Abstract
Background and purpose:
Orally administered withanoside IV (a compound isolated from the roots of Withania somnifera) improved memory deficits in mice with a model of Alzheimer's disease induced by the amyloid peptide Aβ(25-35). Sominone, an aglycone of withanoside IV, was identified as an active metabolite after oral administration of withanoside IV. We aimed to identify receptors or associated molecules of sominone, and to investigate the effects of sominone on memory in normal mice.
Experimental approach:
Phosphorylation levels of 71 molecules were compared between control and sominone-stimulated cortical cultured cells to search for target molecules of sominone. Object location memory and neurite density in the brain were evaluated in sominone-injected mice.
Key results:
Phosphorylation of RET (a receptor for the glial cell line-derived neurotrophic factor, GDNF) was increased in neurons by sominone, without affecting the synthesis and secretion of GDNF. Knockdown of RET prevented sominone-induced outgrowths of axons and dendrites. After a single i.p. injection of sominone into normal mice, they could better memorize scenery information than control mice. Sixty minutes after sominone injection, RET phosphorylation was increased, particularly in the hippocampus of mice. After the memory tests, the densities of axons and dendrites were increased in the hippocampus by sominone administration.
Conclusions and implications:
Sominone could reinforce the morphological plasticity of neurons by activation of the RET pathway and thus enhance memory. Sominone, a compound with low molecular weight, may be a GDNF-independent stimulator of the RET pathway and/or a novel modulator of RET signalling.
Keywords: axon, dendrite, phosphorylation, novel location, RET
Introduction
We previously hypothesized that enhancing brain function requires the reinforcement of neuronal networks, including neurite regeneration and synapse formation; therefore, we have been exploring anti-dementia drugs based on reconstructing neuronal networks in the damaged brain.
We previously investigated the effects of extracts and constituents of Ashwagandha (root of Withania somnifera Dunal), an Ayurvedic tonic medicine, on neurite outgrowth in cultured neurons (Tohda et al., 2000; Kuboyama et al., 2002; 2005; Zhao et al., 2002). Withanoside IV (MW: 782) was isolated from a methanol extract of Ashwagandha, and showed anti-dementia activity after oral administration to a mouse model of Alzheimer's disease (Kuboyama et al., 2006).
We also found that withanoside IV enhanced the growth activity of neurites from injured and/or spared neurons and synaptogenesis in the brain (Kuboyama et al., 2006). Similar effects were observed following spinal cord injury in mice where oral administration of withanoside IV attenuated hindlimb dysfunction, enhanced axonal regrowth and increased peripheral myelin levels (Nakayama and Tohda, 2007).
Withanoside IV is a steroidal saponin conjugated with two glucose residues at position C3, and may be metabolized into a sapogenin, sominone, by enterobacterial β-glucosidases. As expected, ‘sominone’, an aglycone of withanoside IV (Atta-ur-Rahman Jamal and Choudhary, 1992), was identified as the main metabolite in serum after oral administration of withanoside IV (Kuboyama et al., 2006). Sominone induced axonal and dendritic regeneration and synaptic reconstruction in cultures of rat cortical neurons damaged by the amyloid peptide, Aβ(25-35) (Kuboyama et al., 2006). Therefore, withanoside IV may act as a prodrug, with sominone as the active principle at target organs by activating neuronal network formation in vitro. In vivo activities and the underlying mechanism of action of sominone on neurons have not been elucidated yet. If a small molecule such as sominone can facilitate neuronal function in normal neurons, it might also be a promising drug against memory dysfunction in dementia because it activates the remaining neurons. It is also possible that key molecules contributing to neuronal plasticity-based memory formation may be explored by analysing the signal pathways of sominone.
The aims of this study were to verify the memory enhancement activity of sominone and to clarify the target molecule of sominone. We found that sominone stimulated RET, which is a receptor of the glial cell line-derived neurotrophic factor (GDNF) family (nomenclature follows Alexander et al., 2008), exhibiting transmembrane tyrosine kinase activity. Sominone also enhanced spatial memory in rats.
Methods
All experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Sugitani Campus of the University of Toyama and NIH Guidelines on the Care and Use of Laboratory Animals. All protocols were approved by the Committee for Animal Care and Use of Sugitani Campus of the University of Toyama. All efforts were made to minimize the number of animals used.
Preparation of sominone
Sominone was enzymatically prepared from withanoside IV, which was isolated from a methanol extract of Ashwagandha (root of W. somnifera Dunal), as previously described (Zhao et al., 2002). Withanoside IV (10.0 mg) was treated with naringinase (1 g) (Sigma) for 4 days at 37°C in 8 mL phosphate-citrate buffer (pH 5.25) containing 15% ethanol and 10% toluene. The mixture was extracted with ether, and dehydrated by filtration over magnesium sulphate. The extract was evaporated and resolubilized in CH3CN, and purified by preparative HPLC (JASCO HPLC system) with the binary eluent of H2O and CH3CN to give 2.3 mg sominone. The ratios of H2O to CH3CN were 75 to 25 at the start, 65 to 35 at 15 min, 60 to 40 at 40 min, 10 to 90 at 41 min, 10 to 90 at 51 min, 75 to 25 at 60 min and 75 to 25 at 70 min respectively. The chemical structure of sominone was identified by comparing 1H-NMR and 13C-NMR spectra with reported spectra (Atta-ur-Rahman Jamal and Choudhary, 1992).
Primary culture
Embryos were removed from pregnant Sprague-Dawley rats (Japan SLC, Shizuoka, Japan) at 17–19 days of gestation. The cortices were dissected and the dura mater was removed. The tissues were minced and dissociated and then grown in cultures with Neurobasal medium, including 12% horse serum, 0.6% D-glucose and 2 mM L-glutamine on 8-well chamber slides (Falcon, Franklin Lakes, NJ, USA) coated with 50 µg·mL−1 poly-D-lysine at 37°C in a humidified incubator with 10% CO2. When compounds were added, half of the medium in each well was replaced with fresh medium containing 2% B-27 supplement without serum. The time schedules of the experiments are illustrated at the bottom of each respective figure.
Immunocytochemistry
Rat cortical neurons were cultured in 8-well chamber slides at a density of 1.8–2.6 × 105 cells·cm−2. To measure the lengths of axons and dendrites, the cells were treated with 1 µM sominone or vehicle (0.1% DMSO). The cells were fixed with 4% paraformaldehyde and then immunostained with a monoclonal antibody against phosphorylated neurofilament-H (pNF-H; 1:500) as an axonal marker or a polyclonal antibody against microtubule-associated protein 2 (MAP2; 1:500) as a dendritic marker. Alexa Fluor 488-conjugated goat anti-mouse IgG (1:300) and Alexa Fluor 568-conjugated goat anti-rabbit IgG were used as second antibodies. Fluorescent images were captured by a fluorescent microscope (AX-80, Olympus, Tokyo, Japan) at 320 µm × 425 µm, and four images were captured per treatment. The lengths of neurites positive for pNF-H or MAP2 were measured using an image analyser Neurocyte (Kurabo, Osaka, Japan), which automatically traces and measures neurite lengths without measuring cell bodies. The total length of axons or dendrites was divided by cell numbers in an identical area to calculate the average length per cell. The expression level of phosphoRET (pRET) in neurons was quantified using ATTO densitography, as described in the Immunohistochemistry method.
Antibody array
Phosphorylated levels of 71 molecules were compared according to the manufacturer's instructions using human receptor tyrosine kinase phosphorylation antibody array (RayBiotech, GA, USA). In brief, rat cortical neurons were cultured in a 10 cm dish at a density of 1.5 × 105 cells·cm−2. Seven days later, cells were treated with 1 µM sominone or vehicle (0.1% DMSO) for 60 min. Each cell lysate was incubated with an array membrane overnight at 4°C at a protein concentration of 479 µg·mL−1. After washing, membranes were incubated with biotin-conjugated anti-phosphotyrosine overnight at 4°C. The membranes were incubated with HRP-conjugated streptavidin for 2 h at room temperature. Blots on array membranes were treated by detection reagents and detected by chemiluminescence with LAS-1000 plus (Fuji Film, Tokyo, Japan). Blot densities were quantified using an image analyser ATTO densitograph (ATTO, Tokyo, Japan).
siRNA transfection
siRNAs were transfected into rat cortical neurons according to the manufacturer's protocol for DharmaFECT 3 (Dharmacon, Lafayette, CO, USA). In brief, rat cortical neurons were cultured in 8-well chamber slides at a density of 2.6 × 105 cells·cm−2. Seven days later, a serum-free medium containing 1 µL DharmaFECT 3 solution and 0.5 nM siRNA of RET was applied to a well. As a control, siCONTROL (Dharmacon) was used at 0.5 nM. The appropriate concentration of siRNA of RET was determined in preliminary experiments.
Immunoblotting
Cell lysates (40 µg) were prepared by lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 20 mM NaF, 20 mM β-glycerophosphate, 1 mM dithiothreitol, 1 mM Na3VO4, 1 mM PMSF, 4 µg·mL−1 leupeptin, 4 µg·mL−1 aprotinin). Protein concentrations of cell lysates were quantified using the Quick Start Bradford Protein Assay (Bio-Rad, Tokyo, Japan). The lysates were analysed by electrophoresis using 8% SDS-polyacrylamide gel. Separated proteins were transferred electrophoretically to a nitrocellulose membrane, blocked with 5% skim milk solution for 1 h, and then incubated with a RET antibody (clone C-20) overnight at 4°C. After washing, the membrane was incubated with HRP-conjugated donkey anti-goat IgG secondary antibody for 1 h at room temperature. The antigen–antibody complex was visualized using ECL Plus Western blotting detection reagents (Amersham Bioscience, NJ, USA) and detected by chemiluminescence with LAS-1000 plus (Fuji Film). Band densities were determined using an image analyser ATTO densitograph (ATTO).
GDNF ELISA
The GDNF contents in rat cultured cortical cells and conditioned media were measured by ELISA according to the manufacturer's protocol for the GDNF Emax ImmunoAssay System (Promega, WI, USA). In brief, rat cortical neurons were cultured in a 24-well slide at a density of 2.0 × 105 cells·cm−2. Six days later, vehicle solution (0.1% DMSO) or 1 µM sominone was added to cells with a serum-free medium. Fifteen and sixty minutes after stimulation, conditioned media were aspirated and cell lysates were prepared with lysis buffer (50 mM Tris-HCl, pH 7.5, 140 mM NaCl, 1% NP-40, 10 mM EDTA, 20 mM NaF, 20 mM β-glycerophosphate, 1 mM dithiothreitol, 1 mM Na3VO4, 1 mM PMSF, 4 µg·mL−1 leupeptin, 4 µg·mL−1 aprotinin). Protein concentrations of cell lysates were quantified using the Quick Start Bradford Protein Assay (Bio-Rad). An ELISA plate coated with anti-GDNF monoclonal antibody was treated with blocking buffer. Test samples were applied to the plate, and anti-GDNF polyclonal antibody was overlaid. Horseradish peroxidase-conjugated anti-chicken IgY was applied to the plate, and then the colour-developing reaction was measured at 450 nm. GDNF contents in conditioned media and cell lysates were calculated, using a standard curve of GDNF (0, 15.6, 31, 62, 125, 250, 500, 1000 pg·mL−1).
Novel location test
Male ddY mice (6 weeks old, Japan SLC) were housed with free access to food and water, and kept in a controlled environment (22 ± 2°C, 50 ± 5% humidity, 12 h light cycle starting at 7:00 am). The novel location recognition test assessed the ability of mice to recognize the novel spatial arrangement of familiar objects and is related to hippocampal function (Save et al., 1992). Sominone (10 µmol·kg−1) or vehicle solution (10% DMSO in physiological saline) was injected i.p. once into mice (n= 4). For two consecutive days, mice were individually habituated to an open-field box made of polyvinyl chloride (33 cm × 28 cm; height, 26.5 cm) for 10 min. Four days after i.p. injection of sominone, the mice were trained in 10 min training trials, and then tested in 10 min trials, each with a 24 h inter-trial interval (Figure 4B). For the training session, two identical novel objects (glass bottles) were placed at a fixed distance within the open-field box surrounded by different wall coverings. One side of the black wall of the square field had vertical white strips and the opposite side of the wall had white circles. The two objects were placed on the floor of the box. A mouse was placed at the opposite edge of the field, and the number of explorations of each object was recorded during a 10 min period (training session). After the training session, the mice were tested in a location novelty recognition test in which one of the bottles was moved to a different position in the field. The mice were then allowed to explore freely for 10 min and the number of explorations of each object was recorded (test session). An observer, unaware of the treatments, scored exploration times when a mouse touched the object with the nose or forelimbs. Mice did not step onto the bottle and the duration of each exploratory behaviour was very short; therefore, the contact times with an object almost reflected the time spent exploring the object, and assessing contact times may exclude artificial exploratory behaviour such as staying near an object. A preference index, defined as the ratio of the number of times a mouse made contact with an object before replacing (training session) or after replacing (test session) the object to the total number of times the mouse made contact with both objects, was used to measure cognitive function. Locomotor behaviours were evaluated after the novel location test. Distances moved in an open field (circular field with 58 cm diameter) during 10 min were measured.
Figure 4.
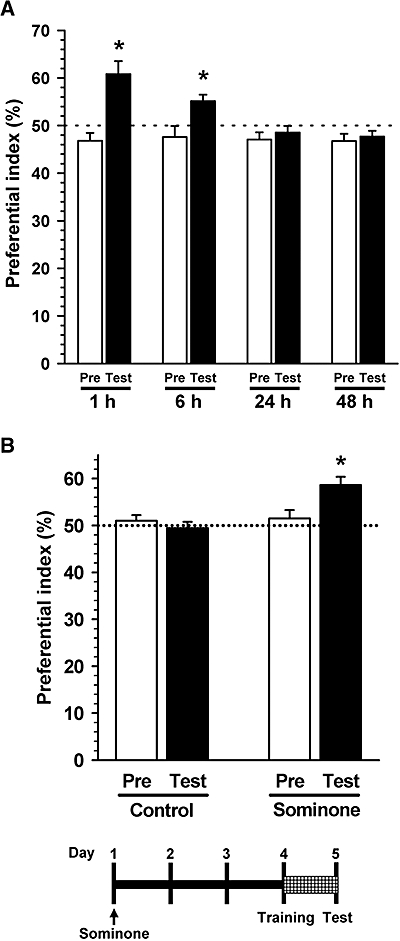
Effect of sominone on location memory in mice. (A) The day after habituation, all mice (ddY, male, 6 weeks old) were given vehicle (10% DMSO in saline, i.p.). Training sessions were carried out 1 h after injection and then test sessions were performed after each interval (1, 6, 24 or 48 h). A preference index was defined as the ratio of the number of times a mouse made contact with a object before moving (training session, Pre) or after moving (test session, Test) to the total number of times the mouse made contact with both objects. *P < 0.05 versus chance (50%), n= 5. (B) Another group of mice was used for the data in (B), independent of those in (A). After habituation, mice (ddY, male, 6 weeks old) were given vehicle solution (10% DMSO in saline, i.p., Control) or sominone (10 µmol·kg−1, i.p.). Four days after injection, a novel location test was carried out. The interval time between the training session and test session was 24 h. *P < 0.05 versus chance (50%), n= 4 (one-sample t-test).
To assess the appropriate interval time, another group of mice were divided into four groups (n= 5), and the test session was conducted 1, 6, 24, 48 h after the training session (Figure 4A). All objects and the arena were thoroughly cleaned with 70% ethanol and then water between trials to remove odours. Behavioural testing was performed in the light phase.
Immunohistochemistry
Immediately after the behaviour test, mice were decapitated under anaesthesia. The brains were quickly removed from the skull and frozen in powdered dry ice. The brains were cut in 12 µm coronal sections using a cryostat (CM3050S; Leica, Heidelberg, Germany), and the slices were fixed with 4% paraformaldehyde and stained with a monoclonal antibody against pNF-H or MAP2. Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 568-conjugated goat anti-rabbit IgG were used as secondary antibodies. Staining and quantification were carried out under identical conditions. Fluorescent images were captured using a fluorescent microscope (AX-80) at 324 µm × 430 µm (areas of the striatum, cerebral cortex, thalamus, hypothalamus, brain stem and cerebellum) or 648 µm × 860 µm (hippocampal area and corpus callosum), and two to five sets of serial brain slices from five mice were used to capture images for each treatment. The measuring points were selected with 4–50 squares (square size: 11 µm × 11 µm for striatum area; 63.3 µm × 63.3 µm for hippocampal area; 31.7 µm × 31.7 µm for cortical area, thalamus area, hypothalamus area, brain stem area and cerebellum area; 126.6 µm × 126.6 µm for corpus callosum) to cover the whole area of each region or subregion (e.g. the molecular layer of the dentate gyrus). Background intensity was determined by staining slices without each first antibody. Fluorescent intensities of immunopositive areas (after subtracting the background intensity) in those squares were quantified using ATTO densitography (ATTO).
Statistical analysis
Statistical comparisons were performed with one-way anova, two-way anova and repeated measures two-way anova followed by the Fisher LSD post hoc test, one-sample t-test or Student's t-test using SigmaStat 3.5 (SYSTAT, CA, USA). Values of P < 0.05 were considered significant. The means of the data are presented together with the SE.
Materials
Neurobasal media and B-27 supplement were purchased from Gibco BRL (Rockville, MD, USA). Recombinant rat nerve growth factor was purchased from R&D Systems (Minneapolis, MN, USA). A monoclonal antibody against pNF-H was purchased from Sternberger Monoclonals (Lutherville, MD, USA). A polyclonal antibody against MAP2a and MAP2b was purchased from Chemicon (Temecula, CA, USA). A monoclonal antibody against MAP2 was purchased from Thermo Fisher Scientific (Fremont, CA, USA). Polyclonal antibodies against pRET (Tyr 1062), RET (clone C-20), RET (clone H-300), GDNF receptor α (GFRα)-1 (clone H-70) and a HRP-conjugated donkey anti-goat IgG antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Alexa Fluor 488-conjugated goat anti-mouse IgG and Alexa Fluor 568-conjugated goat anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR, USA). RET is a transmembrane tyrosine kinase enzyme (Alexander et al., 2008).
Results
Effects of sominone on axonal and dendritic outgrowths
Sominone (0.001–10 µM) was added to cultures of cortical neurons 4 days after seeding, and axon length (Figure 1A) or dendrite length (Figure 1B) was measured after an additional 4 days. The axon and dendrite lengths were significantly increased by sominone in a dose-dependent manner. The maximum dose of sominone was 0.1 µM for axon extension and 1 µM for dendrite extension.
Figure 1.
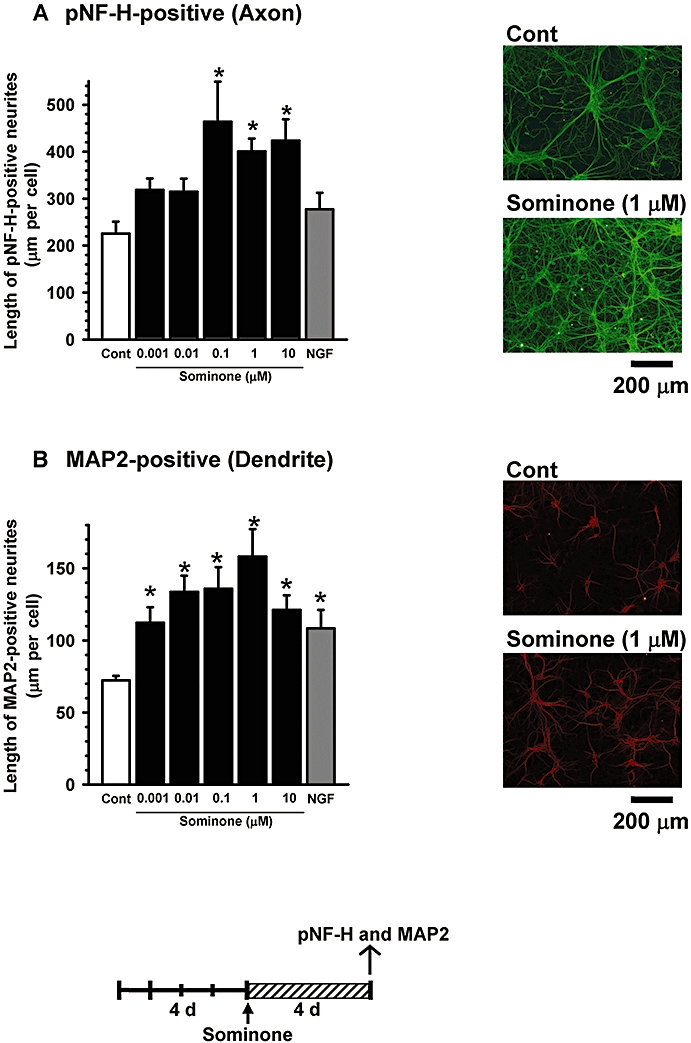
Cortical cells were cultured for 4 days and then treated with vehicle solution (Cont, 0.1% DMSO), 0.001–10 µM sominone (Som) or 100 ng·mL−1 nerve growth factor (NGF). Four days after treatment, the cells were fixed and double-immunostained with an antibody against phosphorylated neurofilament-H (pNF-H) (A) and microtubule-associated protein 2 a and b (MAP2) (B). The lengths of pNF-H-positive neurites and MAP2-positive neurites per cell were quantified for each treatment. *P < 0.05 versus vehicle, n= 6 (one-way anova followed by the Fisher LSD post hoc test). Typical photographs are shown.
Enhancement of the phosphorylation of RET by sominone in cortical neurons
To identify the direct target molecule of sominone, molecules phosphorylated by sominone stimulation were screened using receptor tyrosine kinase phosphorylation antibody array membranes. As a result, the phosphorylation level of RET (rearranged during transfection) was increased 60 min after sominone (1 µM) treatment compared with vehicle-treated cells (124.3% of vehicle-treated cells).
The RET receptor tyrosine kinase is physiologically stimulated by any member of the GDNF family (Durbec et al., 1996; Trupp et al., 1996). A common feature of the receptor complex for GDNF family ligands is the requirement of two receptor subunits, one specialized for transmembrane signalling, the protein RET, and the other involved in ligand binding, the glycosyl phosphatidylinositol-anchored co-receptor GFRα (Airaksinen et al., 1999). The family of GFRα co-receptors comprises four members (GFRα-1–4), which determine ligand specificity. GFRα-1–4 are the preferential co-receptors for GDNF, neurturin, artemin and persephin respectively (Sariola and Saarma, 2003).
To confirm the reproducibility of RET phosphorylation by sominone, the rat cortical culture was stimulated by sominone and immunostained for pRET. We also applied directly recombinant GDNF to the cells to evaluate the effects of a high concentration of GDNF on neurite extension. Eight days after seeding, sominone (1 µM), GDNF (100 ng·mL−1) or vehicle solution was applied to the cells. One series of treated cells was immunostained for pRET, 15 min after drug stimulation. Another series of treated cells was immunostained for pNF-H and MAP2, 4 days after drug stimulation. The dose of GDNF (100 ng·mL−1) is generally effective for neurite extension (Schäfer and Mestres, 1999). Sominone treatment significantly increased the phosphorylation of RET in neurons, although the level of phosphorylation by sominone was more potent than by GDNF (Figure 2A). Both treatments with sominone and GDNF significantly extended axon length (Figure 2B) and dendrite length (Figure 2C).
Figure 2.
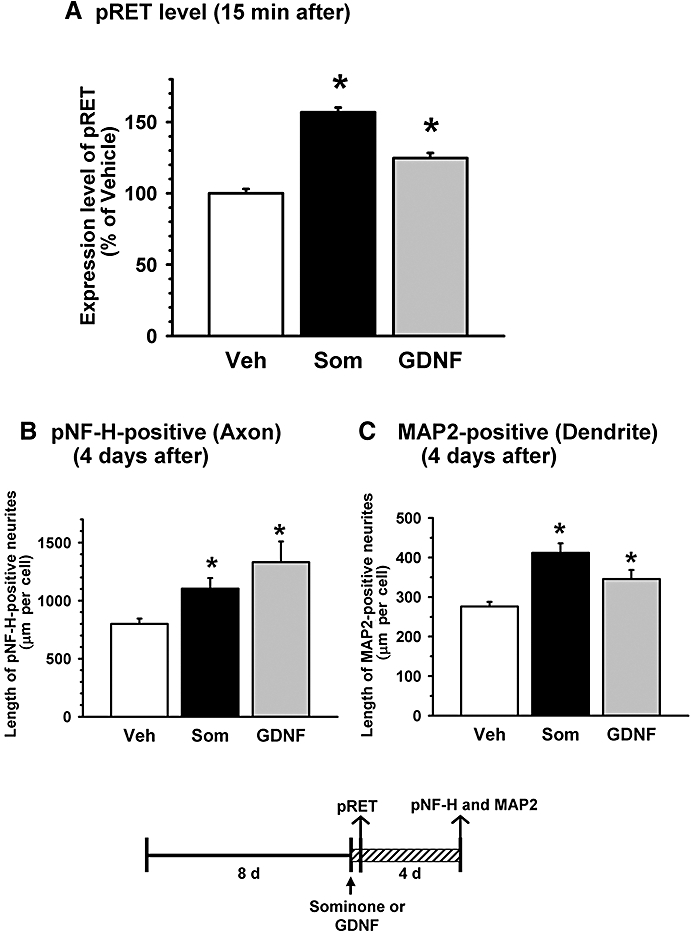
Effects of sominone and glial cell line-derived neurotrophic factor (GDNF) on the phosphorylation of RET and neurite outgrowth. Cortical cells were cultured for 8 days and then treated with vehicle solution (Veh, 0.1% DMSO), 1 µM sominone (Som) or 100 ng·mL−1 GDNF for 4 days. (A) Expression levels of phosphorylated RET in neurons were quantified 15 min after drug stimulation. *P < 0.05 versus Veh, n= 193–229 neurons (one-way anova followed by the Fisher LSD post hoc test). (B and C) Four days after treatment, the cells were fixed and double-immunostained for phosphorylated neurofilament-H (pNF-H) (B) and microtubule-associated protein 2 (MAP2). (C) The lengths of pNF-H-positive neurites and MAP2-positive neurites per cell were quantified for each treatment. *P < 0.05 versus Veh, n= 5.
Expression levels of RET and GFRα-1 were not increased in cortical neurons 60 min after stimulation with sominone (Table 1). Our cortical cell culture was not a pure culture of neurons, but contained several types of glial cells because we aimed to investigate neuronal functions under the influence of glial cells; therefore, the expressions of pRET, RET and GFRα-1 in MAP2-positive neuronal soma were measured to evaluate these levels in neurons.
Table 1.
Expression levels of RET and GFRα-1 in sominone-treated cells
|
60 min after | ||||
|---|---|---|---|---|
| Stimulation | RET expression | n | GFRα-1 expression | n |
| Cont | 100.0 ± 6.32 | 45 | 100.0 ± 1.33 | 253 |
| Som (1 µM) | 91.7 ± 6.85 | 60 | 94.1 ± 2.27 | |
Cortical cells were cultured for 7 days and then treated with vehicle solution (Cont, 0.1% DMSO) or 1 µM sominone (Som) for 60 min. Sixty minutes after treatment, the cells were fixed and immunostained with an antibody against RET or GFRα-1. The numbers of observed neurons are shown as n in the table.
Sominone does not stimulate synthesis and secretion of GDNF, a ligand of RET
We next investigated whether RET phosphorylation by sominone was mediated by GDNF. GDNF was not detected 15 and 60 min after stimulation in conditioned media from control cells and sominone-treated cells (Table 2). Although intracellular GDNF was detected, sominone treatment did not significantly alter GDNF contents in cells after 15 and 60 min.
Table 2.
GDNF contents in sominone-treated cells and conditioned media
| Cell lysate | GDNF (pg·mL−1·mg−1protein) | |
|---|---|---|
| Stimulation | 15 min after | 60 min after |
| Cont | 485.1 ± 56.58 | 527.3 ± 27.45 |
| Som (1 µM) | 504.6 ± 42.62 | 492.8 ± 7.35 |
| Conditioned medium | GDNF (pg·mL−1) | |
| Stimulation | 15 min after | 60 min after |
| Cont | 0 ± 0 | 0 ± 0 |
| Som (1 µM) | 0 ± 0 | 0 ± 0 |
Cortical cells were cultured for 7 days and then treated with vehicle solution (Cont, 0.1% DMSO) or 1 µM sominone (Som) for 60 min. Fifteen and sixty minutes after treatment, conditioned media were aspirated, and cell lysates were prepared. GDNF concentrations in the conditioned media and cell lysates were measured by ELISA. n= 3.
Effects of RET knockdown on sominone-induced axonal and dendritic outgrowths
To investigate the function of RET in sominone-induced neurite extension, we knocked down the level of RET by siRNA transfection. Two days after the transfection of siRNA for RET (0.5 nM), the RET level was markedly reduced in cortical cells (Figure 3A); therefore, 2 days after siRNA transfection, sominone (1 µM) or vehicle solution was applied to the cells, and the medium was replaced with fresh medium without any drugs 1 day later. After further incubation for three additional days, the cells were double-immunostained for pNF-H and MAP2. In cortical neurons after the transfection of control siRNA, axons (Figure 3B) and dendrites (Figure 3C) were significantly extended by sominone treatment; however, the sominone-induced extensions of axons and dendrites were almost completely inhibited in neurons after transfection of RET siRNA. Significant interaction for axon lengths between siRNA treatment and drug treatment was shown in two-way anova[F(1,16) = 8.408, P= 0.01]. Significant interaction of dendrite lengths between siRNA treatment and drug treatment was also shown in two-way anova[F(1,16) = 4.986, P= 0.04]. These data indicate that RET is essential for sominone-induced extensions of axons and dendrites, and at least 1 day treatment with sominone is sufficient to trigger neurite elongation.
Figure 3.
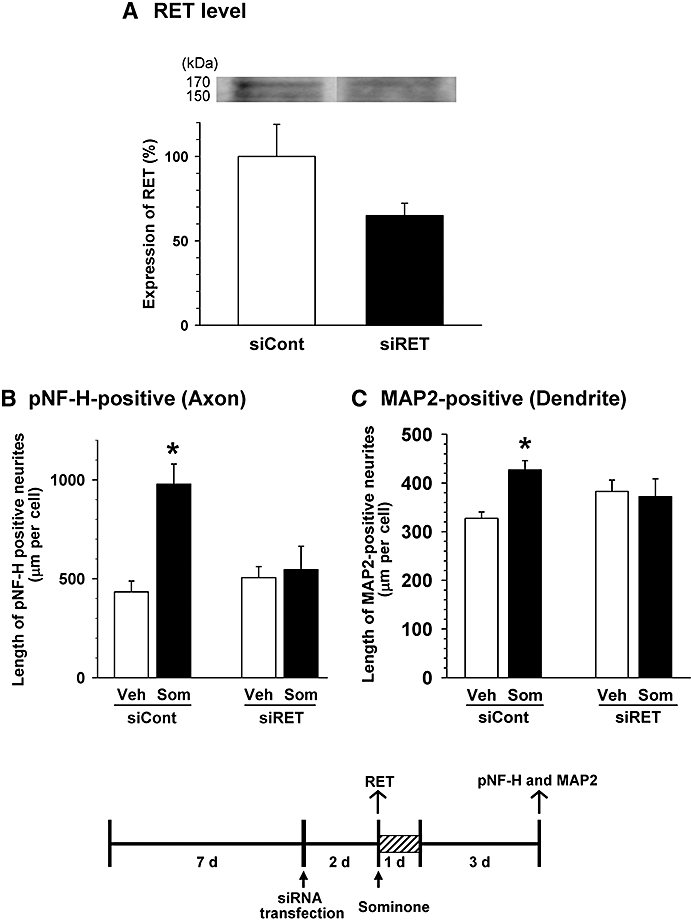
Effect of RET knockdown on sominone-induced neurite outgrowth. (A) siRNA for RET (0.5 nM) or control siRNA (0.5 nM) was transfected into cortical cells 7 days after culture. Two days later, cell lysates were prepared, and the expression levels of RET protein were quantified by immunoblotting. n= 3. (B and C) siRNA for RET (0.5 nM) or control siRNA (0.5 nM) was transfected into cortical cells 7 days after culture. Two days later, cells were incubated with 1 µM sominone or the vehicle (Veh, 0.1% DMSO) for 1 day. Four days after drug administration, cells were fixed and double-immunostained for phosphorylated neurofilament-H (pNF-H) and microtubule-associated protein 2 (MAP2). The lengths of pNF-H-positive neurites (B) and MAP2-positive neurites (C) per cell were quantified for each treatment. *P < 0.05 versus Veh, n= 5 (two-way anova followed by the Fisher LSD post hoc test).
Effects of sominone on novel location memory
To study the effects of sominone on memory enhancement, sominone (10 µmol·kg−1) was injected i.p. once into mice. Four days after injection, an object location test was initiated to assess spatial memory. The dose of sominone, 10 µmol·kg−1, was shown to be the maximal effective dose in our previous experiment. To assess the memory enhancement effect in normal mice, we determined the appropriate interval time between a training session and a test session (Figure 4A), in which mice cannot memorize object location. The value of the preferential index decreased to the chance value (50%) in a time-dependent manner, and index values in the training session and the test session were not significantly different with 24 and 48 h intervals. Statistical analysis by two-way anova also indicated significant interaction between variable sessions and variable intervals [F(3,32) = 5.877, P= 0.003]; therefore, we used the 24 h interval condition in Figure 4B.
Three days after injection of sominone, a novel object recognition test was initiated to assess visual recognition memory. Although sominone-injected mice showed frequent exploratory behaviour for a novel object (54.5 ± 2.8%), this was not significantly greater than by chance (50%). Exploratory behaviour for the novel object in control mice (51.9 ± 3.00%) was also not different from chance (50%). Then, a novel location test was initiated 4 days after sominone injection. When a familiar object moved to a novel location, sominone-injected mice in the test session showed significantly more frequent exploring behaviour compared with the chance performance (Figure 4B); however, control mice showed no specific exploratory behaviour of the object in the novel location. Statistical analysis by repeated measures two-way anova also indicated significant interaction between variable sessions and variable drug treatments [F(1,6) = 8.543, P= 0.027]. Total contact times of control and sominone-treated mice in each 10 min session were not significantly different (training session: control, 46.8 ± 6.59; sominone, 51.8 ± 2.21; test session: control, 39.5 ± 10.47; sominone, 39.8 ± 4.31, n= 4). Locomotor behaviours, which were evaluated by distances moved in an open field (circular field with 58 cm diameter) during 10 min, were not significantly different between the two groups (control, 4333.1 ± 461.3 cm; sominone, 4323.4 ± 242.1 cm, n= 4).
Increases in phosphorylation of RET, and densities of axons and dendrites by sominone in the mouse brain
Expression levels of pRET were significantly increased in the hippocampal CA1 and CA3 and parietal cortex 60 min after sominone injection (10 µmol·kg−1, i.p.) compared with vehicle-injected mice (Figures 5 and 8). The level also tended to increase in the hippocampal dentate gyrus and perirhinal cortex and striatum. There were no significant differences in pRET between control and sominone-treated groups in other brain areas, such as the thalamus, hypothalamus, brain stem and cerebellum.
Figure 5.
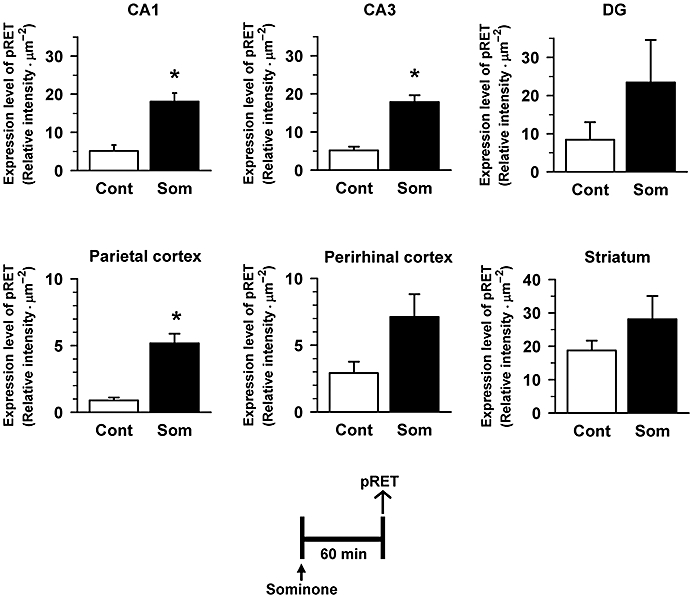
Effect of sominone on phosphorylation of RET in the mouse brain. Sixty minutes after the injection of vehicle solution (10% DMSO in saline, i.p., Cont) or sominone (10 µmol·kg−1, i.p.), brain slices were immunostained with phosphorylated RET (pRET) antibody. pRET-positive areas were quantified in the hippocampal CA1, CA3 and dentate gyrus, and the parietal, perirhinal and frontal cortex. *P < 0.05 versus Cont. n= 3 (Student's t-test).
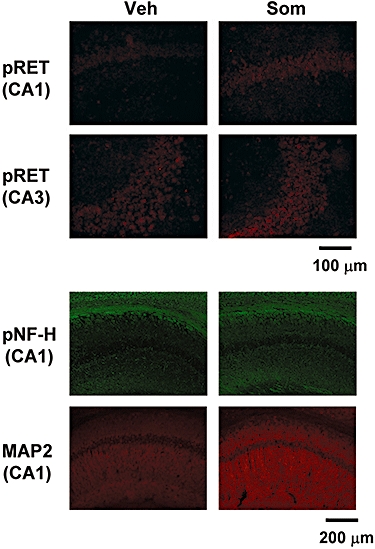
Typical images from hippocampal slices are shown after immunostaining for phosphorylated RET (pRET), phosphorylated neurofilament-H (pNF-H) and microtubule-associated protein 2 (MAP2). Expressions of pRET in CA1 were increased 60 min after the injection of sominone. Expressions of pNF-H and MAP2 were increased 5 days after the injection of sominone.
Immediately after the novel location test, that is, 5 days after sominone injection, brain slices were prepared, and the levels of pNF-H and MAP2 were measured in the brains of mice by immunohistochemistry. The brain regions assessed included three cortical regions (parietal cortex, perirhinal cortex, frontal cortex), seven subregions in three hippocampal regions (CA1, CA3 and dentate gyrus) and the striatum. Expression levels of pNF-H were significantly increased in all subregions of the hippocampus in sominone-injected mice compared with control mice (Figures 6 and 8). In the cortex regions and striatum, significant differences in pNF-H levels were not observed between control mice and sominone-injected mice.
Figure 6.
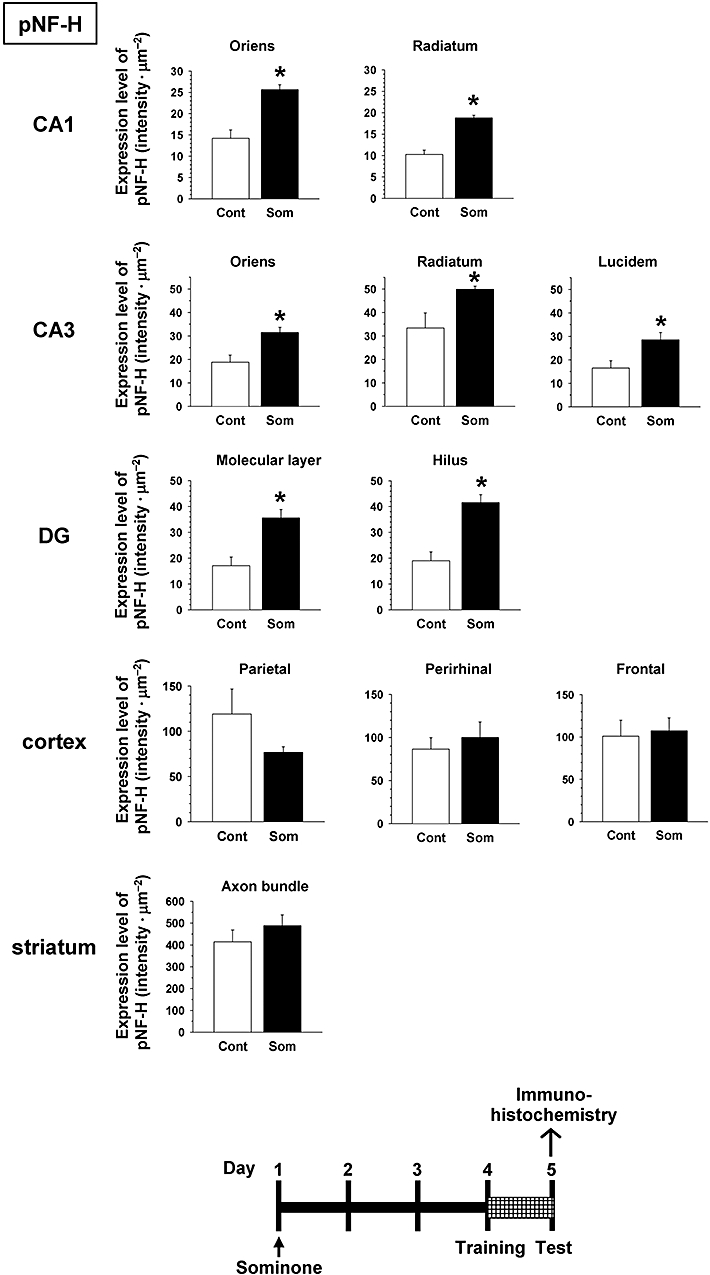
Effect of sominone on axonal density in the mouse brain. After the novel location test (Figure 4), that is, 5 days after the injection of vehicle solution (10% DMSO in saline, i.p., Cont) or sominone (10 µmol·kg−1, i.p.), brain slices were immunostained with phosphorylated neurofilament-H (pNF-H) antibody. pNF-H-positive areas were quantified in the stratum oriens and stratum radiatum in CA1, the stratum oriens, stratum radiatum and stratum lucidum in CA3, the molecular layer and hilus in the dentate gyrus (DG), the parietal cortex, perirhinal cortex, frontal cortex and matrix and axonal bundles in the striatum. *P < 0.05 versus vehicle. n= 4 (Student's t-test).
Expression levels of MAP2 were significantly increased in CA1 subregions of the hippocampus in sominone-injected mice compared with control mice (Figures 7 and 8). The level of MAP2 tended to increase also in CA3 and the dentate gyrus by sominone injection; however, marked differences in MAP2 levels were not observed between control mice and sominone-injected mice in the cortex regions and striatum.
Figure 7.
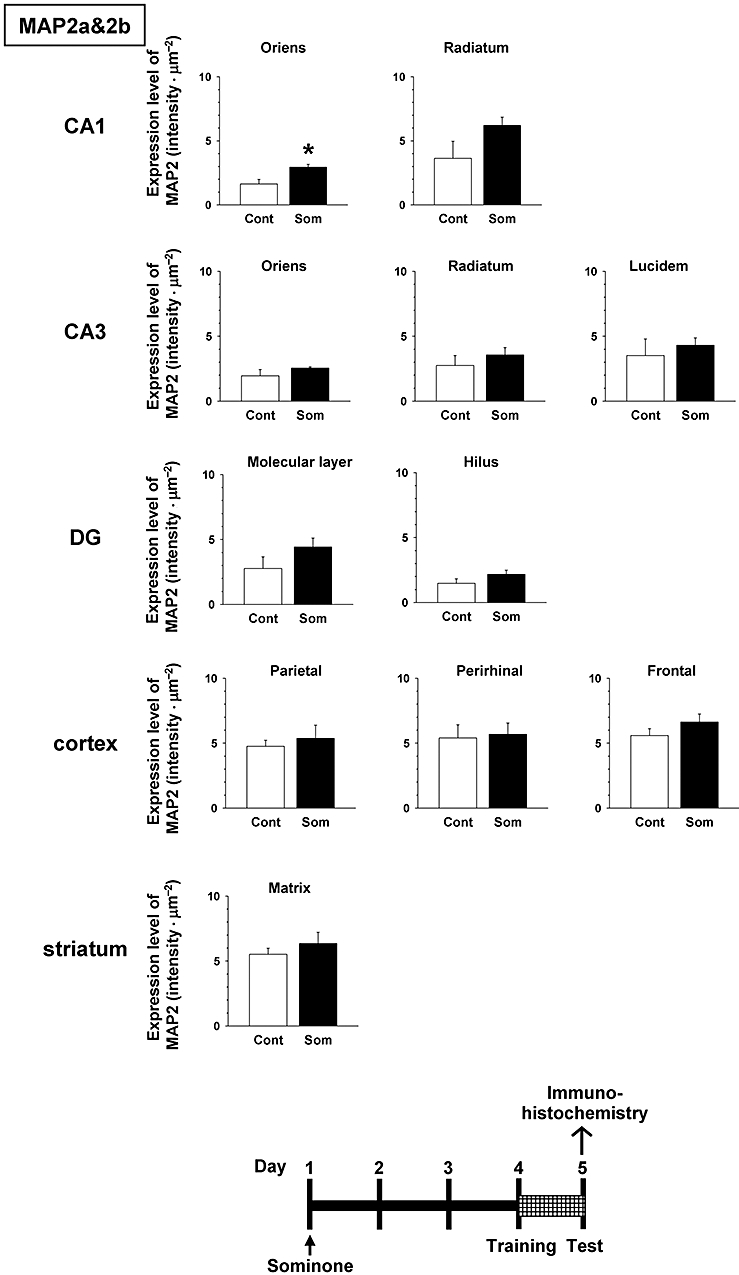
Effect of sominone on dendritic density in the mouse brain. After the novel location test (Figure 4), that is, 5 days after the injection of vehicle solution (10% DMSO in saline, i.p., Cont) or sominone (10 µmol·kg−1, i.p.), brain slices were immunostained with microtubule-associated protein 2 (MAP2) antibody. MAP2-positive areas were quantified in the stratum oriens and stratum radiatum in CA1, the stratum oriens, stratum radiatum and stratum lucidum in CA3, the molecular layer and hilus in the dentate gyrus (DG), the parietal cortex, perirhinal cortex, frontal cortex and matrix in the striatum. *P < 0.05 versus vehicle. n= 4 (Student's t-test).
Discussion and conclusions
We previously showed that sominone significantly induced axonal and dendritic regeneration and synaptic reconstruction in cultures of rat cortical neurons damaged by Aβ(25-35) (Kuboyama et al., 2006). In the present study, we demonstrated for the first time that sominone could enhance object location memory in vivo and that the target molecule of sominone was RET.
We confirmed in this study that treatment with sominone extended the lengths of axons and dendrites of cultured rat cortical neurons also under normal conditions (Figure 1). In vivo, the administration of sominone increased the densities of axons and dendrites in the hippocampus (Figures 6–8). Enhancement of the object location memory (Figure 4) may be due to the up-regulation of neurite proteins induced by sominone. As object recognition tests were carried out before the novel location test, some excitatory signalling in neurons associated with memorization may be added to a net effect of sominone. However, we would primarily like to show the difference between the control group and sominone-treated group.
We needed to clarify why and how sominone was able to extend axons and dendrites. To elucidate the mechanism of sominone, we identified its target molecule as RET, which was shown to be phosphorylated after sominone. Phosphorylation was increased in cultured cortical neurons at least 15 min after the administration of sominone (Figure 2A). Sominone induced RET phosphorylation and outgrowths of axons and dendrites at a similar level to GDNF. The applied dose of GDNF (100 ng·mL−1) is at the upper end of the effective dose range (Schäfer and Mestres, 1999). Therefore, sominone may have high potency for the RET-mediated neurite outgrowth pathway.
The phosphorylation of RET by sominone was also confirmed in the hippocampus and cerebral cortex of mouse brains (Figure 5). Protein sequence homologies of rat, mouse and human RETs are very high (rat–mouse, 93%; rat–human, 84%; mouse–human, 81%). Sominone may activate RET in rat cortical neurons and the mouse brain, although it remains unknown whether sominone directly binds to RET. RET was identified as a GDNF receptor in 1996 (Durbec et al., 1996; Trupp et al., 1996). As GDNF was originally characterized as a survival factor mainly for midbrain dopaminergic neurons (Akerud et al., 1999), the functions of RET have been studied mainly in dopaminergic neurons (Jain et al., 2006; Kramer et al., 2007) and motor neurons (Lu et al., 2003; Gould et al., 2008). However, further investigations indicated that RET was expressed in a broad area of the brain containing the hippocampus and cerebral cortex as well as the substantia nigra (Burazin and Gundlach, 1999), although the expression level is more dense prenatally than in adults (Quartu et al., 2007). The densities of axons and dendrites in the cortex were not significantly increased in sominone-injected mice (Figures 6 and 7), suggesting that expression levels of RET in the cerebral cortex of adult mice might be insufficient for inducing neurite outgrowth, or essential molecules for RET-mediated neurite outgrowth might be insufficient in the adult cerebral cortex.
Cellular changes following phosphorylation of RET include neurite extension (Enomoto et al., 2001; Gustin et al., 2007; Brantley et al., 2008), oligodendrocyte development (Strelau and Unsicker, 1999), synaptic maturation (Bourque and Trudeau, 2000; Baudet et al., 2008) and cell survival (Lin et al., 1993; Airaksinen and Saarma, 2002). RET can activate various signalling pathways, including Ras/extracellular signal-regulated kinase (ERK) (Worby et al., 1996) and phosphatidylinositide-3 kinase (PI3K)/Akt (Murakami et al., 1999). These pathways are activated mainly through tyrosine 1062, which is a binding site for Shc adaptor proteins (Hayashi et al., 2000). After binding Shc to tyrosine 1062, Shc associates with GAB1/2 adaptor proteins and the GRB2/SOS complex, leading to the activation of PI3K/Akt and Ras/ERK signalling pathways respectively. Activation of the Ras/ERK pathway modulates presynaptic plasticity and hippocampus-dependent learning in mice (Kushner et al., 2005). Several groups have reported that local activation of PI3K and the accumulation of phosphatidylinositol 3,4,5-trisphosphate at the tip of one of the immature neurites were important for axon specification and elongation in cultured hippocampal neurons (Shi et al., 2003; Menager et al., 2004). In addition, a correlation of PI3K with learning and memory has been suggested. Infusion of a PI3K inhibitor, LY294002 or wortmannin, into hippocampal CA1 temporarily impairs the retrieval and extinction of contextual memory (Barros et al., 2001; Chen et al., 2005). Intracerebroventricular injection of wortmannin inhibits spatial memory in rats (Liu et al., 2003), and intraamygdala infusion of wortmannin interferes with long-term fear memory (Lin et al., 2001). Our studies using PI3K knockout mice also suggested that PI3K plays a role in the generation and/or maintenance of synapses and myelinated axons in the brain, leading to learning ability (Tohda et al., 2007; 2009;). However, a relationship between RET activation and memory has not been proved directly. There are also several reports concerning GDNF and memory, among which a recent report suggested that the expression of the GDNF transgene in astrocytes improves cognitive deficits in aged rats (Pertusa et al., 2008). However, the activation of RET was not discussed in this report, although increases in neurotransmitters were the suspected main causes of the improvement. In contrast, memory enhancement by sominone is probably mediated by the reinforcement of neurite proteins, in axons and dendrites, which may be induced by RET activation. We are currently studying the effects of sominone on synaptic currents by electrophysiological experiments to evaluate whether the reinforcement of neurite proteins leads to synaptic plasticity. As sominone is a low-molecular compound (MW: 458.6) and has hydrophobic properties, it is possible that sominone can pass through the blood–brain barrier; however, we need to clarify experimentally that sominone could enter the brain through the blood–brain barrier and activate neurons directly.
It is still not known if sominone directly binds to RET in a ligand-like manner or modulates RET conformation. Similarly, the GDNF-independent activation of RET by sominone may require GFRα molecules to transduce signals downstream..
Although this study was performed using normal cultured neurons and normal mice, we had supposed that the effect of sominone might also be useful in the neurodegenerative stage. Our previous study showed that continuous oral administration of withanoside IV, which acts as a prodrug of sominone, improved memory disorders and increased the densities of axons, dendrites and presynapses in the hippocampal CA1 and CA3, temporal cortex and parietal cortex when losses of these neurite proteins had already been induced by Aβ(25-35) injection into the brain (Kuboyama et al., 2006). Therefore, sominone also may have the potential to regenerate damaged neuronal networks and thus may contribute to the amelioration of dementia. As this type of anti-dementia drug has not been developed yet, sominone is quite an attractive candidate as a lead compound for anti-dementia drugs. In future studies we will evaluate the effects of sominone on memory disorders using Alzheimer's disease model transgenic mice.
In conclusion, we found that sominone might reinforce the morphological plasticity of neurons by activation of the RET pathway, independently of GDNF, and enhance memory. Sominone may be an exogenous low-molecular-weight stimulator of the RET pathway and/or a novel modulator of RET signalling.
Acknowledgments
This work was partially supported by Grants-in-Aid for Scientific Research (C) (17500249) from the Japan Society for the Promotion of Science, and a grant for Expansion Program in the Knowledge Cluster Initiative from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Glossary
Abbreviations:
- ERK
Ras/extracellular signal-regulated kinase
- GDNF
glial cell line-derived neurotrophic factor
- GFRα
glycosylphosphatidylinositol-anchored receptor α
- PI3K
phosphatidylinositide-3 kinase
- PMSF
phenylmethylsulphonyl fluoride
Conflict of interest
The authors state no conflicts of interest.
References
- Figure 8.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999;13:313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- Akerud P, Alberch J, Eketjäll S, Wagner J, Arenas E. Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. J Neurochem. 1999;73:70–78. doi: 10.1046/j.1471-4159.1999.0730070.x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn) 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta-ur-Rahman Jamal SA, Choudhary MI. Two new withanolides from Withania somnifera. Heterocycles. 1992;34:689–698. [Google Scholar]
- Barros DM, Mello e Souza T, de Souza MM, Choi H, DeDavid e Silva T, Lenz G, et al. LY294002, an inhibitor of phosphoinositide 3-kinase given into rat hippocampus impairs acquisition, consolidation and retrieval of memory for one-trial step-down inhibitory avoidance. Behav Pharmacol. 2001;12:629–634. doi: 10.1097/00008877-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Baudet C, Pozas E, Adameyko I, Andersson E, Ericson J, Ernfors P. Retrograde signaling onto Ret during motor nerve terminal maturation. J Neurosci. 2008;28:963–975. doi: 10.1523/JNEUROSCI.4489-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Brantley MA, Jr, Jain S, Barr EE, Johnson EM, Jr, Milbrandt J. Neurturin-mediated ret activation is required for retinal function. J Neurosci. 2008;28:4123–4135. doi: 10.1523/JNEUROSCI.0249-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burazin TCD, Gundlach AL. Localization of GDNF/neurturin receptor (c-ret, GFRα-1 and α-2) mRNAs in postnatal rat brain: differential regional and temporal expression in hippocampus, cortex and cerebellum. Mol Brain Res. 1999;73:151–171. doi: 10.1016/s0169-328x(99)00217-x. [DOI] [PubMed] [Google Scholar]
- Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr, Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–3974. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Gould TW, Yonemura S, Oppenheim RW, Ohmori S, Enomoto H. The neurotrophic effects of glial cell line-derived neurotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J Neurosci. 2008;28:2131–2146. doi: 10.1523/JNEUROSCI.5185-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin JA, Yang M, Johnson EM, Jr, Milbrandt J. Deciphering adaptor specificity in GFL-dependent RET-mediated proliferation and neurite outgrowth. J Neurochem. 2007;102:1184–1194. doi: 10.1111/j.1471-4159.2007.04624.x. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, et al. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- Jain S, Golden JP, Wozniak D, Pehek E, Johnson EM, Jr, Milbrandt J. RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J Neurosci. 2006;26:11230–11238. doi: 10.1523/JNEUROSCI.1876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, et al. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:616–628. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama T, Tohda C, Zhao J, Nakamura N, Hattori M, Komatsu K. Axon- or dendrite- predominant outgrowth induced by constituents from Ashwagandha. Neuroreport. 2002;13:1715–1752. doi: 10.1097/00001756-200210070-00005. [DOI] [PubMed] [Google Scholar]
- Kuboyama T, Tohda C, Komatsu K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol. 2005;144:961–971. doi: 10.1038/sj.bjp.0706122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama T, Tohda C, Komatsu K. Withanoside IV and its active metabolite, sominone, attenuate Aβ (25-35)-induced neurodegeneration. Eur J Neurosci. 2006;23:1417–1426. doi: 10.1111/j.1460-9568.2006.04664.x. [DOI] [PubMed] [Google Scholar]
- Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR, et al. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci. 2005;25:9721–9734. doi: 10.1523/JNEUROSCI.2836-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, et al. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdale. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, et al. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J Neurochem. 2003;87:1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Murakami H, Iwashita T, Asai N, Shimono Y, Iwata Y, Kawai K, et al. Enhanced phosphatidylinositol 3-kinase activity and high phosphorylation state of its downstream signalling molecules mediated by ret with the MEN 2B mutation. Biochem Biophys Res Commun. 1999;262:68–75. doi: 10.1006/bbrc.1999.1186. [DOI] [PubMed] [Google Scholar]
- Menager C, Arimura N, Fukata Y, Kaibuchi K. PIP3 is involved in neuronal polarization and axon formation. J Neurochem. 2004;89:109–118. doi: 10.1046/j.1471-4159.2004.02302.x. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Tohda C. Withanoside IV improves hindlimb function by facilitating axonal regrowth and increase in peripheral nervous system myelin level after spinal cord injury. Neurosci Res. 2007;58:176–182. doi: 10.1016/j.neures.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Pertusa M, García-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29:1366–1379. doi: 10.1016/j.neurobiolaging.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Quartu M, Serra PM, Boi M, Ferretti TM, Lai LM, Fiacco MD. Tissue distribution of Ret, GFRalpha-1, GFRalpha-2 and GFRalpha-3 receptors in the human brainstem at fetal, neonatal and adult age. Brain Res. 2007;1173:36–52. doi: 10.1016/j.brainres.2007.07.064. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- Schäfer KH, Mestres P. The GDNF-induced neurite outgrowth and neuronal survival in dissociated myenteric plexus cultures of the rat small intestine decreases postnatally. Exp Brain Res. 1999;125:447–452. doi: 10.1007/s002210050702. [DOI] [PubMed] [Google Scholar]
- Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. doi: 10.1016/s0092-8674(02)01249-7. [DOI] [PubMed] [Google Scholar]
- Strelau J, Unsicker K. GDNF family members and their receptors: expression and functions in two oligodendroglial cell lines representing distinct stages of oligodendroglial development. Glia. 1999;26:291–301. doi: 10.1002/(sici)1098-1136(199906)26:4<291::aid-glia3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Tohda C, Kuboyama T, Komatsu K. Dendrite extension by methanol extract of Ashwagandha (root of Withania somnifera) in SK-N-SH cells. Neuroreport. 2000;11:1981–1985. doi: 10.1097/00001756-200006260-00035. [DOI] [PubMed] [Google Scholar]
- Tohda C, Nakanishi R, Kadowaki M. Learning-deficits and agenesis of synapses and myelinated axons in phosphoinositide-3 kinase-deficient mice. Neurosignals. 2007;15:293–306. doi: 10.1159/000108936. [DOI] [PubMed] [Google Scholar]
- Tohda C, Nakanishi R, Kadowaki M. Hyperactivity, memory deficit and anxiety-related behaviors in mice lacking the p85α subunit of phosphoinositide-3 kinase. Brain Dev. 2009;31:69–74. doi: 10.1016/j.braindev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, et al. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Worby CA, Vega QC, Zhao Y, Chao HH, Seasholtz AF, Dixon JE. Glial cell line-derived neurotrophic factor signals through the RET receptor and activates mitogen-activated protein kinase. J Biol Chem. 1996;271:23619–23622. doi: 10.1074/jbc.271.39.23619. [DOI] [PubMed] [Google Scholar]
- Zhao J, Nakamura N, Hattori M, Kuboyama T, Tohda C, Komatsu K. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull. 2002;50:760–765. doi: 10.1248/cpb.50.760. [DOI] [PubMed] [Google Scholar]


