Abstract
Dysregulated inflammation contributes to disease pathogenesis in both the periphery and the brain. Cytokines are coordinators of inflammation and were originally defined as secreted mediators, released from expressing cells to activate plasma membrane receptors on responsive cells. However, a group of cytokines is now recognized as having dual functionality. In addition to their extracellular effects, these cytokines act inside the nuclei of cytokine-expressing or cytokine-responsive cells. Interleukin-1 (IL-1) family cytokines are key pro-inflammatory mediators, and blockade of the IL-1 system in inflammatory diseases is an attractive therapeutic goal. All current therapies target IL-1 extracellular actions. Here we review evidence that suggests IL-1 family members have dual functionality. Several IL-1 family members have been detected inside the nuclei of IL-1-expressing or IL-1-responsive cells, and intranuclear IL-1 is reported to regulate gene transcription and mRNA splicing. However, further work is required to determine the impact of IL-1 intranuclear actions on disease pathogenesis. The intranuclear actions of IL-1 family members represent a new and potentially important area of IL-1 biology and may have implications for the future development of anti-IL-1 therapies.
Keywords: Interleukin-1, nuclear localization, inflammation, disease, IL-1RA, HMGB1
The dual function cytokine hypothesis
Inflammation is required for the efficient clearance of infections and repair of injured tissue, but dysregulated inflammation contributes to the pathogenesis of major peripheral and central nervous system (CNS) diseases. Inflammation is therefore a current focus for drug development. Cytokines, the coordinators of inflammation, were defined originally as soluble mediators, released from an expressing cell to activate transmembrane receptors on responsive cells. However, some cytokines are now known to have an additional set of intracellular (intracrine) actions, either within the expressing cell or following internalization by a responsive cell, and so can be described as having dual functionality (Re, 2003).
High mobility group box 1 (HMGB1) is the prototypical dual function cytokine. First characterized as a nuclear DNA-binding protein that modifies the interactions of transcription factors with DNA (Thomas and Travers, 2001), HMGB1 was later found to be released actively from monocytes in response to pro-inflammatory stimuli (Gardella et al., 2002). In the periphery, released HMGB1 has pleitropic pro-inflammatory effects, including action as a late mediator of endotoxin lethality in mice (Wang et al., 1999; Lotze and Tracey, 2005). Furthermore, in the brain, blockade of HMGB1 actions by administration of a neutralizing antibody reduces the damage caused by cerebral ischaemia (Liu et al., 2007).
Other cytokines now reported to exhibit dual functionality include interleukin (IL)-16 (Center et al., 2004; Wilson et al., 2005; Zhang et al., 2008) and interferon-γ (Will et al., 1996; Ahmed et al., 2003). Dual function cytokines tend to share certain characteristics, including cytosolic translation, non-classical secretion and nuclear localization in cytokine expressing cells (Cruikshank et al., 2000; Zhang et al., 2001; Ahmed et al., 2003; Lotze and Tracey, 2005).
Interleukin-1 family cytokines are a group of key pro-inflammatory mediators, implicated in the pathogenesis of peripheral and CNS diseases, making the IL-1 system an attractive target for therapeutic intervention. IL-1 family cytokines are assumed to act primarily as secreted mediators, and all current anti-IL-1 therapeutic strategies target these extracellular IL-1 actions. However, several IL-1 family members localize to cell nuclei and, like HMGB1, may have dual functionality. The intranuclear actions of IL-1 family members remain a poorly understood, potentially important area of IL-1 biology, and will be the focus of this review.
The importance of IL-1 family cytokines in peripheral and CNS disease
The best characterized IL-1 family members are the agonists IL-1α (IL-1F1), IL-1β (IL-1F2), IL-18 (IL-1F4) and the naturally occurring antagonist IL-1RA (IL-1F3, Dinarello, 1996). IL-1β is often described as the prototypical pro-inflammatory cytokine. Released in response to local or systemic injury or disease, IL-1β orchestrates host defence responses. IL-1β has wide-ranging effects on gene expression including up-regulating cytokines, acute phase proteins and tissue remodelling enzymes (Dinarello, 1996). As a key mediator of innate immunity, IL-1β is a potent pyrogen (Murakami et al., 1990) and stimulates neutrophilia and the infiltration of circulating leukocytes into inflamed tissues (Pettipher et al., 1986; Ulich et al., 1987). IL-1β also plays an important role in the adaptive immune response by stimulating the development of activated lymphocytes (Gery et al., 1972).
In the periphery, IL-1β is required for the efficient clearance of bacterial infections (Miller et al., 2007). However, IL-1β is also implicated in the pathogenesis of many acute and chronic peripheral diseases. For example, excessive acute activation of the IL-1β system contributes to the multi-organ failure caused by sepsis (Cohen, 2002). Chronic overproduction of IL-1β is crucial in the familial periodic fever syndromes (Church et al., 2008) and can contribute to the growth, vascularization and metastasis of malignant tumours (Voronov et al., 2003; Elaraj et al., 2006; Krelin et al., 2007). In addition, chronically elevated IL-1β levels are implicated in the pathogenesis of rheumatoid arthritis (Kay and Calabrese, 2004) and chronic obstructive pulmonary disease (Chung, 2001).
Endogenous IL-1β expression in the healthy brain is very low (Vitkovic et al., 2000). The majority of central IL-1β actions occur in the context of neuroinflammation, which leads to an up-regulation of IL-1β expression by microglia, the resident CNS macrophage population (Pearson et al., 1999; Vezzani et al., 1999; De Simoni et al., 2000; Mabuchi et al., 2000). Enhanced IL-1β expression is observed in many acute and chronic neurodegenerative diseases, and IL-1β polymorphisms are linked to altered susceptibility to these diseases (Allan et al., 2005).
The importance of IL-1β in brain injury caused by cerebral ischaemia has been firmly established in animals. Administering exogenous IL-1β exacerbates damage caused by focal cerebral ischaemia in rodents (Yamasaki et al., 1995; Loddick and Rothwell, 1996). Blockade of the IL-1β system with a caspase-1 (an IL-1β processing enzyme) inhibitor, IL-1RA, or a neutralizing antibody for IL-1β, also reduces this damage in rats (Relton and Rothwell, 1992; Yamasaki et al., 1995; Ross et al., 2007). In addition, deletion of the genes for both IL-1α and β, or of caspase-1, substantially reduces ischaemic brain damage in mice, whereas deletion of IL-1RA enhances damage (Schielke et al., 1998; Boutin et al., 2001; Pinteaux et al., 2006).
Interleukin-1β is far more widely studied than IL-1α (there are more than 31 000 papers in the Pubmed database, http://www.ncbi.nlm.nih.gov/sites/entrez, on IL-1β, in comparison with less than 9000 on IL-1α, using the following keyword search: ‘IL-1alpha/beta’ OR ‘IL-1 alpha/beta’ OR ‘Interleukin-1alpha/beta’ OR ‘Interleukin-1 alpha/beta’). This may be due to an assumption that as both cytokines bind and activate the same receptor, they are likely to have redundant effects in vivo (Dinarello, 1997). However, comparison of IL-1α- and IL-1β-deficient mice reveals that these cytokines have non-redundant roles in host defence and disease pathogenesis. Tumorigenesis, turpentine-induced fever and defence against bacterial infection are all dependent on IL-1β but not IL-1α (Horai et al., 1998; Krelin et al., 2007; Miller et al., 2007). In contrast, diet-induced weight gain and atherosclerosis are IL-1α- and not IL-1β-dependent (Kamari et al., 2007). T cell-dependent antibody production is IL-1β-dependent, but the activation of T cells in response to contact allergens is IL-1α-dependent (Nakae et al., 2001a; Nakae et al., 2001b). In addition, IL-1α and β have different, complementary roles in host defence against Candida albicans infection (Vonk et al., 2006). Different patterns of IL-1α and β expression, processing and release (Lonnemann et al., 1989; Fenton, 1992; Hacham et al., 2000) may all be important in explaining the non-redundant effects of these two cytokines. In addition, as discussed below, the intranuclear actions of IL-1α and β are likely to be different.
Recently, a further seven IL-1 family ligands (IL-1F5-F11) have been identified through sequence homology (Dunn et al., 2001; Schmitz et al., 2005). IL-33 (IL-1F11) acts as an immunomodulator, promoting TH2-type immune responses, and is implicated in the pathogenesis of asthma, rheumatoid arthritis and cardiovascular disease (Kakkar and Lee, 2008). The roles of IL-1F5-10 in host defence and disease pathogenesis remain poorly understood.
IL-1 family members as released mediators
Interleukin-1 family members are commonly assumed to act primarily following release from IL-1 producing cells, via binding transmembrane IL-1 receptors on responsive cells. Mechanisms of IL-1α and β processing and release, and activation of the classical IL-1 signalling pathway on responsive cells, have been reviewed extensively elsewhere, (Nickel, 2003; Prudovsky et al., 2003; Mariathasan and Monack, 2007; Brikos and O'Neill, 2008) and are summarized here (see Figure 1).
Figure 1.

IL-1 family members as released mediators. IL-1α and β, the two best characterized IL-1 family agonists, are translated in the cytoplasm as 31 kD pro-forms. Pro-IL-1α and β are then proteolytically cleaved by calpain and caspase-1, respectively, to produce the mature proteins. Caspase-1 is activated by recruitment to multimeric inflammasomes, enhancing caspase-1 autoproteolysis. Pro-IL-1α, mature IL-1α and mature IL-1β can all be released from cells and bind to transmembrane IL-1RI (RI) on IL-1-responsive cells. This leads to the recruitment of IL-1RAcP (AcP) to IL-1RI. A multi-protein complex is recruited to the cytoplasmic domain of the receptor dimer, leading to the activation of NFκB and mitogen-activated protein kinases, and to changes in gene expression and RNA stability. IL-1RA, the best developed anti-IL-1 therapeutic agent, acts as a competitive antagonist, binding IL-1RI but failing to recruit IL-1RAcP and activate signal transduction. IL-1, interleukin-1; IL-1RA, IL-1 receptor antagonist; IL-1RI, type I IL-1 receptor; IL-1RAcP, IL-1 receptor accessory protein; NFκB, nuclear factor κB.
Interleukin-1α and β are unusual secreted proteins, in that they are translated in the cytosol, and have no signal sequence to direct them through the endoplasmic reticulum (ER)-Golgi classical pathway of secretion (Rubartelli et al., 1990; Stevenson et al., 1992). Pro-IL-1β (31 kD) is cleaved to release the active 17 kD mature protein by caspase-1 (Thornberry et al., 1992). Caspase-1 activity is regulated by NOD-like receptors, a family of intracellular pattern recognition receptors that detect pathogen- and damage-associated molecular patterns including gout-associated uric acid crystals, cytosolic DNA and bacterial flagellin (Mariathasan and Monack, 2007; Muruve et al., 2008). NOD-like receptor activation leads to oligomerization and recruitment of caspase-1 to a multimeric inflammasome complex, facilitating caspase-1 activation by autoproteolysis (Mariathasan and Monack, 2007).
Pro-IL-1α (31 kD) is released on cell death and, in contrast to pro-IL-1β, can activate IL-1 receptors (Mosley et al., 1987). Pro-IL-1α can also be cleaved by calpains (calcium-dependent proteases, Kobayashi et al., 1990). Heat shock, calcium ionophores and ATP all stimulate the release of mature IL-1αin vitro (Watanabe and Kobayashi, 1994; Perregaux and Gabel, 1998; Mandinova et al., 2003). However, IL-1α release from monocytes is less efficient than IL-1β release in vitro (Lonnemann et al., 1989; Rubartelli et al., 1990), and in vivo many consider IL-1α to be a predominantly intracellular cytokine released only on cell death during severe disease (Dinarello, 1996). This view is supported by the detection of IL-1α-neutralizing autoantibodies in a substantial proportion of healthy humans (5–28%, Saurat et al., 1991; Miossec, 2002). In these individuals, IL-1α-reactive B-cells have evaded immunological tolerance mechanisms that would normally lead to the depletion or inactivation of self antigen-reactive B-cells (Singh and Schwartz, 2006). One explanation for this failure to develop proper immune tolerance to IL-1α is that during immune cell development, IL-1α is retained intracellularly, and so not available extracellularly for identification as a self antigen.
Extracellular IL-1α and β bind and activate the single transmembrane domain type I IL-1 receptor (IL-1RI) on responsive cells (Vigers et al., 1997). Pro-IL-1α, mature IL-1α and mature IL-1β all bind IL-1RI with similar affinity (KD= 1–10 nM) (Dower et al., 1986; Mosley et al., 1987; McMahan et al., 1991). This triggers IL-1 receptor accessory protein (IL-1RAcP) binding to the IL-1RI/IL-1α/β complex (Wesche et al., 1997). A multi-protein signalling complex is then recruited to the active receptor heterodimer. This signalling complex ultimately activates mitogen-activated protein kinases and nuclear factor κB (NFκB), and so stabilizes mRNA and regulates gene transcription [reviewed in Brikos and O'Neill (2008)]. A more rapid IL-1RI/IL-1RAcP-dependent IL-1β signalling pathway has been observed recently in neurones (Viviani et al., 2003; Davis et al., 2006). IL-1β induces changes in neuronal firing rates through neutral sphingomyelinase activation, and downstream Src kinase-mediated phosphorylation of the NMDA receptor subunit NR2B (Viviani et al., 2003). This rapid signalling pathway is implicated in the febrile response to IL-1β (Sanchez-Alavez et al., 2006).
Interleukin-1RA and IL-1RII are both negative regulators of IL-1α/β signalling. IL-1RA is a competitive antagonist at IL-1RI, which binds to IL-1RI but fails to recruit IL-1RAcP and activate signal transduction (Sims, 2002). IL-1RII acts as a decoy receptor, binding IL-1α and β without activating signalling (Colotta et al., 1993; Rauschmayr et al., 1997). As with IL-1 family ligands, there are a number of newly identified members of the IL-1 receptor family (Sims, 2002). Some are known to bind new IL-1 family ligands (IL-1F5-F11), while others remain orphan receptors.
Current anti-IL-1 therapeutic strategies
The importance of IL-1α and β in the pathogenesis of peripheral and CNS diseases makes the IL-1 system an attractive therapeutic target (Ledford, 2007). The best developed anti-IL-1 therapy is IL-1RA, the naturally occurring antagonist that competes with IL-1α and β for IL-1RI. IL-1RA is approved for the treatment of rheumatoid arthritis in the USA (Kay and Calabrese, 2004) and is currently being developed as a treatment for stroke (Emsley et al., 2005). However, IL-1RA has a short plasma half-life and relatively poor brain penetration, so novel anti-IL-1 therapies are still being investigated [reviewed in Braddock and Quinn (2004)]. These new therapies all target the extracellular actions of IL-1α and β, either by inhibiting IL-1β processing and release or by reducing the bioavailability of IL-1α and β in the extracellular space. However, several IL-1 family members localize to cell nuclei and may regulate intranuclear processes such as transcription and RNA splicing. There follows a review of the evidence for intranuclear roles of IL-1 family members (summarized in Figure 2) and the potential implications for future development of anti-IL-1 therapeutics.
Figure 2.
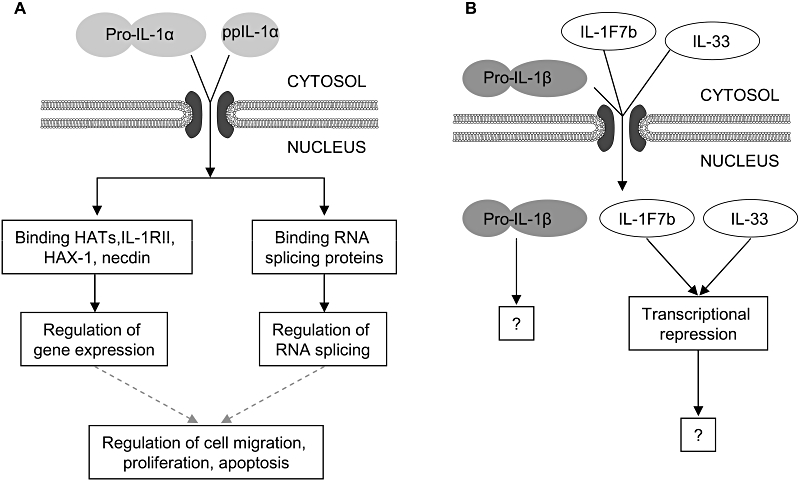
Interleukin (IL)-1 intranuclear actions. (A) Pro-IL-1α and the IL-1α pro-piece (ppIL-1α) are actively imported into the nucleus from the cytosol. Intranuclear pro- and ppIL-1α regulate gene expression through binding histone acetyl transferases (HATs), IL-1RII, HAX-1 and necdin. ppIL-1α also regulates RNA splicing through binding RNA splicing proteins. Changes in gene expression and RNA splicing may be responsible for the intranuclear effects of pro- and ppIL-1α on cell migration, proliferation and apoptosis (broken lines). (B) Pro-IL-1β diffuses passively into cell nuclei, but no intranuclear actions of pro-IL-1β have been reported. IL-33 and IL-1F7b can also enter cell nuclei. Intranuclear IL-33 and IL-1F7b are reported to repress transcription, but the mechanisms involved remain poorly defined.
Intranuclear actions of IL-1α
The most widely reported intranuclear IL-1 family member is IL-1α. Experiments conducted prior to elucidation of the IL-1 classical signalling pathway indicated that radiolabelled IL-1α was internalized and localized to the nucleus of a number of IL-1-responsive cell lines (Mizel et al., 1987; Grenfell et al., 1989; Curtis et al., 1990; Weitzmann and Savage, 1992). Later, it was shown that IL-1 internalization and nuclear localization were not necessary for classical signalling through IL-1RI (Heguy et al., 1991). However, whether IL-1α endocytosis and nuclear localization have any other role (independent of the classical signalling pathway) has not been investigated further.
Intranuclear IL-1α has also been observed in IL-1α expressing cells (see Table 1). The nuclear entry of cytosolic proteins is regulated by the nuclear pore complex (NPC), a large multi-protein complex spanning the nuclear membrane, which allows free diffusion of proteins smaller than ∼50 kD (Paine, 1975; Peters, 1984; Fahrenkrog and Aebi, 2003). Larger nuclear proteins contain one of a range of nuclear localization sequence (NLS) motifs, including the canonical NLS, a short, positively charged sequence of amino acids, allowing their interaction with the nuclear import apparatus. The NLS is bound by cytosolic importins, which facilitate transport across the NPC [reviewed in Stewart (2007)]. This process is driven by gradients of the GDP- and GTP-bound forms of the small GTPase Ran across the nuclear membrane (Izaurralde et al., 1997).
Table 1.
The nuclear localization of IL-1 in IL-1-expressing cells
| Cell type/stimulus | IL-1 isoform | IL-1 nuclear localization | References |
|---|---|---|---|
| Overexpression | |||
| NIH-3T3 cells | pro-IL-1α | +++ | Wessendorf et al. (1993) |
| ppIL-1α | +++ | ||
| Endothelial cell line | pro-IL-1α | +++ | Maier et al. (1994) |
| Mature IL-1α | − | ||
| Perivascular mesangial cells | pro-IL-1α | − | Stevenson et al. (1997) |
| ppIL-1α | +++ | ||
| HEK-293 | ppIL-1α | +++ | Pollock et al. (2003) |
| NIH-3T3 | pro-IL-1α | +++ | Werman et al. (2004) |
| SaOS-2 | pro-IL-1α | +++ | Palmer et al. (2005) |
| NIH-3T3 | pro-IL-1α | + | Sudo et al. (2005) |
| ppIL-1α | +++ | ||
| HEK-293 | pro-IL-1α | +++ | Cheng et al. (2008) |
| COS-7 | pro-IL-1α | +++ | Luheshi et al. (2009) |
| pro-IL-1β | + | ||
| Endogenous expression | |||
| Lipid A-treated human mesangial cells | pro-IL-1α | ++ | Stevenson et al. (1992) |
| pro-IL-1β | +++ | ||
| Untreated brown adipose tissue cells | pro-IL-1α | +++ | Burysek and Houstek (1996) |
| Mature IL-1α | +++ | ||
| Systemic sclerosis fibroblasts | pro-IL-1α | +++ | Kawaguchi et al. (2004) |
| Untreated vascular smooth muscle cells | pro-IL-1α | +++ | Schultz et al. (2007) |
| Chlamydia trachomatis-infected HeLa cells | pro-IL-1α | +++ | Cheng et al. (2008) |
| LPS-treated microglia | pro-IL-1α | +++ | Luheshi et al. (2009) |
| pro-IL-1β | + | ||
Summary of studies reporting nuclear localization of IL-1α and β isoforms, either when overexpressed (transient or stable transfection) or when expressed endogenously. +++, ++, + and − indicate the level of nuclear IL-1 relative to cytosolic IL-1, with +++ indicating a predominantly intranuclear distribution and − an exclusively cytosolic distribution. IL-1 nuclear localization was assessed by cell fractionation, immunocytochemistry and imaging of fluorescent tagged IL-1 fusion proteins.
HEK-293, human embryonic kidney cell line; HeLa, human cervical epithelial cell line; IL-1, interleukin-1; NIH-3T3, murine fibroblast cell line; ppIL-1α, IL-1α pro-piece; SaOS-2, human osteosarcoma cell line.
Both pro-IL-1α and β are small enough (31 kD) to diffuse passively across the NPC. However, Wessendorf et al. (1993) made the surprising discovery that the pro-piece of IL-1α (ppIL-1α) contains a canonical NLS, able to target a β-galactosidase fusion protein to the nucleus. Since this discovery of the IL-1α NLS, nuclear localization of pro-IL-1α and ppIL-1α has been reported both in transfected cells and in cells endogenously expressing IL-1α (see Table 1). Indeed pro-IL-1α appears to be predominantly intranuclear in many of these cell types.
Intranuclear IL-1α is reported to regulate cell proliferation, migration and gene expression (summarized in Table 2). These IL-1α effects have been observed mainly in IL-1α-overexpressing cells and are not inhibited by blockade of extracellular IL-1α actions (using IL-1RA or neutralizing antibodies). The lack of effect of exogenous IL-1α has also been used to exclude involvement of extracellular IL-1α. In some cases, an intranuclear site of action for IL-1α has been more convincingly demonstrated by IL-1α NLS mutagenesis. However, confusion remains as to whether pro-IL-1α or ppIL-1α is the active isoform, the nature of IL-1α intranuclear actions, and the molecular mechanisms through which IL-1α exerts intranuclear effects.
Table 2.
Intranuclear actions of IL-1α
| Cell type | IL-1α isoform | Intranuclear effect |
Evidence that effect is intranuclear |
References | ||||
|---|---|---|---|---|---|---|---|---|
| IL-1RA | neutralizing Ig | Exog. IL-1α | Expr. mature IL-1α | NLS mutation | ||||
| Intranuclear IL-1α effects on proliferation/cell death | ||||||||
| Endothelial cell line | pro-IL-1α | Inhibits proliferation | ✓ | ✗ | ✗ | ✓ | ✗ | Maier et al. (1994) |
| SaOS-2 | pro-IL-1α | Inhibits proliferation | ✓ | ✗ | ✓ | ✗ | ✗ | Palmer et al. (2005) |
| HEK-293, cancer cells | ppIL-1α | Induces apoptosis | ✗ | ✗ | ✗ | ✗ | ✓ | Pollock et al. (2003) |
| SSc and normal fibroblasts | pro-IL-1α | Enhances proliferation | ✓ | ✓ | ✓ | ✗ | ✗ | Kawaguchi et al. (2004) |
| Perivascular mesangial cells | ppIL-1α pro-IL-1α | Causes malignant transformation | ✗ | ✗ | ✗ | ✗ | ✓ | Stevenson et al. (1997) |
| Vascular smooth muscle cells | pro-IL-1α ppIL-1α Mature IL-1α | No effect of intranuclear IL-1α on proliferation | N/A | N/A | N/A | N/A | N/A | Beasley and Cooper (1999) |
| Intranuclear IL-1α effects on gene expression | ||||||||
| Endothelial cell line | pro-IL-1α | Induces PAI-1 and collagenase expression | ✓ | ✗ | ✗ | ✓ | ✗ | Maier et al. (1994) |
| NIH-3T3, COS-7, endothelial cell line | pro-IL-1α ppIL-1α | Induces IL-6, IL-8 and endogenous IL-1α expression Enhances IFNγ or TNFα induction of MIP-2 | ✓ | ✗ | ✗ | ✓ | ✗ | Werman et al. (2004) |
| HeLa, macrophages, HEK-293 | pro-IL-1α | Induces IL-8 expression | ✓ | ✓ | ✗ | ✗ | ✗ | Cheng et al. (2008) |
| SSc and normal fibroblasts | pro-IL-1α | Induces IL-6 and procollagen expression | ✓ | ✓ | ✓ | ✗ | ✗ | Kawaguchi et al. (2004) |
| Intranuclear IL-1α effects on cell migration | ||||||||
| Endothelial cell line | pro-IL-1α | Inhibits migration | ✓ | ✗ | ✗ | ✗ | ✓ | McMahon et al. (1997) |
| Endothelial cell line | pro-IL-1α ppIL-1α | Promotes migration | ✗ | ✗ | ✓ | ✓ | ✗ | Merhi-Soussi et al. (2005) |
Evidence that IL-1α effects described involve intranuclear IL-1α. IL-1RA: cell incubation with IL-1RA does not block effect. Exog. IL-1α: application of exogenous IL-1α to cells does not reproduce effect. Neutralizing Ig: incubation of cells with IL-1α-neutralizing antibody does not block effect. Expr. mature IL-1α: expression of mature IL-1α (lacking the NLS) does not reproduce effect. NLS mutation: mutation of IL-1α NLS blocks the effect.
COS-7, african green monkey kidney fibroblast cell line; HEK-293, human embryonic kidney cell line; HeLa, human cervical epithelial cell line; IFNγ, interferon-γ; IL-1, interleukin-1; IL-1RA, IL-1 receptor antagonist; MIP-2, macrophage inhibitory protein-2; N/A, not applicable, as no intranuclear IL-1α effect observed; NIH-3T3, murine fibroblast cell line; NLS, nuclear localization sequence; PAI-1, plasminogen activator inhibitor-1; ppIL-1α, IL-1α pro-piece; SaOS-2, human osteosarcoma cell line; SSc, systemic sclerosis; TNFα, tumour necrosis factor α.
The confusion surrounding the nature of the intranuclear effects of IL-1α is well demonstrated by the various reported roles of intranuclear IL-1α isoforms on cell proliferation. In endothelial cell lines and a human osteosarcoma cell line (SaOS-2), overexpression of pro-IL-1α inhibits cell proliferation (Maier et al., 1994; Palmer et al., 2005). In addition, in HEK-293 (human embryonic kidney cell line) cells and cancer cell lines, overexpression of intranuclear ppIL-1α causes apoptosis (Pollock et al., 2003). However, in other cell types IL-1α appears to promote cell proliferation. Endogenous expression of pro-IL-1α in fibroblasts promotes fibroblast proliferation in systemic sclerosis (SSc) (Kawaguchi et al., 2004; Abraham and Varga, 2005). Furthermore, in perivascular mesangial cells, ppIL-1α overexpression causes malignant transformation, suggesting a role for ppIL-1α as an oncoprotein (Stevenson et al., 1997). In vascular smooth muscle cells, intranuclear pro-IL-1α and ppIL-1α have no effect on proliferation (Beasley and Cooper, 1999). Some of the variables that may explain the contradictory results observed include cell type, IL-1α isoform (pro- vs. ppIL-1α) and expression system (endogenous expression, stable or transient transfection). The role of endogenous intranuclear IL-1α in regulating cell proliferation in vivo remains unknown.
Intranuclear pro-IL-1α may also regulate cell migration (McMahon et al., 1997; Merhi-Soussi et al., 2005). However, these two papers report opposite effects of pro-IL-1α on cell migration rates, perhaps reflecting differences in the cell migration assay used (migration following culture wounding vs. migration across a transwell membrane).
The most consistently reported effects of intranuclear IL-1α are on gene expression. In HEK-293 cells, and in murine and human fibroblasts, pro-IL-1α overexpression enhances expression of the pro-inflammatory cytokine IL-6 and/or the chemokine IL-8 (Kawaguchi et al., 2004; Werman et al., 2004; Cheng et al., 2008). In endothelial cells, pro-IL-1α overexpression enhances interferon-γ- or tumour necrosis factor α-induced macrophage inhibitory protein 2 expression (Werman et al., 2004). Endothelial cell ppIL-1α overexpression also enhances endogenous IL-1α gene expression, indicating that an intracrine positive feedback loop may operate in these cells (Werman et al., 2004). Endogenous IL-1α has similar intranuclear effects on gene expression. In SSc fibroblasts, endogenous pro-IL-1α enhances IL-6 and collagen expression (Kawaguchi et al., 2004). In addition, in Chlamydia trachomatis-infected HeLa (human cervical epithelial cell line) cells, endogenous pro-IL-1α regulates IL-8 expression (Cheng et al., 2008).
The mechanism by which intranuclear IL-1α regulates gene expression remains unclear. Pro- and ppIL-1α bind histone acetyl transferases (Buryskova et al., 2004), multifunctional enzymes that can regulate transcription by modifying chromatin structure and acetylating transcription factors such as NFκB (Chan and La Thangue, 2001; Chen et al., 2001). This interaction may explain how pro- and ppIL-1α can transactivate gene expression of a Gal-4 reporter when fused to the Gal-4 DNA-binding domain, and can directly activate NFκB- and activator protein-1-dependent transcription (Buryskova et al., 2004; Werman et al., 2004). ppIL-1α also interacts with necdin (an intranuclear suppressor of growth and collagen production), HAX-1 (HS1-associated protein X-1, a ubiquitously expressed protein with poorly defined functions) and intranuclear IL-1RII (Yin et al., 2001; Hu et al., 2003; Kawaguchi et al., 2006). These interactions are implicated in the intranuclear effects of pro-IL-1α on cell proliferation and gene expression in SSc fibroblasts (Hu et al., 2003; Kawaguchi et al., 2006).
In contrast to these reports that intranuclear IL-1α regulates transcription, Pollock et al. (2003) argue that regulation of RNA splicing underlies the pro-apoptotic effects of ppIL-1α. ppIL-1α localizes to nuclear speckles [storage sites for RNA splicing proteins, reviewed in Lamond and Spector (2003)] and not transcription sites in HEK-293 cells. Pollock et al. (2003) demonstrate that ppIL-1α interacts with various RNA splicing proteins, and that a point mutation blocking this interaction inhibits the pro-apoptotic effects of ppIL-1α. ppIL-1α overexpression caused a shift in the alternative splicing of the apoptosis regulatory gene Bcl-X (B-cell lymphoma-X) from the anti-apoptotic Bcl-XL to the pro-apoptotic Bcl-XS isoform, suggesting a mechanism by which this ppIL-1α interaction may promote apoptosis.
To conclude, the most convincing evidence thus far is for a role in regulating gene expression, possibly through an interaction with histone acetyl transferases. Cell-specific differences in the expression and activation of other transcription and splicing factors would help explain the model-dependent impact of intranuclear pro-IL-1α on the expression of specific genes, and on cell proliferation and migration. However, investigations into the roles of intranuclear IL-1α have remained focused on cell lines overexpressing IL-1α. The question remains as to whether similar or entirely novel IL-1α nuclear effects occur in cells expressing endogenous IL-1αin vivo.
icIL-1RA isoforms: regulators of IL-1α intranuclear actions?
Unlike IL-1α and IL-1β, secreted IL-1RA (sIL-1RA, a 17 kD protein variably glycosylated to produce a 22–25 kD protein) has an N-terminal signal sequence that directs its trafficking and secretion through the ER-Golgi. However, three intracellular isoforms of IL-1RA (icIL-1RA 1–3) have been identified that lack this signal sequence and remain intracellular [reviewed in Arend (2002)]. icIL-1RA1 and icIL-1RA2 are generated from alternative transcriptional start sites on the IL-1RA gene. Either one (icIL-1RA1, 18 kD) or two (icIL-1RA2, 25 kD) 5′ exons are transcribed and spliced into an internal splice acceptor site within the first exon of sIL-1RA (Haskill et al., 1991; Muzio et al., 1995). icIL-1RA3 (16 kD) is a truncated variant of sIL-1RA, created either by alternative translational initiation or alternative splicing (Malyak et al., 1998).
The potential actions of these intracellular isoforms remain poorly defined and may be separated into three categories. First, they may be released (Corradi et al., 1995; Levine et al., 1997; Muzio et al., 1999; Wilson et al., 2004; Evans et al., 2006) and act as competitive antagonists for IL-1RI, in a similar manner to sIL-1RA. Recombinant icIL-1RA1 has a similar affinity to sIL-1RA for IL-1RI, whereas icIL-1RA3 has a four- to fivefold lower affinity and so is less likely to act in this way (Malyak et al., 1998). Second, icIL-1RA isoforms expressed in IL-1-responsive cells may antagonize IL-1α- and β-induced signalling through IL-1RI by an intracellular mechanism (Watson et al., 1995; Garat and Arend, 2003; Banda et al., 2005). Banda et al. (2005) report that icIL-1RA1 interacts with the COP9 signalsome, inhibits COP9 signalsome-associated kinases, and so inhibits cytokine gene expression induced by exogenously applied IL-1α in keratinocyte cell lines. Third, icIL-1RA could antagonize the intranuclear actions of IL-1α. For example, Merhi-Soussi et al. (2005) found that stably co-transfecting icIL-1RA1 with either pro-IL-1α or ppIL-1α blocked the effects of pro- or ppIL-1α on cell migration. Pro-IL-1α can regulate icIL-1RA1 gene expression via an intracellular mechanism, suggesting a negative feedback loop to preventing excessive pro-IL-1α intranuclear action (Higgins et al., 1999). The three intracellular isoforms of IL-1RA may thus have intracellular actions, and in particular may alter the intranuclear actions of IL-1α in IL-1α-expressing cells.
Intranuclear IL-1β, IL-33 and IL-1F7b
Pro-IL-1β, in contrast to pro-IL-1α, is commonly viewed as a cytosolic and extracellular cytokine, despite early reports showing the nuclear localization of pro-IL-1β in lipid A-stimulated mesangial cells (Stevenson et al., 1992), and the internalization and nuclear localization of radiolabelled IL-1β by fibroblasts (Qwarnstrom et al., 1988). We have recently found that endogenously expressed pro-IL-1β is intranuclear in cultured microglia (Luheshi et al., 2009). In contrast to pro-IL-1α, pro-IL-1β enters cell nuclei by passive diffusion (Luheshi et al., 2009). Whether pro-IL-1β is intranuclear in other cell types remains unknown.
Many pro-IL-1α intranuclear actions reported in the literature are dependent on ppIL-1α, which shares little sequence homology with ppIL-1β (Maier et al., 1994; McMahon et al., 1997; Stevenson et al., 1997; Pollock et al., 2003; Werman et al., 2004; Merhi-Soussi et al., 2005). In addition, ppIL-1β fails to reproduce the apoptosis-promoting effects of intranuclear ppIL-1α (Pollock et al., 2003). These reports support the hypothesis that pro-IL-1β does not have the same intranuclear actions as pro-IL-1α. Whether pro-IL-1β has no effect on intranuclear processes, or has a separate set of intranuclear actions, remains unknown.
Of the newly identified IL-1 family cytokines, IL-33 (also known as IL-1F11) and IL-1F7b both localize to the nucleus of expressing cells (Carriere et al., 2007; Sharma et al., 2008). IL-1F7b nuclear localization in an overexpressing macrophage cell line correlates with the inhibition of LPS-induced cytokine gene expression (Sharma et al., 2008). IL-33 interacts with chromatin and shows transcriptional repressor activity when overexpressed in cell lines (Carriere et al., 2007). Intranuclear IL-33 is found in resting endothelial cells in vivo, and expression is down-regulated on endothelial activation by pro-inflammatory or angiogenic stimuli (Kuchler et al., 2008; Moussion et al., 2008). This has led some to suggest that transcriptional repression by intranuclear IL-33 helps maintain endothelial cells in a resting state (Kuchler et al., 2008).
Future directions: implications of IL-1 family cytokine dual functionality for future development of anti-IL-1 therapeutics
Thus, in addition to their extracellular effects, several IL-1 family members appear to have intranuclear actions. However, the nature and in vivo consequences of IL-1 family intranuclear actions remain unclear.
The intranuclear mechanisms of action for IL-1α, IL-33, IL-1F7b (and potentially IL-1β) remain poorly described. The most widely studied intranuclear IL-1 family member, IL-1α, appears to regulate gene transcription and RNA splicing. However, further investigation is required into the protein–protein interactions involved in these intranuclear IL-1α actions. In addition, the mechanisms by which IL-1F7b and IL-33 repress transcription remain unknown, and whether IL-1β has any intranuclear effects similarly remains unclear.
Further investigation is also required into the role of IL-1 family member intranuclear actions in vivo. Intranuclear HMGB1 is expressed constitutively in almost all eukaryotic cells, and HMGB1 deficiency is lethal in mice due to the importance of HMGB1 intranuclear actions in development and homeostasis (Calogero et al., 1999).
In contrast to HMGB1, IL-1α and β expression tends to be low in healthy tissues and enhanced by infection or injury (Ulich et al., 1990; Clark et al., 1991). Furthermore, IL-1α- and β-deficient animals develop normally in the absence of an immune challenge (Horai et al., 1998). Thus, intranuclear IL-1α and β actions, like their extracellular actions, are likely to contribute to inflammation during host defence responses or disease pathogenesis. Based on the in vitro studies described above, intranuclear IL-1α may promote abnormal proliferation and excessive collagen production by SSc fibroblasts, contributing to the extensive fibrosis that characterizes the final pathology of this disease (Kawaguchi et al., 2004; Abraham and Varga, 2005; Kawaguchi et al., 2006). However, in vivo evidence to support a role of intranuclear IL-1α or β in any disease is lacking, and identification of conditions under which IL-1α and β localize to cell nuclei in vivo may help identify the potential influence of these intranuclear cytokines on disease progression.
The detection of intranuclear IL-33 in resting endothelial cells indicates that this IL-1 family member may, like HMGB1, play a role in homeostasis (Kuchler et al., 2008; Moussion et al., 2008). However, like IL-1α and β, expression of IL-33 can be induced by pro-inflammatory stimuli (Schmitz et al., 2005; Xu et al., 2008). Whether IL-33 is intranuclear under these conditions remains unknown.
Interleukin-1 family members are key pro-inflammatory cytokines whose extracellular actions are implicated in the pathogenesis of major peripheral and CNS diseases. Current anti-IL-1 therapy is limited to blockade of these extracellular actions. The more recently discovered intranuclear actions of IL-1 family members reviewed here suggest that these cytokines should be considered as dual function mediators. This dual functionality of cytokines represents a novel and potentially biologically important area of cytokine biology. Further investigation will clearly be required to determine the precise nature and importance of IL-1 family intranuclear actions in disease and to assess the therapeutic implications of these intranuclear actions. This could lead to the identification of novel therapeutics to treat inflammatory diseases.
Acknowledgments
We would like to thank Professor Philip Woodman (University of Manchester) for useful discussions. The authors were funded by the British Pharmacological Society (NML), the Medical Research Council (NJR) and the Wellcome Trust (DB, NML).
Glossary
Abbreviations:
- Bcl-X
B-cell lymphoma-X
- CNS
central nervous system
- ER
endoplasmic reticulum
- HAT
histone acetyl transferase
- HMGB1
high mobility group box 1
- icIL-1RA
intracellular IL-1RA
- IL-1
interleukin-1
- IL-1RA
IL-1 receptor antagonist
- IL-1RI
type I IL-1 receptor
- IL-1RAcP
IL-1 receptor accessory protein
- NFκB
nuclear factor κB
- NLS
nuclear localization sequence
- NPC
nuclear pore complex
- sIL-1RA
secreted IL-1RA
- SSc
systemic sclerosis
- ppIL-1
IL-1 pro-piece
Conflict of interest
The authors state no conflicts of interest.
References
- Abraham DJ, Varga J. Scleroderma: from cell and molecular mechanisms to disease models. Trends Immunol. 2005;26:587–595. doi: 10.1016/j.it.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Ahmed CM, Burkhart MA, Mujtaba MG, Subramaniam PS, Johnson HM. The role of IFNgamma nuclear localization sequence in intracellular function. J Cell Sci. 2003;116:3089–3098. doi: 10.1242/jcs.00528. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Banda NK, Guthridge C, Sheppard D, Cairns KS, Muggli M, Bech-Otschir D, et al. Intracellular IL-1 receptor antagonist type 1 inhibits IL-1-induced cytokine production in keratinocytes through binding to the third component of the COP9 signalosome. J Immunol. 2005;174:3608–3616. doi: 10.4049/jimmunol.174.6.3608. [DOI] [PubMed] [Google Scholar]
- Beasley D, Cooper AL. Constitutive expression of interleukin-1alpha precursor promotes human vascular smooth muscle cell proliferation. Am J Physiol. 1999;276:H901–H912. doi: 10.1152/ajpheart.1999.276.3.H901. [DOI] [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov. 2004;3:330–339. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]
- Brikos C, O'Neill LA. Signalling of toll-like receptors. Handb Exp Pharmacol. 2008;183:21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- Burysek L, Houstek J. Multifactorial induction of gene expression and nuclear localization of mouse interleukin 1 alpha. Cytokine. 1996;8:460–467. doi: 10.1006/cyto.1996.0062. [DOI] [PubMed] [Google Scholar]
- Buryskova M, Pospisek M, Grothey A, Simmet T, Burysek L. Intracellular interleukin-1alpha functionally interacts with histone acetyltransferase complexes. J Biol Chem. 2004;279:4017–4026. doi: 10.1074/jbc.M306342200. [DOI] [PubMed] [Google Scholar]
- Calogero S, Grassi F, Aguzzi A, Voigtlander T, Ferrier P, Ferrari S, et al. The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat Genet. 1999;22:276–280. doi: 10.1038/10338. [DOI] [PubMed] [Google Scholar]
- Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center DM, Cruikshank WW, Zhang Y. Nuclear pro-IL-16 regulation of T cell proliferation: p27(KIP1)-dependent G0/G1 arrest mediated by inhibition of Skp2 transcription. J Immunol. 2004;172:1654–1660. doi: 10.4049/jimmunol.172.3.1654. [DOI] [PubMed] [Google Scholar]
- Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Cheng W, Shivshankar P, Zhong Y, Chen D, Li Z, Zhong G. Intracellular interleukin-1alpha mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infect Immun. 2008;76:942–951. doi: 10.1128/IAI.01313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat Clin Pract Rheumatol. 2008;4:34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- Clark BD, Bedrosian I, Schindler R, Cominelli F, Cannon JG, Shaw AR, et al. Detection of interleukin 1 alpha and 1 beta in rabbit tissues during endotoxemia using sensitive radioimmunoassays. J Appl Physiol. 1991;71:2412–2418. doi: 10.1152/jappl.1991.71.6.2412. [DOI] [PubMed] [Google Scholar]
- Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Corradi A, Franzi AT, Rubartelli A. Synthesis and secretion of interleukin-1 alpha and interleukin-1 receptor antagonist during differentiation of cultured keratinocytes. Exp Cell Res. 1995;217:355–362. doi: 10.1006/excr.1995.1097. [DOI] [PubMed] [Google Scholar]
- Cruikshank WW, Kornfeld H, Center DM. Interleukin-16. J Leukoc Biol. 2000;67:757–766. doi: 10.1002/jlb.67.6.757. [DOI] [PubMed] [Google Scholar]
- Curtis BM, Widmer MB, deRoos P, Qwarnstrom EE. IL-1 and its receptor are translocated to the nucleus. J Immunol. 1990;144:1295–1303. [PubMed] [Google Scholar]
- Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006;98:1379–1389. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- Dower SK, Kronheim SR, Hopp TP, Cantrell M, Deeley M, Gillis S, et al. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature. 1986;324:266–268. doi: 10.1038/324266a0. [DOI] [PubMed] [Google Scholar]
- Dunn E, Sims JE, Nicklin MJ, O'Neill LA. Annotating genes with potential roles in the immune system: six new members of the IL-1 family. Trends Immunol. 2001;22:533–536. doi: 10.1016/s1471-4906(01)02034-8. [DOI] [PubMed] [Google Scholar]
- Elaraj DM, Weinreich DM, Varghese S, Puhlmann M, Hewitt SM, Carroll NM, et al. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res. 2006;12:1088–1096. doi: 10.1158/1078-0432.CCR-05-1603. [DOI] [PubMed] [Google Scholar]
- Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans I, Dower SK, Francis SE, Crossman DC, Wilson HL. Action of intracellular IL-1Ra (Type 1) is independent of the IL-1 intracellular signalling pathway. Cytokine. 2006;33:274–280. doi: 10.1016/j.cyto.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B, Aebi U. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat Rev Mol Cell Biol. 2003;4:757–766. doi: 10.1038/nrm1230. [DOI] [PubMed] [Google Scholar]
- Fenton MJ. Review: transcriptional and post-transcriptional regulation of interleukin 1 gene expression. Int J Immunopharmacol. 1992;14:401–411. doi: 10.1016/0192-0561(92)90170-p. [DOI] [PubMed] [Google Scholar]
- Garat C, Arend WP. Intracellular IL-1Ra type 1 inhibits IL-1-induced IL-6 and IL-8 production in Caco-2 intestinal epithelial cells through inhibition of p38 mitogen-activated protein kinase and NF-kappaB pathways. Cytokine. 2003;23:31–40. doi: 10.1016/s1043-4666(03)00182-0. [DOI] [PubMed] [Google Scholar]
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I, Gershon RK, Waksman BH. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972;136:128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenfell S, Smithers N, Miller K, Solari R. Receptor-mediated endocytosis and nuclear transport of human interleukin 1 alpha. Biochem J. 1989;264:813–822. doi: 10.1042/bj2640813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacham M, Argov S, White RM, Segal S, Apte RN. Distinct patterns of IL-1 alpha and IL-1 beta organ distribution–a possible basis for organ mechanisms of innate immunity. Adv Exp Med Biol. 2000;479:185–202. doi: 10.1007/0-306-46831-x_16. [DOI] [PubMed] [Google Scholar]
- Haskill S, Martin G, Van LL, Morris J, Peace A, Bigler CF, et al. cDNA cloning of an intracellular form of the human interleukin 1 receptor antagonist associated with epithelium. Proc Natl Acad Sci USA. 1991;88:3681–3685. doi: 10.1073/pnas.88.9.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heguy A, Baldari C, Bush K, Nagele R, Newton RC, Robb RJ, et al. Internalization and nuclear localization of interleukin 1 are not sufficient for function. Cell Growth Differ. 1991;2:311–315. [PubMed] [Google Scholar]
- Higgins GC, Wu Y, Postlethwaite AE. Intracellular IL-1 receptor antagonist is elevated in human dermal fibroblasts that overexpress intracellular precursor IL-1 alpha. J Immunol. 1999;163:3969–3975. [PubMed] [Google Scholar]
- Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wang S, Zhang Y, Feghali CA, Dingman JR, Wright TM. A nuclear target for interleukin-1alpha: interaction with the growth suppressor necdin modulates proliferation and collagen expression. Proc Natl Acad Sci USA. 2003;100:10008–10013. doi: 10.1073/pnas.1737765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E, Kutay U, von Kobbe C, Mattaj IW, Gorlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamari Y, Werman-Venkert R, Shaish A, Werman A, Harari A, Gonen A, et al. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195:31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, McCarthy SA, Watkins SC, Wright TM. Autocrine activation by interleukin 1alpha induces the fibrogenic phenotype of systemic sclerosis fibroblasts. J Rheumatol. 2004;31:1946–1954. [PubMed] [Google Scholar]
- Kawaguchi Y, Nishimagi E, Tochimoto A, Kawamoto M, Katsumata Y, Soejima M, et al. Intracellular IL-1alpha-binding proteins contribute to biological functions of endogenous IL-1alpha in systemic sclerosis fibroblasts. Proc Natl Acad Sci USA. 2006;103:14501–14506. doi: 10.1073/pnas.0603545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 2004;43(Suppl. 3):iii2–iii9. doi: 10.1093/rheumatology/keh201. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proc Natl Acad Sci USA. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krelin Y, Voronov E, Dotan S, Elkabets M, Reich E, Fogel M, et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- Kuchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, et al. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol. 2008;173:1229–1242. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol. 2003;4:605–612. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- Ledford H. Fever pitch. Nature. 2007;450:600–601. doi: 10.1038/450600a. [DOI] [PubMed] [Google Scholar]
- Levine SJ, Wu T, Shelhamer JH. Extracellular release of the type I intracellular IL-1 receptor antagonist from human airway epithelial cells: differential effects of IL-4, IL-13, IFN-gamma, and corticosteroids. J Immunol. 1997;158:5949–5957. [PubMed] [Google Scholar]
- Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21:3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- Lonnemann G, Endres S, Van der Meer JW, Cannon JG, Koch KM, Dinarello CA. Differences in the synthesis and kinetics of release of interleukin 1 alpha, interleukin 1 beta and tumor necrosis factor from human mononuclear cells. Eur J Immunol. 1989;19:1531–1536. doi: 10.1002/eji.1830190903. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Luheshi NM, Rothwell NJ, Brough D. The dynamics and mechanisms of interleukin-1alpha and beta nuclear import. Traffic. 2009;10:16–25. doi: 10.1111/j.1600-0854.2008.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- McMahan CJ, Slack JL, Mosley B, Cosman D, Lupton SD, Brunton LL, et al. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon GA, Garfinkel S, Prudovsky I, Hu X, Maciag T. Intracellular precursor interleukin (IL)-1alpha, but not mature IL-1alpha, is able to regulate human endothelial cell migration in vitro. J Biol Chem. 1997;272:28202–28205. doi: 10.1074/jbc.272.45.28202. [DOI] [PubMed] [Google Scholar]
- Maier JA, Statuto M, Ragnotti G. Endogenous interleukin 1 alpha must be transported to the nucleus to exert its activity in human endothelial cells. Mol Cell Biol. 1994;14:1845–1851. doi: 10.1128/mcb.14.3.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyak M, Guthridge JM, Hance KR, Dower SK, Freed JH, Arend WP. Characterization of a low molecular weight isoform of IL-1 receptor antagonist. J Immunol. 1998;161:1997–2003. [PubMed] [Google Scholar]
- Mandinova A, Soldi R, Graziani I, Bagala C, Bellum S, Landriscina M, et al. S100A13 mediates the copper-dependent stress-induced release of IL-1alpha from both human U937 and murine NIH 3T3 cells. J Cell Sci. 2003;116:2687–2696. doi: 10.1242/jcs.00471. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Merhi-Soussi F, Berti M, Wehrle-Haller B, Gabay C. Intracellular interleukin-1 receptor antagonist type 1 antagonizes the stimulatory effect of interleukin-1alpha precursor on cell motility. Cytokine. 2005;32:163–170. doi: 10.1016/j.cyto.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- Miossec P. Anti-interleukin 1alpha autoantibodies. Ann Rheum Dis. 2002;61:577–579. doi: 10.1136/ard.61.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel SB, Kilian PL, Lewis JC, Paganelli KA, Chizzonite RA. The interleukin 1 receptor. Dynamics of interleukin 1 binding and internalization in T cells and fibroblasts. J Immunol. 1987;138:2906–2912. [PubMed] [Google Scholar]
- Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, et al. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987;262:2941–2944. [PubMed] [Google Scholar]
- Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N, Sakata Y, Watanabe T. Central action sites of interleukin-1 beta for inducing fever in rabbits. J Physiol. 1990;428:299–312. doi: 10.1113/jphysiol.1990.sp018213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Muzio M, Polentarutti N, Sironi M, Poli G, De GL, Introna M, et al. Cloning and characterization of a new isoform of the interleukin 1 receptor antagonist. J Exp Med. 1995;182:623–628. doi: 10.1084/jem.182.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Polentarutti N, Facchetti F, Peri G, Doni A, Sironi M, et al. Characterization of type II intracellular IL-1 receptor antagonist (IL-1ra3): a depot IL-1ra. Eur J Immunol. 1999;29:781–788. doi: 10.1002/(SICI)1521-4141(199903)29:03<781::AID-IMMU781>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Nakae S, Asano M, Horai R, Iwakura Y. Interleukin-1 beta, but not interleukin-1 alpha, is required for T-cell-dependent antibody production. Immunology. 2001a;104:402–409. doi: 10.1046/j.1365-2567.2001.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Naruse-Nakajima C, Sudo K, Horai R, Asano M, Iwakura Y. IL-1 alpha, but not IL-1 beta, is required for contact-allergen-specific T cell activation during the sensitization phase in contact hypersensitivity. Int Immunol. 2001b;13:1471–1478. doi: 10.1093/intimm/13.12.1471. [DOI] [PubMed] [Google Scholar]
- Nickel W. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem. 2003;270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- Paine PL. Nucleocytoplasmic movement of fluorescent tracers microinjected into living salivary gland cells. J Cell Biol. 1975;66:652–657. doi: 10.1083/jcb.66.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G, Trolliet S, Talabot-Ayer D, Mezin F, Magne D, Gabay C. Pre-interleukin-1alpha expression reduces cell growth and increases interleukin-6 production in SaOS-2 osteosarcoma cells: differential inhibitory effect of interleukin-1 receptor antagonist (icIL-1Ra1) Cytokine. 2005;31:153–160. doi: 10.1016/j.cyto.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Pearson VL, Rothwell NJ, Toulmond S. Excitotoxic brain damage in the rat induces interleukin-1beta protein in microglia and astrocytes: correlation with the progression of cell death. Glia. 1999;25:311–323. [PubMed] [Google Scholar]
- Perregaux DG, Gabel CA. Post-translational processing of murine IL-1: evidence that ATP-induced release of IL-1 alpha and IL-1 beta occurs via a similar mechanism. J Immunol. 1998;160:2469–2477. [PubMed] [Google Scholar]
- Peters R. Nucleo-cytoplasmic flux and intracellular mobility in single hepatocytes measured by fluorescence microphotolysis. EMBO J. 1984;3:1831–1836. doi: 10.1002/j.1460-2075.1984.tb02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettipher ER, Higgs GA, Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci USA. 1986;83:8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinteaux E, Rothwell NJ, Boutin H. Neuroprotective actions of endogenous interleukin-1 receptor antagonist (IL-1ra) are mediated by glia. Glia. 2006;53:551–556. doi: 10.1002/glia.20308. [DOI] [PubMed] [Google Scholar]
- Pollock AS, Turck J, Lovett DH. The prodomain of interleukin 1alpha interacts with elements of the RNA processing apparatus and induces apoptosis in malignant cells. FASEB J. 2003;17:203–213. doi: 10.1096/fj.02-0602com. [DOI] [PubMed] [Google Scholar]
- Prudovsky I, Mandinova A, Soldi R, Bagala C, Graziani I, Landriscina M, et al. The non-classical export routes: FGF1 and IL-1alpha point the way. J Cell Sci. 2003;116:4871–4881. doi: 10.1242/jcs.00872. [DOI] [PubMed] [Google Scholar]
- Qwarnstrom EE, Page RC, Gillis S, Dower SK. Binding, internalization, and intracellular localization of interleukin-1 beta in human diploid fibroblasts. J Biol Chem. 1988;263:8261–8269. [PubMed] [Google Scholar]
- Rauschmayr T, Groves RW, Kupper TS. Keratinocyte expression of the type 2 interleukin 1 receptor mediates local and specific inhibition of interleukin 1-mediated inflammation. Proc Natl Acad Sci USA. 1997;94:5814–5819. doi: 10.1073/pnas.94.11.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re RN. The intracrine hypothesis and intracellular peptide hormone action. Bioessays. 2003;25:401–409. doi: 10.1002/bies.10248. [DOI] [PubMed] [Google Scholar]
- Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Ross J, Brough D, Gibson RM, Loddick SA, Rothwell NJ. A selective, non-peptide caspase-1 inhibitor, VRT-018858, markedly reduces brain damage induced by transient ischemia in the rat. Neuropharmacology. 2007;53:638–642. doi: 10.1016/j.neuropharm.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc Natl Acad Sci USA. 2006;103:2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurat JH, Schifferli J, Steiger G, Dayer JM, Didierjean L. Anti-interleukin-1 alpha autoantibodies in humans: characterization, isotype distribution, and receptor-binding inhibition–higher frequency in Schnitzler's syndrome (urticaria and macroglobulinemia) J Allergy Clin Immunol. 1991;88:244–256. doi: 10.1016/0091-6749(91)90335-l. [DOI] [PubMed] [Google Scholar]
- Schielke GP, Yang GY, Shivers BD, Betz AL. Reduced ischemic brain injury in interleukin-1 beta converting enzyme-deficient mice. J Cereb Blood Flow Metab. 1998;18:180–185. doi: 10.1097/00004647-199802000-00009. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Schultz K, Murthy V, Tatro JB, Beasley D. Endogenous Interleukin-1{alpha} promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2927–H2934. doi: 10.1152/ajpheart.00700.2006. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kulk N, Nold MF, Graf R, Kim SH, Reinhardt D, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180:5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- Sims JE. IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol. 2002;14:117–122. doi: 10.1016/s0952-7915(01)00306-5. [DOI] [PubMed] [Google Scholar]
- Singh NJ, Schwartz RH. Primer: mechanisms of immunologic tolerance. Nat Clin Pract Rheumatol. 2006;2:44–52. doi: 10.1038/ncprheum0049. [DOI] [PubMed] [Google Scholar]
- Stevenson FT, Torrano F, Locksley RM, Lovett DH. Interleukin 1: the patterns of translation and intracellular distribution support alternative secretory mechanisms. J Cell Physiol. 1992;152:223–231. doi: 10.1002/jcp.1041520202. [DOI] [PubMed] [Google Scholar]
- Stevenson FT, Turck J, Locksley RM, Lovett DH. The N-terminal propiece of interleukin 1 alpha is a transforming nuclear oncoprotein. Proc Natl Acad Sci USA. 1997;94:508–513. doi: 10.1073/pnas.94.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114. [DOI] [PubMed] [Google Scholar]
- Sudo M, Kobayashi Y, Watanabe N. Presence of a cytoplasmic retention sequence within the human interleukin-1alpha precursor. Zoolog Sci. 2005;22:891–896. doi: 10.2108/zsj.22.891. [DOI] [PubMed] [Google Scholar]
- Thomas JO, Travers AA. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem Sci. 2001;26:167–174. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Ulich TR, del Castillo J, Keys M, Granger GA, Ni RX. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha-induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987;139:3406–3415. [PubMed] [Google Scholar]
- Ulich TR, Guo KZ, Irwin B, Remick DG, Davatelis GN. Endotoxin-induced cytokine gene expression in vivo. II. Regulation of tumor necrosis factor and interleukin-1 alpha/beta expression and suppression. Am J Pathol. 1990;137:1173–1185. [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Conti M, De LA, Ravizza T, Moneta D, Marchesi F, et al. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–5065. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers GP, Anderson LJ, Caffes P, Brandhuber BJ. Crystal structure of the type-I interleukin-1 receptor complexed with interleukin-1beta. Nature. 1997;386:190–194. doi: 10.1038/386190a0. [DOI] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C. ‘Inflammatory’ cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk AG, Netea MG, van Krieken JH, Iwakura Y, Van der Meer JW, Kullberg BJ. Endogenous interleukin (IL)-1 alpha and IL-1 beta are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Kobayashi Y. Selective release of a processed form of interleukin 1 alpha. Cytokine. 1994;6:597–601. doi: 10.1016/1043-4666(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Watson JM, Lofquist AK, Rinehart CA, Olsen JC, Makarov SS, Kaufman DG, et al. The intracellular IL-1 receptor antagonist alters IL-1-inducible gene expression without blocking exogenous signaling by IL-1 beta. J Immunol. 1995;155:4467–4475. [PubMed] [Google Scholar]
- Weitzmann MN, Savage N. Nuclear internalisation and DNA binding activities of interleukin-1, interleukin-1 receptor and interleukin-1/receptor complexes. Biochem Biophys Res Commun. 1992;187:1166–1171. doi: 10.1016/0006-291x(92)91319-l. [DOI] [PubMed] [Google Scholar]
- Werman A, Werman-Venkert R, White R, Lee JK, Werman B, Krelin Y, et al. The precursor form of IL-1alpha is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesche H, Korherr C, Kracht M, Falk W, Resch K, Martin MU. The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinases (SAP kinases) J Biol Chem. 1997;272:7727–7731. doi: 10.1074/jbc.272.12.7727. [DOI] [PubMed] [Google Scholar]
- Wessendorf JH, Garfinkel S, Zhan X, Brown S, Maciag T. Identification of a nuclear localization sequence within the structure of the human interleukin-1 alpha precursor. J Biol Chem. 1993;268:22100–22104. [PubMed] [Google Scholar]
- Will A, Hemmann U, Horn F, Rollinghoff M, Gessner A. Intracellular murine IFN-gamma mediates virus resistance, expression of oligoadenylate synthetase, and activation of STAT transcription factors. J Immunol. 1996;157:4576–4583. [PubMed] [Google Scholar]
- Wilson HL, Francis SE, Dower SK, Crossman DC. Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation. J Immunol. 2004;173:1202–1208. doi: 10.4049/jimmunol.173.2.1202. [DOI] [PubMed] [Google Scholar]
- Wilson KC, Cattel DJ, Wan Z, Rahangdale S, Ren F, Kornfeld H, et al. Regulation of nuclear Prointerleukin-16 and p27(Kip1) in primary human T lymphocytes. Cell Immunol. 2005;237:17–27. doi: 10.1016/j.cellimm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–680. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- Yin H, Morioka H, Towle CA, Vidal M, Watanabe T, Weissbach L. Evidence that HAX-1 is an interleukin-1 alpha N-terminal binding protein. Cytokine. 2001;15:122–137. doi: 10.1006/cyto.2001.0891. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kornfeld H, Cruikshank WW, Kim S, Reardon CC, Center DM. Nuclear translocation of the N-terminal prodomain of interleukin-16. J Biol Chem. 2001;276:1299–1303. doi: 10.1074/jbc.M008513200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tuzova M, Xiao ZX, Cruikshank WW, Center DM. Pro-IL-16 recruits histone deacetylase 3 to the Skp2 core promoter through interaction with transcription factor GABP. J Immunol. 2008;180:402–408. doi: 10.4049/jimmunol.180.1.402. [DOI] [PubMed] [Google Scholar]


