Abstract
Background and purpose:
As adenosine 5′-triphosphate (ATP) is one of the inhibitory mediators of the bladder outflow region, this study investigates the possible release of ATP or related purines in response to electrical field stimulation (EFS) and the purinoceptor(s) involved in nerve-mediated relaxations of the pig urinary bladder neck.
Experimental approach:
Urothelium-denuded and intact phenylephrine-precontracted strips were mounted in organ baths containing physiological saline solution at 37°C and gassed with 95% O2 and 5% CO2 for isometric force recordings.
Key results:
EFS, in the presence of atropine, guanethidine and NG-nitro-L-arginine, and exogenous purines, produced frequency- and concentration-dependent relaxations respectively. Adenosine 5′-diphosphate (ADP) and adenosine were more potent than ATP in producing relaxation, while uridine 5′-triphosphate, uridine 5′-diphosphate and α,β-methylene ATP were less effective. The non-selective P2 antagonist suramin, and the P2Y1 and P1 receptor blockers 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium and 8-(p-sulphophenyl)theophylline, respectively, inhibited the responses to EFS and ATP. The P1 agonist's potency was: 5′-N-ethylcarboxamidoadenosine (NECA)>4-2[[6-amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzene propanoic acid hydrochloride>2-chloro-N6-cyclopentyladenosine>-2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-b-D-ribofuranuronamide = adenosine. 4-(-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol, an A2A antagonist, reduced the relaxations to EFS, adenosine and NECA. In urothelium-intact samples, relaxations to EFS and purines were smaller than in urothelium-denuded preparations. Neuronal voltage-gated Na+ channels blockade failed to modify ATP relaxations. At basal tension, EFS- and ATP-induced contractions were resistant to desensitization or blockade of P2X1 and P2X3 receptors.
Conclusions and implications:
ATP is involved in the non-adrenergic, non-cholinergic, non-nitrergic inhibitory neurotransmission in the pig bladder neck, producing relaxation largely through muscle A2A receptors after breakdown to adenosine, and P2Y1 receptors after breakdown to ADP. Antagonists of these receptors may be useful for urinary incontinence treatment produced by intrinsic sphincteric deficiency.
Keywords: EFS, NANC inhibitory neurotransmission, ATP, ADP, adenosine, P2Y1- and A2A-receptors, pig urinary bladder neck
Introduction
adenosine 5′-triphosphate (ATP) (Sigma, St Louis, MO, USA) and the related purines adenosine 5′-diphosphate (ADP; Sigma) and adenosine (Sigma) are signalling molecules that mediate different biological actions via at least two main classes of membrane receptors denoted as P1 and P2 purinoceptors (Burnstock, 1978). ATP acts at P2 receptors, which are subdivided into ligand-gated ion channels P2X receptors and G protein-coupled P2Y receptors (Ralevic and Burnstock, 1998). Currently, seven P2X subtypes (P2X1, P2X2, P2X3, P2X4 P2X5, P2X6 and P2X7) and eight P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) are recognized, including receptors that are sensitive to purines and pyrimidines (Burnstock, 2007). P2Y receptors can also be divided on the basis of their endogenous agonists into adenine nucleotide-preferring (P2Y1, P2Y11, P2Y12and P2Y13) and uracil nucleotide-preferring (P2Y2, P2Y4, P2Y6and P2Y14) receptors (Abbracchio et al., 2006). Adenosine interacts through four subtypes of P1 receptors (A1, A2A, A2B and A3) that have been cloned and characterized based on molecular structure, pharmacology and mechanisms of G protein-mediated signalling mechanisms (Ralevic and Burnstock, 1998; Fredholm et al., 2000).
The role of ATP as a neurotransmitter in the lower urinary tract is well established (Burnstock, 2001). Thus, in the urinary bladder of several species, the atropine-resistant component of the excitatory neurotransmission has been ascribed to the endogenous release of ATP acting at P2X receptors in detrusor muscle (Burnstock, 1972; 1978; Kasakov and Burnstock, 1982; Levin et al., 1986). In addition, ATP produces a dual effect (contraction and relaxation) on smooth muscle via activation of P2X and P2Y receptors respectively (Bolego et al., 1995; McMurray et al., 1998). In contrast to its dual excitatory and inhibitory action in the bladder, ATP only causes relaxation via P2Y receptors in the urethra of several species, including pig (Werkström and Andersson, 2005) and hamster (Pinna et al., 1998), where the main inhibitory neurotransmitter is nitric oxide. In the bladder neck, limited information exists about the role of purinergic signalling in regulating muscle tone. Relaxation to ATP and adenosine in pig (Hills et al., 1984) and ATP relaxation mediated via P2Y receptors in mini-pig (Tong et al., 1997) bladder neck have been reported. As this structure together with the proximal urethra constitute the urine outflow region of the bladder (De Groat, 2006), knowledge of the transmitters involved in the control of smooth muscle tone is essential for the maintenance of urinary continence (English et al., 1999). Therefore, the current study investigates the role of ATP and/or related purines in the non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmission, as well as the receptor(s) involved in these relaxations in the pig urinary bladder neck.
Methods
Dissection and mounting
Adult pigs of either sex with no lesions in their urinary tract were selected from the local slaughterhouse. Urinary bladders were removed immediately after the animals had been killed, and kept in chilled physiological saline solution (PSS) at 4°C. The adjacent connective and fatty tissues were removed with care, and strips were dissected out from the bladder neck as previously described (Hernández et al., 2006b). Urothelium-intact and -denuded strips 4–6 mm long and 2–3 mm wide were suspended horizontally, with one end connected to an isometric force transducer (Grass FT 03C, Grass Instrument Co., Quincy, MA, USA) and the other one to a micrometer screw, in 5 mL organ baths containing PSS at 37°C gassed with carbogen (95% O2 and 5% CO2) to obtain a final pH of 7.4. The signal was continuously recorded on a polygraph (Graphtec Multicorder MC 6621, Hugo Sachs Elektronik, March-Hugstetten, Germany). Passive tension of 2 g was applied to the strips, and they were allowed to equilibrate for 60 min.
Experimental procedure
The contractile ability of the strips was determined by exposing them to potassium-rich (124 mM) PSS (KPSS). Noradrenergic neurotransmission, muscarinic receptors and NO synthase were blocked by pre-incubation of the strips with guanethidine (10 µM), atropine (1 µM) and NG-nitro-L-arginine (L-NOARG, 100 µM) (Sigma), respectively, for 1 h, replacing the solution every 20 min, and these drugs were present throughout the experiment. Under these conditions and after 1 µM phenylephrine (PhE)-induced tone (Sigma), relaxations to electrical field stimulation (EFS) and purinoceptor agonists were carried out. EFS was performed by delivering rectangular pulses (1 ms duration, 1–16 Hz, 20 s trains, with constant current output adjusted to 75 mA), at 4 min intervals, from a CS20 stimulator (Cibertec, Barcelona, Spain). In the experiments to investigate the effect of phentolamine on the relaxations to EFS and exogenous ATP, the strips were precontracted with the thromboxane analogue 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U46619; Sigma) (0.1 µM). A first frequency- and concentration-response curve (FRC and CRC, respectively) to EFS and purinoceptor agonists was performed, the bath solution was changed every 20 min during a total period of 80 min, and the preparations were incubated for 30 min with the P1 and P2 purinoceptor- or neuronal voltage-gated Na+ channel-blocker, tetrodotoxin (TTX; Sigma), and then a second relaxation FRC or CRC was constructed. Control curves were run in parallel. To study the possible involvement of purinergic signalling on excitatory neurotransmission, contractions to EFS (8 and 16 Hz) and ATP (1 mM) were performed on basal tension of samples pre-incubated with L-NOARG (100 µM) and phentolamine (0.1 µM) to block NO synthase and α-adrenoceptors respectively.
Calculations and statistics
For each concentration-response relaxation curve to ATP, and P1 and P2 purinoceptor agonists, the drug concentration required to give 20 (EC20) or 50% (EC50) relaxation of the PhE-induced contraction was estimated by computerized non-linear regression analysis (GraphPad Prism, San Diego, CA, USA.). The sensitivity of the drugs is expressed in terms of pEC20 or pEC50, where pEC20 or pEC50is defined as the negative logarithm of EC20[pEC20 = −log EC20 (M)] or EC50[pEC50 = −log EC50 (M)]. Differences were analysed by Student's t-test for paired and unpaired observations, and by analysis of variance and a posteriori Bonferroni method for multiple comparisons. Differences were considered significant with a probability level of P < 0.05.
Drugs and solutions
The drug/molecular target nomenclature agrees to British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008). The following drugs were used: adenosine, ADP, 6-N,N-diethyl-D-b,g-dibromomethyleneATP trisodium salt (ARL67156), ATP, atropine, guanethidine, α,β-methylene ATP (α,β-meATP), L-NOARG, PhE, pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) (PPADS), 8-(p-sulphophenyl)theophylline (8-SPT), suramin, U46619, uridine 5′-diphosphate (UDP), uridine 5′-triphosphate (UTP) and TTX, all from Sigma. 1-2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-b-D-ribofuranuronamide (2-Cl-IB-MECA), 2-chloro-N6-cyclopentyladenosine (2-Cl-cyclopentyladenosine), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), 4-2[[6-amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzene propanoic acid hydrochloride (CGS21680), clopidogrel, 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium (MRS2179), 2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridine carboxaldehyde (MRS2211), N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide (MRS1220), 5′-N-ethylcarboxamidoadenosine (NECA), 4-(2,3,6,7-tetrahydro-2,6-dioxo-1-propyl-1H-purin-8-yl)-benzenesulphonic acid potassium salt (PSB1115), 2′, 3′-O-(2,4,6-trinitrophenyl)adenosine-5′-triphosphate tetratriethylammonium (TNP-ATP) and 4-(-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol (ZM241385) were all from Tocris (Bristol, UK). CGS21680, clopidogrel, 2-Cl-IB-MECA, 2-Cl-cyclopentyladenosine, DPCPX, MRS1220, NECA, PSB1115, 8-SPT and ZM241385 were dissolved in dimethyl sulphoxide. The other drugs were dissolved in distilled water. The solvents used had no effect on the contractility of the bladder neck preparations. The composition of PSS was (mM): NaCl 119, KCl 4.6, MgCl2 1.2, NaHCO3 24.9, glucose 11, CaCl2 1.5, KH2PO4 1.2 and ethylenediamine tetra-acetic acid 0.027. The solution was maintained at 37°C and continuously gassed with 95% O2 and 5% CO2 to maintain pH at 7.4.
The CS20 stimulator was obtained from Cibertec.
Results
Urothelium-denuded strips of pig urinary bladder neck were allowed to equilibrate to a passive tension of 1.7 ± 0.1 g (n = 136, preparations from 59 pigs using 2–3 strips from each animal). Under these conditions, KPSS (124 mM) produced a contraction of 1.9 ± 0.2 g (n = 136). The strips were precontracted with 1 µM PhE, which induced a sustained contraction above basal tension of 1.6 ± 0.3 g.
Relaxations induced by EFS, ATP and P2 purinoceptor agonists
EFS (1–16 Hz), in the presence of atropine, guanethidine and L-NOARG, evoked frequency-dependent relaxations (relaxation of 74 ± 7% of the PhE-induced contraction, n = 87, at 16 Hz) (Figure 1A). The non-NO, NANC relaxations evoked by EFS at 1, 2, 4, 8 and 16 Hz (as percentage of the maximal relaxation) were of 5 ± 4%, 23 ± 6%, 57 ± 6%, 89 ± 4% and 100 ± 0%, respectively, these responses being reproducible in a second nerve stimulation curve. ATP and P2 receptor agonists induced relaxation in a concentration-dependent manner, ADP showing a higher relaxant potency versus that exhibited by ATP. UTP, UDP and α,β-meATP were less effective (Figure 1A,B, Table 1).
Figure 1.
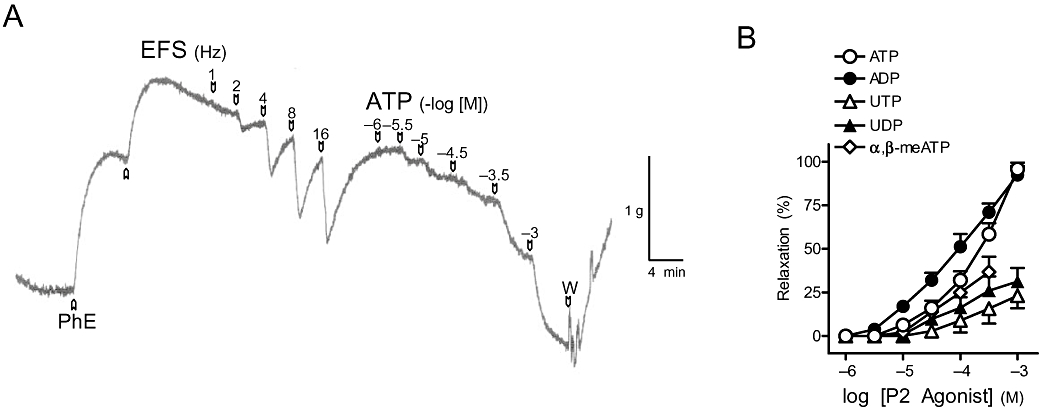
(A) Isometric force recordings showing the relaxations evoked by electrical field stimulation (EFS, 1 ms duration, 1–16 Hz, 20 s trains) and exogenous adenosine 5′-triphosphate (ATP, 1 µM–1 mM) on 1 µM phenylephrine (PhE)-precontracted pig urinary bladder neck strips treated with guanethidine (10 µM), atropine (0.1 µM) and NG-nitro-L-arginine (100 µM). Vertical bar shows tension in grams, and horizontal bar shows time in minutes. (B) Log concentration-response relaxation curves to exogenous ATP and P2 purinoceptor agonists. Results are expressed as a percentage of the PhE-induced contraction and represent mean ± SEM of 6–12 preparations. α,β-meATP, α,β-methylene adenosine 5′-triphosphate; ADP, adenosine 5′-diphosphate; UDP, uridine 5′-diphosphate; UTP, uridine 5′-triphosphate.
Table 1.
Relaxation induced by P2 purinoceptor agonists in the pig urinary bladder neck
| n | pEC20 | R (%) | |
|---|---|---|---|
| ATP | 12 | 4.3 ± 0.1 | 95.8 ± 3.7 |
| ADP | 9 | 4.8 ± 0.1* | 92.4 ± 5.1 |
| UTP | 7 | 3.2 ± 0.1*# | 23.2 ± 7.2*# |
| UDP | 7 | 3.9 ± 0.2*#Ω | 31.3 ± 7.8*# |
| α,β-meATP | 6 | 4.2 ± 0.1#Ω | 36.7 ± 8.9*#Ω |
Results are expressed as mean ± SEM of n experiments.
*#Ω P < 0.05 versus ATP, ADP and UTP respectively (analysis of variance followed by Bonferroni method).
α,β-meATP, α,β-methylene adenosine 5′-triphosphate; ADP, adenosine 5′-diphosphate; ATP, adenosine 5′-triphosphate; pEC20 = −log EC20, where EC20 is the concentration of agonist producing 20% relaxation of phenylephrine (PhE)-induced contraction; R is the relaxation, expressed as a percentage of the PhE-induced contraction, evoked at the highest concentration of agonist used: ATP, 1 mM; ADP, 1 mM; UTP, 1 mM; UDP, 1 mM; and α,β-meATP, 300 µM; UDP, uridine 5′-diphosphate; UTP, uridine 5′-triphosphate.
Effect of TTX on relaxations to EFS and exogenous purines
TTX (1 µM), a neuronal voltage-gated Na+ channel blocker, abolished the relaxations induced by EFS but failed to modify the responses to ATP, ADP and adenosine (Table 5).
Table 5.
Effects of intact urothelium and of blockade of neuronal voltage-gated Na+ channels with TTX on relaxations evoked by ATP, ADP and adenosine
|
ATP |
|||
|---|---|---|---|
| n | pEC50 | R (%) | |
| Urothelium denuded | 7 | 3.7 ± 0.2 | 92.3 ± 4.1 |
| Urothelium intact | 7 | 3.4 ± 0.2* | 79.5 ± 9.7 |
| Control | 7 | 3.9 ± 0.1 | 91.7 ± 4.4 |
| TTX (1 µM) | 7 | 3.9 ± 0.1 | 88.8 ± 5.1 |
| ADP | |||
| Urothelium denuded | 6 | 4.0 ± 0.1 | 97.9 ± 2.1 |
| Urothelium intact | 6 | 3.6 ± 0.1* | 89.1 ± 8.7 |
| Control | 6 | 4.0 ± 0.1 | 90.0 ± 4.7 |
| TTX (1 µM) | 6 | 4.1 ± 0.1 | 88.9 ± 4.5 |
| Adenosine | |||
| Urothelium denuded | 7 | 4.1 ± 0.1 | 95.5 ± 4.1 |
| Urothelium intact | 7 | 3.8 ± 0.1* | 85.6 ± 5.5 |
| Control | 7 | 4.0 ± 0.1 | 93.0 ± 5.0 |
| TTX (1 µM) | 7 | 4.0 ± 0.1 | 92.8 ± 4.7 |
Results are expressed as mean ± SEM of n experiments.
P < 0.05 versus control (unpaired and paired t-tests in case of urothelium and TTX respectively).
ADP, adenosine 5′-diphosphate; ATP, adenosine 5′-triphosphate; pEC50 = −log EC50, where EC50 is the concentration of agonist producing 50% relaxation of phenylephrine (PhE)-induced contraction; R is the relaxation, expressed as a percentage of the PhE-induced contraction, evoked at the highest concentration of agonist used: ATP, 1 mM; ADP, 1 mM; and adenosine, 1 mM; TTX, tetrodotoxin.
Effects of P2 and P1 receptor antagonists on relaxations to EFS and purinoceptor agonists
Suramin (100 µM) (Figure 2A) and PPADS (30 µM) (Figure 2B), non-selective P2 receptor antagonists, reduced the relaxations evoked by 1–8 Hz EFS. However, responses to 16 Hz were not changed by these antagonists. The relaxations to exogenous ATP were inhibited by suramin (Figure 2C) but not by PPADS (Figure 2D) (Table 2). This antagonist, however, blocked the relaxations to ADP (Table 2). MRS2179 (10 µM), a P2Y1 receptor antagonist, inhibited the relaxations to EFS, ADP and ATP (Figure 3A–C). These responses were not changed by the selective P2Y12 and P2Y13receptor antagonists, clopidogrel (10 µM) and MRS2211 (10 µM) (Table 2). 8-SPT (100 µM), a P1 purinoceptor antagonist, reduced the relaxations to EFS by about 20–30%, depending on the frequency of stimulation (Figure 4A,B) and ATP (Figure 4A,D, Table 2) respectively. 8-SPT (100 µM) plus MRS2179 (10 µM) produced an inhibition higher than that evoked by 8-SPT alone on relaxations to both EFS (Figure 4C) and exogenous ATP (Figure 4E, Table 2).
Figure 2.
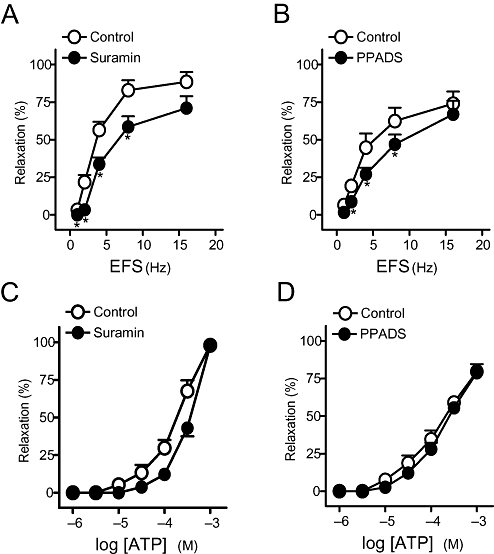
(A,B) Frequency-response and (C,D) log concentration-response relaxation curves to electrical field stimulation (EFS) and exogenous adenosine 5′-triphosphate (ATP), respectively, on 1 µM phenylephrine (PhE)-precontracted pig urinary bladder neck strips treated with guanethidine (10 µM), atropine (0.1 µM) and NG-nitro-L-arginine (100 µM), in control conditions and in the presence of suramin (100 µM) (A,C) and pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid) (PPADS) (30 µM) (B,D). Results are expressed as a percentage of the PhE-induced contraction and represent mean ± SEM of seven to eight preparations. *P < 0.05 versus control (paired t-test).
Table 2.
Effects of blockers of P2, P2Y1and P1 purinoceptors on relaxations evoked by ATP, and of blockers of P1, P2Y1, P2Y12 and P2Y13receptors on responses induced by ADP
|
ATP |
|||
|---|---|---|---|
| n | pEC50 | R (%) | |
| Control | 8 | 3.7 ± 0.1 | 98.1 ± 1.4 |
| Suramin (100 µM) | 8 | 3.4 ± 0.1* | 97.9 ± 2.9 |
| Control | 7 | 3.9 ± 0.1 | 79.7 ± 3.0 |
| PPADS (30 µM) | 7 | 3.8 ± 0.2 | 78.8 ± 5.8 |
| Control | 7 | 3.7 ± 0.1 | 95.5 ± 3.7 |
| MRS2179 (10 µM) | 7 | – | 80.0 ± 5.1* |
| Control | 7 | 4.1 ± 0.1 | 92.9 ± 4.7 |
| 8-SPT (100 µM) | 7 | 3.8 ± 0.1* | 77.1 ± 6.5 |
| Control | 6 | 4.1 ± 0.1 | 92.1 ± 4.5 |
| MRS2179 + 8-SPT | 6 | 3.7 ± 0.1* | 85.5 ± 9.1 |
| ADP | |||
| Control | 9 | 4.1 ± 0.1 | 87.9 ± 4.0 |
| 8-SPT (100 µM) | 9 | 4.0 ± 0.2 | 88.1 ± 3.5 |
| Control | 6 | 4.1 ± 0.1 | 94.9 ± 4.3 |
| PPADS (30 µM) | 6 | – | 78.0 ± 7.0* |
| Control | 7 | 4.0 ± 0.1 | 97.9 ± 2.0 |
| MRS2179 (10 µM) | 7 | – | 75.1 ± 9.7* |
| Control | 6 | 4.2 ± 0.1 | 92.0 ± 6.1 |
| Clopidogrel (10 µM) | 6 | 4.2 ± 0.1 | 88.7 ± 5.0 |
| Control | 7 | 4.0 ± 0.1 | 89.7 ± 5.5 |
| MRS2211 (10 µM) | 7 | 3.9 ± 0.1 | 91.0 ± 5.1 |
Results are expressed as mean ± SEM of n experiments.
P < 0.05 versus control (paired t-test).
8-SPT, 8-(p-sulphophenyl)theophylline; ADP, adenosine 5′-diphosphate; ATP, adenosine 5′-triphosphate; MRS2179, 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium; MRS2211, 2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridine carboxaldehyde; pEC50 = −log EC50, where EC50 is the concentration of agonist producing 50% relaxation of phenylephrine (PhE)-induced contraction; PPADS, pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid); R is the relaxation, expressed as a percentage of the PhE-induced contraction, evoked at the highest concentration of agonist used: ATP, 1 mM; and ADP, 1 mM.
Figure 3.
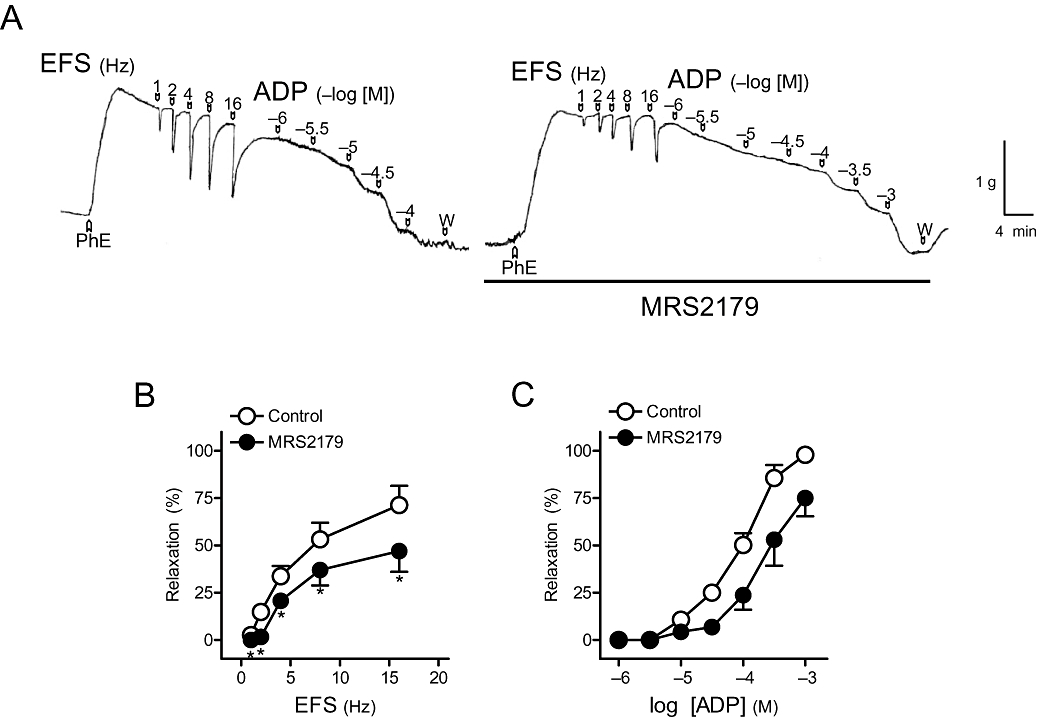
(A) Isometric force recordings showing the relaxations evoked by electrical field stimulation (EFS, 1 ms duration, 1–16 Hz, 20 s trains) and exogenous adenosine 5′-diphosphate (ADP) (1–100 µM), in the absence or presence of 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium (MRS2179) (10 µM), on 1 µM phenylephrine (PhE)-precontracted pig urinary bladder neck strips treated with guanethidine (10 µM), atropine (0.1 µM) and NG-nitro-L-arginine (100 µM). Vertical bar shows tension in grams, and horizontal bar shows time in minutes. (B) Frequency-response and (C) log concentration-response relaxation curves to EFS and exogenous ADP, respectively, in control conditions and in the presence of MRS2179 (10 µM). Results are expressed as a percentage of the PhE-induced contraction and represent mean ± SEM of seven preparations. *P < 0.05 versus control (paired t-test).
Figure 4.
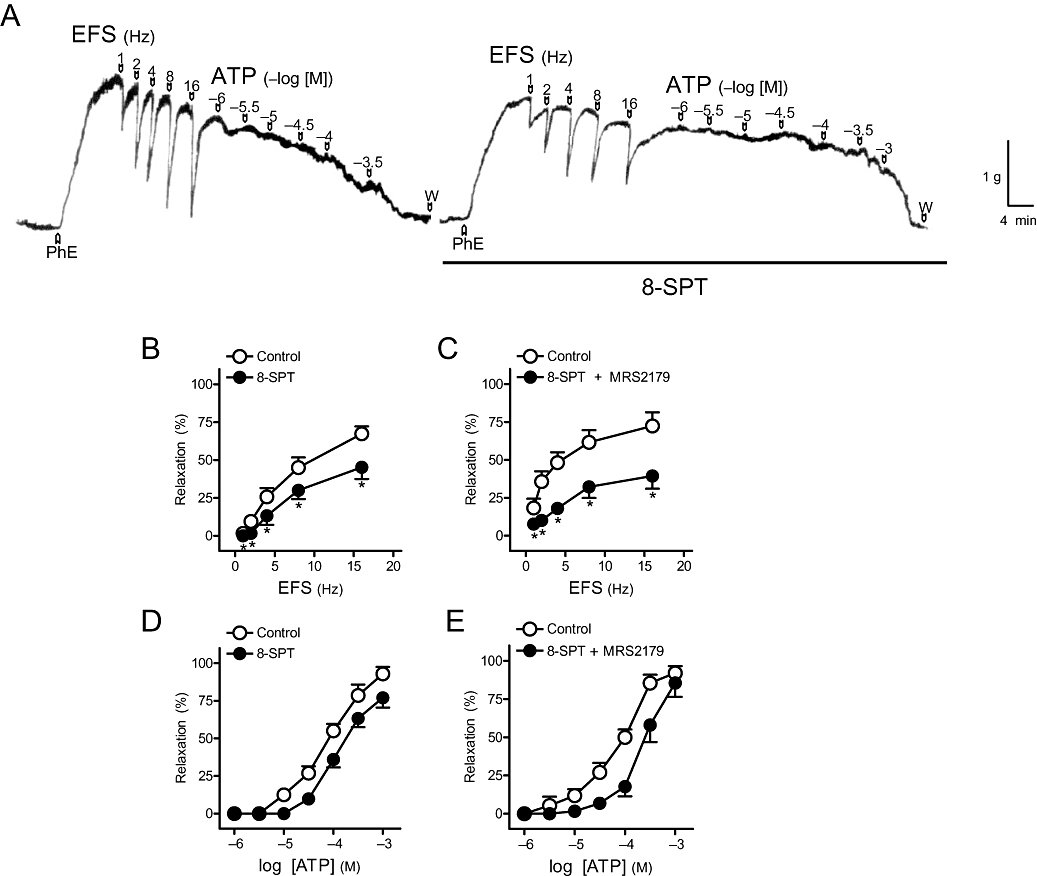
(A) Isometric force recordings showing the relaxations evoked by electrical field stimulation (EFS, 1 ms duration, 1–16 Hz, 20 s trains) and exogenous adenosine 5′-triphosphate (ATP) (1 µM–0.3 mM), in the absence or presence of 8-(p-sulphophenyl)theophylline (8-SPT) (100 µM), on 1 µM phenylephrine (PhE)-precontracted pig urinary bladder neck strips treated with guanethidine (10 µM), atropine (0.1 µM) and NG-nitro-L-arginine (100 µM). Vertical bar shows tension in grams, and horizontal bar shows time in minutes. (B,C) Frequency-response and (D,E) log concentration-response relaxation curves to EFS and exogenous ATP, respectively, in control conditions and in the presence of 8-SPT (100 µM) (B, D) and 8-SPT plus 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium (MRS2179) (10 µM) (C,E). Results are expressed as a percentage of the PhE-induced contraction and represent mean ± SEM of six to seven preparations. *P < 0.05 versus control (paired t-test).
Relaxations induced by adenosine and P1 purinoceptor agonists
Adenosine and P1 receptor agonists induced relaxations in a concentration-dependent manner, with the following order of potency: NECA>CGS21680>2-Cl-cyclopentyladenosine>2-Cl-IB-MECA = adenosine (Figure 5, Table 3).
Figure 5.
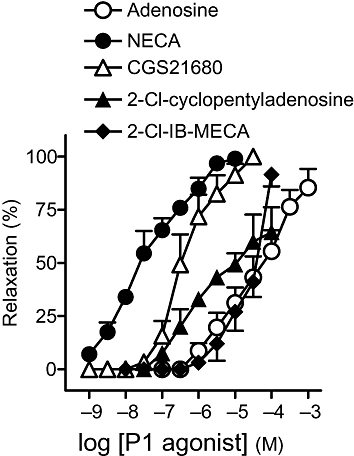
Log concentration-response relaxation curves to adenosine and P1 purinoceptor agonists. Results are expressed as a percentage of the phenylephrine-induced contraction and represent mean ± SEM of 6–10 preparations. 2-Cl-cyclopentyladenosine, 2-chloro-N6-cyclopentyladenosine; 2-Cl-IB-MECA, 1-2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-b-D-ribofuranuronamide; CGS21680, 4-2[[6-amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzene propanoic acid hydrochloride; NECA, 5′-N-ethylcarboxamidoadenosine.
Table 3.
Relaxation induced by P1 purinoceptor agonists
| n | pEC50 | R (%) | |
|---|---|---|---|
| Adenosine | 10 | 4.3 ± 0.2 | 85.4 ± 8.8 |
| NECA | 8 | 7.5 ± 0.1* | 99.1 ± 0.9 |
| 2-Cl-cyclopentyladenosine | 6 | 5.2 ± 0.2*# | 64.4 ± 11.8*# |
| CGS21680 | 8 | 6.4 ± 0.1*#Ω | 100.0 ± 0.0Ω |
| 2-Cl-IB-MECA | 8 | 4.5 ± 0.1#ΩΨ | 91.6 ± 5.5Ω |
Results are expressed as mean ± SEM of n experiments.
*#ΩΨP < 0.05 versus adenosine, NECA, 2-Cl-cyclopentyladenosine and CGS21680 respectively (analysis of variance followed by Bonferroni method).
2-Cl-cyclopentyladenosine, 2-chloro-N6-cyclopentyladenosine; 2-Cl-IB-MECA, 1-2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-b-D-ribofuranuronamide; CGS21680, 4-2[[6-amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzene propanoic acid hydrochloride; NECA, 5′-N-ethylcarboxamidoadenosine; pEC50 = −log EC50, where EC50 is the concentration of agonist producing 50% relaxation of phenylephrine (PhE)-induced contraction; R is the relaxation, expressed as a percentage of the PhE-induced contraction, evoked at the highest concentration of agonist used: adenosine, 1 mM; NECA, 10 µM; 2-Cl-cyclopentyladenosine, 100 µM; CGS21680, 30 µM; and 2-Cl-IB-MECA, 100 µM.
Effects of A1, A2A, A2B and A3 receptor antagonists on relaxations to EFS, adenosine and NECA
ZM241385 (0.1 µM), a selective A2A receptor antagonist, reduced relaxations to EFS (Figure 6A,B), adenosine (Figure 6A,C, Table 4) and NECA (Figure 6D, Table 4), whereas DPCPX (10 µM), PSB1115 (0.1 µM) and MRS1220 (10 µM), A1, A2B and A3receptor antagonists, respectively, failed to modify these responses.
Figure 6.
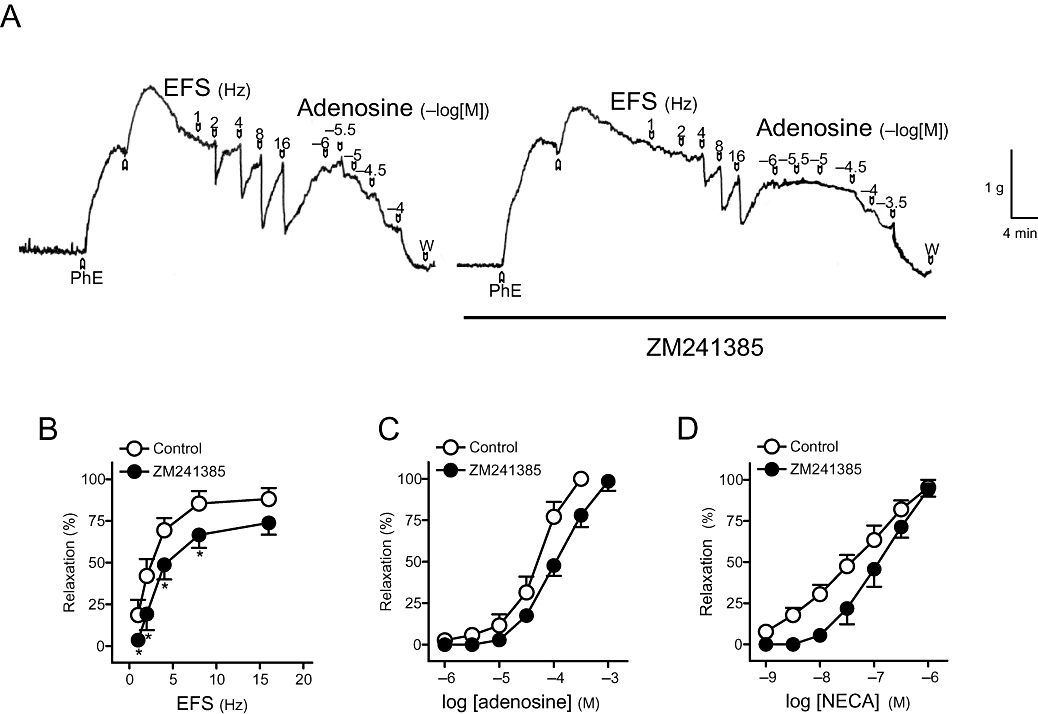
(A) Isometric force recordings showing the relaxations evoked by electrical field stimulation (EFS, 1 ms duration, 1–16 Hz, 20 s trains) and exogenous adenosine (1–100 µM), in the absence or presence of 4-(-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol (ZM241385) (0.1 µM), on 1 µM phenylephrine (PhE)-precontracted pig urinary bladder neck strips treated with guanethidine (10 µM), atropine (0.1 µM) and NG-nitro-L-arginine (100 µM). Vertical bar shows tension in grams, and horizontal bar shows time in minutes. (B) Frequency-response and (C,D) log concentration-response relaxation curves to EFS, adenosine and 5′-N-ethylcarboxamidoadenosine (NECA), respectively, in control conditions and in the presence of ZM241385 (0.1 µM). Results are expressed as a percentage of the PhE-induced contraction and represent mean ± SEM of seven to eight preparations. *P < 0.05 versus control (paired t-test).
Table 4.
Effects of blockers of A1, A2A, A2B and A3purinoceptors on relaxations evoked by adenosine and NECA
|
Adenosine |
NECA |
|||||
|---|---|---|---|---|---|---|
| n | pEC50 | R (%) | n | pEC50 | R (%) | |
| Control | 6 | 3.9 ± 0.1 | 100 ± 0 | 6 | 7.2 ± 0.1 | 92.0 ± 4.9 |
| DPCPX (10 µM) | 6 | 3.8 ± 0.1 | 97.1 ± 2.3 | 6 | 7.3 ± 0.1 | 85.1 ± 7.7 |
| Control | 7 | 4.2 ± 0.1 | 100 ± 0 | 8 | 7.4 ± 0.1 | 95.3 ± 4.7 |
| ZM241385 (0.1 µM) | 7 | 3.9 ± 0.1* | 98.5 ± 5.7 | 8 | 6.9 ± 0.1* | 94.1 ± 4.3 |
| Control | 6 | 4.0 ± 0.1 | 84.8 ± 9.7 | 6 | 7.4 ± 0.1 | 93.9 ± 4.0 |
| PSB1115 (0.1 µM) | 6 | 4.0 ± 0.1 | 82.6 ± 6.3 | 6 | 7.4 ± 0.1 | 89.9 ± 7.1 |
| Control | 6 | 4.0 ± 0.1 | 100 ± 0 | 6 | 7.1 ± 0.1 | 92.0 ± 5.8 |
| MRS1220 (10 µM) | 6 | 4.0 ± 0.1 | 100 ± 0 | 6 | 7.1 ± 0.1 | 93.2 ± 5.0 |
Results are expressed as mean ± SEM of n experiments.
P < 0.05 versus control (paired t-test).
DPCPX, 8-cyclopentyl-1,3-dipropylxanthine; MRS1220, N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide; NECA, 5′-N-ethylcarboxamidoadenosine; pEC50 = −log EC50, where EC50 is the concentration of agonist producing 50% relaxation of phenylephrine (PhE)-induced contraction; PSB1115, 4-(2,3,6,7-tetrahydro-2,6-dioxo-1-propyl-1H-purin-8-yl)-benzenesulphonic acid potassium salt; R is the relaxation, expressed as a percentage of the PhE-induced contraction, evoked at the highest concentration of agonist used: adenosine, 1 mM; and NECA, 1 µM; ZM241385, 4-(-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol.
Effect of intact urothelium on relaxations to EFS and exogenous purines
In samples with intact urothelium, the relaxations to EFS (Figure 7A), ATP (Figure 7B, Table 5), ADP (Figure 7C, Table 5) and adenosine (Figure 7D, Table 5) were reduced versus those evoked in denuded urothelium samples.
Figure 7.
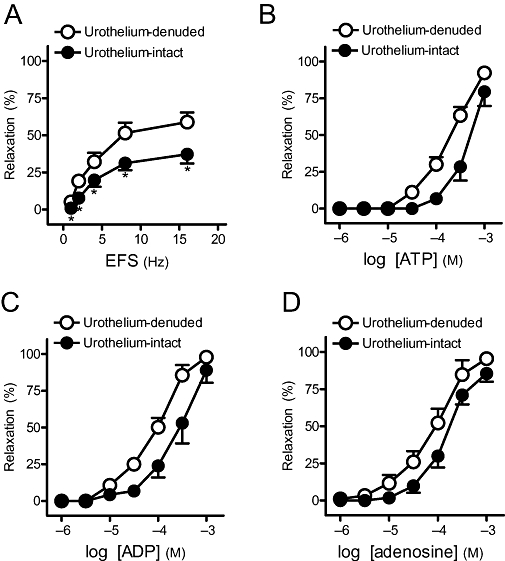
(A) Frequency-response and (B,C,D) log concentration-response relaxation curves to adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP) and adenosine, respectively, in urothelium-denuded and urothelium-intact strips. Results are expressed as a percentage of the phenylephrine-induced contraction and represent mean ± SEM of six to seven preparations. *P < 0.05 versus control (unpaired t-test). EFS, electrical field stimulation.
Effect of ecto-nucleoside triphosphate diphosphohydrolase type 2 (NTPDase2) and α-adrenoceptor blockade on relaxations to EFS and ATP
An inhibitor of NTPDase2, ARL67156 (100 µM), failed to modify the relaxations to EFS and ATP. Thus, relaxations of 69.9 ± 5.0% and 72.1 ± 5.1% were obtained at 16 Hz of EFS (P > 0.05, n = 6, paired t-test) in the absence or presence of ARL67156 respectively. ATP (1 mM) produced relaxations of 93.3 ± 6.5% and 91.0 ± 5.7%, and pEC50values of 3.5 ± 0.1 and 3.6 ± 0.1 (P > 0.05, n = 7, paired t-test) in the absence or presence of ARL67156 respectively.
Strips treated with phentolamine (0.1 µM) showed relaxations to EFS and ATP similar to that exhibited in preparations incubated with guanethidine (10 µM). Thus, relaxations of 63 ± 4.5% and 67 ± 5.1% were obtained at 16Hz of EFS (P > 0.05, n = 7, paired t-test) in the presence of phentolamine and guanethidine respectively. ATP (1 mM) relaxation in the presence of phentolamine was 87.5 ± 7.0%, with a pEC50value of 3.7 ± 0.1, while that in the presence of guanethidine was 93.3 ± 4.9%, with a pEC50value of 3.8 ± 0.1 (P > 0.05, n = 7, paired t-test, when comparing the values obtained in the presence of phentolamine or guanethidine).
Effects of desensitization and blockade of P2X1 and/or P2X3 receptors on contractions induced by EFS and ATP on basal tone
In 41% of the preparations, in addition to the relaxations induced by NANC nerve stimulation, a fast-twitch contractile component was seen. For this reason, we studied the possible involvement of P2X receptors in contractions to nerve stimulation. Thus, on strips at low tone pretreated with phentolamine (0.1 µM) and L-NOARG (100 µM) to block α-adrenoceptors and NO synthase, respectively, EFS (8 and 16 Hz) and ATP (1 mM) induced contractions that were not changed by the desensitization or blockade of P2X1and/or P2X3 receptors with α,β-meATP (10 µM) or TNP-ATP (30 µM) (Table 6). The EFS-evoked contractions were abolished by TTX, thus indicating their neurogenic nature.
Table 6.
Effects of desensitization or blockade of P2X1and/or P2X3 receptors with α,β-meATP or TNP-ATP, respectively, on contractions evoked by EFS (1 ms, 8 and 16 Hz, 20 s trains) and ATP (1 mM) on basal tone of pig bladder neck strips pretreated with phentolamine (0.1 µM) and NG-nitro-L-arginine (100 µM) to block α-adrenoceptors and nitric oxide synthase respectively
| n |
EFS (Hz) |
ATP (mM) |
||
|---|---|---|---|---|
| 8 | 16 | 1 | ||
| Control | 9 | 18 ± 5 | 24 ± 4 | 5 ± 2 |
| α,β-meATP (10 µM) | 9 | 18 ± 5 | 21 ± 4 | 4 ± 2 |
| Control | 9 | 12 ± 3 | 19 ± 4 | 4 ± 1 |
| TNP-ATP (30 µM) | 9 | 12 ± 3 | 20 ± 4 | 4 ± 1 |
Results are expressed as percentage of the potassium-rich physiological saline solution (124 mM)-induced tone and represent the mean ± SEM of n preparations.
α,β-meATP, α,β-methylene adenosine 5′-triphosphate; ATP, adenosine 5′-triphosphate; EFS, electrical field stimulation; TNP-ATP, 2′, 3′-O-(2,4,6-trinitrophenyl)adenosine-5′-triphosphate tetratriethylammonium.
Discussion and conclusions
Our results suggest that ATP is involved in the non-nitrergic, NANC inhibitory neurotransmission in the pig bladder neck, producing relaxation through muscle A2A receptors, after the breakdown of ATP to adenosine, and P2Y1 receptors, after the breakdown of ATP to ADP.
NO (Thornbury et al., 1992; Hernández et al., 2007; 2008;) and peptides, such as pituitary adenylate cyclase-activating polypeptide (PACAP) (Hernández et al., 2006a,b;), are involved in NANC inhibitory neurotransmission of the bladder neck, producing relaxation of smooth muscle through neuronal or perhaps non-neuronal mechanisms. In addition to these agents, a large NANC nerve stimulation-evoked relaxation resistant to NO synthase or vasoactive intestinal peptide (VIP)/PACAP receptor inhibitors has also been described, thus indicating the involvement of other mediator(s) in such relaxations (Hernández et al., 2007; 2008;). In the present study, suramin, a non-selective P2 purinoceptor antagonist, reduced relaxations to both EFS and exogenous ATP, suggesting that ATP and/or its breakdown products could mediate part of the non-nitrergic NANC inhibitory neurotransmission in the pig bladder neck. PPADS, another P2 receptor antagonist, reduced the relaxations to EFS, but it did not significantly change the responses to ATP, although the reason for this is not clear at this time.
ATP and acetylcholine (ACh) released from postganglionic parasympathetic nerves are involved in excitatory neurotransmission in detrusor, and they are responsible for the initiation and maintenance of micturition respectively (Hoyle, 1994; Burnstock, 2001). In our study, the finding that the relaxations to non-nitrergic NANC nerve stimulation were sensitive to TTX, but unchanged by guanethidine or phentolamine, noradrenergic neurotransmission and α-adrenoceptor blockers, respectively, suggests that ATP or related purines are involved in the NANC inhibitory neurotransmission and could be released from parasympathetic nerves in bladder neck. The fact that the relaxations to exogenous ATP and related purines were resistant to TTX, a neuronal voltage-gated Na+ channel blocker, together with the results from the experiments performed on urothelium-denuded samples, suggests that these responses are produced through non-neuronal mechanisms involving activation of purinoceptors located on the smooth muscle. These results agree with those found in the hamster (Pinna et al., 1998) and pig (Werkström and Andersson, 2005) urethra where the relaxations to ATP were mediated by muscle receptors.
The major P2Y receptor that has been found in the pig bladder neck preparations appears to be P2Y1. In our study, the higher relaxant potency of ADP versus that of ATP would indicate mediation by P2Y1, P2Y12 and/or P2Y13 (Bodor et al., 2003; Marteau et al., 2003; Waldo and Harden, 2004). The inhibitory effect of the P2Y1 receptor antagonist MRS2179 on relaxations induced by EFS, ATP and ADP suggests the involvement of P2Y1 receptors in the response to endogenously released purinergic neurotransmitters and exogenously added purines. These results agree with those found in hamster proximal urethra, where P2Y1 receptors were shown to be involved in ATP-induced relaxations (Pinna et al., 2005). The mediation through P2Y12 and P2Y13 receptors of the relaxations to ADP seems to be unlikely as clopidogrel and MRS2211, P2Y12 and P2Y13 receptor antagonists (Von Kügelgen, 2006), respectively, did not change the relaxations induced by EFS or ADP. The human P2Y11receptor is selectively activated by ATP and fails to respond to ADP (Communi et al., 1997). These results are consistent with those reported in both vascular and non-vascular preparations. Relaxation induced by adenine nucleotides acting directly on the smooth muscle has been previously reported in the rabbit coronary artery (Corr and Burnstock, 1994) and in the hamster proximal urethra (Pinna et al., 2005). ADP has also been recently found to induce relaxation in pig coronary artery via adenosine P1 receptors (Rayment et al., 2007). In our study, 8-SPT failed to modify the relaxations induced by ADP, which excludes the involvement of adenosine receptors in these responses. The fact that UTP and UDP were less effective in producing relaxations (acting as partial agonists with maximal responses of 23 and 31% respectively) in comparison with those evoked by ATP and ADP seems to rule out the possibility that P2Y2, P2Y4, P2Y6 and P2Y14 receptors mediate this response. These results agree with those found in the hamster urethra where UTP induced a small response (Pinna et al., 2005), and female pig urethra where UTP had no relaxant effect (Werkström and Andersson, 2005).
In the pig bladder neck, adenosine was found to show a higher relaxant potency than that exhibited by ATP. Adenosine may be generated from the hydrolysis of ATP by the action of ecto-nucleotidases, which sequentially dephosphorylate ATP to its corresponding nucleosides, which in turn can act at P1 receptors (Ralevic and Burnstock, 1998). In our study, ARL67156, an inhibitor of NTPDase2 (Iqbal et al., 2005), failed to modify the relaxations to EFS and ATP, which suggest that this inhibitor is not very effective in this preparation, as the adenosine receptor antagonist 8-SPT inhibited relaxations to EFS and exogenous ATP, suggesting that ATP is acting via P1 receptors following breakdown to adenosine. This is also suggested by the higher inhibition of EFS observed in the presence of 8-SPT plus MRS2179 versus that in the presence of 8-SPT or MRS2179 alone. This could indicate that adenosine does have a significant role in the signal transduction pathway of the endogenously released purinergic neurotransmitter and that, in addition to the relaxations to ATP mediated via P2Y1, part of these responses are produced via adenosine, acting on P1 purinoceptors in the pig bladder neck. These results are in contrast with those found in the hamster urethra where 8-SPT did not change the relaxations to ATP, thus suggesting that ATP was not acting via adenosine after enzymatic breakdown (Pinna et al., 2005).
Adenosine and adenosine receptors are thought to play a critical role in regulating urinary bladder function, especially in conditions of bladder ischaemia or during obstruction-induced changes in function that underlie lower urinary tract symptoms (Bratslavsky et al., 1999). In the urinary tract, both relaxant and contractile effects involving different adenosine receptor subtypes have been reported. Thus, adenosine promotes relaxation of rat and dog urinary bladder (Suzuki and Kokubun, 1994), and pig intravesical ureter (Hernández et al., 1999), through activation of different A2 receptor subtypes, whereas in the cat bladder, adenosine produces contractions via the A1 subtype (Yang et al., 2000). In our study of the pig bladder neck, the fact that the stable adenosine analogue NECA was more potent than adenosine suggests that this purine did not need to be internalized to relax bladder neck smooth muscle. These results are in contrast with those found in the pig urethra where NECA exhibited a poor relaxant effect in comparison to that evoked by adenosine (Werkström and Andersson, 2005). In pig bladder neck, the rank order of potencies in producing relaxation (NECA>CGS21680>2-Cl-cyclopentyladenosine>2-Cl-IB-MECA = adenosine) is consistent with that defining the A2A receptor subtype (Feoktistov and Biaggioni, 1993). The fact that NECA and CGS21680 exhibited higher potency versus that evoked by 2-Cl-cyclopentyladenosine and 2-Cl-IB-MECA, A1 and A3receptor agonists, respectively, indicates the involvement of A2A receptors. This suggestion was confirmed by the finding that the A2A receptor antagonist, ZM241385, inhibited the relaxations to EFS, adenosine and NECA. The lack of effect of the A1, A2B and A3receptor antagonists seems to rule out the possible involvement of these subtypes in the adenosine-evoked relaxation. Therefore, the purinergic system plays an important role in the inhibitory transmission of pig bladder neck, producing relaxation through activation of a heterogeneous population of muscle P2Y1 and A2Areceptors. However, a large part of the relaxant response to nerve stimulation was resistant to the blockade of these receptors, suggesting the existence of a non-purinergic, non-nitrergic NANC component in the inhibitory neurotransmission in the pig bladder neck. Further studies will be performed in order to identify the nature of the neurotransmitter(s) involved in these responses.
In a set of experiments with intact urothelium, relaxation responses to EFS, ATP, ADP and adenosine were considerably smaller than those in urothelium-denuded strips. These results could indicate the possible release of excitatory mediators from urothelium of bladder neck. In the urinary bladder, the release of ATP, ACh and NO from bladder urothelium has been demonstrated (Ferguson et al., 1997; Birder et al., 1998; Wessler et al., 1998; Vlaskovska et al., 2001; Yoshida et al., 2006; Kullmann et al., 2008). Moreover, adenosine is also synthesized in bladder urothelium, and different functional P1 receptors are expressed on urothelial cells (Yu et al., 2006). An NO-dependent inhibitory factor released from the urothelium of hamster urethra has also been reported (Pinna et al., 1996).
The presence of an additional contractile component in response to NANC nerve stimulation could be indicative of the involvement of P2X receptors. Purinergic signalling involves rapidly desensitizing P2X receptors, P2X1or P2X3 receptors, and purinoceptors that are relatively insensitive to desensitization, P2X2and P2X4receptors (Ralevic and Burnstock, 1998; North, 2002). However, the presence of P2X1and/or P2X3 receptors is unlikely as ATP induced only a small contraction at basal tone. Also, these responses and the contractions evoked by nerve stimulation were resistant to TNP-ATP, a P2X1 and P2X3 receptor blocker, as well as to P2X receptor desensitization with α,β-meATP. These results seem to rule out the involvement of purines in the excitatory neurotransmission of the bladder neck.
In conclusion, the present results suggest that ATP is involved in the non-nitrergic, NANC inhibitory neurotransmission in the pig bladder neck, producing relaxation through muscle A2A receptors, after breakdown of ATP to adenosine, and P2Y1 receptors, after breakdown of ATP to ADP. Selective antagonists of these receptors could be useful in the therapy of urinary incontinence produced by intrinsic sphincteric deficiency.
Acknowledgments
The authors wish to thank Prof James Malone-Lee from the Department of Urology of The Whittington Hospital of London, and Dr Héctor Zerpa from The Royal Veterinary College, University of London, for their valuable scientific collaboration. Dr Medardo Hernández was supported by a grant from the Universidad Complutense de Madrid.
Glossary
Abbreviations:
- 2-Cl-cyclopentyladenosine
2-chloro-N6-cyclopentyladenosine
- 2-Cl-IB-MECA
1-2-chloro-6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-b-D-ribofuranuronamide
- 8-SPT
8-(p-sulphophenyl)theophylline
- α,β-meATP
α,β-methylene adenosine 5′-triphosphate
- ADP
adenosine 5′-diphosphate
- ARL67156
6-N,N-diethyl-D-b,g-dibromomethyleneATP trisodium salt
- ATP
adenosine 5′-triphosphate
- CGS21680
4-2[[6-amino-9-(N-ethyl-b-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzene propanoic acid hydrochloride
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- L-NOARG
NG-nitro-L-arginine
- MRS1220
N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide
- MRS2179
2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium
- MRS2211
2-[(2-chloro-5-nitrophenyl)azo]-5-hydroxy-6-methyl-3-[(phosphonooxy)methyl]-4-pyridine carboxaldehyde
- NECA
5′-N-ethylcarboxamidoadenosine
- PhE
phenylephrine
- PPADS
pyridoxal phosphate-6-azo(benzene-2,4-disulphonic acid)
- PSB1115
4-(2,3,6,7-tetrahydro-2,6-dioxo-1-propyl-1H-purin-8-yl)-benzenesulphonic acid potassium salt
- TNP-ATP
2′, 3′-O-(2,4,6-trinitrophenyl)adenosine-5′-triphosphate tetratriethylammonium
- TTX
tetrodotoxin
- U46619
9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α
- UDP
uridine 5′-diphosphate
- UTP
uridine 5′-triphosphate
- ZM241385
4-(-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl) phenol
Conflict of interest
None.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII. Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- Bodor ET, Waldo GL, Hooks SB, Corbitt J, Boyer JL, Harden TK. Purification and functional reconstitution of the human P2Y12 receptor. Mol Pharmacol. 2003;64:1210–1216. doi: 10.1124/mol.64.5.1210. [DOI] [PubMed] [Google Scholar]
- Bolego C, Pinna C, Abbracchio MP, Cattabeni F, Puglisi L. The biphasic response of rat vesical smooth muscle to ATP. Br J Pharmacol. 1995;114:1557–1562. doi: 10.1111/j.1476-5381.1995.tb14939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratslavsky G, Whitbeck C, Horan P, Levin RM. Effects of in vivo ischemia on contractile responses of rabbit bladder to field stimulation, carbachol, ATP and KCl. Pharmacology. 1999;59:221–226. doi: 10.1159/000028323. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L, editors. Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approach. New York: Raven Press; 1978. pp. 107–118. [Google Scholar]
- Burnstock G. Purinergic signalling in lower urinary tract. In: Abbracchio MP, Williams M, editors. Handbook of Experimental Pharmacology, Volume 151/I. Purinergic and Pyrimidinergic Signalling I – Molecular, Nervous and Urinogenitary System Function. Berlin: Springer-Verlag; 2001. pp. 423–515. [Google Scholar]
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D, Govaerts C, Parmentier M, Boeynaems JM. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- Corr L, Burnstock G. Analysis of P2-purinoceptor subtypes on the smooth muscle and endothelium of rabbit coronary artery. J Cardiovasc Pharmacol. 1994;23:709–715. doi: 10.1097/00005344-199405000-00004. [DOI] [PubMed] [Google Scholar]
- De Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol. 2006;147:S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English SF, Amundsen CL, McGuire EJ. Bladder neck competency at rest in women with incontinence. J Urol. 1999;161:578–580. [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I. Characterization of adenosine receptors in human erythroleukemia cells and platelets: further evidence for heterogeneity of adenosine A2 receptor subtypes. Mol Pharmacol. 1993;43:909–914. [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Hernández M, Barahona MV, Bustamante S, García-Sacristán A, Orensanz LM. A2B adenosine receptors mediate relaxation of the pig intravesical ureter: adenosine modulation of non adrenergic non cholinergic excitatory neurotransmission. Br J Pharmacol. 1999;126:969–978. doi: 10.1038/sj.bjp.0702386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M, Barahona MV, Recio P, Benedito S, Martínez AC, Rivera L, et al. Neuronal and smooth muscle receptors involved in the PACAP- and VIP-induced relaxations of the pig urinary bladder neck. Br J Pharmacol. 2006a;149:100–109. doi: 10.1038/sj.bjp.0706832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M, Barahona MV, Recio P, Bustamante S, Benedito S, Rivera L, et al. PACAP 38 is involved in the non-adrenergic non-cholinergic inhibitory neurotransmission in the pig urinary bladder neck. Neurourol Urodyn. 2006b;25:490–497. doi: 10.1002/nau.20287. [DOI] [PubMed] [Google Scholar]
- Hernández M, Barahona MV, Recio P, Navarro-Dorado J, Bustamante S, Benedito S, et al. Role of neuronal voltage-gated K(+) channels in the modulation of the nitrergic neurotransmission of the pig urinary bladder neck. Br J Pharmacol. 2008;153:1251–1258. doi: 10.1038/sj.bjp.0707669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M, Recio P, Barahona MV, Bustamante S, Peña L, Martínez AC, et al. Pre-junctional alpha(2)-adrenoceptors modulation of the nitrergic transmission in the pig urinary bladder neck. Neurourol Urodyn. 2007;26:578–583. doi: 10.1002/nau.20368. [DOI] [PubMed] [Google Scholar]
- Hills J, Meldrum LA, Klarskov P, Burnstock G. A novel non-adrenergic non-cholinergic nerve-mediated relaxation of the pig bladder neck: an examination of possible neurotransmitter candidates. Eur J Pharmacol. 1984;99:287–293. doi: 10.1016/0014-2999(84)90135-3. [DOI] [PubMed] [Google Scholar]
- Hoyle CH. Non-adrenergic, non-cholinergic control of the urinary bladder. World J Urol. 1994;12:233–244. doi: 10.1007/BF00191202. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Vollmayer P, Braun N, Zimmermann H, Müller CE. A capillary electrophoresis method for the characterization of ecto-nucleoside triphosphate diphosphohydrolases (NTPDases) and the analysis of inhibitors by in-capillary enzymatic microreaction. Purinergic Signal. 2005;1:349–358. doi: 10.1007/s11302-005-8076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L, Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982;86:291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Kullmann FA, Artim DE, Birder LA, De Groat WC. Activation of muscarinic receptors in rat bladder sensory pathways alters reflex bladder activity. J Neurosci. 2008;28:1977–1987. doi: 10.1523/JNEUROSCI.4694-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RM, Ruggieri MR, Wein AJ. Functional effects of the purinergic innervation of the rabbit urinary bladder. J Pharmacol Exp Ther. 1986;236:452–457. [PubMed] [Google Scholar]
- McMurray G, Dass N, Brading AF. Purinoceptor subtypes mediating contraction and relaxation of marmoset urinary bladder smooth muscle. Br J Pharmacol. 1998;123:1579–1586. doi: 10.1038/sj.bjp.0701774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau F, Le Poul E, Communi D, Labouret C, Savi P, Boeynaems JM, et al. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pinna C, Glass R, Knight GE, Bolego C, Puglisi L, Burnstock G. Purine- and pyrimidine-induced responses and P2Y receptor characterization in the hamster proximal urethra. Br J Pharmacol. 2005;144:510–518. doi: 10.1038/sj.bjp.0706047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna C, Puglisi L, Burnstock G. ATP and vasoactive intestinal polypeptide relaxant response in hamster isolated proximal urethra. Br J Pharmacol. 1998;124:1069–1074. doi: 10.1038/sj.bjp.0701908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna C, Ventura S, Puglisi L, Burnstock G. A pharmacological and histochemical study of hamster urethra and the role of urothelium. Br J Pharmacol. 1996;119:655–662. doi: 10.1111/j.1476-5381.1996.tb15723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rayment SJ, Ralevic V, Barrett DA, Cordell R, Alexander SPH. A novel mechanism of vasoregulation: ADP-induced relaxation of the porcine isolated coronary artery is mediated via adenosine release. FASEB J. 2007;21:577–585. doi: 10.1096/fj.06-7050com. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kokubun S. Subtypes of purinoceptors in rat and dog urinary bladder smooth muscles. Br J Pharmacol. 1994;112:117–122. doi: 10.1111/j.1476-5381.1994.tb13039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornbury KD, Hollywood MA, McHale NG. Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol. 1992;451:133–144. doi: 10.1113/jphysiol.1992.sp019157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong YC, Hung YC, Cheng JT. Evidence of P2Y-purinoceptor mediated bladder neck smooth muscle post-contractile relaxation in the male mini-pig. Neurosci Lett. 1997;225:181–184. doi: 10.1016/s0304-3940(97)00212-7. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, et al. P2X3 knockout mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- Werkström V, Andersson KE. ATP- and adenosine-induced relaxation of the smooth muscle of the pig urethra. BJU Int. 2005;96:1386–1391. doi: 10.1111/j.1464-410X.2005.05853.x. [DOI] [PubMed] [Google Scholar]
- Wessler I, Kirkpatrick CJ, Racke K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther. 1998;77:59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- Yang SJ, An JY, Shim JO, Park CH, Huh IH, Sohn UD. The mechanism of contraction by 2-chloroadenosine in cat detrusor muscle cells. J Urol. 2000;163:652–658. [PubMed] [Google Scholar]
- Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, et al. Non-neuronal cholinergic system in human bladder urothelium. Urology. 2006;67:425–430. doi: 10.1016/j.urology.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Yu W, Zacharia LC, Jackson EK, Apodaca G. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol. 2006;291:C254–C265. doi: 10.1152/ajpcell.00025.2006. [DOI] [PubMed] [Google Scholar]


