Abstract
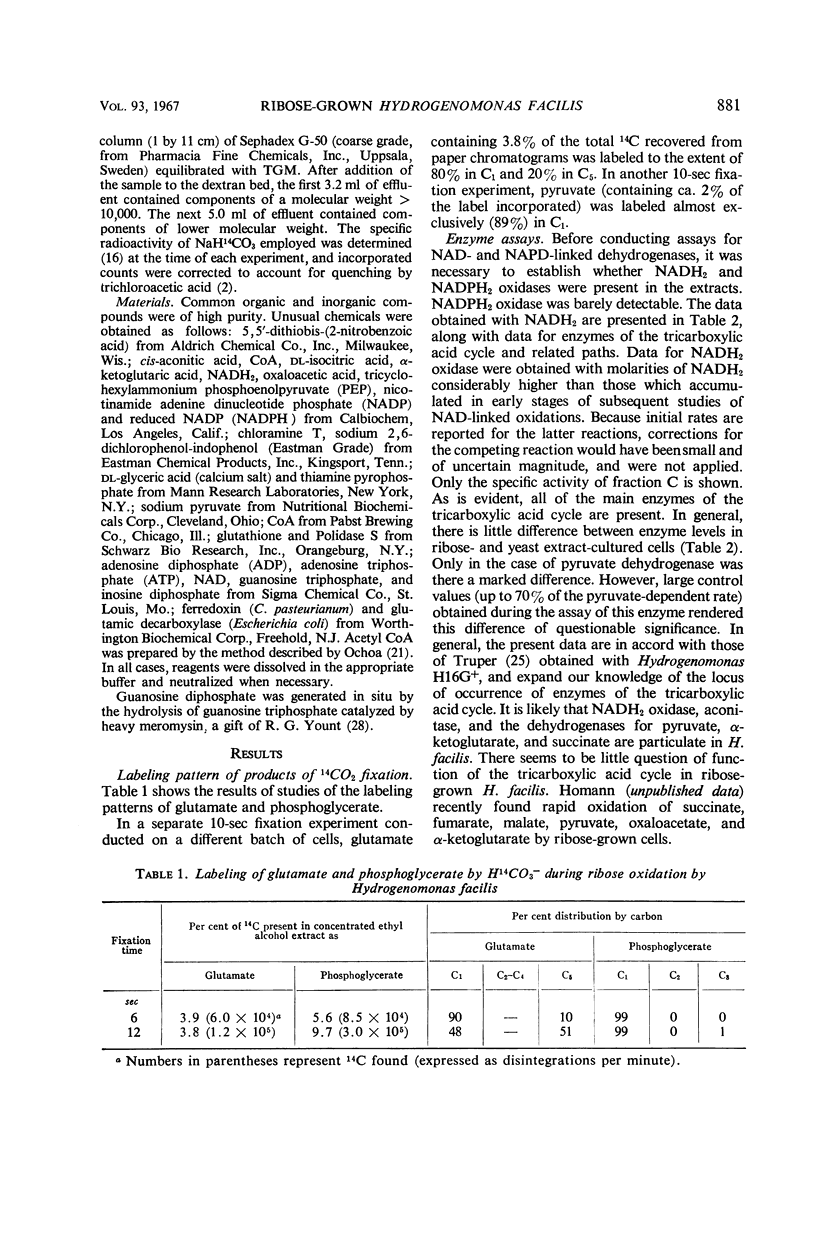
Exposure of ribose-grown Hydrogenomonas facilis to 14CO2 for 6 to 12 sec during ribose oxidation resulted in labeling of a number of compounds, three of which were glutamate, phosphoglycerate, and pyruvate. Phosphoglycerate and pyruvate were labeled almost exclusively in C1, suggesting operation of the reductive pentose phosphate cycle. Glutamate was labeled initially to the extent of 90% in C1 and 10% in C5, and this was followed by a concentration of radioisotope in C5. All of the enzymes of the tricarboxylic acid cycle were detectable in ribose-grown cells, and, in general, specific activities were similar to those found in yeast extract-grown cells. Reduced nicotinamide adenine dinucleotide oxidase, aconitase, and the dehydrogenases for pyruvate, α-ketoglutarate, and succinate appeared to be of particulate origin. In addition to enzymes of the tricarboxylic acid cycle, an acetyl coenzyme A-stimulated phosphoenolpyruvate carboxylase was found, as was isocitrate lyase. Possible participation of these catalysts in glutamate synthesis is discussed.
Full text
PDF



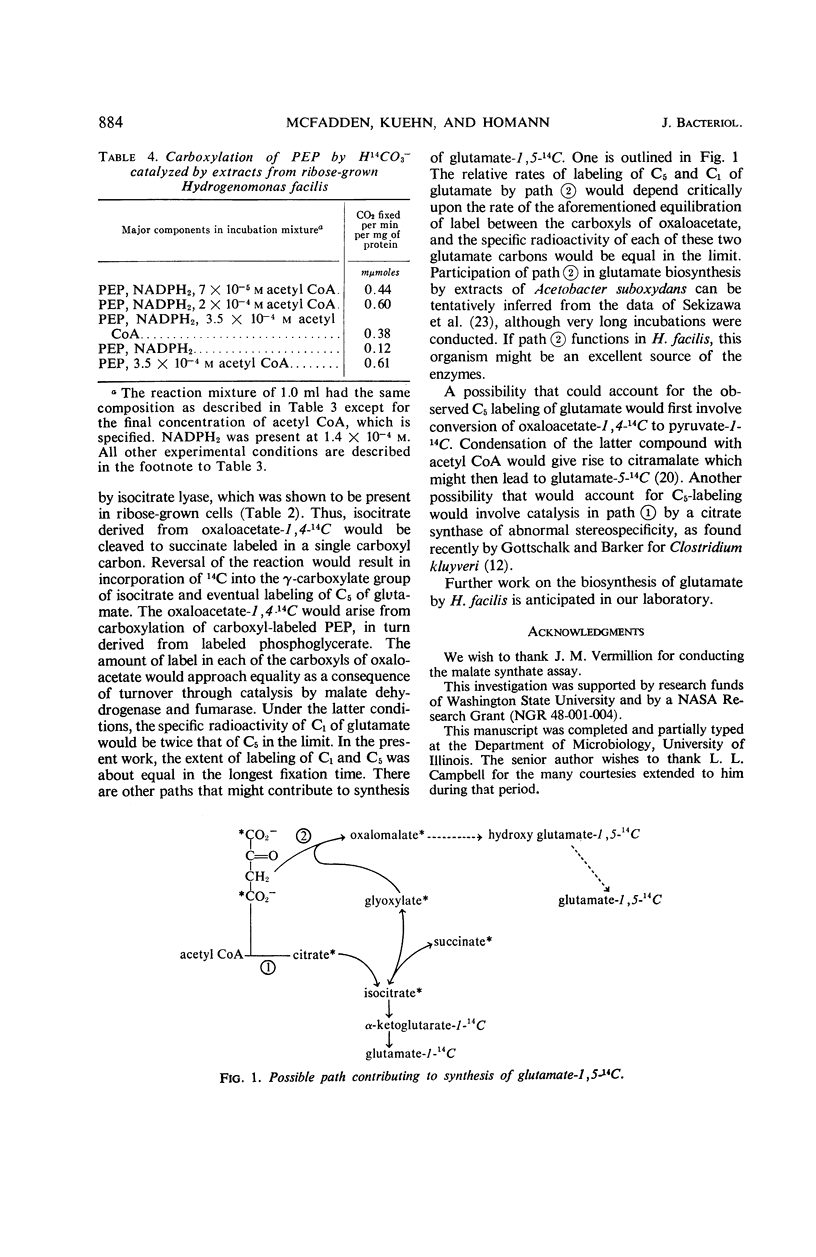

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONOFF S. Metabolism of soybean leaves. III. The organic acids produced in short-time photosynthesis. Arch Biochem Biophys. 1951 Jul;32(2):237–248. doi: 10.1016/0003-9861(51)90269-x. [DOI] [PubMed] [Google Scholar]
- BRONZINI D., DELUCA G. CONSIDERAZIONI CLINICO-STATISTICHE SULLE ANESTESIE GENERALI ESEGUITE NEI TAGLI CESAREI NELL'OSPEDALE CIVILE DI MATERA. Minerva Ginecol. 1964 Mar 15;16:197–198. [PubMed] [Google Scholar]
- BUCHANAN B. B., BACHOFEN R., ARNON D. I. ROLE OF FERREDOXIN IN THE REDUCTIVE ASSIMILATION OF CO2 AND ACETATE BY EXTRACTS OF THE PHOTOSYNTHETIC BACTERIUM, CHROMATIUM. Proc Natl Acad Sci U S A. 1964 Sep;52:839–847. doi: 10.1073/pnas.52.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Evans M. C. The synthesis of alpha-ketoglutarate from succinate and carbon dioxide by a subcellular preparation of a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1212–1218. doi: 10.1073/pnas.54.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALVIN M. The path of carbon in photosynthesis. Science. 1962 Mar 16;135(3507):879–889. doi: 10.1126/science.135.3507.879. [DOI] [PubMed] [Google Scholar]
- CANOVAS J. L., KORNBERG H. L. FINE CONTROL OF PHOSPHOPYRUVATE CARBOXYLASE ACTIVITY IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Jan;96:169–172. doi: 10.1016/0005-2787(65)90624-6. [DOI] [PubMed] [Google Scholar]
- COHEN S. Studies on D-ribulose and its enzymatic conversion to D-arabinose. J Biol Chem. 1953 Mar;201(1):71–84. [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- GEROK W., WALLER H. D. Eine manometrische Methode zur quantitativen Bestimmung von Aminosäuren. Klin Wochenschr. 1956 Dec 15;34(47-48):1284–1288. doi: 10.1007/BF01477516. [DOI] [PubMed] [Google Scholar]
- Gottschalk G., Barker H. A. Synthesis of glutamate and citrate by Clostridium kluyveri. A new type of citrate synthase. Biochemistry. 1966 Apr;5(4):1125–1133. doi: 10.1021/bi00868a003. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The effect of inorganic salts on the ketone decomposition of oxaloacetic acid. Biochem J. 1942 Apr;36(3-4):303–305. doi: 10.1042/bj0360303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFADDEN B. A., HOMANN H. R. CHARACTERISTICS AND INTERMEDIATES OF SHORT-TERM C-14-O-2 INCORPORATION DURING RIBOSE OXIDATION BY HYDROGENOMONAS FACILIS. J Bacteriol. 1965 Mar;89:839–847. doi: 10.1128/jb.89.3.839-847.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFADDEN B. A., HOMANN H. R. QUANTITATIVE STUDIES OF THE EFFECT OF ORGANIC SUBSTRATES AND 2,4-DINITROPHENOL ON HETEROTROPHIC CARBON DIOXIDE FIXATION IN HYDROGENOMONAS FACILIS. J Bacteriol. 1963 Nov;86:971–977. doi: 10.1128/jb.86.5.971-977.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. Feedback inhibition of phosphoenolpyruvate carboxylase of Salmonella. Biochem Biophys Res Commun. 1965 Dec 9;21(5):503–508. doi: 10.1016/0006-291x(65)90412-2. [DOI] [PubMed] [Google Scholar]
- Maragoudakis M. E., Sekizawa Y., King T. E., Cheldelin V. H. Glutamate biosynthesis in Acetobacter suboxydans. VI. Formation from acetate plus pyruvate. Biochemistry. 1966 Aug;5(8):2646–2653. doi: 10.1021/bi00872a024. [DOI] [PubMed] [Google Scholar]
- McFadden B. A., Tu C. C. Regulation of autotrophic and heterotrophic carbon dioxide fixation in Hydrogenomonas facilis. J Bacteriol. 1967 Mar;93(3):886–893. doi: 10.1128/jb.93.3.886-893.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODGERS K. Estimation of succinic acid in biological materials. Biochem J. 1961 Aug;80:240–244. doi: 10.1042/bj0800240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizawa Y., Maragoudakis M. E., King T. E., Cheldelin V. H. Glutamate biosynthesis in an organism lacking a Krebs tricarboxylic acid cycle. V. Isolation of alpha-hydroxy-gamma-ketoglutarate (HKG) in Acetobacter suboxydans. Biochemistry. 1966 Jul;5(7):2392–2398. doi: 10.1021/bi00871a032. [DOI] [PubMed] [Google Scholar]
- TOWERS G. H., MORTIMER D. C. The role of keto acids in photosynthetic carbon dioxide assimilation. Can J Biochem Physiol. 1956 May;34(3):511–519. [PubMed] [Google Scholar]
- Trüper H. G. Tricarboxylic acid cycle and related enzymes in Hydrogenomonas strain H16G+ grown on various carbon sources. Biochim Biophys Acta. 1965 Dec 16;111(2):565–568. doi: 10.1016/0304-4165(65)90074-7. [DOI] [PubMed] [Google Scholar]
- WACHSMAN J. T., BARKER H. A. Tracer experiments on glutamate fermentation by Clostridium tetanomorphum. J Biol Chem. 1955 Dec;217(2):695–702. [PubMed] [Google Scholar]
- YOUNT R. G., KOSHLAND D. E., Jr Properties of the O18 exchange reaction catalyzed by heavy meromyosin. J Biol Chem. 1963 May;238:1708–1713. [PubMed] [Google Scholar]



