Abstract
Background and purpose:
The effects of centrally administered cannabinoids on body core temperature (Tc) and the contribution of endogenous cannabinoids to thermoregulation and fever induced by lipopolysaccharide (LPS) (Sigma Chem. Co., St. Louis, MO, USA) were investigated.
Experimental approach:
Drug-induced changes in Tc of male Wistar rats were recorded over 6 h using a thermistor probe (Yellow Springs Instruments 402, Dayton, OH, USA) inserted into the rectum.
Key results:
Injection of anandamide [(arachidonoylethanolamide (AEA); Tocris, Ellisville, MO, USA], 0.01–1 µg i.c.v. or 0.1–100 ng intra-hypothalamic (i.h.), induced graded increases in Tc (peaks 1.5 and 1.6°C at 4 h after 1 µg i.c.v. or 10 ng i.h.). The effect of AEA (1 µg, i.c.v.) was preceded by decreases in tail skin temperature and heat loss index (values at 1.5 h: vehicle 0.62, AEA 0.48). Bell-shaped curves were obtained for the increase in Tc induced by the fatty acid amide hydrolase inhibitor [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate (Cayman Chemical Co., Ann Arbor, MI, USA) (0.001–1 ng i.c.v.; peak 1.9°C at 5 h after 0.1 ng) and arachidonyl-2-chloroethylamide (ACEA; Tocris) (selective CB1 agonist; 0.001–1 µg i.c.v.; peak 1.4°C 5 h after 0.01 µg), but (R,S)-(+)-(2-Iodo-5-nitrobenzoyl)-[1-(1-methyl-piperidin-2-ylmethyl)-1H-indole-3-yl] methanone (Tocris) (selective CB2 agonist) had no effect on Tc. AEA-induced fever was unaffected by i.c.v. pretreatment with 6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indole-3-yl](4-methoxyphenyl) methanone (Tocris) (selective CB2 antagonist), but reduced by i.c.v. pretreatment with N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251; Tocris) (selective CB1 antagonist). AM251 also reduced the fever induced by ACEA or LPS.
Conclusions and implications:
The endogenous cannabinoid AEA induces an integrated febrile response through activation of CB1 receptors. Endocannabinoids participate in the development of the febrile response to LPS constituting a target for antipyretic therapy.
Keywords: anandamide, ACEA, AM251, AM630, AM1241, URB597, capsazepine, fever, LPS
Introduction
Fever is a multi-mediated response that integrates the complex physiological defence strategy of the host that follows the recognition of invading microbial or non-microbial organisms as foreign by immune cells. Recognition of the invader activates the production and release of a diverse array of peptidic and non-peptidic mediators, which includes many cytokines, corticotropin releasing factor (CRF), endothelins (Dinarello, 1984; Dinarello et al., 1986; Helle et al., 1988; Rothwell, 1990; Fabricio et al., 1998), endogenous opioids (Fraga et al., 2008) and prostaglandins (Dinarello et al., 1991; Fraga et al., 2008). Such mediators activate mechanisms that ultimately increase the thermoregulatory set-point located in the hypothalamus to promote the febrile response (Zeisberger, 1999; Roth and Souza, 2001 for review).
The recreational, medicinal and ritualistic uses of Cannabis sativa date back thousands of years, but it was only in the 1960s that the plant's main active constituent, Δ9-tetrahydrocannabinol (Δ9-THC), was isolated and structurally characterized (Mechoulam and Gaoni, 1965a,b;). Since then, it has been found that Δ9-THC largely mimics the effects of the lipidic endocannabinoid anandamide [arachidonoylethanolamide (AEA)] (Devane et al., 1992) and 2-arachidonoylglicerol (Mechoulam et al., 1995), which promote widespread effects through activation of two specific G protein-coupled cannabinoid receptors, namely CB1 (Matsuda et al., 1990) and CB2 receptors (Munro et al., 1993) respectively. These receptors can signal inhibitory effects on adenylyl cyclase and calcium channel activities, and activate mitogen-activated protein kinases and potassium channels (Mackie, 2008). CB1 receptors are heterogeneously distributed within the central nervous system (CNS) and can account for several of the characteristic pharmacological properties of cannabinoid receptor agonists (Pertwee, 2005; 2008; for review). CB2 receptors are present mainly in the periphery, have been detected in cells of the immune system and are responsible for the immunoregulatory effects of cannabinoids (Pertwee, 1997; Howlett, 2002). In addition, AEA can also interact with, and stimulate, capsaicin-sensitive transient potential receptor vanilloid 1 (TRPV1) receptors (Rice et al., 2002; Pertwee, 2005). Inactivation of endocannabinoids is dependent on two mechanisms, reuptake and degradation (Beltramo et al., 1997; Hillard and Campbell, 1997; Hillard et al., 1997). The fatty acid amide hydrolase (FAAH) is the main enzyme responsible for the degradation of AEA to arachidonic acid and ethanolamide (Cravatt et al., 1996; Goparaju et al., 1999a,b;).
It has long been recognized that rodents treated with cannabinoids display four characteristic symptoms: analgesia, catalepsy, hypoactivity and hypothermia (Lomax and Campbell, 1971; Pertwee, 2005 for review). Despite the prominence of hypothermia [i.e. a reduction in body core temperature (Tc)], this symptom is only observed in animals administered high doses of Δ9-THC. In fact, at lower doses, Δ9-THC increases rather than decreases Tc (Sofia, 1972; Fennessy and Taylor, 1977). As to the effects of endocannabinoids on Tc, Crawley et al. (1993) and Chaperon and Thiebot (1999) have shown that systemic administration of AEA induces a dose-dependent reduction in Tc. The effects of central administration of natural and synthetic cannabinoids on Tc have also been explored. In this regard, dose-dependent hypothermia has been observed following microinjections of Δ9-THC into the anterior hypothalamic/preoptic area (AH/POA) in mice (Fitton and Pertwee, 1982), or of the synthetic cannabinoid agonists [(BaR)-trans-3-(1,1-dimethylheptyl)-6α,7,10,10α-tetrahydro-1-hydroxy-6,5-dimethyl-βH-dibenzo-[b,d]pyran-9-methanol] (HU-210) (Ovadia et al., 1995) or [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-pyrrolo[3,2,1ij] quinolin-6-one] (WIN-55212-2) in rats (Rawls et al., 2002). The latter study suggested that CB1 receptors within the AH/POA are responsible for the signalling of cannabinoid-induced hypothermia, as the effect of WIN-55212-2 was dose-dependently antagonized by intramuscular treatment with [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride] (SR141716A), a selective CB1 receptor antagonist.
However, the effects of centrally administered endogenous cannabinoids on Tc responses and the role of CB1 and CB2 receptors in bringing about their putative effects are currently unknown. The present study was designed to examine this issue, and also the possible involvement of central endocannabinoids and their receptors in thermoregulation and the febrile response to LPS.
Methods
Animals
Male Wistar rats weighing 180–200 g, housed 4–5 per cage at 24 ± 1°C under a 12:12 h light–dark cycle (lights on at 7 h, 0 min) with free access to food chow and tap water were used in this study. All experiments were approved by the institution's Ethics Committee for Research On Laboratory Animal Use and are in accordance with the guidelines set by the US National Institute of Health and Brazilian legislation.
Intracerebral cannula implantation and microinjections
Under anaesthesia with sodium pentobarbitone (Sigma Chem. Co., St. Louis, MO, USA) (40 mg·kg−1, i.p.), a permanent 22-gauge stainless-steel guide cannula (0.8 mm outer diameter, 10 mm long) was stereotaxically implanted into the right lateral ventricle at coordinates: 1.6 mm lateral to the midline, 1.5 mm posterior to bregma and 2.5 mm under the brain surface, and the incisor bar was lowered 2.5 mm below the horizontal zero (Paxinos et al., 1985). In other rats, for intra-hypothalamic (i.h.) injection, a 24-gauge stainless-steel guide cannula (0.55 mm outer diameter, 15 mm long) was stereotaxically implanted unilaterally into the AH/POA. Its stereotaxic coordinates were: 0.6 mm lateral to the midline, 7.7 mm anterior to the interaural line and 6.5 mm under the brain surface, and the incisor bar was lowered 3.0 mm below the horizontal zero (Paxinos et al., 1985). Cannulae were fixed to the skull with jeweller's screws embedded in dental acrylic cement. All procedures were conducted under aseptic conditions, and the rats were treated with oxytetracycline hydrochloride (400 mg·kg−1, i.m.) and allowed to recover for 1 week prior to experimental use.
Pyrogenic stimuli were all injected between 10:00 and 11:00 A.M. Microinjections into the lateral ventricle (i.c.v.) and into the AH/POA (i.h.) were made aseptically using a 30-gauge needle connected by polyethylene (PE 10) tubing to a 25 or 10 µL Hamilton syringe (Sigma Aldrich, St. Louis, MO, USA) respectively. The needle protruded 2 mm beyond the cannula tip, and a 2 µL (i.c.v.) or 200 nL (i.h.) volume was injected slowly (over 1 min) with Hamilton syringe. For i.h. injections the syringe was coupled to a microinfusion pump (model KDS101, KD Scientific, EUA, Holliston, MA, USA). After the injection, the needle remained in place for 30 s before it was withdrawn to prevent backflow of the injection fluid through the cannula.
After the experiments, rats received a microinjection of Evan's blue (2.5%) either into the lateral ventricle (5 µL) or into the POA (500 nL), as appropriate, followed by an overdose of pentobarbitone, and were perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde. Each brain was removed, stored in the same fixative for 6 h, kept in 30% sucrose overnight and cut in 40 µm coronal sections on freezing microtome to assess, under light microscopy, the position of the cannulae and respective sites of perfusion. Animals showing cannula misplacement or blockage upon injection, or abnormal body weight gain patterns during the post-implantation period were excluded from the study.
Temperature measurements
Tc was measured by inserting a thermistor probe (Yellow Springs Instruments 402, Dayton, OH, USA) 4 cm into the rectum for 1 min, at 30 min intervals, for up to 6 h. For each measurement, the animal was picked up gently and held by the experimenter during temperature measurements, without removing the animal from its home cage. This procedure was performed at least twice on the day before the experiment to minimize temperature changes secondary to handling. On the day of the experiment, the basal Tc of each animal was estimated four times, at 30 min intervals, before any injection. Only animals displaying a mean basal Tc between 36.8 and 37.2°C were selected for the study. The experiments were conducted during the light cycle in a temperature-controlled room of 28 ± 1°C, the thermoneutral zone for rats (Gordon, 1990; Romanovsky et al., 2002). In some experiments, tail skin temperature (Ts) was measured, without removing the rats from their home cages, by attaching a thermistor probe to the lateral surface of the most distal third of the tail for 1 min, at 30 min intervals, up to 6 h. The thermistor probe was fixed and isolated from the changes of ambient temperature (Ta) by a thermal isolating tape laying over a piece of micropore to avoid tail skin irritation. On the day of the experiment, the basal Ts of each animal was determined four times, at 30 min intervals, before any injection. Only animals displaying a basal Ts between 32.0 and 33.0°C were selected for the study.
To indirectly estimate the changes in the peripheral vasomotor tone, the heat loss index (HLI) was calculated to eliminate direct influences of both Ta and Tc on Ts, according to the formula:
The value of HLI can vary from 0 (maximal vasoconstriction) to 1 (maximal vasodilatation) (Romanovsky et al., 2002).
Experimental design
In the first set of experiments, we simultaneously evaluated the effects of i.c.v. injections of AEA on Tc and Ts, to enable calculation of the HLI. The effect of i.h. injections of AEA was also evaluated on Tc only.
The next set of experiments investigated the effects of i.c.v. injections of [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate (URB597; Cayman Chemical Co., Ann Arbor, MI, USA) (an inhibitor of FAAH), arachidonyl-2-chloroethylamide (ACEA; Tocris) (a selective CB1 receptor agonist) and (R,S)-(+)-(2-Iodo-5-nitrobenzoyl)-[1-(1-methyl-piperidin-2-ylmethyl)-1H-indole-3-yl] methanone (AM1241; Tocris) (a selective CB2 receptor agonist) on Tc.
Another set of experiments was designed to confirm the identities of the receptors implicated in febrile responses induced by AEA and ACEA. The animals were pretreated (i.c.v.) with N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251; Tocris) (a selective CB1 receptor antagonist), 6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indole-3-yl](4-methoxyphenyl) methanone (AM630; Tocris) (a selective CB2 receptor antagonist) or capsazepine (CPZ; Sigma Chem. Co.) (an antagonist of TRPV1 – or vanilloid – receptors) 30 min before i.c.v. administration of AEA or ACEA.
A final experiment was designed to investigate the contribution of central cannabinoid CB1 receptor-operated mechanisms to the febrile response induced by LPS. The animals were pretreated (i.c.v.) with AM251 30 min before the i.p. administration of LPS.
Statistical analysis
For data analysis of changes in Tc and Ts, the baseline temperatures before any treatment were calculated for each animal by averaging the last three temperature measurements before any injection, and all subsequent temperatures were expressed as changes from the mean basal core temperature value (ΔTc). HLI was calculated as described earlier. All data are reported as means ± SEM and were analysed for statistical significance by two-way analysis of variance followed by Bonferroni's test. The data were analysed using Prism Software (Graph-Pad, San Diego, CA, USA). Significance was set at P < 0.05.
Drugs and materials
AEA (anandamide,), ACEA, AM1241, AM251 and AM630, all purchased from Tocris (Ellisville, MO, USA), and URB597 (Cayman Chemical Co.) were all diluted in saline containing propylene glycol (10%) and Tween 80 (1%). CPZ (Sigma Chem. Co., St. Louis, MO, USA) was diluted in saline containing ethanol (20%) and cremophor (20%). Lipopolysaccharide (LPS, Escherichia coli 0111:B4, Sigma Chem. Co.) was diluted in saline. Sodium pentobarbitone (Sigma Chem. Co.); microinfusion pump (KD Scientific); thermistor probe (Yellow Springs Instruments, model 402).
Results
Central effects of AEA on Tc
Figure 1A shows that the i.c.v. administration of AEA at doses of 0.01, 0.1, 1 and 10 µg (injected in 2 µL) induced long-lasting and dose-dependent increases in Tc that started at 2 h after administration and peaked around 5 h. As the increase in Tc induced by 1 µg of AEA was clearly maximal, this dose was selected to assess the simultaneous effects of the endocannabinoid on Tc and Ts. The experiments showed that the increase in Tc induced by i.c.v. AEA was clearly preceded (and accompanied) by significant and sustained reductions in Ts (Figure 1C), which resulted in decreases in calculated HLI (Figure 1D). Thus, AEA evoked an integrated thermoregulatory response comprising increases in Tc concomitant with cutaneous vasoconstriction. Importantly, i.h. injections of AEA (0.1, 1, 10 and 100 ng, in 0.2 µL) into the AH/POA promoted dose-dependent increases in Tc, with a maximal effect at 10 ng (Figure 1B). The onset (1 h) of the response to i.h. AEA and the time elapsed to reach a plateau (2–3 h) were shorter than those observed following i.c.v. injection.
Figure 1.
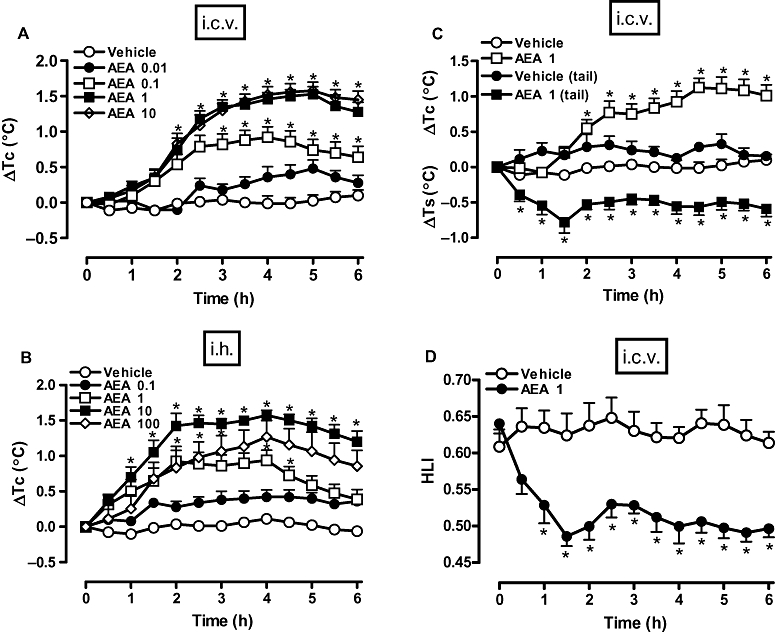
Anandamide [arachidonoylethanolamide (AEA), i.c.v. and i.h.] increased core temperature (Tc) and reduced tail skin temperature (Ts) and heat loss index (HLI). AEA (or vehicle) was injected either by the i.c.v. route (in 2 µL), at 0.01, 0.1, 1 or 10 µg (A), or at 1 µg (C) or by the intra-hypothalamic (i.h.) route (in 0.2 µL), at 0.1, 1, 10 or 100 ng (B). Values of HLI in (D) were calculated from results depicted in (C), as described in Methods. Each value is the mean ± SEM of the change in Tc [changes from the mean basal core temparature value (ΔTc), in °C] observed in 6–10 rats in (A) and (B), or of the change in Tc or Ts (ΔTc or ΔTs, respectively, in °C) (C) and HLI (D) observed in 7–10 rats. *P < 0.05 compared to vehicle-treated group.
Effects of URB597 (FAAH inhibitor), ACEA (CB1 agonist) and AM1241 (CB2 agonist) on Tc
The i.c.v. administration of URB597 or ACEA (both at 0.001–1 µg, in 2 µL) induced bell-shaped increases in Tc. The dose of 0.1 µg URB597 induced the greatest increase in Tc, which started 1.5 h after administration and peaked at 5 h (Figure 2A). The most effective dose of ACEA was 0.01 µg, which raised Tc significantly at 3 h after injection to attain a peak at 5 h (Figure 2B). However, the i.c.v. administration of the selective CB2 receptor agonist AM1241 (0.01–1 µg, in 2 µL) did not affect Tc values significantly (Figure 2C).
Figure 2.
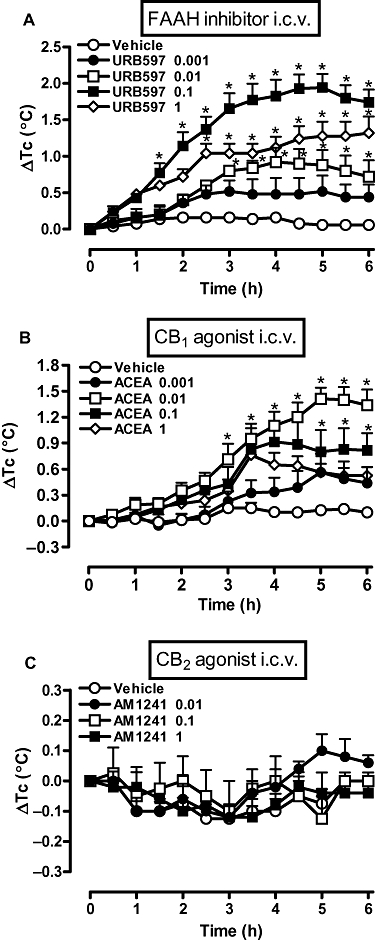
A fatty acid amide hydrolase (FAAH) inhibitor (URB597) and a CB1 receptor agonist [arachidonyl-2-chloroethylamide (ACEA)] increased core temparature (Tc), but a CB2 receptor agonist (AM1241) did not. Rats received i.c.v. injections of (A) URB597 (0.001, 0.01, 0.1 or 1 ng), (B) ACEA (0.001, 0.01, 0.1 or 1 µg) or (C) AM1241 (0.01, 0.1 or 1 µg), or an equal volume of the corresponding vehicle (2 µL). Each value is the mean ± SEM of the change in Tc [changes from the mean basal core temperature value (ΔTc), in °C] observed in 6–9 rats. *P < 0.05 compared to vehicle-treated group. AM1241, (R,S)-(+)-(2-Iodo-5-nitrobenzoyl)-[1-(1-methyl-piperidin-2-ylmethyl)-1H-indole-3-yl] methanone; URB597, [3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate.
Effect of AM251 (CB1 antagonist), AM630 (CB2 antagonist) and CPZ (TRPV1 antagonist) on fever induced by AEA and ACEA
Prior i.c.v. treatment with AM251 (1, 5 and 10 µg) inhibited the development of the febrile response to AEA (1 µg, i.c.v.), being most effective at the dose of 5 µg (Figure 3A). At the latter dose, AM251 also attenuated substantially the fever induced by ACEA (0.01 µg, i.c.v.; Figure 3B). However, neither AM630 (10 µg, i.c.v.) nor CPZ (1, 3 and 10 µg, i.c.v.) influenced the fever induced by AEA (Figure 3C,D respectively). Importantly, Figure 3 also shows that i.c.v. injections of AM251, AM630 or CPZ alone (each at the highest doses) failed to alter the Tc of control animals.
Figure 3.
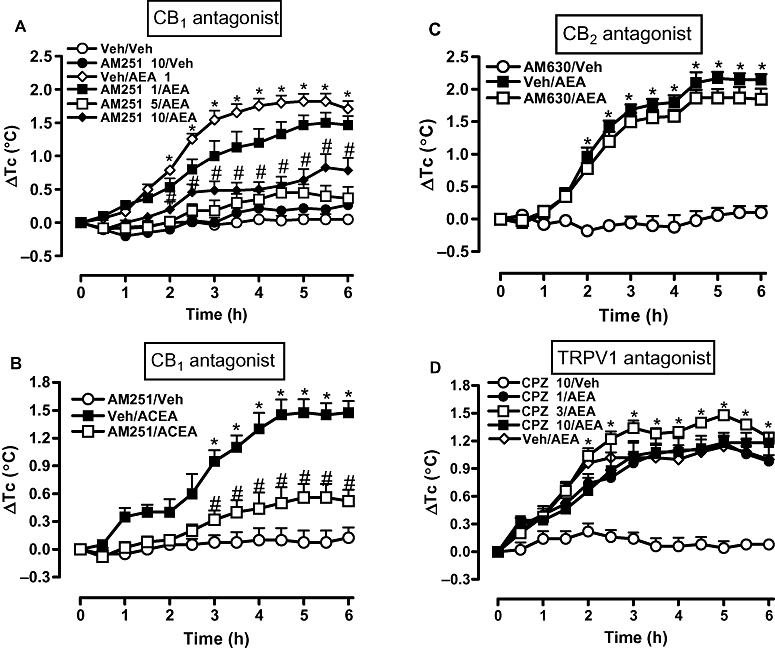
A selective antagonist of CB1 receptors (AM251), but not of CB2 receptors (AM630) or TRPV1 receptors [capsazepine (CPZ)], attenuated fever induced by arachidonoylethanolamide (AEA) and another CB1 receptor agonist [arachidonyl-2-chloroethylamide (ACEA)]. Rats received an i.c.v. injection of (A) AM251 at 1, 5 or 10 µg or (B) at 5 µg, (C) AM630 at 10 µg, (D) CPZ at 1, 3 or 10 µg, or an equal volume of corresponding vehicles (Veh, 2 µL) 30 min before i.c.v. injection of (A,C,D) AEA (1 µg), (B) ACEA (0.01 µg) or vehicle alone (2 µL). Each value is the mean ± SEM of the change in Tc [changes from the mean basal core temperature value (ΔTc), in °C] observed in 6–9 rats. *P < 0.05 compared to AM251/Veh (A,B), AM630/Veh (C) or CPZ/Veh (D) group. #P < 0.05 compared to Veh/AEA (A,C,D) or Veh/ACEA (B) group. AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; AM630, 6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indole-3-yl](4-methoxyphenyl) methanone; TRPV1, transient receptor potential vanilloid 1.
Effect of AM251 (CB1 antagonist) on fever induced by LPS
Systemic administration of LPS (50 µg·kg−1, i.p.) induced an increase in Tc that started 1 h after injection, peaked at 2.5 h and remained at this level until 6 h. Pretreatment of the animals with the selective CB1 receptor antagonist AM251 (5 µg, i.c.v.) promoted a marked and long-lasting decrease in the fever induced by LPS, from the 1.5 h time point onwards (Figure 4). It is noteworthy that, besides inhibiting the magnitude of LPS-induced fever by about 50–70%, AM251 also delayed the onset of the response to LPS and the time to its peak effect. A higher dose of AM251 (10 µg) did not inhibit the response to LPS to a greater extent than 5 µg (results not shown).
Figure 4.
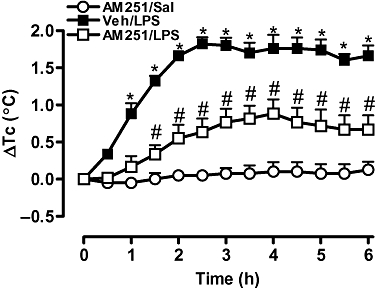
The selective CB1 receptor antagonist AM251 reduced the febrile response induced by lipopolysaccharide (LPS). Rats received an i.c.v. injection of AM251 (5 µg) or equal volume of the vehicle (Veh, 2 µL) 30 min before i.p. injection of LPS (50 µg·kg−1) or saline (Sal) (1 mL·kg−1). Each value is the mean ± SEM of the change in Tc [changes from the mean basal core temperature value (ΔTc), in °C] observed in 6–7 rats. *P < 0.05, #P < 0.05 when compared to AM251/Sal or Veh/LPS group respectively. AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide.
Discussion
The present study shows that central administration of the endocannabinoid AEA promotes dose-dependent increases in Tc in rats, which appears to integrate a genuine febrile response. The effect of AEA on Tc was mimicked by i.c.v. injection of a selective CB1 receptor agonist and prevented by treatment with a CB1 receptor antagonist, whereas ligands selective for CB2 receptors were inactive. Moreover, as blockade of AEA metabolism, by use of an inhibitor of FAAH, increased Tc, and LPS-induced fever was attenuated by blockade of central CB1 receptors, central endocannabinoids appear to play a relevant physiological role in thermoregulation.
As cited in the Introduction, several studies have reported that systemic, i.c.v. or i.h. treatment with exogenous cannabinoids induces robust hypothermic responses in rodents (Holtzman et al., 1969; Lomax and Campbell, 1971; Taylor and Fennessy, 1977; Rawls et al., 2002), which are likely to be subsequent to stimulation of CB1 receptors (Rawls et al., 2002). Likewise, systemic (i.p.) AEA administration in rats was reported to cause solely hypothermic responses (Crawley et al., 1993; Chaperon and Thiebot, 1999), which are blocked by i.p. treatment with the CB1 receptor antagonist SR141716A (Costa et al., 1999). Conversely, i.p. and p.o. administration of Δ1-THC (Sofia, 1972) or i.v. Δ9-THC injection (Taylor and Fennessy, 1977) was found to cause dual effects in rats, that is, increases in Tc at lower doses, but profound hypothermia following higher doses. The current study, which appears to be the first to assess Tc responses to a centrally administered endocannabinoid, detected clear-cut sustained and dose-related increases in Tc following i.c.v. injection of AEA. Although CB2 receptors are generally absent from the majority of brain cells, CB1 receptors are widely, but heterogeneously, expressed throughout the CNS (Matsuda et al., 1990; Munro et al., 1993; Pertwee, 2005). Importantly, the hypothalamus is particularly enriched with CB1 receptors (Tsou et al., 1998), especially in the lateral hypothalamic area and preoptic anterior nucleus (Moldrich and Wenger, 2000), the latter being critically implicated in thermoregulation (Boulant, 2000). In agreement with this evidence, we observed that AEA was about 10-fold more potent in promoting increases in Tc when microinjected directly into the AH/POA than when the i.c.v. route of administration was employed.
By measuring Tc and Ts concomitantly, we also determined whether the increase in Tc induced by i.c.v. AEA constituted an integrated thermoregulatory response (i.e. fever) or was merely the result of hyperthermia. Indeed, the long-lasting increase in Tc caused by AEA (1 µg, i.c.v.) was preceded and accompanied by a reduction in Ts, which led to a sustained reduction of the calculated HLI, a reliable means of discriminating fever from hyperthermia (Romanovsky et al., 2002). Therefore, AEA-treated rats clearly developed an integrated response comprising an increase in Tc associated with a significant decrease in heat dissipation (i.e. reduction in Ts) due to tail skin vasoconstriction, as is characteristic of the febrile response phenomenon in these species (Romanovsky et al., 2002).
Even though AEA displays higher affinity for the CB1 receptors, it can also interact with the CB2 and TRPV1 (vanilloid) receptors (Matsuda et al., 1990; Munro et al., 1993; Rice et al., 2002; Pertwee, 2005). We thus attempted to carefully evaluate which of these targets was relevant for the development of AEA-induced fever. Our findings demonstrate that the fever induced by i.c.v. AEA was sensitive to dose-dependent inhibition by prior treatment with the CB1 receptor antagonist AM251, but not the CB2 receptor antagonist AM630. Moreover, i.c.v. injections of the CB1 receptor agonist ACEA caused graded increases in Tc over a broad dose range, whereas similar treatment with the CB2 receptor agonist AM1241 (up to 1 µg) did not change Tc. Finally, the development of AEA-induced fever was not modified by prior i.c.v. administration of CPZ, a TRPV1 receptor antagonist (Valenzano and Sun, 2004). Therefore, the evidence collected points to a pivotal role of CB1 receptors in the signalling mechanisms that promote the AEA-induced fever response.
It is perhaps important to comment that i.c.v. ACEA yielded a bell-shaped dose-response curve for its Tc increasing effect, whereas AEA did not. This difference could be ascribed to: (i) the limited range of AEA doses chosen for the curve impeded the detection of the curve's downward deflection portion; (ii) at higher doses, ACEA (unlike AEA) may loose its selectivity towards CB1 receptors and act on additional AEA-insensitive targets; and/or (3) i.c.v. injection of high doses of CB1 receptor agonists might stimulate hypothermic responses, as reported to occur in rats following systemic injection of endocannabinoids (Crawley et al., 1993; Chaperon and Thiebot, 1999; Costa et al., 1999). Further studies are needed to address each of these possibilities.
To investigate if central endocannabinoids participate in thermoregulation, we assessed the effects of an inhibitor of FAAH (URB597), the main enzyme responsible for AEA degradation (Cravatt et al., 1996; Goparaju et al., 1999a), on Tc, as well as the influence of the selective CB1 receptor antagonist AM251 on fever induced by an exogenous pyrogen (LPS). Indeed, i.c.v. administration of URB597 (0.001–1 ng) caused graded increases in Tc, even though 1 ng induced a smaller effect than 0.1 ng. The reasons why this dose-response curve to URB597 displays a bell-shaped pattern remain to be clarified. Nonetheless, as inhibition of FAAH would be expected to increase endogenous AEA concentration in the synaptic cleft to enhance stimulation of CB1 receptors, these data favour the hypothesis that endocannabinoids contribute to Tc regulation.
However, the febrile response to systemic (i.p.) LPS injection was reduced by prior i.c.v. injection of the CB1 receptor antagonist AM251, at a dose that did not alter Tc in control rats per se. This constitutes strong functional evidence for the participation of the central endocannabinoid system in mediating, at least partially and via CB1 receptor-operated mechanisms, the fever promoted by this exogenous pyrogen. A recent study (Benamar et al., 2007) also reported that systemic administration of the selective CB1 receptor antagonist SR141716A prevented the development of fever induced by LPS (50 µg·kg−1, i.p.) in rats. Surprisingly, the same study found that i.p. injections of the CB1 selective agonist WIN-55212-2 or Δ9-THC, at doses that did not cause hypothermia per se, also inhibited the febrile response to LPS, and that the antipyretic effect of WIN-55212-2 (which also reduced plasma interleukin-6 levels) was prevented by SR141716A. The authors suggested that their unexpected findings could perhaps be due to a partial agonistic activity of SR141716A at CB1 receptors. The different findings, concerning Tc changes induced by systemic (Benamar et al., 2007) and i.c.v. (present study) injections of CB1 receptor agonists and antagonists, might indicate that central and peripheral endocannabinoids have distinct modulatory functions in the thermoregulatory changes induced by pyrogens.
In summary, the current study has shown that i.c.v. injection of a CB1 (but not CB2) receptor agonist causes sustained increases in Tc. This effect is associated with simultaneous peripheral vasoconstriction and a reduction in HLI, thus characterizing an integrated febrile response. Importantly, as central injection of an inhibitor of AEA catabolism raised Tc, and LPS-induced fever was sensitive to attenuation by central CB1 receptor blockade, brain endocannabinoids seem to be implicated in pyrexia. We are presently engaged in studies to determine at what level(s) endocannabinoids are recruited to take part in the complex central signalling mechanisms triggered by LPS to provoke fever. Such studies could help establish the potential of central CB1 receptors as a new relevant target for antipyretic therapy.
Acknowledgments
This study was supported by Conselho Nacional de Pesquisa (CNPq 304627/2007-0) and Fundação de Amparo a Pesquisa de São Paulo (2007/04791-1). D.F. was the recipient of a CNPq doctoral scholarship.
Glossary
Abbreviations:
- ACEA
arachidonyl-2-chloroethylamide
- AEA (anandamide)
arachidonoylethanolamide
- AH/POA
anterior hypothalamic/preoptic area
- AM1241
(R,S)-(+)-(2-Iodo-5-nitrobenzoyl)-[1-(1-methyl-piperidin-2-ylmethyl)-1H-indole-3-yl] methanone
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- AM630
6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indole-3-yl](4-methoxyphenyl) methanone
- CPZ
capsazepine
- CRF
corticotropin releasing factor
- Δ9-THC
Δ9-tetrahydrocannabinol
- ΔTc
changes from the mean basal core temperature value
- FAAH
fatty acid amide hydrolase
- HLI
heat loss index
- HU-210
[(BaR)-trans-3-(1,1-dimethylheptyl)-6α,7,10,10α-tetrahydro-1-hydroxy-6,5-dimethyl-βH-dibenzo-[b,d]pyran-9-methanol]
- i.h.
intra-hypothalamic
- LPS
lipopolysaccharide
- SR141716A
[N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride]
- Ta
ambient temperature
- Tc
core temperature
- TRPV1
transient receptor potential vanilloid 1
- Ts
tail skin temperature
- URB597
[3-(3-carbamoylphenyl)phenyl] N-cyclohexylcarbamate
- WIN-55212-2
[(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-pyrrolo[3,2,1ij] quinolin-6-one]
Conflict of interest
None.
References
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Benamar K, Yondorf M, Meissler JJ, Geller EB, Tallarida RJ, Eisenstein TK, et al. A novel role of cannabinoids: implication in the fever induced by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 2007;320:1127–1133. doi: 10.1124/jpet.106.113159. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31(Suppl. 5):S157–S161. doi: 10.1086/317521. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Costa B, Vailati S, Colleoni M. SR 141716A, a cannabinoid receptor antagonist, reverses the behavioural effects of anandamide-treated rats. Behav Pharmacol. 1999;10:327–331. doi: 10.1097/00008877-199905000-00009. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav. 1993;46:967–972. doi: 10.1016/0091-3057(93)90230-q. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1. Rev Infect Dis. 1984;6:51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Cannon JG, Mancilla J, Bishai I, Lees J, Coceani F. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Res. 1991;562:199–206. doi: 10.1016/0006-8993(91)90622-3. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, et al. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricio AS, Silva CA, Rae GA, D'Orleans-Juste P, Souza GE. Essential role for endothelin ET(B) receptors in fever induced by LPS (E. coli) in rats. Br J Pharmacol. 1998;125:542–548. doi: 10.1038/sj.bjp.0702075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennessy MR, Taylor DA. The effect of delta9-tetrahydrocannabinol on body temperature and brain amine concentrations in the rat at different ambient temperatures. Br J Pharmacol. 1977;60:65–71. doi: 10.1111/j.1476-5381.1977.tb16748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton AG, Pertwee RG. Changes in body temperature and oxygen consumption rate of conscious mice produced by intrahypothalamic and intracerebroventricular injection of Δ9-tetrahydrocannabinol (Δ9-THC) Br J Pharmacol. 1982;75:409–414. doi: 10.1111/j.1476-5381.1982.tb08802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga D, Machado RR, Fernandes LC, Souza GE, Zampronio AR. Endogenous opioids: role in prostaglandin-dependent and -independent fever. Am J Physiol Regul Integr Comp Physiol. 2008;294:R411–R420. doi: 10.1152/ajpregu.00465.2007. [DOI] [PubMed] [Google Scholar]
- Goparaju SK, Kurahashi Y, Suzuki H, Ueda N, Yamamoto S. Anandamide amidohydrolase of porcine brain: cDNA cloning, functional expression and site-directed mutagenesis(1) Biochim Biophys Acta. 1999a;1441:77–84. doi: 10.1016/s1388-1981(99)00143-2. [DOI] [PubMed] [Google Scholar]
- Goparaju SK, Ueda N, Taniguchi K, Yamamoto S. Enzymes of porcine brain hydrolyzing 2-arachidonoylglycerol, an endogenous ligand of cannabinoid receptors. Biochem Pharmacol. 1999b;57:417–423. doi: 10.1016/s0006-2952(98)00314-1. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Thermal biology of the laboratory rat. Physiol Behav. 1990;47:963–991. doi: 10.1016/0031-9384(90)90025-y. [DOI] [PubMed] [Google Scholar]
- Helle M, Brakenhoff JP, De Groot ER, Aarden LA. Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988;18:957–959. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Campbell WB. Biochem and pharmacology of arachidonylethanolamide, a putative endogenous cannabinoid. J Lipid Res. 1997;38:2383–2398. [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Jarrahian A, Campbell WB. Accumulation of N-arachidonoylethanolamine (anandamide) into cerebellar granule cells occurs via facilitated diffusion. J Neurochem. 1997;69:631–638. doi: 10.1046/j.1471-4159.1997.69020631.x. [DOI] [PubMed] [Google Scholar]
- Holtzman D, Lovell RA, Jaffe JH, Freedman DX. 1-delta9-tetrahydrocannabinol: neurochemical and behavioral effects in the mouse. Science. 1969;163:1464–1467. doi: 10.1126/science.163.3874.1464. [DOI] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Lomax P, Campbell C. Phenitrone and marihuana induced hypothermia. Experientia. 1971;27:1191–1192. doi: 10.1007/BF02286922. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl. 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. Hashish. IV. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron. 1965a;21:1223–1229. doi: 10.1016/0040-4020(65)80064-3. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. A total synthesis of dl-delta-1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc. 1965b;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Ovadia H, Wohlman A, Mechoulam R, Weidenfeld J. Characterization of the hypothermic effect of the synthetic canabinnoid HU-210 in the rat. Relation to the adrenergic system and endogenous pyrogens. Neuropharmacology. 1995;34:175–180. doi: 10.1016/0028-3908(94)00133-d. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;168:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–159. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Rice AS, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot Essent Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Roth J, Souza GEP. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res. 2001;34:301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Central activation of thermogenesis by prostaglandins: dependence on CRF. Horm Metab Res. 1990;22:616–618. doi: 10.1055/s-2007-1004986. [DOI] [PubMed] [Google Scholar]
- Sofia RD. A paradoxical effect for 1 -tetrahydrocannabinol on rectal temperature in rats. Res Commun Chem Pathol Pharmacol. 1972;4:281–288. [PubMed] [Google Scholar]
- Taylor DA, Fennessy MR. Biphasic nature of the effects of Δ9-tetrahydrocannabinol on body temperature and brain amines of the rat. Eur J Pharmacol. 1977;46:93–99. doi: 10.1016/0014-2999(77)90244-8. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Sun Q. Current perspectives on the therapeutic utility of VR1 antagonists. Curr Med Chem. 2004;11:3185–3202. doi: 10.2174/0929867043363686. [DOI] [PubMed] [Google Scholar]
- Zeisberger E. From humoral fever to neuroimmunological control of fever. J Therm Biol. 1999;24:287–326. [Google Scholar]


