Hill coefficients from capsaicin to 6-paradol is not understood (Table 1), but probably does not simply mean that ‘n’ molecules are needed to activate the channel.
Our results show that 6-shogaol and 6-paradol activate cinnamaldehyde (TRPA1) sensing DRGs and such TRPA1 activation was confirmed in heterologously expressed cells. Interestingly, these compounds stimulated both TRPA1 and TRPV1 channels in a dose-dependent manner, with TRPV1 being about 100-fold more potent (Figure 4B and D); most likely because of the better fit of the vanilloid moiety into the TRPV1 binding pocket.
Bandell et al. (2004) found that 8-gingerol was a TRPA1 agonist. The gingerols, shogaols and paradols differ from the non-TRPA1 agonist, capsaicin, mainly by the amide moiety in the alkyl chain, suggesting that the phenol core is not sufficient to confer TRPA1 specificity.
Role of the cis C6 double bond in the structure–activity-relationship of α-SOH on TRPA1 and TRPV1
To determine the structure–activity relationship defining the α-SOH recognition properties of TRPA1 and TRPV1 channels, we investigated the role of the double bonds in the polyenic chain using the synthetic analogues I–IV.
α-SOH and its analogues are TRPA1 and TRPV1 agonists with a diminished efficacy compared with cinnamaldehyde and capsaicin respectively (Figure 4). The inability of these alkylamides to produce total activation of the channels may arise from the presence of multiple closed states, receptor desensitization or shorter open times (Lape et al., 2008).
For α-SOH, our data show that the cis C6 bond is critical for the activity at TRPA1, but not at TRPV1 (Figure 4A and C). In this regard, the fully saturated (I) and α,β unsaturated (II) α-SOH analogues produce small TRPA1 responses while the cis C6 di-unsaturated (III) and cis C5 mono (IV) analogues stimulated TRPA1 to almost the same extent as α-SOH (Figure 4A), thereby highlighting the role of the cis double bond in the molecule's alkyl chain. Although we did not test the cis C6 mono-unsaturated analogue, our data show that the cis C5 compound activates TRPA1 and TRPV1 with similar potency to compound III, suggesting that the placement of this unsaturation at either C5 or C6 produces similar effects on the channels. In regard to TRPV1 stimulation, small differences in efficacy were observed for the other mono-unsaturated and fully saturated compounds (Figure 4C). These small changes are consistent with decreases in hydrophobicity or molecular flexibility of the tested compounds as α-SOH, being the most unsaturated, is also the most potent. Taken together, the observed structure–activity relationships show that α-SOH is recognized differently by TRPA1 and TRPV1 channels.
α,β Unsaturation of alkylamides does not provide TRPA1 specificity and is only partly required in shogaols to activate TRPA1
Thiol-reactive chemicals from mustard, garlic and cinnamon activate TRPA1 by covalent modification of N-terminal cysteine residues (Hinman et al., 2006; Macpherson et al., 2007). In contrast to its cis isomer, the C6 trans hydroxy-β-sanshool contains an α,β conjugated bond but does not stimulate TRPA1 (Koo et al., 2007). The weak effect on TRPA1 of the α,β unsaturated analogue (II) was unexpected (Figures 4A and 5E) because all other tested compounds with α,β unsaturation are TRPA1 agonists (Macpherson et al., 2007). The weak response of II does not seem to be due to hampered membrane permeation as another mono-unsaturated molecule with the same chain length (IV) and hydrophobicity stimulated TRPA1 through the N-terminal cysteines (Figures 4A and 5F).
We have made the important observation that covalent bonding via intracellular cysteines at the electrophilic carbonyl (Figure S4) occurs with all tested TRPA1 reactive alkylamides (Figure 5D). Indeed, independent of the degree of unsaturation of these compounds, GSH forms covalent adducts with the alkylamide tested (Figure S4). However, TRPA1 activity cannot be rationalized just in terms of covalent binding to a reagent as the configuration of the cis C6 unsaturation in the alkylamides also determines their effect on TRPA1 (Figure 4A).
TRPV1 reactivity to pungent chemicals did not require covalent binding at the intracellular cysteine C158
Pungent extracts from onion and garlic that stimulate both TRPA1 and TRPV1 channels act on TRPV1 by covalent modification of one cysteine residue of rat TRPV1, C157A (Salazar et al., 2008). By analogy, we looked for similar effects of the sanshools and the hydroxyarylalkanones. However, among the molecules that covalently bind to TRPA1, none activated TRPV1 through its reactive cysteine (Figure 6).
Possible physiological implications
In regard to the tingling sensation evoked by α-SOH, it is unlikely that its molecular basis is due to TRPA1 stimulation as many TRPA1 agonists do not produce this sensation. Recently, it has been suggested that the tingling could come from inhibitory effects of α-SOH on voltage-gated channels in tactile fibres containing two-pore potassium channels (Bautista et al., 2008). Other sanshools (δ,γ) stimulate TRPV1 and cause burning sensations (Sugai et al., 2005b), indicating a clear TRPV1 contribution to this sensation. These aversive responses are supported by our behavioural studies showing that knocking out TRPV1 abolished the aversion to analogue I and α-SOH. We would suggest that the sensory properties of the synthetic analogues I–IV would elicit burning whereas only compounds III and IV might be perceived as tingling.
Sichuan oil is rich in linalool, which stimulates TRPA1 but not TRPV1 (Figure 4B and D). Our sensory trial revealed that linalool produces neither burning nor tingling sensations but elicited a weak but unpleasant taste and a strong floral odour. Clearly, the absence of pungency of this compound raises the question as to why linalool that activates TRPA1 is not pungent. One possibility is that like many hydrophobic compounds, it can affect channels including voltage-gated sodium channels that would reduce its pungency (Lundbaek et al., 2004).
To conclude, we found that the detection of a pungent taste in molecules from Sichuan and Melegueta peppers is mediated, at least in part, by TRPA1 and TRPV1, and their implication may rationalize the pungent properties of both the alkylamides and hydroxyarylalkanones. Finally, while TRPV1 stimulation by these molecules operates through non-covalent binding, TRPA1 responses present complex interactions involving covalent and non-covalent gating.
Glossary
Abbreviations:
- 2-APB
2-aminoethyl diphenyl borate
- DRG
dorsal root ganglia
- GSH
glutathione
- HEK
human embryonic kidney
- KCNK
two pore potassium channels
- MTSEA
2-aminoethyl methanethiosulphonate hydrobromide
- α-SOH
hydroxy-α-sanshool
- TRP
transient receptor potential
- TRPA1
transient receptor potential ankyrin 1
- TRPM8
transient receptor potential melastatin 8
- TRPV1
transient receptor potential vanilloid 1
Conflict of interest
The authors state no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 1H NMR (360 MHz, CDCl3, TMS as reference) of purified Sichuan extract.
Figure S2 13C NMR (90 MHz, CDCl3, TMS as reference) of purified Sichuan extract.
Figure S3 HPLC Chromatogram of purified Sichuan extract.
Figure S4 ESI-MS spectra of glutathione alone or adducts formed with selected compounds. (A–L) ESI-MS spectra of GSH alone (A) or products of reactions of GSH and each molecule (B–L). GSH alone exists as a monomer, dimer and trimer in solution MH+, m/z (308, 614, 921). GSH forms no adduct with several TRPA1 agonists (2-APB, linalool, cis-6-nonenal and 6-paradol). When reacted with the remaining TRPA1 agonists (cinnamaldehyde, α-SOH, I–IV, 6-shogaol), adducts of predicted mass are observed as outlined on each spectrum. Note that GSH does not form adduct with cis-6-nonenal whereas they are observed for compounds I and IV, suggesting that the carbonyl of the amide is the electron acceptor for the nucleophile.
Figure S5 Chemical structures of capsaicin, 6-gingerol, 6-shogaol and 8-gingerol. The vanilloids capsaicin, 6-gingerol, 6-shogaol and 8-gingerol stimulate TRPV1 and share a common phenol core. However, only capsaicin among all these compounds does not activate TRPA1.
Figure S6 Analysis of TRP and KCNK channels expression in DRG neurons by quantitative PCR after 1 (D1) and 7 days (D7) of culture. The values are shown as amount of RNA relative to β-actin RNA. After 1 day of culture, the expression levels of the KCNK channels and TRPA1 are in the same order of magnitude, whereas TRPV1 level of expression is about 1000- fold higher than the other channels. After 7 days of culture, most genes are up-regulated. In particular, TRPA1 is up-regulated by a factor 55. Means ± SEM (n = 3).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Reference
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edn. 2008 revision. Br J Pharmacol. 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Sigal YM, Milstein AD, Garrison JL, Zorn JA, Tsuruda PR, et al. Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci. 2008;11:772–779. doi: 10.1038/nn.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141:737–745. doi: 10.1038/sj.bjp.0705652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Dedov VN, Tran VH, Duke CC, Connor M, Christie MJ, Mandadi S, et al. Gingerols: a novel class of vanilloid receptor (VR1) agonists. Br J Pharmacol. 2002;137:793–798. doi: 10.1038/sj.bjp.0704925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- Govindarajan VS. Ginger–chemistry, technology, and quality evaluation: part 1. Crit Rev Food Sci Nutr. 1982;17:1–96. doi: 10.1080/10408398209527343. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Morita A, Iwasawa T, Kobata K, Sekiwa Y, Morimitsu Y, et al. A nonpungent component of steamed ginger–[10]-shogaol–increases adrenaline secretion via the activation of TRPV1. Nutr Neurosci. 2006;9:169–178. doi: 10.1080/110284150600955164. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Koo JY, Jang Y, Cho H, Lee CH, Jang KH, Chang YH, et al. Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur J Neurosci. 2007;26:1139–1147. doi: 10.1111/j.1460-9568.2007.05743.x. [DOI] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–727. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett. 1998;134:163–168. doi: 10.1016/s0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- Lundbaek JA, Birn P, Hansen AJ, Sogaard R, Nielsen C, Girshman J, et al. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J Gen Physiol. 2004;123:599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Nagatomo K, Kubo Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc Natl Acad Sci USA. 2008;105:1737–1738. doi: 10.1073/pnas.0809769105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Riera CE, Vogel H, Simon SA, le Coutre J. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R626–R634. doi: 10.1152/ajpregu.00286.2007. [DOI] [PubMed] [Google Scholar]
- Salazar H, Llorente I, Jara-Oseguera A, Garcia-Villegas R, Munari M, Gordon SE, et al. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Sugai E, Morimitsu Y, Kubota K. Quantitative analysis of sanshool compounds in Japanese pepper (Xanthoxylum piperitum DC.) and their pungent characteristics. Biosci Biotechnol Biochem. 2005a;69:1958–1962. doi: 10.1271/bbb.69.1958. [DOI] [PubMed] [Google Scholar]
- Sugai E, Morimitsu Y, Iwasaki Y, Morita A, Watanabe T, Kubota K. Pungent qualities of sanshool-related compounds evaluated by a sensory test and activation of rat TRPV1. Biosci Biotechnol Biochem. 2005b;69:1951–1957. doi: 10.1271/bbb.69.1951. [DOI] [PubMed] [Google Scholar]
- Tackie A, Dwuma-Badu D, Ayim JSK, Dabrat T, Knapp JE, Slakin DJ. Hydroxyphenyl alkanones from afromum melegueta. Phytochemistry. 1975;14:853–854. [Google Scholar]
- Witte DG, Cassar SC, Masters JN, Esbenshade T, Hancock AA. Use of a fluorescent imaging plate reader–based calcium assay to assess pharmacological differences between the human and rat vanilloid receptor. J Biomol Screen. 2002;7:466–475. doi: 10.1177/108705702237679. [DOI] [PubMed] [Google Scholar]
- Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


