Abstract
Background and purpose:
Our team previously demonstrated that diabetes induces a deterioration in vascular dynamics, in parallel with the enhanced formation of advanced glycation end products. The aim of this study was to determine whether prevention of the arterial stiffening by pyridoxamine in diabetes is associated with inhibition of the pathogenic glycation on aortic collagen.
Experimental approach:
Diabetes was induced in rats by a single tail vein injection with 55 mg·kg−1 steptozotocin (STZ). After induction of hyperglycaemia, animals were treated for 8 weeks with pyridoxamine (1 g·L−1 in drinking water) and compared with the age-matched untreated diabetic controls. Pulse wave reflection along the vasculature was derived using the impulse response function of the filtered aortic input impedance spectra.
Key results:
Treatment of this experimental diabetes with pyridoxamine resulted in a significant increase in wave transit time and a decrease in wave reflection factor, indicating that pyridoxamine attenuates the diabetes-induced augmentation in systolic load of the left ventricle coupled to its arterial system. Meanwhile, pyridoxamine therapy ameliorated the diabetes-related cardiac hypertrophy, as evidenced by the reduction in ratio of the left ventricular weight to body weight. Glycation-derived modification of aortic collagen was also found to be attenuated by administration of pyridoxamine to the STZ-induced diabetic rats.
Conclusions and implications:
Pyridoxamine imparts significant protection against the diabetes-induced deterioration in pulsatile arterial load imposed on the heart, at least partly through inhibition of the formation of advanced glycation end products and their accumulation on aortic collagen of the STZ-treated rats.
Keywords: advanced glycation end products, aortic input impedance, streptozotocin-diabetic rats, pulse wave reflection, pyridoxamine
Introduction
Protein damage by the formation of advanced glycation end products (AGEs) is considered one of the pathogenic mechanisms of diabetic complications (Ahmed and Thornalley, 2007; Swaminathan and Shah, 2008). Persistent hyperglycaemia, dyslipidaemia and oxidative stress can act in concert to induce the AGE formation and to promote cardiovascular inflammation, fibrosis and injury (Bucala, 1997; Baynes and Thorpe, 1999; Brownlee, 2005; Thomas et al., 2005). Despite their complexity and widespread pathological distribution, AGEs produce the formation of covalent cross-links between proteins, which are thought to be one of the central underlying processes by which they cause damage (Bucala, 1997). The pathological cross-linking of long-lived proteins such as collagen can affect tissue remodelling and result in loss of elasticity. Therefore, the diabetes-related increase in AGE formation and accumulation on aortic collagen may contribute to the development of certain physical changes of the vasculature.
Therapeutic interventions for reducing AGE formation should target AGE formation by reducing cross-link formation. Aminoguanidine is perhaps the best-known and experimentally, the most widely used inhibitor of AGE formation. Unfortunately, its application in the clinical setting is limited due to serious side effects, such as induction of autoantibody formation, rapid progressive glomerulonephritis and anaemia (Singh et al., 2001). Another promising agent under investigation is pyridoxamine (PM), one of the three natural forms of vitamin B6 (Culbertson et al., 2003). The mechanism of action of PM includes: (i) scavenging a range of toxic carbonyl species derived from glucose and lipid peroxidation (Nagaraj et al., 2002; Voziyan et al., 2002; Amarnath et al., 2004); (ii) inhibiting the post-Amadori step of the Maillard reaction by sequestering catalytic redox metal ions (Voziyan et al., 2003); and (iii) blocking oxidative degradation of Amadori intermediate (Takatori et al., 2004). In toxicity studies, PM was well tolerated and showed a favourable safety profile with no reported adverse effects (Degenhardt et al., 2002; Williams et al., 2003). Thus, the combination of those multiple activities, along with PM safety, indicate that it is a promising drug candidate for treatment of diabetic complications (Voziyan and Hudson, 2005).
Our team previously demonstrated that rats with diabetes have augmented vascular load imposed on the heart, in parallel with the enhanced AGE formation on aortic collagen (Chang et al., 2006). We hypothesize that PM may prevent the diabetes-related arterial stiffening and cardiac hypertrophy, possibly through its ability to block the formation of AGEs. So, the accurate characterization of long-term treatment of the diabetic syndrome with PM on the left ventricular (LV) afterload is of significance. This can be accomplished by measurement of the aortic input impedance that is the frequency relationship between pulsatile pressure and flow signals recorded in the ascending aorta (Milnor, 1989; Nichols and O'Rourke, 2005). Herein, the study focused on investigating the effects of PM on physical properties of the arterial system in streptozotocin (STZ)-induced diabetes in rats, using the vascular impedance analysis. Glycation-derived modification of aortic collagen was also detected by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) to verify the hypothesis.
Methods
Animals and catheterization
Male Wistar rats at 2 months were randomly divided into four groups (n = 12 in each group) as follows: (i) normal controls (NC); (ii) STZ-diabetic rats (DM); (iii) NC treated with PM (NC + PM); and (iv) DM treated with PM (DM + PM). Diabetes was induced in animals by a single tail vein injection with 55 mg·kg−1 STZ in 0.1 M citrate buffer (pH 4.5). After comfirmation of the development of hyperglycaemia (2 days later) by blood glucose determination using a surestep Test Strip, rats were randomly allocated into a vehicle-treated diabetic group, and a treatment group receiving PM in drinking water of 1 g·L−1. Animals were studied 8 weeks after exposure to PM to determine its effects on the pulsatile nature of blood flows in the diabetic arteries. All rats were allowed free access to Purina chow and water and housed two to three per cage in a 12 h light/dark cycle animal room. The animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the National Taiwan University.
General surgical procedures and measurement of the haemodynamic variables in anaesthetized rats have been described previously (Chang et al., 2003). In brief, animals were anaesthetized with sodium pentobarbital (50 mg·kg−1, i.p.), placed on a heating pad, intubated and ventilated with a rodent respirator. The chest was opened through the second intercostal space of the right side. An electromagnetic flow probe (internal circumference 8 mm) was positioned around the ascending aorta to record the pulsatile aortic flow. A high-fidelity pressure catheter was used to measure the pulsatile aortic pressure via isolated carotid artery of the right side. The electrocardiogram (ECG) of lead II was recorded with a Gould ECG/Biotach amplifier. The selective pressure and flow signals of 5–10 beats were averaged in the time domain, using the peak R wave of ECG as a fiducial point. Timing between the pressure and flow signals, due to spatial distance between the flow probe and proximal aortic pressure transducer, was corrected by a time domain approach, in which the foot of the pressure waveform was realigned with that of the flow (Mitchell et al., 1994). The resulting pressure and flow signals were subjected to further vascular impedance analysis.
Aortic input impedance spectra
The aortic input impedance spectra (Zi) could be obtained from the ratio of ascending aortic pressure harmonics to the corresponding flow harmonics, using a standard Fourier series expansion technique (Milnor, 1989; Chang et al., 2003; Nichols and O'Rourke, 2005). Total peripheral resistance of the systemic circulation (Rp) was calculated as mean aortic pressure divided by mean aortic flow. The aortic characteristic impedance (Zc) was computed by averaging high-frequency moduli of the aortic input impedance data points (4th–10th harmonics) (Huijberts et al., 1993; Gaballa et al., 1999). Taking Zc into consideration, we calculated the systemic arterial compliance C at mean aortic pressure Pm by expanding the two-element (Liu et al., 1986) into the three-element Windkessel model, which accounts for a nonlinear exponential pressure–volume relationship:
 |
SV is the stroke volume; K is the ratio of total area under the aortic pressure curve to the diastolic area (Ad); b is the coefficient in the pressure–volume relation (−0.0131 ± 0.009 in aortic arch); Pi is the pressure at the time of incisura and Pd is the end-diastolic pressure.
The wave transit time (τ) can be computed by the impulse response of the filtered Zi. This was accomplished by the inverse transformation of Zi after multiplication of the first 12 harmonics by a Dolph–Chebychev weighting function with the order 24 (Laxminarayan et al., 1978). Meanwhile, the time domain reflection factor (Rf) can be derived as the amplitude ratio of backward-to-forward peak pressure wave by the method proposed by Westerhof et al. (1972). Therefore, both the wave transit time and the wave reflection factor characterize the wave reflection phenomenon in the vasculature.
Gel electrophoresis
Method for measuring collagen glycation has been proposed by Turk et al. (1999). Collagen samples from aortic walls, previously digested by pepsin, proteinase K and collagenase, were investigated by SDS-PAGE on Mini PROTEAN 3 System. This was carried out using a 4% stacking and a 10% separating gel, running buffer system (Tris-HCl, PH 8.3/SDS/glycine), and Coomassie blue staining. Each lane was loaded with 20 µg protein from two to three rats.
Statistics
Results are expressed as means ± SEM. Because cardiac output is significantly related to body shape, this variable was normalized to body weight (BW) when comparison was made between the DM and the age-matched controls. Other haemodynamic variables derived from blood flow were also normalized to BW to detect the effects of PM on these parameters with or without diabetes. A two-way anova was used to determine the effects of diabetes and PM on the physical properties of the rat arterial system. Simple effect analysis was used when a significant interaction between diabetes and PM occurred. Differences among means within levels of a factor were determined by Tukey's honestly significant difference method. Significant differences were assumed at the level of P < 0.05.
Materials
Citrate buffer and PM, Sigma Chemical Co. (St. Louis, MO, USA); surestep Test Strip, Lifescan Inc. (Milpitas, CA, USA); rodent respirator (Model 131), New England Medical Instruments (Medway, MA, USA); electromagnetic flow probe (model 100 series), Carolina Medical Electronics (King, NC, USA); high-fidelity pressure catheter (model SPC 320, size 2F), Millar Instruments (Houston, TX, USA); Gould ECG/Biotach amplifier, Gould Electronics (Cleveland, OH, USA); Mini PROTEAN 3 System, Bio-Rad Lab (Hercules, CA, USA).
Results
Table 1 shows the effects of PM on blood glucose level, BW, left ventricular weight (LVW) and aortic pressure profile in the DM. The high glucose level in the diabetic animals did not change in response to PM treatment. After exposure to PM, the diabetic rats showed a significant fall in LVW, but did not differ in BW from the untreated diabetic animals. The diabetes-related increase in LVW/BW ratio was attenuated by administration of PM to the rats with insulin deficiency. Neither diabetes nor PM had a significant effect on the aortic pressure profile, nor was there a diabetes × PM interaction for the arterial blood pressure.
Table 1.
Effects of diabetes and pyridoxamine on blood glucose level, body weight, left ventricular weight and aortic pressure profile in male Wistar rats
| Variable | NC (n = 12) | NC+ PM (n = 12) | DM (n = 12) | DM + PM (n = 12) |
|---|---|---|---|---|
| Glucose (mg·mL−1) | 0.982 ± 0.011 | 1.004 ± 0.032 | 4.502 ± 0.130 | 4.305 ± 0.209 |
| BW (g) | 433.3 ± 13.1 | 420.0 ± 11.5 | 274.8 ± 11.1† | 296.3 ± 16.4 |
| LVW (g) | 0.813 ± 0.019 | 0.722 ± 0.023 | 0.688 ± 0.032† | 0.609 ± 0.029‡ |
| LVW/BW (mg·g−1) | 1.88 ± 0.02 | 1.72 ± 0.03 | 2.51 ± 0.07† | 2.07 ± 0.04‡ |
| Ps (mmHg) | 112.5 ± 2.7 | 106.0 ± 2.5 | 111.5 ± 3.8 | 103.3 ± 2.0 |
| Pd (mmHg) | 87.3 ± 3.1 | 77.6 ± 3.0 | 84.2 ± 4.0 | 73.2 ± 2.1 |
| Pm (mmHg) | 101.1 ± 2.9 | 92.5 ± 2.7 | 99.1 ± 3.7 | 89.9 ± 2.0 |
All values are expressed as means ± SEM.
Significant difference (P < 0.05) from the control group (NC).
Significant difference (P < 0.05) from the STZ-diabetic group (DM).
BW, body weight; LVW, left ventricular weight; Ps, systolic aortic pressure; Pd, diastolic aortic pressure; Pm, mean aortic pressure; PM, pyridoxamine; STZ, streptozotocin.
Figure 1 depicts the aortic input impedance spectra from a DM + PM compared with those of an untreated diabetic animal. After exposure to PM, the diabetic rat showed no significant changes in moduli at lower harmonics and in averaging high-frequency moduli of the aortic characteristic impedance. Figure 2 shows the impulse response function curve derived from the filtered aortic input impedance spectra shown in Figure 1. Treatment of the diabetic syndrome with PM induced an increase in wave transit time (time difference between the appearance of the reflected peak and the initial peak), suggesting that PM attenuates the diabetes-induced abnormality in timing of the pulse wave reflection.
Figure 1.

Aortic input impedance spectra derived from the pressure and flow signals measured in the ascending aorta. After exposure to PM, the diabetic rat showed no significant changes in moduli at lower harmonics or in the average high-frequency moduli of the characteristic impedance of the aorta. STZ, streptozotocin; PM, pyridoxamine; Rp, total peripheral resistance; Zc, aortic characteristic impedance.
Figure 2.

Impulse response function curve derived from the filtered aortic input impedance spectra shown in Figure 1. The thick arrow shows the discrete reflection peak from the body circulation, and the thin arrow demonstrates the initial peak as a reference. One half of the time difference between the appearance of the reflected peak and the initial peak approximates the wave transit time in the lower body circulation. PM attenuated the diabetes-induced abnormality in timing of the pulse wave reflection, as evidenced by the increase in wave transit time. STZ, streptozotocin; PM, pyridoxamine.
Figure 3 shows the effects of diabetes and PM on the static haemodynamic data, including basal heart rate (HR), cardiac output (CO), stroke volume (SV) and total peripheral resistance (Rp). The diabetes-related decline in HR was prevented by administration of PM to the rats treated with STZ (Figure 3A). By contrast, the significant increase in both CO (Figure 3B) and SV (Figure 3C) and decrease in Rp (Figure 3D) associated with diabetes were not affected by the PM treatment. Meanwhile, PM therapy exerted no effects on those static haemodynamic variables in NC.
Figure 3.

Effects of diabetes and PM on basal heart rate (HR in A), cardiac output (CO in B), stroke volume (SV in C) and total peripheral resistance (Rp in D). The diabetes-related decline in HR was prevented by administration of PM to the STZ-treated rats (A). By contrast, the significant increase in both CO (B) and SV (C) and a decrease in Rp (D) associated with diabetes were not affected by the PM treatment. PM also had no effects on these static haemodynamic variables in NC. NC, normal controls; DM, STZ-diabetic rats; PM, pyridoxamine; STZ, streptozotocin.
Figure 4 depicts the effects of diabetes and PM on the pulsatile nature of blood flows in arteries in terms of aortic characteristic impedance (Zc), aortic compliance (Cm), wave transit time (τ) and wave reflection factor (Rf). The diabetes-derived fall in Zc (Figure 4A) and rise in Cm (Figure 4B) were not modified by administration of PM to the rats treated with STZ. By contrast, PM therapy attenuated the diabetes-related deterioration in pulse wave reflection, as evidenced by both the increase of 21.1% in τ (P < 0.01) (Figure 4D) and the decrease of 33.7% in Rf (P < 0.01) (Figure 4C). The increased τ associated with the decreased Rf indicate that PM prevents the diabetes-induced early return of the augmented backward wave from the peripheral circulation, diminishing the systolic load of the left ventricle coupled to its arterial system. On the other hand, the oscillatory components of the ventricular afterload, including Zc, Cm, τ and Rf, were not modified by administration of PM to the NC.
Figure 4.

Effects of diabetes and PM on the characteristic impedance (Zc in A) of the aorta, systemic arterial compliance at mean aortic pressure (Cm in B), wave reflection factor (Rf in C), and wave transit time (τ in D). Zc and Cm were, respectively, decreased and increased with diabetes, and these alterations were not significantly affected by PM treatment. By contrast, the diabetes-induced deterioration in Rf and τ were prevented by administration of PM to the STZ-treated rats. PM had no effects on the pulsatile haemodynamic variables in NC. NC, normal controls; DM, STZ-diabetic rats; PM, pyridoxamine; STZ, streptozotocin.
Figure 5 demonstrates the SDS-PAGE electrophoresis profiles of aortic collagen from the rats studied. Collagen samples obtained 8 weeks after the administration of STZ displayed molecular weight fragments between 26 and 34 kDa that are similar to those of aortic collagen samples from the long-term diabetic animals (Turk et al., 1999; Chang et al., 2006). The reduction of diabetic AGE content relative to the untreated diabetic controls induced by PM therapy was calculated by (AGEDM+PM − AGEDM)/AGEDM. After exposure to PM for 8 weeks, the DM showed a significant decline of 38.3 ± 2.7% in glycation-derived modification on aortic collagen (P < 0.01).
Figure 5.
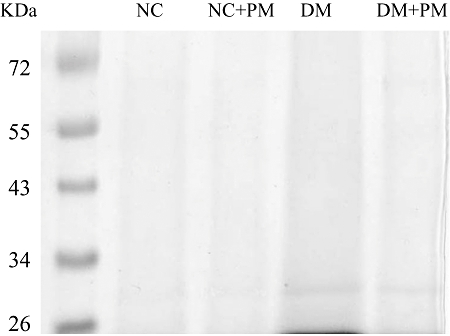
SDS-PAGE electrophoresis profiles of aortic collagen from the animals studied. Collagen samples were previously digested by pepsin, proteinase K and collagenase. Samples were loaded onto 10% separating gel and stained with Coomassie blue. Lane 1: molecular weight marker; lane 2: NC, lane 3: NC treated with PM; lane 4: DM; lane 5: DM treated with PM. Diabetic collagen samples displayed molecular weight fragments between 26 and 34 kDa that are much higher than those from the age-matched controls. The glycated aortic collagen was decreased in the STZ-diabetic rats treated with PM for 8 weeks. NC, normal controls; DM, STZ-diabetic rats; PM, pyridoxamine; SDS-PAGE, sodium dodecyl sulphate polyacrylamide gel electrophoresis; STZ, streptozotocin.
Discussion
Our team previously showed that the diabetes-induced aortic stiffening and cardiac hypertrophy were significantly improved by aminoguanidine (Chang et al., 2006). However, the clinical use of this drug to treat diabetic complications is limited due to its serious side effects (Singh et al., 2001). By contrast, PM is a natural compound that minimizes toxicity, especially the immune response in treatment of diabetic nephropathy and diabetic retinopathy (Voziyan and Hudson, 2005; Swaminathan and Shah, 2008). In this study, our significant findings were that PM targets the larger Windkessel vessels and wave reflection phenomena by inhibiting the formation and accumulation of AGEs on aortic collagen in diabetes.
PM has no beneficial effects on the resistance to blood flows in the diabetic arteries
The DM exhibited isobaric vasodilatation, an increase in blood flow that occurs in the absence of any significant change in arterial blood pressure, resulting in a decrease in total peripheral vascular resistance (Rp) (Figure 3D). It has been shown that metabolic parameters, such as oxidative protein modification, nitric oxide (NO) and peroxynitrite, have the potential to cause contractile dysfunction of the vascular smooth muscle cells in diabetic arteries (Snyder and Bredt, 1992; Tilton et al., 1993; Stadler et al., 2005). Contractile dysfunction of the diabetic resistance vessels may lengthen the vascular smooth muscle cells, causing an increase in arteriolar diameter and thus a fall in Rp (Pieper, 1999). Although PM, by blocking AGEs, could act as a preventive agent in diabetic vascular complications (Voziyan and Hudson, 2005; Ahmed and Thornalley, 2007), no beneficial effects of PM on resistance to blood flows in arteries were observed in this experimental diabetes. This result was similar to the findings that aminoguanidine exerts no significant effects on disordered endothelial-dependent vasodilatation in diabetic animals (Hill and Ege, 1994; Pieper et al., 1996; Crijns et al., 1998).
PM prevents the diabetes-induced arterial stiffening and glycation reaction on aortic collagen
With isobaric vasodilatation, inactivation of the vascular smooth muscle cells has the potential to elevate the elastic modulus of the aortic wall and contribute to a fall in aortic distensibility (elasticity) (Milnor, 1989). Contractile dysfunction of the diabetic aortas probably lengthens the aortic smooth muscle cells, resulting in an increase in aortic lumen diameter that can cause a fall in Zc (Figure 4A). Thus, there is a problem with using Zc to describe the aortic distensibility in this experimental diabetes due to its inverse relation to the lumen radius squared of the tube (Milnor, 1989; Nichols and O'Rourke, 2005; Chang et al., 2006). By contrast, being relatively independent of body shape, wave transit time (τ), which is inversely related to pulse wave velocity, could be derived to describe the aortic distensibility: the stiffer the aortic wall, the shorter the wave transit time and vice versa (Milnor, 1989).
The ability of AGEs to form the cross-links on aortic collagen is one of the central underlying processes responsible for the increased arterial stiffening in diabetes (Brownlee et al., 1988). PM administered to the STZ-treated rats for 8 weeks retarded the diabetes-induced fall in aortic distensibility, as evidenced by the increase of 21.1% in τ (Figure 4D). Prevention of the diabetes-related aortic stiffness by PM treatment parallels the reduction of AGE accumulation on collagen in the wall of the elastic reservoir (Figure 5). This result is supported by the findings that AGEs have an important role in vascular dynamics and inhibition of the AGE formation improves aortic distensibility in STZ-induced diabetes in rats (Huijberts et al., 1993; Chang et al., 2006).
Just as the elastic modulus is an expression employed to characterize the material properties, distensibility is a term used to describe the elastic behaviour of a hollow vessel. Compliance and distensibility are quite different, for compliance is equal to distensibility times volume (Guyton, 1992). Herein, the DM showed an increase in aortic compliance at Pm (Figure 4B). The decreased distensibility associated with the increased compliance suggests that volume expansion in the vasculature may exist in animals with diabetes. The volume expansion in DM is supported by other reports in the literature (Zatz and Brenner, 1986; Tomlinson et al., 1992). PM administered to the diabetic animals for 8 weeks produced no significant alteration in Cm. Thus, the diabetes-related abnormality in volume expansion could be prevented by PM, as manifested by the increased distensibility associated with the unchanged Cm.
PM attenuates the diabetes-induced augmentation in LV systolic load and cardiac hypertrophy
In diabetes changes in timing and magnitude of the pulse wave reflection do impair the loading condition for the left ventricle coupled to the arterial system (O'Rourke et al., 1987; Chang et al., 2006). As mentioned earlier, a reduction in τ was detected in rats with insulin deficiency, suggesting that diabetes may cause an early return of the pulse wave reflection from the peripheral circulation. Treatment of the STZ-diabetic animals with PM for 8 weeks retarded this early return of the pulse wave reflection (Figure 4D). Meanwhile, this diabetic syndrome contributed to a significant rise in Rf (Figure 4C), indicating that the heavy reflection intensity occurs in rats administered STZ. After exposure to PM, the diabetic animals showed a significant fall of 33.7% in Rf, suggesting that the heavy reflection phenomenon is alleviated. The increased τ associated with the decreased Rf indicate that PM, by diminishing the AGE content on aortic collagen in diabetes, improves the systolic loading condition for the left ventricle coupled to its arterial system. Meanwhile, ratio of the LVW to BW was reduced by PM treatment, suggesting that prevention of the diabetes-related cardiac hypertrophy may correspond to the drug-induced decline in vascular load imposed on the heart.
Limitations
Because the aortic input impedance cannot be measured in conscious animals, it is difficult to evaluate the effects of pentobarbital anaesthesia on rats treated with STZ and on animals administered PM. In this study, the results pertained only to measurements made in the open-chest rat with anaesthesia. This setting may induce changes in arterial pressure profiles and may introduce reflex effects not found in the closed-chest setting. Just how much the anaesthesia and thoractomy affect the pulsatile haemodynamics in rats is uncertain. However, studies with other animal models suggest that the effects are small relative to the biological and experimental variability between animals (Cox, 1974).
Taken together, the DM exhibited detrimental effects on the physical properties of the vasculature as well as the pulse wave reflection from the peripheral circulation. After PM treatment, an increase in τ suggests that the drug may prevent the diabetes-induced decline in aortic distensibility, in parallel with the reduction of glycated aortic collagen. The increased τ associated with the decreased Rf indicate that PM can attenuate the diabetes-related augmentation in LV systolic load, preventing the cardiac muscle from hypertrophy in animals with insulin deficiency. We conclude that PM therapy ameliorates the vascular complications observed in STZ-induced diabetes in rats, at least partly through inhibition of the AGE formation and its accumulation on collagen in the arterial walls.
Acknowledgments
This study was supported by Grants from the National Taiwan University Hospital and Dr. Chih-Hsien Wang (NTUH-97-N976).
Glossary
Abbreviations:
- AGEs
advanced glycation end products
- BW
body weight (g)
- C
systemic arterial compliance (µL·kg−1·mmHg−1)
- CO
cardiac output (mL·kg−1·min−1)
- HR
basal heart rate (beats·min−1)
- LVW
left ventricular weight (g)
- NO
nitric oxide
- Pd
diastolic aortic pressure (mmHg)
- Pm
mean aortic pressure (mmHg)
- Ps
systolic aortic pressure (mmHg)
- PM
pyridoxamine
- Rf
wave reflection factor
- Rp
total peripheral resistance (mmHg·min·kg·mL−1)
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- STZ
streptozotocin
- SV
stroke volume (mL·kg−1·beat−1)
- Zc
aortic characteristic impedance (mmHg·min·kg·mL−1)
- Zi
aortic input impedance spectra (mmHg·min·kg·mL−1)
- τ
wave transit time (ms)
Conflict of interest
The authors state no conflict of interest.
References
- Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications. Diabetes Obes Metab. 2007;9:233–245. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- Amarnath V, Amarnath K, Amarnath K, Davies S, Roberts LJ., Jr Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem Res Toxicol. 2004;17:410–415. doi: 10.1021/tx0300535. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Bucala R. Lipid and lipoprotein modification by GEA's: role in atherosclerosis. Exp Physiol. 1997;82:327–337. doi: 10.1113/expphysiol.1997.sp004028. [DOI] [PubMed] [Google Scholar]
- Chang KC, Hsu KL, Tseng YZ. Effects of diabetes and gender on mechanical properties of the arterial system in rats: aortic impedance analysis. Exp Biol Med. 2003;228:70–78. doi: 10.1177/153537020322800110. [DOI] [PubMed] [Google Scholar]
- Chang KC, Hsu KL, Tseng CD, Lin YD, Cho YL, Tseng YZ. Aminoguanidine prevents arterial stiffening and cardiac hypertrophy in streptozotocin-induced diabetes in rats. Br J Pharmacol. 2006;147:944–950. doi: 10.1038/sj.bjp.0706684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RH. Three-dimensional mechanics of arterial segments in vitro methods. J Appl Physiol. 1974;36:381–384. doi: 10.1152/jappl.1974.36.3.381. [DOI] [PubMed] [Google Scholar]
- Crijns FR, Struijker-Boudier HA, Wolffenbuttel BH. Arteriolar reactivity in conscious diabetic rats. Influence of aminoguanidine treatment. Diabetes. 1998;47:918–923. doi: 10.2337/diabetes.47.6.918. [DOI] [PubMed] [Google Scholar]
- Culbertson SM, Vassilenko EI, Morrison LD, Ingold KU. Paradoxical impact of antioxidants on post-amadori glycoxidation: counterintuitive increase in the yields of pentosidine and N(ε)-carboxymethyllysine using a novel multifunctional pyridoxamine derivative. J Biol Chem. 2003;278:38384–38394. doi: 10.1074/jbc.M305099200. [DOI] [PubMed] [Google Scholar]
- Degenhardt TP, Khalifah RG, Klaich GM, Schotzinger RJ. Pharmacokinetics, tolerability and biologic activity of oral pyridorin™, a novel age inhibitor, in patients with type 1 diabetes and diabetic nephropathy. J Am Soc Nephrol. 2002;13:652A. [Google Scholar]
- Gaballa MA, Raya TE, Hoover CA, Goldman S. Effects of endothelial and inducible nitric oxide synthases inhibition on circulatory function in rats after myocardial infarction. Cardiovasc Res. 1999;42:627–635. doi: 10.1016/s0008-6363(98)00343-5. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Wonsiewicz MJ. Human Physiology and Mechanisms of Disease. Philadelphia, PA: Saunders; 1992. Overview of the circulation; medical physics of pressure, flow, resistance, and vascular compliance; p. 115. p. [Google Scholar]
- Hill MA, Ege EA. Active and passive mechanical properties of isolated arterioles from STZ-induced diabetic rats. Effect of aminoguanidine treatment. Diabetes. 1994;43:1450–1456. doi: 10.2337/diab.43.12.1450. [DOI] [PubMed] [Google Scholar]
- Huijberts MS, Wolffenbuttel BH, Boudier HA, Crijns FR, Kruseman AC, Poitevin P, et al. Aminoguanidine treatment increases elasticity and decreases fluid filtration of large arteries from diabetic rats. J Clin Invest. 1993;92:1407–1411. doi: 10.1172/JCI116716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan S, Sipkema P, Westerhof N. Characterization of the arterial system in the time domain. IEEE Trans Biomed Eng. 1978;25:177–184. doi: 10.1109/TBME.1978.326244. [DOI] [PubMed] [Google Scholar]
- Liu A, Brin KP, Yin FCP. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am J Physiol. 1986;251:H588–H600. doi: 10.1152/ajpheart.1986.251.3.H588. Heart Circ Physiol 20. [DOI] [PubMed] [Google Scholar]
- Milnor WR. Hemodynamics. Baltimore, MD: Williams & Wilkins Co; 1989. [Google Scholar]
- Mitchell GF, Pfeffer MA, Westerhof N, Pfeffer JM. Measurement of aortic input impedance in rats. Am J Physiol. 1994;267:H1907–H1915. doi: 10.1152/ajpheart.1994.267.5.H1907. Heart Circ Physiol 36. [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Sarkar P, Mally A, Biemel KM, Lederer MO, Padayatti PS. Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys. 2002;402:110–119. doi: 10.1016/S0003-9861(02)00067-X. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries. London: Arnold; 2005. [Google Scholar]
- O'Rourke MF, Avolio AP, Nichols WW. Left ventricular-systemic arterial coupling in humans and strategies to improve coupling in disease states. In: Yin FCP, editor. Ventricular/Vascular Coupling. New York: Springer-Verlag; 1987. pp. 1–19. [Google Scholar]
- Pieper GM. Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia. 1999;42:204–213. doi: 10.1007/s001250051140. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Moore-Hilton G, Roza AM. Evaluation of the mechanism of endothelial dysfunction in the genetically-diabetic BB rat. Life Sci. 1996;58:147–152. doi: 10.1016/0024-3205(95)02360-7. [DOI] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Snyder T, Bredt A. Biological roles of nitric oxide. Sci Am. 1992;5:68–77. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- Stadler K, Jenei V, Somogyi A, Jakus J. Beneficial effects of aminoguanidine on the cardiovascular system of diabetic rats. Diabetes Metab Res Rev. 2005;21:189–196. doi: 10.1002/dmrr.501. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Shah SV. Novel approaches targeted toward oxidative stress for the treatment of chronic kidney disease. Curr Opin Nephrol Hy. 2008;17:143–148. doi: 10.1097/MNH.0b013e3282f4e539. [DOI] [PubMed] [Google Scholar]
- Takatori A, Ishii Y, Itagaki S, Kyuwa S, Yoshikawa Y. Amelioration of the beta-cell dysfunction in diabetic APA hamster by antioxidants and AGE inhibitor treatments. Diabetes Metab Res Rev. 2004;20:211–218. doi: 10.1002/dmrr.428. [DOI] [PubMed] [Google Scholar]
- Thomas MC, Baynes JW, Thorpe SR, Cooper ME. The role of AGEs and AGE inhibitors in diabetic cardiovascular disease. Curr Drug Targets. 2005;6:453–474. doi: 10.2174/1389450054021873. [DOI] [PubMed] [Google Scholar]
- Tilton RG, Chang K, Hasan KS, Smith SR, Petrash JM, Misko TP, et al. Prevention of diabetic vascular dysfunction by guanidines: inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993;42:221–232. doi: 10.2337/diab.42.2.221. [DOI] [PubMed] [Google Scholar]
- Tomlinson KC, Gardiner SM, Hebden RA, Bennett T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol Rev. 1992;44:103–150. [PubMed] [Google Scholar]
- Turk Z, Mišur I, Turk N, Benko B. Rat tissue collagen modified by advanced glycation: Correlation with duration of diabetes and glycemic control. Clin Chem Lab Med. 1999;37:813–820. doi: 10.1515/CCLM.1999.122. [DOI] [PubMed] [Google Scholar]
- Voziyan PA, Hudson BG. Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci. 2005;62:1671–1681. doi: 10.1007/s00018-005-5082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voziyan PA, Metz TO, Baynes JW, Hudson BG. A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J Biol Chem. 2002;277:3397–3403. doi: 10.1074/jbc.M109935200. [DOI] [PubMed] [Google Scholar]
- Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, et al. Modification of proteins in vitro by physiological levels of glucose: pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. J Biol Chem. 2003;278:46616–46624. doi: 10.1074/jbc.M307155200. [DOI] [PubMed] [Google Scholar]
- Westerhof N, Sipkema P, Vanden Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- Williams ME, Bolton WK, Degenhardt TP, Schotzinger RJ. A phase 2 clinical trial of pyridoxamine (Pyridorin™) in type 1 and type 2 diabetic patients with overt nephropathy (PYR-206) J Am Soc Nephrol. 2003;14:7A. [Google Scholar]
- Zatz R, Brenner BM. Pathogenesis of diabetic microangiopathy: the hemodynamic view. Am J Med. 1986;80:443–453. doi: 10.1016/0002-9343(86)90719-9. [DOI] [PubMed] [Google Scholar]


