Abstract
Background and purpose:
Gaboxadol has been in development for treatment of chronic pain and insomnia. The clinical use of gaboxadol has revealed that adverse effects seem related to peak serum concentrations. The aim of this study was to investigate the mechanism of intestinal absorption of gaboxadol in vitro and in vivo.
Experimental approach:
In vitro transport investigations were performed in Caco-2 cell monolayers. In vivo pharmacokinetic investigations were conducted in beagle dogs. Gaboxadol doses of 2.5 mg·kg−1 were given either as an intravenous injection (1.0 mL·kg−1) or as an oral solution (5.0 mL·kg−1).
Key results:
Gaboxadol may be a substrate of the human proton-coupled amino acid transporter, hPAT1 and it inhibited the hPAT1-mediated L-[3H]proline uptake in Caco-2 cell monolayers with an inhibition constant Ki of 6.6 mmol·L−1. The transepithelial transport of gaboxadol was polarized in the apical to basolateral direction, and was dependent on gaboxadol concentration and pH of the apical buffer solution. In beagle dogs, the absorption of gaboxadol was almost complete (absolute bioavailability, Fa, of 85.3%) and Tmax was 0.46 h. Oral co-administration with 2.5–150 mg·kg−1 of the PAT1 inhibitor, L-tryptophan, significantly decreased the absorption rate constant, ka, and Cmax, and increased Tmax of gaboxadol, whereas the area under the curve and clearance of gaboxadol were constant.
Conclusions and implications:
The absorption of gaboxadol across the luminal membrane of the small intestinal enterocytes is probably mediated by PAT1. This knowledge is useful for reducing gaboxadol absorption rates in order to decrease peak plasma concentrations.
Keywords: Pharmacokinetics, hPAT1 (SLC36A1), gaboxadol, tryptophan, modified intestinal absorption, drug delivery, Caco-2 cells, dog
Introduction
The GABAA receptor agonist gaboxadol [4, 5, 6, 7-tetrahydroisoxazolo (5,4-c) pyridine-3-ol; THIP] has been in clinical development for the treatment of insomnia (Lancel and Faulhaber, 1996; Ebert et al., 2006). At therapeutically relevant concentrations gaboxadol exclusively activates the extra-synaptic GABAA receptors (Ebert and Wafford, 2006) and has therefore been characterized as a selective extra-synaptic GABAA agonist (Nutt, 2005). Earlier preclinical studies with radiolabelled gaboxadol have shown that gaboxadol is almost completely absorbed (84–93%) after oral administration in rat, mouse and human (Schultz et al., 1981). Single gaboxadol doses of 10 or 20 mg, taken as a tablet 30 min. before bedtime, have been shown to improve sleep maintenance and enhance slow wave sleep in humans (Lundahl et al., 2007). Nevertheless, development of dose-related adverse events has been reported, in a small open-labelled study in patients with moderate to severe chronic pain, with morning dosing and intramuscular administration of 5–30 mg gaboxadol. These adverse events were closely correlated to peak serum concentrations, leading the authors to conclude that if the compound was to be used in the treatment of pain in cancer, peak serum concentrations above 0.55 µmol·L−1 should be avoided (Kjaer and Nielsen, 1983). Obviously some adverse events are related to the specific disease; however, the need for reduced serum peak concentrations may very well be relevant for other indications of gaboxadol as well. The intestinal absorption mechanism for gaboxadol across the intestinal epithelium is still unknown.
The human proton-dependent amino acid transporter 1 (hPAT1) of the solute carrier family SLC36 (SLC36A1), is an absorptive intestinal transporter for small zwitterionic amino acids and amino acid mimetics such as GABA (Thwaites et al., 1995b). The PAT1 cDNA was first cloned as the lysosomal amino acid transporter 1 (LYAAT1) from rat brain in 2001 by Sagnéet al. (2001), and later from mouse intestine (Boll et al., 2002) and Caco-2 cells (Chen et al., 2003). hPAT1 mRNA expression has been detected in most parts of the human gastrointestinal tract with maximal expression in small intestinal tissues (Chen et al., 2003; Anderson et al., 2004). hPAT1 has a relatively broad substrate specificity, and drug substances such as vigabatrin (Abbot et al., 2006) and D-cycloserine (Thwaites et al., 1995a) have also been shown to be transported via the transporter. Furthermore, is has been demonstrated that 5-hydroxytryptamine, L-tryptophan and tryptamine are effective inhibitors of amino acid transport via PAT1 (Metzner et al., 2005). Recently, two GABA analogues, muscimol, a GABAA receptor agonist, and 4,5,6,7,-tetrahydroisoxazolo(4,5-c)-pyridin-3-ol (THPO), a GABA uptake inhibitor, were also identified as substrates for hPAT1 (Larsen et al., 2008). Both analogues are heterocyclic compounds containing a 3-isoxazolol moiety, which has been shown to function as a carboxylic acid bioisostere for binding to the GABA receptors and transporters present in the brain (Corey et al., 1994; Krogsgaard-Larsen et al., 1994) and now also for hPAT1 substrate recognition. Based upon these findings and the structural similarities of muscimol, THPO and gaboxadol, it is hypothesized that gaboxadol is a substrate for PAT1 and that PAT1 is involved in the in vivo absorption of gaboxadol.
The aim of the present study was to identify the intestinal transport mechanism of gaboxadol in vitro and in vivo. During the in vitro studies it was found that gaboxadol was a ligand for hPAT1. Further transport studies suggested that hPAT1-mediated transport of gaboxadol across the apical membrane was important for the overall transepithelial transport. Therefore, a further aim was to investigate if PAT1 could be utilized for modifying the intestinal absorption of gaboxadol and subsequently alter the pharmacokinetic profile of gaboxadol. A pharmaceutical formulation approach based on co-administration of gaboxadol and tryptophan was successfully applied to decrease the absorption rate constant of gaboxadol.
Methods
Cell culture experiments
Protocols for cell culturing and in vitro experiments were as previously described (Larsen et al., 2008). Caco-2 cells of passages 20 through 29 were seeded onto Transwell™ inserts (1.12 cm2, 0.4 µm pore size) and experiments were conducted on day 25–28 after seeding. The apical uptake and the transepithelial transport of gaboxadol across Caco-2 cell monolayers in apical to basolateral direction (A–B) and basolateral to apical direction (B–A) were measured in Hanks' balanced salt solution (HBSS) buffers. In all experiments, buffer applied to the basolateral side was pH 7.4. Unless otherwise stated, buffer applied in the apical chamber was adjusted to pH 6.0 after the addition of gaboxadol hydrochloride or 35 mmol·L−1 tryptophan. The transport of 0.34, 3.5 or 7.0 mmol·L−1 gaboxadol was investigated. These concentrations were selected based on a single bedtime oral dose of 15 mg gaboxadol to humans, which provides an approximate luminal concentration of 0.34 mmol·L−1, and the obtained gaboxadol affinity for hPAT1. Apical uptake experiments were initiated by adding fresh apical HBSS medium with 12.5 nmol·L−1 (0.5 µCi) L-(3H)proline and 0–30 mmol·L−1 gaboxadol or 0–35 mmol·L−1 tryptophan to the apical chamber. The apical uptake experiments were terminated after 5 min. Samples were analysed by scintillation counting.
In vivo experiments
Absorption of gaboxadol in dogs
All animal care and experimental studies were approved by the Animal Welfare Committee, appointed by the Danish Ministry of Justice, and were carried out in compliance with EC Directive 86/609/EEC, the Danish law regulating experiments on animals and NIH Guidelines for the Care and Use of Laboratory Animals. Six full-grown male beagle dogs (body weight 15.9–21.7 kg) were selected and allocated into a Roman quadrant design and assigned to receive all the six formulations of gaboxadol hydrochloride randomly during 6 weeks. The dogs were fasted for 20–24 h before the initiation of the experiment and fed again 10 h after the administration. The gaboxadol dose was given either as an intravenous injection (1.0 mL·kg−1) or as an oral solution given by gavage (5.0 mL·kg−1) directly into the stomach using a soft tube. All dogs received 2.5 mg·kg−1 gaboxadol. In addition to gaboxadol, the oral formulations contained 0, 2.5, 10, 50 or 150 mg·kg−1 of tryptophan to ensure simultaneous co-administration of the two compounds. All solutions were adjusted to a pH of 5.2, and osmolarity was checked with a Vapro vapor pressure osmometer (model 552O, Wescor Inc., Logan, UT, USA), the intravenous solutions were adjusted to iso-osmolarity with glucose. Blood samples (2 mL) were taken from the cephalic vein by individual venepuncture and collected into Eppendorf tubes containing 200 IE heparin as an anticoagulant. Samples were collected before administration of gaboxadol and after 5, 15, 30, 60, 90 min, and 2, 3, 4, 6, 8 and 10 h after gaboxadol administration. The plasma was harvested immediately by centrifugation for 15 min at 2200 g and 4–8°C and stored at −80°C until further analysis. The animals had a 6-day washout period between treatments.
Investigation of gastric emptying in dog
A protocol similar to the one described earlier using paracetamol as a marker was used to evaluate the influence of tryptophan on the gastric emptying rate in dogs. Six dogs (body weight 16.1–21.5 kg) were selected and randomly allocated to receive three formulations of paracetamol in a crossover study. The dogs received 50 mg·kg−1 paracetamol as an intravenous injection (1 mL·kg−1) or as an oral solution (5 mL·kg−1) containing 2.5 mg·kg−1 gaboxadol and 0 or 150 mg·kg−1 tryptophan. Fasting of the dogs, drug administration, blood sampling and washout were done as described earlier.
Analytical methods
Quantification of gaboxadol in plasma and buffer: Gaboxadol was extracted from plasma and buffer samples by liquid extraction. 100 µL HBSS or plasma samples were mixed with 25 µL internal standard (d4-gaboxadol) and 25 µL purified water. Protein precipitation was carried out by addition of 400 µL cold acetonitrile. After centrifugation at 10 000 g for 15 min, 425 µL of supernatant was transferred to glass tubes and evaporated to dryness under nitrogen at 45°C. The samples were redissolved in 80 µL of methanol/acetonitrile (30:70), whirl-mixed for 10 min and centrifuged for 3 min at 3300× g. Gaboxadol was subsequently quantified by hydrophilic interaction chromatography followed by tandem mass spectrometry (MS/MS) detection using a protocol modified from Kall et al. (2007). The liquid chromatography (LC) system comprised by an Agilent 1100 series pump and degasser. An Asahipak amino column, (NH2P-50, 150 × 2 mm) from Phenomenex (Torrance, CA, USA) was used with a mobile phase of 20.0 mmol·L−1 ammonium acetate (pH 4): acetonitrile (30:70) and a flow rate of 0.2 mL·min−1. Twenty-microlitre samples were injected onto the column, which was kept at room temperature. The total run time was 10 min with the first 5 min of elution let to waste. The elution time of gaboxadol on the column was approximately 8 min. The MS/MS system used consisted of a Sciex API 4000 MS/MS detector with a Turbo Ion Spray and Turbo V source (Applied Biosystems, Foster City, CA, USA). The signals were linear between 0.5 and 2500 ng·mL−1, and the limit of quantification by this procedure was 0.5 ng·mL−1. The software was from Analyst™ (Applied Biosystems, version 4.0).
Quantification of paracetamol in plasma samples: 100 µL plasma were added to 60 mg sodium chloride, 50 µL purified water and 25 µL internal standard solution (25 µg·mL−1 theophylline in water) before protein precipitation and extraction with 1000 µL ethyl acetate was performed. The Eppendorf tubes were mixed for 20 s on a vortex mixer and centrifuged in a Labofuge 400 from Function Line (Heraeus Instruments, Hanau, Germany) at room temperature for 5 min at 6000 g. The supernatant was evaporated to dryness under a stream of nitrogen at 60°C, and subsequently the residue was resolved in 100 µL of mobile phase. Twenty-five microlitres was injected into the column. Analysis was performed in Merck–Hitachi equipment with a Merck–Hitachi L-4250 UV detector at a wavelength of 245 nm. The column was an XBridge C18, 3.5 µm, 4.6 × 150 mm and the Merck L-5025 column thermostat was heated to 40°C. The mobile phase consisted of 0.025 M phosphate buffer (pH 6.0), acetonitrile (95:5), and the flow rate was 1.0 mL·min−1. The limit of quantification by this procedure was 0.7 µg·mL−1 and the assay was linear between 0.7 µg·mL−1 and 147 µg·mL−1.
Data analysis
The flux, J, of gaboxadol (mass·time−1·area−1) across the Caco-2 cell monolayer was calculated from Fick's first law. The mass of substrate, Q, accumulating in the receptor compartment per time, t, and as a function of area, A, at steady-state conditions was as follows
| (1) |
where C is the initial concentration of the gaboxadol in the donor chamber and Papp is the apparent permeability coefficient of gaboxadol across the cell monolayer. Sink conditions were confirmed by assuring that solubility was not exceeded and no more than 10% of the initial concentration of gaboxadol was accumulated in the receptor chamber. The steady state flux values of gaboxadol were calculated as mean flux values from 20 to 100 min. The 50% inhibitory concentration (IC50) value of tryptophan was the concentration at which the apical uptake of proline (a prototypical hPAT1 substrate), U, was reduced to 50% of the control value. This value was determined using the equation
| (2) |
where U is the uptake of proline (0–100%) at concentration [I] of the inhibitor, in this case tryptophan. Umin is the minimal uptake of proline (at the highest value of [I]), and Umax is the control uptake ([I]= 0), both given as % values. IC50 values were converted to Ki values assuming competitive kinetics as described by Cheng and Prusoff (1973):
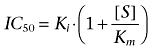 |
(3) |
[S] is the gaboxadol or tryptophan concentration, Km is the Michaelis constant for proline uptake (mmol·L−1). All results are expressed as mean ± standard error of the mean (SEM) of 3–4 independent cell passages. The data were fitted using non-linear regression in order to allow IC50 values to be obtained by GraphPad Prism software (version 4.02; GraphPad Prism Software Inc., San Diego, CA, USA).
Pharmacokinetic parameters from the in vivo studies on beagle dogs were calculated using WinNonlin Professional (version 5.2, Pharsight Corporation, Mountain View, CA, USA). The plasma concentration profiles of both gaboxadol and paracetamol after intravenous dosing were fitted to a two-compartment model whereas a non-compartmental model was used to analyse plasma concentration profiles from oral dosing. The area under the curve (AUC) was determined using the linear trapezoidal method and extrapolating the last measured plasma concentration to infinity. The size of the residual varied between 0.54 and 1.5% of the AUC. The analysis of the elimination rate constant was obtained by linear regression of at least three time points towards the end of the experiment. The bioavailability, Fa, of gaboxadol from the oral solutions was calculated for the individual animal using the following equation (Rowland and Tozer, 1995)
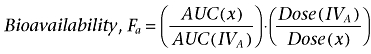 |
(4) |
where AUC(IVA) is the area under the curve of treatment A, the intravenous gaboxadol administration in the absence of tryptophan, x is one of the oral solutions containing gaboxadol and 0–150 mg·kg−1 tryptophan, B-F. Results are expressed as mean ± SEM of the six animals.
In the present study, numerical deconvolution as shown by Langenbucher (Langenbucher, 1982) was used and approximated as:
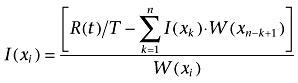 |
(5) |
where T is the time interval, I(Xk) and W(Xk) the average input rate and weight between the times Xk−1 and Xk The plasma concentration profiles obtained in the present study were used for numerical deconvolution supplemented with some terminal plasma concentrations estimated by linear regression, as the method requires a constant time interval. The input function I(t) represents the absorption process, whereas R(t) is the obtained plasma concentration following oral administration, and W(t) is the plasma concentration following the IV dose (Langenbucher, 1982).
The mean cumulative fraction of absorbed gaboxadol and paracetamol per time unit were calculated by deconvolution of the obtained plasma concentrations, which provided a dynamic profile of the theoretical first-order absorption process as 0–100% absorbed for all treatments in every single animal. The cumulative absorption of paracetamol is used to estimate the gastric emptying rate (Calbet and MacLean, 1997; Sunesen et al., 2005). Profiles of both paracetamol and gaboxadol absorption were used to estimate the rate coefficient of absorption (ka). ka was found as the slope of the deconvolution curves when converted to a first-order function by the equation:
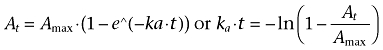 |
(6) |
At was obtained from the deconvolution profiles and was the cumulative fraction gaboxadol/acetaminophen absorbed at a given time t and Amax is 100%. The mean slope and SEM were obtained by linear regression analysis of the linear absorption curves from six animals (At vs. time) performed in GraphPad Prism.
Statistical analysis
Statistical analysis was performed in Sigma Stat (version 3.0.1, SPSS Inc. Chicago, IL, USA) using a Students t-test or a one-way analysis of variance (ANOVA) followed by multiple comparisons versus control group (Holm-Sidak method), *P < 0.05, **P < 0.01, ***P < 0.001.
Materials
The chemicals were from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Caco-2 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and Dulbecco's modified Eagle's medium (DMEM) was obtained from Life Technologies (Høje Taastrup, DK). HBSS with calcium and magnesium was purchased from GIBCO, Invitrogen (Paisley, UK) and 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) from Applichem (Darmstadt, Germany). Cell culture plastic ware was obtained from Corning Inc., Life Sciences (PA, USA). (2,3,4,5-3H)-L-Proline (80 Ci·mmol−1) was purchased from Larodan, (Malmö, Sweden). Gaboxadol hydrochloride [4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol hydrochloride] and the deutero-substituted form d4-gaboxadol [4,4,5,5-tetradeutero-6,7-dihydro-isoxazolo(5,4-c)pyridin-3-ol hydrochloride] were synthesized by H. Lundbeck A/S (Valby, Denmark). Heparin, 5000 IE·mL−1, was purchased from LEO (Ballerup, Denmark). Orthophosphoric acid 85%, disodium hydrogen phosphate, potassium hydrogen phosphate and acetic acid were obtained from Merck (Darmstad, Germany). All chemicals were of analytical reagent grade.
Results
Characterization of gaboxadol transport via hPAT1 in vitro
The interaction between gaboxadol and hPAT1 was investigated by measuring the apical uptake of the hPAT1 substrate proline into Caco-2 cell monolayers in the presence of increasing gaboxadol concentrations (Figure 1). Gaboxadol decreased apical proline uptake in Caco-2 cell monolayers with an estimated inhibitor affinity (Ki value) of 6.6 mmol·L−1. Similarly, the known PAT1 inhibitor, tryptophan, also decreased the apical uptake of proline with a Ki value of 7.7 mmol·L−1. The transepithelial (A-B) flux of gaboxadol transport across Caco-2 cell monolayers was investigated at three apical concentrations (0.34, 3.5 and 7.0 mmol·L−1). The mean Papp values of gaboxadol transport were 8.1 × 10−6 cm·s−1, 6.1 × 10−6 cm·s−1 and 5.6 × 10−6 cm·s−1 for 0.34, 3.5 and 7 mmol·L−1 gaboxadol, respectively (Figure 2). Thus, the gaboxadol permeability across Caco-2 cell monolayers decreased with increasing gaboxadol concentrations (P < 0.05). The gaboxadol transport across Caco-2 cell monolayers using an apical concentration of 3.5 mmol·L−1 gaboxadol was investigated in the presence of 35 mmol·L−1 tryptophan, with or without a pH gradient, and bidirectional (Figure 2). Transport of gaboxadol in the A–B direction was approximately five times higher than in the B–A direction (P < 0.005). The presence of tryptophan reduced the permeability of gaboxadol by 53% (to a Papp of 2.9 × 10−6 cm·s−1, P < 0.005). In the absence of a proton gradient across the monolayer, the permeability of gaboxadol was reduced by 82% to 1.1 × 10−6 cm·s−1 (P < 0.005). Gaboxadol permeability in the presence of tryptophan, in absence of a proton gradient, and in the B–A direction was similar to the permeability of [3H]-mannitol (Papp of 1.6 ± 0.36 × 10−6 cm·s−1). Furthermore, the presence of gaboxadol and tryptophan in the transport experiments did not change the permeability of metoprolol or mannitol transport across Caco-2 cell monolayers. The permeabilities of mannitol and metoprolol were 1.6 ± 0.36 × 10−6 cm·s−1 and 6.9 ± 0.99 × 10−6 cm·s−1, respectively. In summary, the transepithelial gaboxadol transport across Caco-2 cell monolayers was pH-dependent, could be inhibited by tryptophan and was polarized in the A-B direction. Together these observations suggest that hPAT1 mediates gaboxadol transport across the luminal membrane of human intestinal epithelial cells, and that this transport step to a large degree determines the resulting transepithelial transport of gaboxadol.
Figure 1.
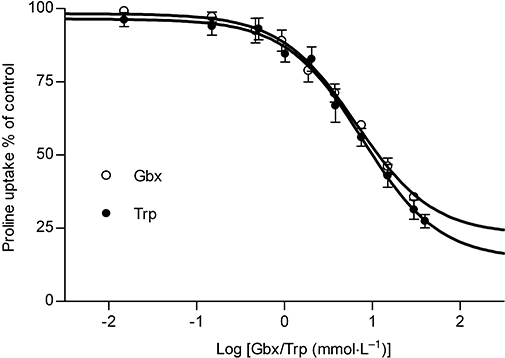
Inhibition of apical L-[3H]proline uptake via hPAT1 by 0.015–30 mmol·L−1 gaboxadol (Gbx) and 0.03–40 mmol·L−1 tryptophan (Trp) in Caco-2 cell monolayers at days 25–28 after seeding. The apical concentration of L-[3H]proline was 12.5 nmol·L−1 (1 µCi·mL−1). Uptake of L-proline was measured for 5 min with an apical pH of 6.0 and a basolateral pH of 7.4. Values are means ± standard error of the mean (SEM) of 3–4 independent cell passages. Fifty percent inhibitory concentration (IC50) values (pIC50± SEM) gaboxadol 6.6 mmol·L−1 (2.18 ± 0.08), tryptophan 7.7 mmol·L−1 (2.11 ± 0.08).
Figure 2.
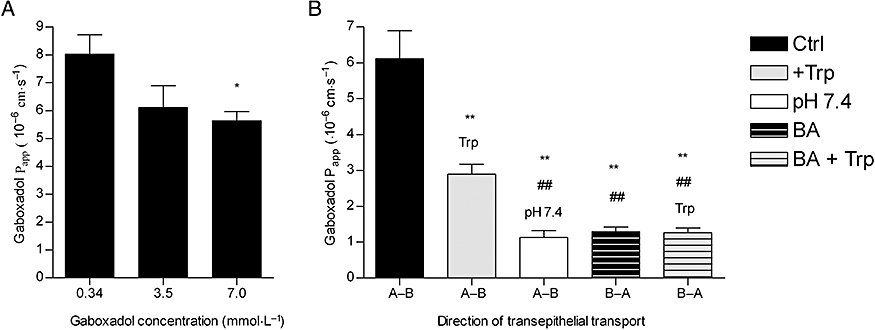
Transepithelial transport of 0.34, 3.5 and 7 mmol·L−1 gaboxadol across Caco-2 cell monolayers. (A) Apparent permeability coefficients, Papp, calculated from flux data (A–B) shown as a function of apical gaboxadol concentration. The apical pH was 6.0 and basolateral pH was 7.4. Statistically significant difference from the Papp of 0.34 mmol·L−1 gaboxadol observed by Student's t-test, *P < 0.05. (B) Papp of 3.5 mmol·L−1 gaboxadol at various conditions. Papp of 3.5 mmol·L−1 gaboxadol was measured in absence or presence of 35 mmol·L−1 tryptophan (Trp) at an apical pH of 6.0 and basolateral pH of 7.4 or at pH 7.4 at both sides. Each data point represents the mean ± standard error of the mean obtained in 3–4 independent cell passages. Statistically significant difference observed by Student's t-test: difference from 3.5 mmol·L−1 gaboxadol (A-B), **P < 0.005 or difference from 3.5 mmol·L−1 gaboxadol in presence of tryptophan (Trp; A–B), ##P < 0.005.
Pharmacokinetic analysis of oral gaboxadol absorption in dog
Gaboxadol plasma concentration profiles following oral or intravenous administration of 2.5 mg·kg−1 gaboxadol in beagle dogs were monitored over 10 h (Figure 3). The bioavailability, Fa, of gaboxadol following oral administration in dog was high (over 80%) (Table 1). Oral co-administration of 2.5–150 mg·kg−1 tryptophan did not change the AUC of gaboxadol significantly, and the mean relative bioavailability of the formulations varied between 75 (10 mg·kg−1 tryptophan) and 86.1% (2.5 mg·kg−1 tryptophan). Also, the elimination rate constant (ke) and the clearance (CL) of gaboxadol did not change with co-administration of tryptophan. However, co-administration of 150 mg·kg−1 tryptophan decreased the maximal gaboxadol plasma concentration, Cmax, from 2502 to 1419 ng·mL−1, that is, 57%. Furthermore, the time required to reach the maximal plasma concentration, Tmax, was increased from 0.46 h to 1.5 h (P < 0.01). The Cmax values of the five dose groups were subsequently fitted to a dose-response curve (Figure 4), which indicated a direct interaction between gaboxadol absorption and tryptophan concentration. The in vivo IC50 value of tryptophan on gaboxadol Cmax was estimated to be 12.6 mg·kg−1, which is equivalent to a concentration of 12.3 mmol·L−1 tryptophan (not corrected for dilution in gastric and intestinal fluids).
Figure 3.

Plasma concentration versus time profiles after intravenous (insert) or oral dosing of gaboxadol to male beagle dogs during 10 h. The dogs received 2.5 mg·kg−1 gaboxadol intravenously or orally (2.5 mg·kg−1; 3.5 mmol·L−1) with tryptophan. Tryptophan was given as a co-administration together with gaboxadol. Data shown are means ± standard error of the mean (n= 6).
Table 1.
Pharmacokinetic parameters of gaboxadol in male beagle dogs
| Group | A (IV) | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Tryptophan (mg·kg−1) | 0 | 0 | 2.5 | 10 | 50 | 150 |
| ke (h−1) | 1.02 ± 0.14 | 0.46 ± 0.02 | 0.50 ± 0.03 | 0.44 ± 0.03 | 0.50 ± 0.03 | 0.48 ± 0.07 |
| AUC (h·ng·mL−1) | 5618 ± 377 | 4715 ± 248 | 4760 ± 350 | 4032 ± 339 | 4294 ± 211 | 4405 ± 801 |
| Tmax (h) | – | 0.46 ± 0.12 | 0.35 ± 0.13 | 0.54 ± 0.15 | 0.46 ± 0.12 | 1.50 ± 0.39** |
| Cmax (ng·mL−1) | 5489 ± 404 | 2502 ± 43 | 2473 ± 178 | 1868 ± 114** | 1662 ± 37*** | 1419 ± 161*** |
| Clearance (mL·h−1·kg−1) | 456 ± 32 | 538 ± 29 | 537 ± 34 | 645 ± 59 | 589 ± 26 | 700 ± 152 |
| Fa | – | 85.3 ± 5.7 | 86.1 ± 6.7 | 75.0 ± 10.4 | 78.2 ± 6.3 | 79.7 ± 14.5 |
Pharmacokinetic parameters of gaboxadol in male beagle dogs after intravenous (A) and oral (B–F) administration of 2.5 mg·kg−1 gaboxadol co-administered with increasing amounts of tryptophan. Results are expressed as mean ± standard error of the mean (n= 6).
P < 0.01,
P < 0.001, significantly different; one-way anova followed by multiple comparisons versus control group.
Figure 4.
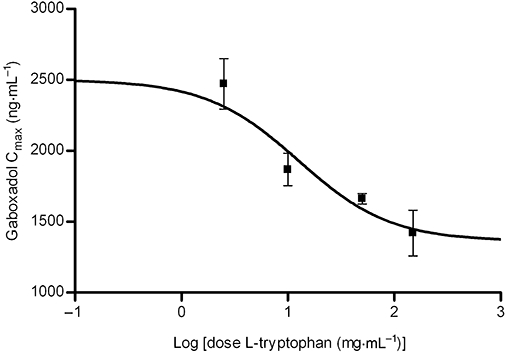
Dose-response curves for the maximal plasma concentration (Cmax) of gaboxadol after co-administration of an oral solution of 2.5 mg·kg−1 gaboxadol and increasing concentrations of tryptophan. Maximum of the curve is the mean Cmax (2502 ng·mL−1) of the control group that was dosed with gaboxadol alone. Each point is determined separately as mean ± standard error of the mean in six dogs.
Absorption rate constants of gaboxadol and paracetamol
Co-administration of increasing tryptophan doses gradually changed the mean cumulative fraction of absorbed gaboxadol as seen in the deconvolution profiles in Figure 5A. The absorption of gaboxadol in the presence of 150 mg·kg−1 tryptophan was significantly decreased at time points 0.5–1.25 h compared with the absorption of gaboxadol alone. An oral dose of 91.5 ± 3.3% of paracetamol was absorbed after 60 min (Figure 5B) indicating that gastric emptying happens mainly within the first hour after administration. Co-administration of 150.0 mg·kg−1 of tryptophan did not significantly change the gastric emptying rate, as the fraction of absorbed paracetamol in the absence or presence of tryptophan was not significantly different at the time points tested. The pharmacokinetic parameters Tmax, AUC and CL of plasma paracetamol concentrations were not significantly different from parameters obtained after co-administration of paracetamol and tryptophan (results not shown). Based on the profiles shown in Figure 5A, the absorption rate constant, ka, of gaboxadol were calculated and these are depicted as a function of the logarithmic tryptophan dose in Figure 6A. The ka of gaboxadol was decreased by co-administration of tryptophan with an in vivo IC50 value on gaboxadol absorption of 10.3 mg·kg−1, which corresponds to an oral solution with a concentration of 10.1 mmol·L−1 tryptophan. Figure 6B shows that 150 mg·kg−1 of the PAT1 inhibitor tryptophan significantly decreased the absorption rate constant of gaboxadol (P < 0.01), whereas it had no significant effect on the absorption rate constant of paracetamol.
Figure 5.
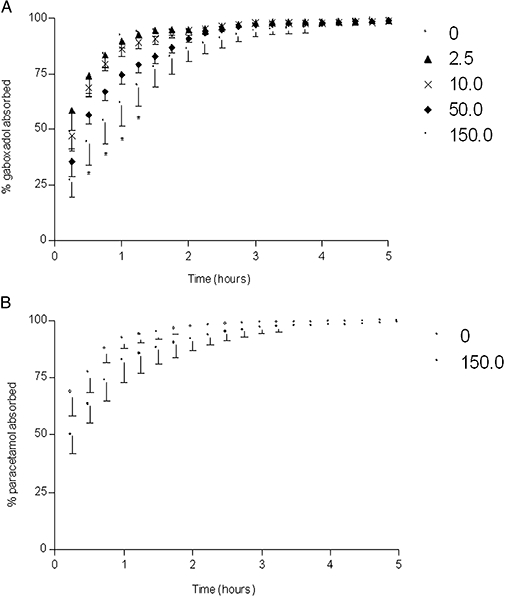
Deconvolution profiles of gaboxadol absorption (A) and paracetamol absorption (B; gastric emptying) following oral administration. (A) The dogs received orally gaboxadol (5.0 mL·kg−1, 2.5 mg·kg−1) together with 0–150 mg·kg−1 tryptophan given as a co-administration. (B) The dogs received 2.5 mg·kg−1 of gaboxadol with 50 mg·kg−1 of paracetamol with or without tryptophan. Data points are mean ± standard error of the mean (n= 6). Significantly different *, P < 0.05 using Student's t-test.
Figure 6.
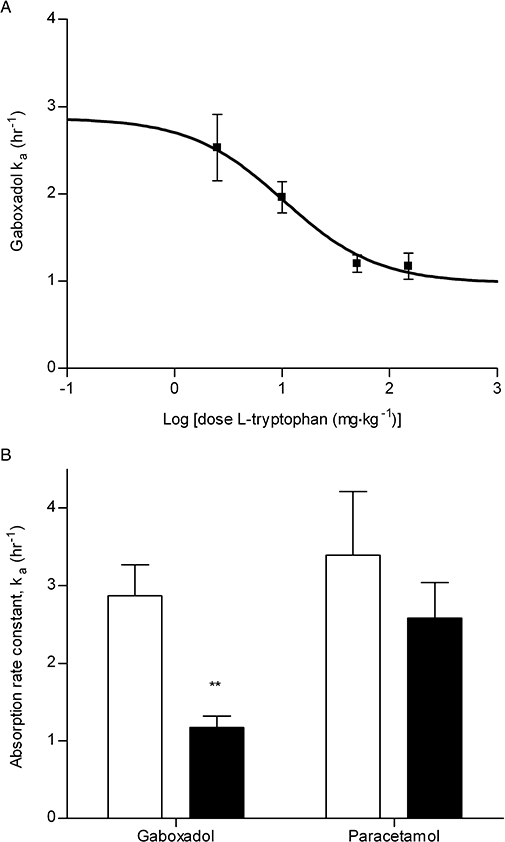
(A) Dose-response curves for the estimated absorption rate constant (ka) of gaboxadol after co-administration of an oral solution of 2.5 mg·kg−1 gaboxadol with increasing concentrations of tryptophan. The maximum of the fitted curve is the mean ka at zero tryptophan concentration, 2.87%·h−1. (B) Absorption rate constants, ka, of gaboxadol and paracetamol in the absence or presence of 150 mg·kg−1 tryptophan. Each point is determined separately as mean ± standard error of the mean in six dogs.
Discussion and conclusions
The high oral bioavailability of gaboxadol was shown decades ago (Schultz et al., 1981). However, the reason for the high intestinal permeability of gaboxadol, despite its unfavourable physiochemical properties, has never been investigated. The interaction of PAT1 with other structurally similar GABA analogues (Larsen et al., 2008) led to the hypothesis investigated in the present study, namely that PAT1 transport proteins could be involved in the intestinal absorption of gaboxadol. The present study suggests for the first time the involvement of the proton-coupled amino acid transporter PAT1 in the intestinal absorption of gaboxadol in vitro and in vivo. Based on this, a formulation approach was used to reduce gaboxadol absorption rates in order to decrease peak plasma concentrations. The present study is to our knowledge the first report, where PAT1 has been shown to be relevant in the in vivo absorption of a drug.
Gaboxadol is a substrate for the proton-coupled amino acid transporter, hPAT1, in Caco-2 cell monolayers
Gaboxadol inhibited the apical uptake of the hPAT1 substrate proline in Caco-2 cell monolayers with a Ki value of 6.6 mmol·L−1. This affinity is comparable with those recently observed for other hPAT1 substrates such as GABA (3.1 mmol·L−1) and the GABA analogues muscimol (1.7 mmol·L−1) and THPO (11.3 mmol·L−1) (Larsen et al., 2008). The affinity of tryptophan was found to be quite similar to gaboxadol, that is, 7.7 mmol·L−1. Metzner et al. (2005) previously characterized tryptophan as an inhibitor of PAT1, and reported a Ki value of 4.7 mmol·L−1 for proline uptake via hPAT1 in Caco-2 cells. Considering minor differences in the proline affinities for hPAT1 between the two laboratories; Metzner et al. reports a Kt of 1.4 mmol·L−1 whereas Larsen et al. reports a Km of 3.6 mmol·L−1, respectively, the affinities of tryptophan for hPAT1 are quite comparable between the two studies (Metzner et al., 2005; Larsen et al., 2008). In accordance with results published by other groups (Thwaites et al., 1993; Metzner et al., 2005), we found that the majority of apical proline transport in Caco-2 cells was mediated by hPAT1, and no evidence of other sodium-dependent or sodium-independent transporters of proline was observed (Larsen et al., 2008). Other amino acid transporters such as the apical sodium-dependent amino acid transporters B0 (B0AT1), B0,+ (ATB0,+) and system (ASC) (ASCT2) are not likely to be involved in the transport of gaboxadol. They are all present in Caco-2 cells but their translocation of substrate is not proton-coupled. Furthermore, these transporters are characterized by higher affinity values for their substrates than observed for PAT1, for example, ASC (ASCT2), approximately 100 µmol·L−1 (Uchiyama et al., 2005); B0 (B0AT1), 500–700 µmol·L−1 (Broer et al., 2004) and B0,+ (ATB0,+), approximately 150 µmol·L−1 (Hatanaka et al., 2002).
The transepithelial transport of gaboxadol across Caco-2 cell monolayers was polarized in the apical to basolateral direction. Gaboxadol transport could be inhibited by tryptophan and was dependent on the pH of the apical donor solution. Furthermore, the Papp of gaboxadol in the apical–basolateral direction decreased with increasing gaboxadol concentration. This is consistent with transport of gaboxadol via hPAT1, and this pathway accounted for approximately 80% of the total transepithelial transport. The amino acid transport system b0,+ has been identified in the small intestine of mouse and in Caco-2 cells, where it accounts for 15–85% of the total transport of alanine and arginine respectively (Wenzel et al., 2001; Dave et al., 2004). However, tryptophan binding to system b0,+ has not been unequivocally shown (Su et al., 1992; Tate et al., 1992), and furthermore cationic amino acids, zwitterionic amino acids and cystine have µmol·L−1 affinities for system b0,+ (Palacin, 1994). Therefore, if gaboxadol is transported via system b0,+ to any significant degree, it should be evident in Caco-2 cells. In vitro the transepithelial gaboxadol transport was furthermore pH-dependent, and PAT1 is the only currently known proton-coupled amino acid transporter in the intestine. The permeability of gabapentin (also a zwitterionic γ-amino analogue) in rat small intestine was shown to be proton independent (Nguyen et al., 2007), hence different apical transport mechanisms exist for gaboxadol and gabapentin. The results indicate that gaboxadol is a substrate for hPAT1 in Caco-2 cell monolayers, and that hPAT1 mediates gaboxadol transport across the luminal membrane of the intestinal enterocytes, which appear to be important for the resulting transepithelial transport. The mechanism for gaboxadol efflux across the basolateral membrane is still unknown.
In vivo absorption of gaboxadol in dogs
The in vivo absorption of gaboxadol in dogs occurred rapidly following oral administration with a Tmax of approximately 0.46 h and a high bioavailability of 85%. These observations are consistent with previous studies on the oral absorption in humans showing a gaboxadol Tmax of approximately 0.5 h and a bioavailability >90% (Schultz et al., 1981; Lund et al., 2006). Once absorbed, gaboxadol is mainly excreted in the urine in the form of gaboxadol, whereas a minor fraction is excreted in the form of a glucuronic acid conjugate comprising of 2–7% in rat and mouse and 30–35% in two human subjects (Schultz et al., 1981; Lund et al., 2006; Shadle et al., 2006). Collectively, this indicates that in dogs gaboxadol is quickly and completely absorbed, probably in the proximal small intestine, with a minimal post-absorptive metabolism.
In vivo absorption of gaboxadol following co-administration of tryptophan
Co-administration of the hPAT1 inhibitor tryptophan had a dose-dependent effect on the absorption profile of gaboxadol resulting in a decreased Cmax and an increased Tmax. A reduction in the gaboxadol absorption rate could be caused by an alteration of the gastric emptying rate. In humans, the rate of gastric emptying decreases as a function of the number of calories in an ingested meal (Calbet and MacLean, 1997; Sunesen et al., 2005), and also in dogs the composition of meals has been shown to prolong gastric emptying (Mizuta et al., 1990). To rule out that the observed effect on gaboxadol absorption was a result of altered gastric emptying, the cumulative absorption curve of paracetamol, which is often used as a marker of gastric emptying (Calbet and MacLean, 1997; Sunesen et al., 2005), was investigated in the presence of tryptophan. The gastric emptying of paracetamol was not significantly affected by high doses of tryptophan. However, a significant effect of tryptophan on the ka of gaboxadol was observed. As co-administration of tryptophan changed gaboxadol Tmax, Cmax and ka whereas the Fa, ke and the AUC were constant, the effect of tryptophan is likely to be a result of interaction between tryptophan and gaboxadol at the site of absorption, and not due to changes in gastric emptying. From other studies it is known that gaboxadol has a low degree of plasma protein binding and is not metabolized by cytochrome P-450s (Lund et al., 2006). Thus, on the basis of the findings in vitro suggesting that hPAT1 mediates the majority of the luminal gaboxadol transport in Caco-2 monolayers, it seems likely that also the in vivo observation can be explained by a PAT1-mediated gaboxadol absorption in dogs, which is reduced by the co-administration of tryptophan. The observed in vivo affinity values of tryptophan inhibition of gaboxadol intestinal transport were estimated from either the effect of tryptophan on gaboxadol Cmax or on the intestinal absorption rate constant, ka. This gave IC50 values of 10.1 and 12.6 mmol·L−1, respectively. As discussed earlier, the in vitro affinity of hPAT1 for tryptophan measured as inhibition of proline transport via hPAT1 was 7.7 mmol·L−1 in Caco-2 cells. The IC50 values are surprisingly close to each other considering that tryptophan inhibition is measured for two different compounds (proline and gaboxadol), and that the in vivo transport is composed of not only luminal transport but appearance in the systemic circulation, which encounters the transfer of gaboxadol across several membranes. hPAT1 substrates are characterized by having affinities in the millimolar range, and the transporter, which is expressed along the entire intestinal tract, has a high capacity (Chen et al., 2003). The molar dosing ratio between gaboxadol and tryptophan was up to 1:41, and as they both have comparable affinities for hPAT1, the reduced Cmax and ka may be due to the competitive interaction between gaboxadol and tryptophan at the site of absorption, that is, at the PAT1 protein in the luminal membrane of the small intestinal enterocytes. The maximal plasma concentration thus occurs with a prolonged Tmax, but due to the excessive capacity and the intestinal expression of PAT1, the absorption fraction remains unchanged as the absorption proceeds along the length of the intestine. The peak plasma concentration of gaboxadol may thus be reduced by modifying the absorption process as illustrated here, or through a more classical sustained release formulation approach as suggested earlier (Kjaer and Nielsen, 1983).
In conclusion, the present study shows for the first time that the high permeability of gaboxadol across Caco-2 cell monolayers is most likely due to PAT1-mediated transport across the luminal membrane resulting in a high transepithelial transport. The in vitro gaboxadol transport kinetics and the pharmacokinetics observed in dogs support the conclusion that PAT1 mediates transport of gaboxadol across the mucosal membrane both in vitro as well as in vivo. In addition, the present study indicates that it is possible to exploit transporter activity in order to modify or control the intestinal absorption of drug substances. The formulation design provides a simple basis for decreasing peak plasma concentration of gaboxadol, while maintaining a high bioavailability. This may aid in reducing side effects related to high plasma peak concentrations.
Acknowledgments
The authors wish to acknowledge the work of technicians: Jesper Tolstrup Jensen from ADME Discovery at H. Lundbeck A/S for assistance with preparations and the LC-MS/MS, Bettina Dinitzen, Maria Læssøe Pedersen and Birgitte Eltong from Faculty of Pharmaceutical Sciences, University of Copenhagen for culturing the Caco-2 cells and also the personnel in the animal facilities at H. Lundbeck A/S for the skilful handling of the dogs.
Glossary
Abbreviations:
- ASC
amino acid transport system ASC
- DMEM
Dulbecco's modified Eagle's medium
- gaboxadol/THIP
[4,5,6,7-tetrahydroisoxazolo (5,4-c) pyridine-3-ol]
- HBSS
Hanks' balanced salt solution
- hPAT1
human proton-coupled amino acid transporter 1
- MES
2-(N-morpholino)ethanesulphonic acid
- THPO
4,5,6,7,-tetrahydroisoxazolo(4,5-c)-pyridin-3-ol
Conflict of interest
The authors state no conflict of interest.
References
- Abbot EL, Grenade DS, Kennedy DJ, Gatfield KM, Thwaites DT. Vigabatrin transport across the human intestinal epithelial (Caco-2) brush-border membrane is via the H+-coupled amino-acid transporter hPAT1. Br J Pharmacol. 2006;147:298–306. doi: 10.1038/sj.bjp.0706557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, et al. H+/Amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology. 2004;127:1410–1422. doi: 10.1053/j.gastro.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Boll M, Foltz M, Rubio-Aliaga I, Kottra G, Daniel H. Functional characterization of two novel mammalian electrogenic proton-dependent amino acid cotransporters. J Biol Chem. 2002;277:22966–22973. doi: 10.1074/jbc.M200374200. [DOI] [PubMed] [Google Scholar]
- Broer A, Klingel K, Kowalczuk S, Rasko JE, Cavanaugh J, Broer S. Molecular cloning of mouse amino acid transport system B0, a neutral amino acid transporter related to Hartnup disorder. J Biol Chem. 2004;279:24467–24476. doi: 10.1074/jbc.M400904200. [DOI] [PubMed] [Google Scholar]
- Calbet JA, MacLean DA. Role of caloric content on gastric emptying in humans. J Physiol. 1997;498:553–559. doi: 10.1113/jphysiol.1997.sp021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fei YJ, Anderson CM, Wake KA, Miyauchi S, Huang W, et al. Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J Physiol. 2003;546:349–361. doi: 10.1113/jphysiol.2002.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Corey JL, Guastella J, Davidson N, Lester HA. GABA uptake and release by a mammalian cell line stably expressing a cloned rat brain GABA transporter. Mol Membr Biol. 1994;11:23–30. doi: 10.3109/09687689409161026. [DOI] [PubMed] [Google Scholar]
- Dave MH, Schulz N, Zecevic M, Wagner CA, Verrey F. Expression of heteromeric amino acid transporters along the murine intestine. J Physiol. 2004;558:597–610. doi: 10.1113/jphysiol.2004.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Wafford KA. Benzodiazepine receptor agonist and insomnia: is subtype selsctivity lost in translation? Drug Discov Today. 2006;3:547–554. [Google Scholar]
- Ebert B, Wafford KA, Deacon S. Treating insomnia: current and investigational pharmacological approaches. Pharmacol Ther. 2006;112:612–629. doi: 10.1016/j.pharmthera.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Huang W, Nakanishi T, Bridges CC, Smith SB, Prasad PD, et al. Transport of D-serine via the amino acid transporter ATB0,+ expressed in the colon. Biochem Biophys Res Commun. 2002;291:291–295. doi: 10.1006/bbrc.2002.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kall MA, Fu I, Dige T, Vallano P, Woolf E, Jorgensen M. Development and validation of a selective and sensitive bioanalytical procedure for the quantitative determination of gaboxadol in human plasma employing mixed mode solid phase extraction and hydrophilic interaction liquid chromatography with tandem mass spectroscopic detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;858:168–176. doi: 10.1016/j.jchromb.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Nielsen H. The analgesic effect of the GABA-agonist THIP in patients with chronic pain of malignant origin. A phase-1-2 study. Br J Clin Pharmacol. 1983;16:477–485. doi: 10.1111/j.1365-2125.1983.tb02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Frolund B, Jorgensen FS, Schousboe A. GABAA receptor agonists, partial agonists, and antagonists. Design and therapeutic prospects. J Med Chem. 1994;37:2489–2505. doi: 10.1021/jm00042a001. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J. The GABAA agonist THIP (gaboxadol) increases non-REM sleep and enhances delta activity in the rat. Neuroreport. 1996;7:2241–2245. doi: 10.1097/00001756-199609020-00036. [DOI] [PubMed] [Google Scholar]
- Langenbucher F. Numerical convolution/deconvolution as a tool for correlating in vitro with in vivo drug availability. Pharm Ind. 1982;44:1166–1172. [Google Scholar]
- Larsen M, Larsen BB, Frolund B, Nielsen CU. Transport of amino acids and GABA analogues via the human proton-coupled amino acid transporter, hPAT1: characterization of conditions for affinity and transport experiments in Caco-2 cells. Eur J Pharm Sci. 2008;35:86–95. doi: 10.1016/j.ejps.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Lund J, Helboe T, Mengel H. Absorption, metabolism and excretion profile of gaboxadol in humans. Sleep. 2006;29:A41. [Google Scholar]
- Lundahl J, Staner L, Staner C, Loft H, Deacon S. Short-term treatment with gaboxadol improves sleep maintenance and enhances slow wave sleep in adult patients with primary insomnia. Psychopharmacology (Berl) 2007;195:139–146. doi: 10.1007/s00213-007-0866-0. [DOI] [PubMed] [Google Scholar]
- Metzner L, Kottra G, Neubert K, Daniel H, Brandsch M. Serotonin, L-tryptophan, and tryptamine are effective inhibitors of the amino acid transport system PAT1. FASEB J. 2005;19:1468–1473. doi: 10.1096/fj.05-3683com. [DOI] [PubMed] [Google Scholar]
- Mizuta H, Kawazoe Y, Haga K, Ogawa K. Effects of meals on gastric emptying and small intestinal transit times of a suspension in the beagle dog assessed using acetaminophen and salicylazosulfapyridine as markers. Chem Pharm Bull (Tokyo) 1990;38:2224–2227. doi: 10.1248/cpb.38.2224. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Smith DE, Fleisher D. PEPT1 enhances the uptake of gabapentin via trans-stimulation of b0,+ exchange. Pharm Res. 2007;24:353–360. doi: 10.1007/s11095-006-9155-6. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Making sense of GABA(A) receptor subtypes: is a new nomenclature needed? J Psychopharmacol. 2005;19:219–220. doi: 10.1177/0269881105055100. [DOI] [PubMed] [Google Scholar]
- Palacin M. A new family of proteins (rBAT and 4F2hc) involved in cationic and zwitterionic amino acid transport: a tale of two proteins in search of a transport function. J Exp Biol. 1994;196:123–137. doi: 10.1242/jeb.196.1.123. [DOI] [PubMed] [Google Scholar]
- Rowland M, Tozer TN. Clinical Pharmacokinetics Concepts and Applications. 3rd edn. Philadelphia, PA: Lippincott, Williams & Wilkins; 1995. [Google Scholar]
- Sagné C, Agulhon C, Ravassard P, Darmon M, Hamon M, El Mestikawy S, et al. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc Natl Acad Sci USA. 2001;98:7206–7211. doi: 10.1073/pnas.121183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz B, Aaes-Jorgensen T, Bogeso KP, Jorgensen A. Preliminary studies on the absorption, distribution, metabolism, and excretion of THIP in animal and man using 14C-labelled compound. Acta Pharmacol Toxicol (Copenh) 1981;49:116–124. doi: 10.1111/j.1600-0773.1981.tb00879.x. [DOI] [PubMed] [Google Scholar]
- Shadle C, Rainakrishnan R, Gargano C, Fu I, Luo R, Alexander R, et al. Assessment of dose proportionality, absolute bioavailability, and tolerability of gaboxadol in healthy young adults. Sleep. 2006;29:A40–A41. [Google Scholar]
- Su TZ, Logsdon CD, Oxender DL. Chinese hamster ovary mRNA-dependent, Na+-independent L-leucine trasport in Xenopus laevis oocytes. Mol Cell Biol. 1992;12:5281–5287. doi: 10.1128/mcb.12.12.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunesen VH, Vedelsdal R, Kristensen HG, Christrup L, Mullertz A. Effect of liquid volume and food intake on the absolute bioavailability of danazol, a poorly soluble drug. Eur J Pharm Sci. 2005;24:297–303. doi: 10.1016/j.ejps.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Tate SS, Yan N, Udenfriend S. Expression cloning of a Na+-independent neutral amino acid transporter from rat kidney. Proc Natl Acad Sci USA. 1992;89:1–5. doi: 10.1073/pnas.89.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GT, Cook MJ, Hirst BH, Simmons NL. H+-coupled (Na+-independent) proline transport in human intestinal (Caco-2) epithelial cell monolayers. FEBS Lett. 1993;333:78–82. doi: 10.1016/0014-5793(93)80378-8. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Armstrong G, Hirst BH, Simmons NL. D-cycloserine transport in human intestinal epithelial (Caco-2) cells: mediation by a H+-coupled amino acid transporter. Br J Pharmacol. 1995a;115:761–766. doi: 10.1111/j.1476-5381.1995.tb14998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GT, Simmons NL. The role of the proton electrochemical gradient in the transepithelial absorption of amino acids by human intestinal Caco-2 cell monolayers. J Membr Biol. 1995b;145:245–256. doi: 10.1007/BF00232716. [DOI] [PubMed] [Google Scholar]
- Uchiyama T, Matsuda Y, Wada M, Takahashi S, Fujita T. Functional regulation of Na+-dependent neutral amino acid transporter ASCT2 by S-nitrosothiols and nitric oxide in Caco-2 cells. FEBS Lett. 2005;579:2499–2506. doi: 10.1016/j.febslet.2005.03.065. [DOI] [PubMed] [Google Scholar]
- Wenzel U, Meissner B, Doring F, Daniel H. PEPT1-mediated uptake of dipeptides enhances the intestinal absorption of amino acids via transport system b0,+ J Cell Physiol. 2001;186:251–259. doi: 10.1002/1097-4652(200102)186:2<251::AID-JCP1027>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]


