Abstract
Glucagon-like peptide-1 (GLP-1) is an incretin hormone secreted by the small intestine in response to nutrient ingestion. It has wide-ranging effects on glucose metabolism, including stimulation of insulin release, inhibition of glucagon secretion, reduction of gastric emptying and augmentation of satiety. Importantly, the insulinotropic actions of GLP-1 are uniquely dependent on ambient glucose concentrations, and it is this particular characteristic which has led to its recent emergence as a treatment for type 2 diabetes. Although the major physiological function of GLP-1 appears to be in relation to glycaemic control, there is growing evidence to suggest that it may also play an important role in the cardiovascular system. GLP-1 receptors (GLP-1Rs) are expressed in the heart and vasculature of both rodents and humans, and recent studies have demonstrated that GLP-1R agonists have wide-ranging cardiovascular actions, such as modulation of heart rate, blood pressure, vascular tone and myocardial contractility. Importantly, it appears that these agents may also have beneficial effects in the setting of cardiovascular disease (CVD). For example, GLP-1 has been found to exert cardioprotective actions in experimental models of dilated cardiomyopathy, hypertensive heart failure and myocardial infarction (MI). Preliminary clinical studies also indicate that GLP-1 infusion may improve cardiac contractile function in chronic heart failure patients with and without diabetes, and in MI patients after successful angioplasty. This review will discuss the current understanding of GLP-1 biology, examine its emerging cardiovascular actions in both health and disease and explore the potential use of GLP-1 as a novel treatment for CVD.
Keywords: glucagon-like peptide-1, glucagon-like peptide-1 receptor, incretin, diabetes, cardiac ischaemia, heart failure, vasodilatation, sympathetic activation
Introduction
The global prevalence of diabetes mellitus is increasing at an exponential rate, and it is estimated that the number of affected individuals will rise to 300 million within the next 20 years (King et al., 1998). Patients with diabetes are characterized by an increased risk of developing both microvascular complications, such as retinopathy, nephropathy and neuropathy, and atherosclerotic macrovascular disease, which may lead to the development of peripheral vascular disease, stroke and ischaemic/hypertensive heart failure (Clements and Bell, 1985). A significant proportion of diabetic patients may also develop diabetic cardiomyopathy in the absence of such aetiological factors, which is associated with a high incidence of congestive heart failure (Fang et al., 2004; Asbun and Villarreal, 2006). Cardiovascular disease (CVD) is the leading cause of mortality in the UK, accounting for premature death in up to 40% of the population, and patients with diabetes are characterized by a significantly elevated risk compared to normoglycaemic individuals (Garcia et al., 1974; Scandinavian Simvastatin Survival Study Group, 1994). Indeed, the Framingham Heart Study found that heart failure was twice as common in diabetic men, and five times as common in diabetic women aged 45–74 compared to the normal population, and that this association was even stronger in younger patients (Kannel and McGee, 1979).
Therapeutic agents such as statins, angiotensin-converting enzyme inhibitors and β-blockers have been demonstrated to cause a significant reduction in the incidence of CVD, and to exert morbidity and mortality benefits (The SOLVD Investigators, 1992; Scandinavian Simvastatin Survival Study Group, 1994; Colucci et al., 2007). However, there remains a substantial incidence of CVD in optimally treated patients, especially those with underlying pathologies, such as diabetes. Thus, there is an ongoing search for more effective therapeutic alternatives. One such candidate may be glucagon-like peptide-1 (GLP-1), a peptide hormone which forms the basis of a recently approved therapy for controlling hyperglycaemia in type 2 diabetes (Kendall et al., 2005). Interestingly, recent evidence suggests that in addition to its established glucose-lowering actions, GLP-1 may also exert several beneficial actions on the cardiovascular system. This review will discuss the emerging cardiovascular actions of GLP-1 and its potential as a treatment for CVD in both diabetic and non-diabetic patients.
The biology of GLP-1
Synthesis and secretion of GLP-1 in humans
The scientific study of GLP-1(7–36) amide originated almost 30 years ago with the cloning of a ‘glucagon-related peptide’ from the pre-proglucagon gene in anglerfish cDNA (Lund et al., 1981). Subsequent sequence analysis performed in numerous species indicated that there is complete conservation of the GLP-1 amino acid sequence in mammals (Bell et al., 1983; Heinrich et al., 1984). GLP-1(7–36) amide is a 30 amino acid polypeptide transcribed on the proglucagon gene, which is located on the long arm of chromosome 2 and is expressed in both pancreatic α-cells and intestinal L-cells (White and Saunders, 1986). However, the expression product is differentially processed between these cell types due to tissue-specific expression of pro-hormone convertase enzymes (Dhanvantari et al., 1996). The majority of active GLP-1 occurs in the form of two equipotent isotypes: a minor form, known as GLP-1(7–37) (transcribed from the proglucagon 78–108), which accounts for approximately 20% of active GLP-1, and a major form, GLP-1(7–36) amide (transcribed from proglucagon 78–107; herein referred to as GLP-1), which accounts for most of the other 80% of active GLP-1 (Orskov et al., 1994). Production of these isoforms results from post-translational processing of the proglucagon gene product that occurs in the L-cells (Figure 1). The greatest density of L-cells occurs in the distal ileum and colon, where they act as the major endogenous source of GLP-1 (Kervran et al., 1987; Eissele et al., 1992). L-cells are open-type epithelial endocrine cells which are juxtaposed with the intestinal lumen, where they appear to respond to local nutrient signals (Gribble, 2008). They are also in close contact with nervous and vascular tissues of the intestine, and a number of neurotransmitters and endocrine hormones are believed to play a role in the regulation of GLP-1 secretion.
Figure 1.
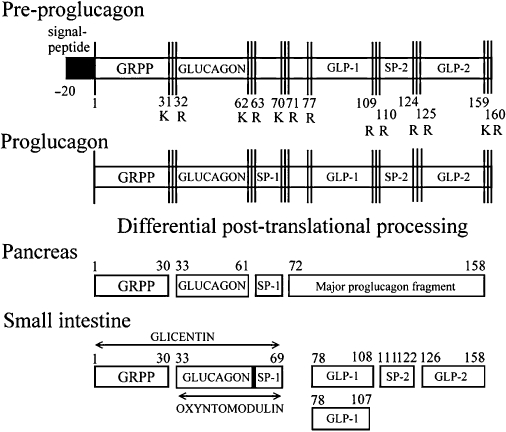
Structure and post-translational processing of the pre-proglucagon gene product. Pre-proglucagon is transcribed and processed to form glicentin, oxyntomodulin, GLP-1(7–36) amide, GLP-1(7–37) and GLP-2 in intestinal L-cells. GRPP, glicentin-related polypeptide; SP1, spacer peptide 1; SP2, spacer peptide 2.
The primary stimulus for GLP-1 secretion is enteral nutrient ingestion. Indeed, consumption of an equicaloric test meal of either carbohydrate, fat or protein has been shown to stimulate GLP-1 secretion in human subjects (Elliott et al., 1993). Furthermore, the same authors reported that an oral glucose load (75 g) leads to increased plasma GLP-1 concentrations (Elliott et al., 1993). Secretion of GLP-1 largely depends upon the specific nutrient composition of the meal, and it has been reported that a particular caloric threshold or nutrient delivery rate must be reached in order to trigger significant secretion (Schirra et al., 1996). For an extensive overview of nutrient, neural and endocrine factors involved in GLP-1 secretion, the reader is directed towards an excellent recent review paper (Dubé and Brubaker, 2004).
Clearance and metabolism of GLP-1
GLP-1 is thought to be eliminated from the circulation by up to three separate mechanisms: renal clearance, hepatic clearance and degradation in the circulation. Renal clearance of GLP-1 is well documented in studies which have measured GLP-1 levels in plasma or renal filtrates from uremic patients (Orskov et al., 1992), anaesthetized pigs (Deacon et al., 1996) and rats undergoing nephrectomy or uretal ligation (Ruiz-Grande et al., 1993). Significant hepatic extraction of GLP-1 has also been observed in pigs following exogenous systemic infusion (Deacon et al., 1996).
Following its release into the circulation, GLP-1 undergoes rapid enzymatic degradation by its primary endogenous inactivator, dipeptidyl peptidase-4 (DPP-4) (Deacon, 2004). This physiologically ubiquitous enzyme rapidly degrades GLP-1(7–36) amide to GLP-1(9–36) amide by the removal of an N-terminal dipeptide. The resultant metabolite has a 1000-fold lower affinity for the GLP-1 receptor (GLP-1R) and is characterized by a complete lack of insulinotropic activity (Knudsen and Pridal, 1996; Deacon et al., 2002; Green et al., 2004c). Thus, the glucose-lowering activity of GLP-1 is relatively short lived, with a circulating half-life of ∼2 min. This rapid inactivation of GLP-1 was clearly demonstrated by a study in human subjects which reported that 30 min after subcutaneous GLP-1 injection, inactive GLP-1(9–36) amide accounted for 78% of total immunoreactive GLP-1 (Deacon et al., 1995). Interestingly, it appears that GLP-1 may undergo further enzymatic break-down subsequent to the action of DPP-4. For example, multiple degradation fragments have been observed following incubation of GLP-1 with both purified human neutral endopeptidase (neprilysin) (Hupe-Sodmann et al., 1995) and a neprilysin activity-containing pancreatic β-cell line (Hupe-Sodmann et al., 1997), suggesting that this enzyme may also be involved in the metabolism of GLP-1. However, a recent study indicated that up to 50% of native GLP-1 entering the circulation may be directly degraded by neprilysin (Plamboeck et al., 2005), indicating that a significant proportion of the GLP-1 breakdown may occur independently of DPP-4, although this remains controversial.
The GLP-1R
First sequenced in rat pancreatic tissue, the GLP-1R is a G-protein coupled receptor that consists of 463 amino acids (Thorens, 1992). The GLP-1R is ubiquitously expressed and has been detected in tissues such as pancreatic islets (Orskov and Poulsen, 1991), heart (Wei and Mojsov, 1995), aorta (Green et al., 2008), lung (Kanse et al., 1988), gastric glands (Uttenthal and Blazquez, 1990) and parts of the central and peripheral nervous system (Shimizu et al., 1987; Kanse et al., 1988; Wei and Mojsov, 1995).
In 1993, Thorens and Waeber subsequently isolated and cloned the human GLP-1R from a pancreatic cDNA library. Further studies revealed that the human GLP-1R has 90% sequence homology to the rat GLP-1R, and that its gene is localized to chromosome 6p21 (Stoffel et al., 1993). Interestingly, this membrane-spanning receptor has been found to share sequence similarities with several other receptors, including those for parathyroid hormone, calcitonin, growth hormone-releasing hormone, pituitary adenylate cyclase-activating polypeptide (PACAP), glucose-dependent insulinotropic polypeptide (GIP), secretin, vasoactive intestinal peptide (VIP) and glucagon (Kieffer and Habener, 1999). The GLP-1R, (illustrated in Figure 2) comprises eight hydrophobic domains, seven of which span the membrane, with a further extracellular N-terminal domain. GLP-1 and similar agonists have been demonstrated to bind to the receptor in an orthosteric manner. However, recent studies employing both a small molecule GLP-1R agonist and molecular modelling of the three-dimensional structure of the receptor, have also identified a non-allosteric activation site (Knudsen et al., 2007; Lin and Wang, 2009).
Figure 2.
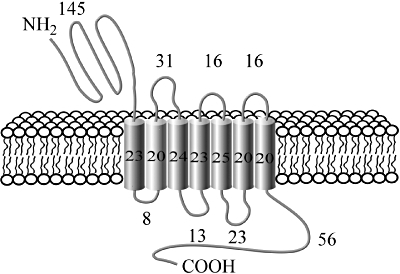
Illustration of the structure of the glucagon-like peptide-1 receptor (GLP-1R) including the number of amino acid residues in each segment. The GLP-1R is a seven-domain, membrane-spanning G-protein coupled receptor comprised of 463 amino acids, which is expressed in tissues such as heart, aorta, pancreas, kidney, lung and the central and peripheral nervous systems.
It is well established that activation of the GLP-1R stimulates the production of cyclic AMP via the action of adenylate cyclase. Cyclic AMP signalling may then be further amplified and diversified via the activation of several downstream factors, such as protein kinase A and cyclic AMP-regulated guanine nucleotide exchange factors (Holz, 2004). Agonism of the GLP-1R is also known to be associated with phosphorylation of the cyclic AMP response binding element, elevation of intracellular calcium, inhibition of voltage-dependent potassium channels, and activation of a number of kinases, including extracellular signal-regulated kinase (ERK)1/2, protein kinase C, phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinase (MAPK) and protein kinase B (Buteau et al., 2001; Arnette et al., 2003; Kang et al., 2007).
The GLP-1R has an extremely high affinity for GLP-1 which it selectively binds at nanomolar concentrations, while other peptides of the glucagon superfamily, such as glucagon, PACAP, GIP and VIP, bind either poorly or not at all. Interestingly, exendin-4(1–39), a peptide which was originally isolated from the venom of the Arizona desert lizard, Heloderma suspectum, which shares 50% structural homology with GLP-1, has also been found to be a potent GLP-1R agonist, binding with comparable affinity to GLP-1 (Goke et al., 1993; Thorens et al., 1993). Exendin-4(1–39) mimics almost every documented physiological action of GLP-1; however, it is becoming apparent that it does exert some effects which are not strictly related to GLP-1. For example, infusion of GLP-1, but not exendin-4, into the rat portal vein has been found to activate vagal afferent nerves (Nishizawa et al., 2000). Conversely, studies in 3T3-L1 adipocytes reported exendin-4, but not GLP-1, to improve insulin sensitivity via a PI3K-dependent mechanism (Idris et al., 2002). It is unclear why these differences between the actions of GLP-1 and exendin-4 exist, although it has been speculated that they may occur via an unidentified, functionally distinct receptor (Nishizawa et al., 2000; Burcelin et al., 2001; Idris et al., 2002). Importantly, exendin-4 possesses the unique characteristic of being resistant to enzymatic degradation by the endogenous inactivator of GLP-1, DPP-4, which has led to its recent exploitation as a treatment for the hyperglycaemia associated with type 2 diabetes (Kendall et al., 2005).
Physiological actions of GLP-1
A wide range of biological actions for GLP-1 (summarized in Figure 3) have been reported in several experimental systems from in vitro cell lines to human subjects (Green et al., 2004b; Drucker, 2006). Many of these effects relate to the efficient storage, utilization and disposal of glucose and other nutrients. GLP-1 is one of two physiological hormones that meet the criteria of an ‘incretin’, that is, released from the intestine in response to nutrients with the ability to stimulate insulin secretion at physiologically relevant concentrations (Creutzfeldt, 1979). GLP-1 exerts a potent insulin-releasing effect on pancreatic β-cells (Hargrove et al., 1995), while inhibiting the release of glucagon from α-cells (Creutzfeldt et al., 1996). The most remarkable feature of the insulin-releasing action of GLP-1 is that it occurs in a glucose-dependent manner (Hargrove et al., 1995), and it is this particular characteristic which prompted widespread interest in its potential as an anti-diabetic treatment (Green and Flatt, 2007). Indeed, it is now well established that GLP-1-induced insulin secretion leads to significant postprandial glucose lowering in both diabetic animal models (Doyle et al., 2001; Xiao et al., 2001; Green et al., 2003; 2004a;) and patients with type 2 diabetes (Gutniak et al., 1994; Juntti-Berggren et al., 1996; Nauck et al., 1996).
Figure 3.
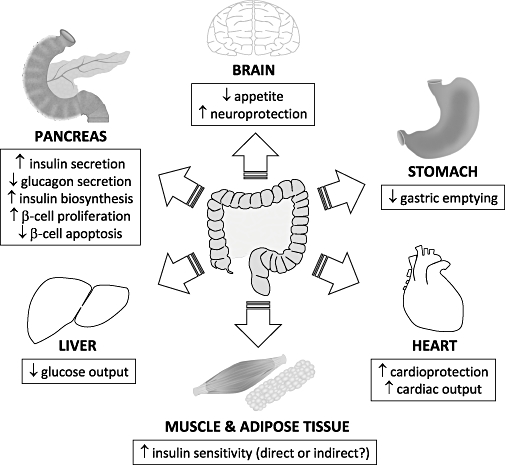
Physiological effects of glucagon-like peptide-1 receptor agonists. Glucagon-like peptide-1 administration exerts diverse biological actions on a number of human target organs, such as the pancreas, heart, brain, liver, stomach, muscle and adipose tissue.
Therapeutic applications of GLP-1
Drug therapies targeting the GLP-1R offer several new possibilities for the treatment of type 2 diabetes. There is persuasive clinical evidence that prolonged therapy with GLP-1 analogues/mimetics exerts beneficial actions on cardiovascular risk factors such as hyperglycaemia, dyslipidaemia and body weight in diabetic subjects (Juntti-Berggren et al., 1996; Madsbad et al., 2004; DeFronzo et al., 2005; Suzuki et al., 2007; Zinman et al., 2007). These effects are likely to occur subsequent to the multiple glucose-lowering actions of GLP-1, such as stimulation of satiety and energy expenditure, reduction of gastric emptying and development of conditioned taste aversion (Turton et al., 1996; Willms et al., 1996; Thiele et al., 1997; Hwa et al., 1998). Other reported anti-diabetic actions of GLP-1 include the improvement of insulin sensitivity (related to both direct and indirect effects on muscle and adipose tissues, although these have yet to be demonstrated in humans) (Sandhu et al., 1999; Gedulin et al., 2005; Green et al., 2006), reduction in hepatic glucose output (Larsson et al., 1997) and possible protective and regenerative actions on the pancreatic β-cell (Buteau et al., 2003; Drucker, 2003; Liu et al., 2004; Gedulin et al., 2005).
The major advantage of GLP-1 over conventional anti-diabetic therapies, such as sulphonylureas, is that its insulinotropic actions are dependent on ambient glucose concentrations, thus mitigating the risks of hypoglycaemia (Hargrove et al., 1995; Green and Flatt, 2007). Consequently, a range of GLP-1 analogue and mimetic compounds are currently in development and are gradually making their way towards the clinic (Table 1). GLP-1 analogues are those peptides which closely resemble the GLP-1 amino acid sequence (e.g. liraglutide) (Flatt et al., 2009), whereas mimetics are compounds with alternative structures that seek to mimic the actions of GLP-1 (e.g. exendin-4, small molecule agonist Boc5) (Chen et al., 2007; Knudsen et al., 2007). Indeed, inhibitors of DPP-4 (known as gliptins, e.g. sitagliptin, alogliptin), which prevent physiological inactivation of endogenous GLP-1 thereby boosting its activity, have been used for several years to control hyperglycaemia in type 2 diabetes (see recent reviews: Green et al., 2007; Flatt et al., 2009).
Table 1.
Examples of glucagon-like peptide-1 (GLP-1) analogues/mimetics which are approved or being investigated for clinical therapeutic application
| Name | Company | Structure | Status |
|---|---|---|---|
| Exenatide (Exendin, Byetta®) | Amylin/Lilly | Exendin (twice-daily subcutaneous injection) | Approved and launched |
| Liraglutide (NN2211, Victoza®) | NovoNordisk | GLP-1-fatty acid (once-daily subcutaneous injection) | Approval pending |
| Taspoglutide (BIM-51077) | Ipsen/Roche | Long-acting GLP-1 analogue | Recruiting for phase III |
| Exenatide-LAR (Exendin-LAR) | Amylin/Lilly | Exendin long-acting release (once-weekly subcutaneous injection) | Phase II |
| Naliglutide (Albiglutide) | GlaxoSmithKline | GLP-1-albumin complex | Phase II |
| MKC253 | MannKind | GLP-1-technospheres for inhalation | Phase I completed |
Early research sought to develop GLP-1 analogues (closely resemble the GLP-1 amino acid sequence) which were resistant to endogenous degradation by DPP-4, while retaining potent activation of the GLP-1R and thereby extending the in vivo half-life to around 4 h (Green et al., 2004b). In the search to obtain a once-daily formulation, it was discovered that the half-life of GLP-1 peptides could be further protracted (>12 h) through methods such as acylation, PEGylation or the attachment of chemical linkers (Green and Flatt, 2007). At present, no GLP-1 analogues are clinically available, although regulatory approval of liraglutide (Victoza®, NovoNordisk), an acylated form of GLP-1, is expected in 2009. However, the GLP-1 mimetic compound, Byetta® (exenatide, Eli Lilly) was successfully launched in the USA in 2005 and subsequently in the UK in 2007.
Initial pre-clinical findings with Byetta® in patients with type 2 diabetes have now been supported by substantial clinical evidence indicating that it exerts several beneficial actions on glucose metabolism. These studies have reported that type 2 diabetic patients chronically treated with Byetta® demonstrate marked increases in first- and second-phase insulin secretion, suppression of postprandial glucagon secretion, significant reductions in postprandial hyperglycaemia and improved basal glycaemic control (Kolterman et al., 2003; Fehse et al., 2005; Nauck et al., 2007; Zinman et al., 2007). Furthermore, in clinical trials Byetta® was reported to cause significant reductions in both plasma HbA1c levels and body weight (Buse et al., 2004; Nauck et al., 2007).
Clinical approval of several further GLP-1 compounds is expected within the next few years (Flatt et al., 2009) (summarized in Table 1). It is hoped that these will offer additional benefits to those of Byetta®, such as longer duration of action, more desirable methods of administration (compared to twice-daily injection) and possible additional functionalities (Flatt et al., 2009). For example, it now appears that the half-life of certain GLP-1-based therapies may be further extended. In this regard, exenatide-LAR, which constitutes a microsphere-encapsulated suspension of exendin-4 administered via once weekly subcutaneous injection, has been developed, and a preliminary phase II trial has indicated that it is effective in patients with type 2 diabetes. Other research efforts are also being invested in the direction of GLP-1-based therapies which may be administered via alternative, more desirable routes, such as inhalation or oral administration (Chen et al., 2007; Knudsen et al., 2007).
Interestingly, it appears that the therapeutic potential of GLP-1 may extend to other degenerative conditions in addition to type 2 diabetes. For example, GLP-1 has been reported to confer neuroprotective effects in both Alzheimer's and Huntington's diseases (Perry and Greig, 2004; Holscher and Li, 2008; Martin et al., 2009), and to exert several direct cardioprotective actions (Bose et al., 2005b; Nikolaidis et al., 2005a; Sokos et al., 2006).
GLP-1 in the cardiovascular system
Although the major physiological function of GLP-1 appears to be in relation to glycaemic control, GLP-1Rs have also been found in a variety of extra-pancreatic tissues. Interestingly, GLP-1Rs have been reported to be widely expressed in the heart and vasculature of both rodents and humans, with specific localization in vascular smooth muscle, cardiomyocytes, endocardium and coronary endothelium/smooth muscle (Wei and Mojsov, 1995; Bullock et al., 1996), suggesting that GLP-1 may play an important role in the cardiovascular system. Indeed, recent work from several different laboratories, including our own, have reported GLP-1R agonists to exert wide ranging cardiovascular effects, such as modulation of heart rate, blood pressure, vascular tone and myocardial contractility (Barragan et al., 1994; Vila Petroff et al., 2001; Yamamoto et al., 2002; Green et al., 2008). Importantly, beneficial actions of these agents on CVD have also been reported in both experimental models and in human patients, either in the presence or absence of diabetes (Nikolaidis et al., 2004a; Nystrom et al., 2004; Ozyazgan et al., 2005; Sokos et al., 2006).
Effects of GLP-1 on blood pressure and heart rate
In normal rodents, it is well established that acute and chronic treatment with both GLP-1 and exendin-4 significantly increases blood pressure and heart rate in a dose-dependent manner within the picomolar to nanomolar range (Barragan et al., 1994; Bojanowska and Stempniak, 2000; Yamamoto et al., 2002; Isbil-Buyukcoskun and Gulec, 2004; Gardiner et al., 2006). However, the data from larger animal and human studies are less clear. Although acute infusion of GLP-1 in conscious calves has also been reported to significantly increase heart rate, it has no effect on blood pressure (Edwards et al., 1997). Similarly, a 2 h GLP-1 infusion in pigs (Kavianipour et al., 2003), and both short- and long-term administration of GLP-1 in humans have been found to have no detectable chronotropic or pressor effects (Thrainsdottir et al., 2004; Sokos et al., 2006; 2007;). These findings may suggest a species-specific effect of GLP-1, although it should be noted that the larger animal and human studies generally employed lower concentrations of GLP-1, within the low picomolar range, compared to the rodent studies which mostly administered large supra-physiological doses given as bolus injections. Interestingly, two groups have independently demonstrated a biphasic effect of GLP-1 in response to low-dose bolus administration, characterized by an initial increase in blood pressure followed by a prolonged hypotension which persists for over 30 min (Barragan et al., 1994; Bojanowska and Stempniak, 2000). It appears increasingly likely that this effect may be mediated by its breakdown product, GLP-1(9–36), as accumulating evidence indicates that this metabolically inactive peptide may play an active beneficial role in the cardiovascular system (see later sections) (Nikolaidis et al., 2005b; Ban et al., 2008; Green et al., 2008; Sonne et al., 2008).
The precise mechanisms underlying the reported effects of GLP-1 on blood pressure and heart rate are yet to be fully established. It appears that they may be mediated via the GLP-1R as exendin(9–39) was found to prevent GLP-1 and exendin-4 induced increases in blood pressure and heart rate after both central and peripheral administration in the rat (Barragan et al., 1996; 1999;). Furthermore, gene-modified mice lacking the GLP-1R have been reported to exhibit a reduced resting heart rate compared to wild-type controls (Gros et al., 2003), supporting a role for endogenous GLP-1 in cardiovascular control. However, in an experimental in vivo rat model, a mesenteric vasoconstriction in response to acute exendin-4 infusion was found to persist in the face of GLP-1R antagonism with exendin(9–39), suggesting that a component of this effect may occur independently of the classical GLP-1R (Gardiner et al., 2006). The involvement of different downstream signalling pathways in the tachycardic and pressor effects of GLP-1 is also the subject of some debate. It was initially suggested that they occurred independently of α- or β-adrenoceptor activation (Barragan et al., 1994). However, subsequent studies concluded that these actions were largely mediated via β-adrenoceptor-dependent activation of the autonomic nervous system (Yamamoto et al., 2002; Gardiner et al., 2006), although it was later reported that β-adrenoceptor blockade with propranolol actually enhanced the pressor effect of GLP-1 (Gardiner et al., 2008). Both central and peripheral administration of GLP-1 have been found to induce Fos-like immunoreactivity in autonomic regulatory sites, including medullary cathecholamine neurons in the area postrema, suggesting neuronal regulation of these cardiovascular actions of GLP-1 (Yamamoto et al., 2002; 2003;). However, recent studies have reported an autonomic-independent exendin-4-induced vasoconstriction which does not involve angiotensin II, vasopressin, neuropeptide Y, endothelin or vasoconstrictor prostanoids (Gardiner et al., 2008), and that cholinergic activation may also be involved in GLP-1-induced increases in blood pressure and heart rate (Isbil-Buyukcoskun and Gulec, 2004).
In addition to the established effects of GLP-1 on blood pressure and heart rate in normal rodents, GLP-1 has also been demonstrated to exert beneficial effects in the pathological situation. Acute infusion of GLP-1 was found to restore blood pressure in hypovolaemic rats after experimental haemorrhage via stimulation of the neurohypophysial hormones, oxytocin and vasopressin (Bojanowska and Stempniak, 2002). In addition, chronic treatment with GLP-1 significantly attenuated the development of hypertension in Dahl salt-sensitive rats (Yu et al., 2003), although these changes were attributed to its diuretic and natriuretic actions, rather than secondary to improvement in insulin resistance or direct central effects. Interestingly, chronic treatment of type 2 diabetic patients with the stable GLP-1 mimetic, exendin-4, and the GLP-1 analogue, liraglutide, have been reported to be associated with beneficial effects on both systolic and diastolic blood pressure without affecting heart rate, although it is likely that these changes occurred secondary to parallel improvements in several cardiovascular risk factors (Klonoff et al., 2008; Garber et al., 2009). As diabetic patients are frequently characterized by activation of the sympathetic nervous system and hypertension, which is an established risk factor for CVD (Bell, 2003), it is imperative that the precise nature of the sympathetic actions of GLP-1 and the mechanisms underlying these effects are fully understood.
Effect of GLP-1 on vascular function
In addition to its established effects on blood pressure and heart rate, GLP-1 has also been reported to have a direct vasorelaxant action in isolated rat vessels, in pulmonary artery, femoral artery and aorta (Golpon et al., 2001; Nystrom et al., 2005; Green et al., 2008), and in mouse mesenteric artery (Ban et al., 2008). Interestingly, although the GLP-1 mimetic, exendin-4, has also been demonstrated to cause dose-dependent relaxation of isolated rat aorta, the magnitude of the response was markedly less than that observed with GLP-1 (Green et al., 2008), and exendin-4 failed to produce vasodilatation in isolated mouse mesenteric artery (Ban et al., 2008). Acute treatment with GLP-1 has also been demonstrated to increase endothelium-dependent blood flow in both the forearm of healthy non-diabetic human subjects (Basu et al., 2007) and the brachial artery of type 2 diabetic patients with stable coronary artery disease (Nystrom et al., 2004), although the latter study reported no significant effects of GLP-1 in normal healthy subjects. Importantly, it appears that the beneficial effects of GLP-1 on vascular function may also extend to the setting of CVD. Chronic treatment with both GLP-1 and exendin-4 was found to restore streptozotocin diabetes-induced impairment of endothelial function and vascular contraction in an experimental rat model (Ozyazgan et al., 2005). Furthermore, continuous chronic infusion of GLP-1 in spontaneously hypertensive heart failure-prone rats has been reported to result in a significant increase in cardiac output in the absence of any changes in blood pressure, suggesting that GLP-1 may cause peripheral vasodilatation in this situation (Poornima et al., 2008). However, in both of these studies, it was unclear whether the reported effects occurred via a direct action of GLP-1 or secondary to modulation of glucose metabolism. Interestingly, an ex vivo study conducted in isolated aortic rings indicated that GLP-1 significantly attenuated endothelial dysfunction in vessels from Dahl salt-sensitive rats (Yu et al., 2003), suggesting that the beneficial vascular effects of GLP-1 may indeed occur directly and independently of its established insulinotropic actions.
It may appear rather disappointing that the reported vasodilatory effects of GLP-1 do not translate into potentially beneficial hypotensive effects in vivo due to the central tachycardic and pressor actions of GLP-1, which have been previously discussed (Yamamoto et al., 2002; Gardiner et al., 2006). However, it is conceivable that local vasodilatation of peripheral tissues combined with increases in blood pressure may still confer some benefit to diabetic patients by improving perfusion to compromised tissues, thus alleviating or preventing some of the associated microvascular complications. This possibility is an exciting development; however, it is clear that a delicate balance exists between the central and peripheral vascular effects of GLP-1, which is complex and warrants further investigation.
Although it is well established that GLP-1 exerts beneficial actions on vascular function, the underlying mechanisms are less clear. Some studies have suggested that the vasorelaxant actions of GLP-1 are both endothelium and nitric oxide dependent (Golpon et al., 2001; Ban et al., 2008), whereas others have indicated that these effects may occur via other pathways, such as activation of KATP channels and cyclic AMP (Nystrom et al., 2005; Green et al., 2008). It has also been suggested that the vascular actions of GLP-1 may be mediated via the classical GLP-1R, as they were found to be abolished in the presence of the established GLP-1R antagonist, exendin(9–39) (Nystrom et al., 2005). However, it has recently been reported that both exendin(9–39) and metabolically inactive GLP-1(9–36) are themselves capable of causing vascular relaxation (Ban et al., 2008; Green et al., 2008). Indeed, relaxation responses to both native GLP-1 and GLP-1(9–36) were found to persist in gene-modified mice lacking the GLP-1R (Ban et al., 2008), indicating that there may be more than one type of GLP-1R in the cardiovascular system or that GLP-1 is capable of exerting receptor-independent effects. Although the precise mechanisms of its vascular actions remain unclear, it appears that GLP-1 may be beneficial in attenuating the endothelial dysfunction which underlies many of the cardiovascular complications of diabetes (Nystrom et al., 2004).
Cardiac actions of GLP-1
In addition to its effects on systemic haemodynamics and vascular function, GLP-1 also appears to play an important role in the heart. Gene-modified mice lacking a functional GLP-1R demonstrate diastolic dysfunction, increased left ventricular (LV) wall thickness and impaired cardiac reserve compared to wild-type controls, suggesting that GLP-1 may play an essential role in the control of normal cardiac structure and function (Gros et al., 2003). Furthermore, studies in both isolated perfused rat hearts and cardiac myocytes suggest that exogenous GLP-1 may inhibit myocardial contractility under basal conditions, despite being associated with elevated intracellular cyclic AMP (Vila Petroff et al., 2001; Zhao et al., 2006). This effect was found to be mediated via the GLP-1R, as it was completely reversed in the presence of exendin(9–39), and occurred secondary to decreased myofilament Ca2+ responsiveness resulting from intracellular acidification (Vila Petroff et al., 2001). It is interesting to note that GLP-1-induced increases in intracellular cyclic AMP appear to have a negative inotropic action, whereas β-adrenoceptor stimulation is known to cause opposite contractile effects, also secondary to elevated cyclic AMP production (Lohse et al., 2003). This striking observation may indicate specific compartmentalization of cyclic AMP/protein kinase A pathways between β-adrenergic and GLP-1 signalling, which could have significant implications for modulation of other important processes within the cardiac myocyte by GLP-1.
GLP-1 and cardiac ischaemia
The majority of studies on the potential beneficial role of GLP-1 in CVD have focused on its actions in the ischaemic heart and its apparent ability to protect cardiac myocytes from ischaemic damage. Several different groups using various experimental models have reported that acute GLP-1 treatment exerts beneficial effects after ischaemia and successful reperfusion. Most of the studies to date have employed models of ex vivo isolated rodent Langendorff heart perfusion with short periods of ischaemia (30–45 min) and reperfusion (30–120 min), and have universally demonstrated that both GLP-1 and exendin-4 significantly reduce infarct size and enhance the recovery of contractile function after transient coronary artery occlusion (Bose et al., 2005a,b; Zhao et al., 2006; Ban et al., 2008; Sonne et al., 2008). Importantly, similar findings have also been reported in vivo. Acute treatment with GLP-1 (in the presence of the DPP-4 inhibitor, valine pyrrolidide) after a short period of ischaemia (30 min) in the rat was found to significantly protect against infarct development after a 2 h reperfusion (Bose et al., 2005a). In an experimental canine model, infusion of GLP-1 during a period of 24 h reperfusion after brief coronary artery occlusion (10 min) was also demonstrated to result in significant improvement in LV regional wall motion and relaxation, although it had no effect on systemic haemodynamics or global systolic function (Nikolaidis et al., 2005a). However, a recent study employing an in vivo porcine model of ischaemia–reperfusion, found that extended treatment with exendin-4 during a 3 day period after 75 min ischaemia significantly decreased infarct size and improved recovery of both systolic and diastolic function (Timmers et al., 2009). In contrast, an earlier study using the same experimental preparation found that treatment with native GLP-1 over a much shorter period of reperfusion had no effect on infarct size, although it did significantly decrease interstitial levels of pyruvate and lactate (Kavianipour et al., 2003).
Interestingly, a recent study has indicated that GLP-1 may also confer beneficial effects on the ischaemic heart in the setting of diabetes. Pretreatment of both normoglycaemic and streptozotocin-induced diabetic mice with the GLP-1 analogue, liraglutide, for a period of 7 days was found to significantly decrease infarct size and the incidence of cardiac rupture after chronic myocardial infarction (MI) (Noyan-Ashraf et al., 2009). This was associated with improvements in survival and cardiac output, although these most likely occurred secondary to the reported benefits on early post-MI remodelling. More interesting was the finding that chronic treatment with liraglutide was found to confer cardioprotection and survival advantages over and above those of the insulin sensitizer, metformin, despite equivalent effects on glycaemic control. Importantly, it appears that these experimental findings may also extend to the clinical situation. In a small non-randomized study of predominantly non-diabetic patients with acute MI undergoing primary percutaneous coronary intervention, short-term infusion of GLP-1 for 72 h was demonstrated to significantly improve LV ejection fraction, and both global and regional wall motion (Nikolaidis et al., 2004b). In addition, a randomized study conducted in a similar number of patients with preserved LV function undergoing coronary artery bypass grafting, found that GLP-1 treatment for 12 h pre- and 36 h post-surgery improved glycaemic control while lowering the requirement for high-dose insulin or inotropes (Sokos et al., 2007). Although further more detailed and chronic studies are clearly required to confirm these initial observations, this nonetheless indicates that GLP-1 may hold potential therapeutic benefit for the treatment of ischaemic heart disease.
The precise mechanisms underlying the beneficial effects of GLP-1 in cardiac ischaemia have yet to be established. However, several experimental studies indicate that they occur independently of effects on glucose metabolism and may involve activation of cyclic GMP/cyclic AMP-dependent pathways and pro-survival kinases such as PI3K, Akt, glycogen synthase kinase-3β, p70s6 kinase, ERK1/2 and p38 MAPK (Bose et al., 2005a; 2007; Zhao et al., 2006; Ban et al., 2008; Xie et al., 2008; Noyan-Ashraf et al., 2009). It has also been suggested that GLP-1 may exert its protective effects on the ischaemic myocardium, at least partly, via beneficial actions on cardiomyocyte apoptosis, oxidative stress and endogenous antioxidant defence mechanisms (Bose et al., 2005a; Xie et al., 2008; Noyan-Ashraf et al., 2009; Timmers et al., 2009). The role of the GLP-1R in the ischaemic heart appears to be particularly interesting. It has been reported that the protective effects of both GLP-1 and exendin-4 against ex vivo ischaemia–reperfusion injury, and the GLP-1 analogue, liraglutide, against MI-induced cardiomyocyte apoptosis, are completely abolished by the established GLP-1R antagonist, exendin(9–39) (Bose et al., 2005a; Sonne et al., 2008; Noyan-Ashraf et al., 2009), suggesting that the early remodelling changes that occur after ischaemia are mediated exclusively via the GLP-1R. However, a couple of recent studies suggest that GLP-1 may improve functional recovery in the ischaemic heart via mechanisms independent of the established GLP-1R, which may involve its ‘inactive’ metabolite GLP-1(9–36). The beneficial effect of both exendin-4 and GLP-1 on cardiac contractile function after experimental ischaemia–reperfusion injury observed in wild-type mice was found to be both resistant to exendin(9–39) and to persist in gene-modified mice lacking a functional GLP-1R (Ban et al., 2008; Sonne et al., 2008). Furthermore, acute treatment with GLP-1(9–36) upon reperfusion (but not before the onset of ischaemia) resulted in an improvement in functional recovery, which occurred independently of the GLP-1R (Ban et al., 2008; Sonne et al., 2008). Interestingly, the beneficial effects of GLP-1 on cardiac functional recovery observed in hearts from GLP-1R mice were abolished by the DPP-4 inhibitor, sitagliptin, suggesting that these effects were mediated by its breakdown product, GLP-1(9–36) (Ban et al., 2008). Taken together, these experiments not only provide compelling evidence for the existence of receptor-independent pathways and/or an unidentified GLP-1R within the heart, but also suggest possible divergence of the mechanisms underlying GLP-1 effects on the ischaemic myocardium. This brings forward the intriguing possibility of selective therapeutic targeting of different aspects of the ischaemic phenotype, although significant further research is clearly required before this may become a reality. It also raises the important question as to whether, in the context of beneficial effects of GLP-1 on the cardiovascular system, it may actually be better not to inhibit DPP-4. In this regard, it is interesting to note that the potential use of DPP-4 inhibitors, such as sitagliptin, as a therapeutic strategy to augment endogenous GLP-1 in CVD remains unexplored.
GLP-1 and heart failure
Although most of the research to date concerning the potential therapeutic application of GLP-1 in CVD has focused on cardiac ischaemia, several recent experimental and clinical studies have also reported favourable functional effects of GLP-1 in failing hearts. Short-term infusion with recombinant GLP-1 over 48 h has been demonstrated to significantly improve LV systolic and diastolic function, and increase myocardial insulin sensitivity and glucose uptake in a canine model of rapid pacing-induced dilated cardiomyopathy (Nikolaidis et al., 2004a). Interestingly, GLP-1(9–36) was found to exert similar beneficial effects to native GLP-1 in this model (Nikolaidis et al., 2005b), supporting the growing suggestion that the metabolically inactive form of GLP-1 may play an active role in the cardiovascular system. Furthermore, spontaneously hypertensive heart failure-prone rats (characterized by obesity, insulin resistance, hypertension and dilated cardiomyopathy), treated chronically with GLP-1 from 9 months of age (when they begin to progress to advanced heart failure and death) exhibited preserved cardiac contractile function, increased myocardial glucose uptake, improved survival and a significant reduction in cardiac myocyte apoptosis (Poornima et al., 2008). Although this study also reported GLP-1 to stimulate myocardial glucose uptake in the failing myocardium, it was unclear whether its beneficial effects on contractile function occurred due to a direct cardiac action or secondary to its established insulinotropic effects.
Importantly, these experimental data are supported by preliminary clinical studies indicating that GLP-1 may also improve LV contractile function in patients with chronic heart failure. An early investigation conducted in a small number of type 2 diabetic patients with chronic heart failure found that short-term GLP-1 infusion for 3 days tended to improve both systolic and diastolic function, although these changes did not reach statistical significance (Thrainsdottir et al., 2004). However, longer-term GLP-1 treatment (5 weeks) in both diabetic and normoglycaemic chronic heart failure patients (New York Heart Association class III and IV) was reported to significantly improve LV ejection fraction, myocardial oxygen consumption and functional status, whereas no effect of GLP-1 was observed in patients with normal cardiac function (Sokos et al., 2006). Although these preliminary clinical studies provide some encouragement for the potential use of GLP-1 in the treatment of heart failure, it is clear that significant further research is required to confirm these initial observations, investigate the underlying mechanisms and explore possible interactions with current heart failure therapies.
Conclusions
There is no doubt that GLP-1-related drug compounds have proven efficacy in the treatment of hyperglycaemia associated with type 2 diabetes. However, it now appears that these agents also exert beneficial effects on the cardiovascular system, which may open up additional therapeutic avenues for the use of GLP-1 compounds in the treatment of CVD in both normal and diabetic patients. However, at present the precise nature and mechanisms underlying these potentially beneficial actions remain largely unknown and appear to be complex. For example, recent research has supported the existence of GLP-1 pathways independent of the classical GLP-1R and/or the existence of multiple GLP-1Rs within the cardiovascular system, and several studies have suggested that the metabolically inactive form of GLP-1, GLP-1(9–36), may also play a significant cardiovascular role. Although preliminary short-term clinical studies with GLP-1 have been encouraging, it is clear that further basic mechanistic research together with longer-term clinical investigations and meta-analyses are required before any potential therapeutic benefits may be realized.
Acknowledgments
The authors' work is supported by the British Heart Foundation, Diabetes UK and the Medical Research Council.
Glossary
Abbreviations:
- CVD
cardiovascular disease
- DPP-4
dipeptidyl peptidase-4
- ERK
extracellular signal-regulated kinase
- GIP
glucose-dependent insulinotropic peptide
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- LV
left ventricular
- MAPK
mitogen-activated protein kinase
- MI
myocardial infarction
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PI3K
phosphoinositide 3-kinase
- VIP
vasoactive intestinal peptide
Conflicts of interest
The authors state that they have no conflicts of interest.
References
- Arnette D, Gibson TB, Lawrence MC, January B, Khoo S, McGlynn K, et al. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic β cells. J Biol Chem. 2003;278:32517–32525. doi: 10.1074/jbc.M301174200. [DOI] [PubMed] [Google Scholar]
- Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7–36) amide in rats. Am J Physiol Endocrinol Metab. 1994;266:E459–E466. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- Barragan JM, Rodriguez RE, Eng J, Blazquez E. Interactions of exendin-(9–39) with the effects of glucagon-like peptide-1-(7–36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept. 1996;67:63–68. doi: 10.1016/s0167-0115(96)00113-9. [DOI] [PubMed] [Google Scholar]
- Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7–36) amide on arterial blood pressure in rats. Am J Physiol Endocrinol Metab. 1999;277:E784–E791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- Bell DSH. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304:368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- Bojanowska E, Stempniak B. Effects of centrally or systemically injected glucagon-like peptide-1(7–36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul Pept. 2000;91:75–81. doi: 10.1016/s0167-0115(00)00119-1. [DOI] [PubMed] [Google Scholar]
- Bojanowska E, Stempniak B. Effects of glucagon-like peptide-1(7–36) amide on neurohypophysial and cardiovascular functions under hypo- or normotensive hypovolaemia in the rat. J Endocrinol. 2002;172:303–310. doi: 10.1677/joe.0.1720303. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005a;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Yellon DM. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005b;19:9–11. doi: 10.1007/s10557-005-6892-4. [DOI] [PubMed] [Google Scholar]
- Bose A, Mocanu M, Carr R, Yellon D. Myocardial ischaemia–reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc Drugs Ther. 2007;21:253–256. doi: 10.1007/s10557-007-6030-6. [DOI] [PubMed] [Google Scholar]
- Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes. 2001;50:1720–1728. doi: 10.2337/diabetes.50.8.1720. [DOI] [PubMed] [Google Scholar]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M. Protein kinase Cζ activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes. 2001;50:2237–2243. doi: 10.2337/diabetes.50.10.2237. [DOI] [PubMed] [Google Scholar]
- Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52:124–132. doi: 10.2337/diabetes.52.1.124. [DOI] [PubMed] [Google Scholar]
- Chen D, Liao J, Li N, Zhou C, Liu Q, Wang G, et al. A nonpeptidic agonist of glucagon-like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci USA. 2007;104:943–948. doi: 10.1073/pnas.0610173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements RS, Jr, Bell DS. Complications of diabetes. Prevalence, detection, current treatment, and prognosis. Am J Med. 1985;79:2–7. doi: 10.1016/0002-9343(85)90503-0. [DOI] [PubMed] [Google Scholar]
- Colucci WS, Kolias TJ, Adams KF, Armstrong WF, Ghali JK, Gottlieb SS, et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the reversal of ventricular remodeling with toprol-XL (REVERT) trial. Circulation. 2007;116:49–56. doi: 10.1161/CIRCULATIONAHA.106.666016. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7–36) amide in type I diabetic patients. Diabetes Care. 1996;19:580–586. doi: 10.2337/diacare.19.6.580. [DOI] [PubMed] [Google Scholar]
- Deacon CF. Circulation and degradation of GIP and GLP-1. Horm Metab Res. 2004;36:761–765. doi: 10.1055/s-2004-826160. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol Endocrinol Metab. 1996;271:E458–E464. doi: 10.1152/ajpendo.1996.271.3.E458. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Plamboeck A, Moller S, Holst JJ. GLP-1-(9–36) amide reduces blood glucose in anesthetized pigs by a mechanism that does not involve insulin secretion. Am J Physiol Endocrinol Metab. 2002;282:E873–E879. doi: 10.1152/ajpendo.00452.2001. [DOI] [PubMed] [Google Scholar]
- Defronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Seidah NG, Brubaker PL. Role of prohormone convertases in the tissue-specific processing of proglucagon. Mol Endocrinol. 1996;10:342–355. doi: 10.1210/mend.10.4.8721980. [DOI] [PubMed] [Google Scholar]
- Doyle ME, Greig NH, Holloway HW, Betkey JA, Bernier M, Egan JM. Insertion of an N-terminal 6-aminohexanoic acid after the 7 amino acid position of glucagon-like peptide-1 produces a long-acting hypoglycemic agent. Endocrinology. 2001;142:4462–4468. doi: 10.1210/endo.142.10.8410. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptide-1 and the islet β-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dubé PE, Brubaker PL. Nutrient, neural and endocrine control of glucagon-like peptide secretion. Horm Metab Res. 2004;36:755–760. doi: 10.1055/s-2004-826159. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Edwards AV, Bloom SR. Cardiovascular and pancreatic endocrine responses to glucagon-like peptide-1(7–36) amide in the conscious calf. Exp Physiol. 1997;82:709–716. doi: 10.1113/expphysiol.1997.sp004059. [DOI] [PubMed] [Google Scholar]
- Eissele R, Göke R, Willemer S, Harthus HP, Vermeer H, Arnold R, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1(7–36) amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, et al. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:5991–5997. doi: 10.1210/jc.2005-1093. [DOI] [PubMed] [Google Scholar]
- Flatt PR, Bailey CJ, Green BD. Recent advances in antidiabetic drug therapies targeting the enteroinsular axis. Curr Drug Metab. 2009;10:125–137. doi: 10.2174/138920009787522124. [DOI] [PubMed] [Google Scholar]
- Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- Garcia MJ, Mcnamara PM, Gordon T, Kannel WB. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–111. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006;316:852–859. doi: 10.1124/jpet.105.093104. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Autonomic nervous system-dependent and -independent cardiovascular effects of exendin-4 infusion in conscious rats. Br J Pharmacol. 2008;154:60–71. doi: 10.1038/bjp.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, et al. Exenatide (exendin-4) improves insulin sensitivity and β-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005;146:2069–2076. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting β-cells. J Biol Chem. 1993;268:19650–19655. [PubMed] [Google Scholar]
- Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7–36) amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–86. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- Green BD, Flatt PR. Incretin hormone mimetics and analogues in diabetes therapeutics. Best Pract Res Clin Endocrinol Metab. 2007;21:497–516. doi: 10.1016/j.beem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Green BD, Gault VA, Irwin N, Mooney MH, Bailey CJ, Harriott P, et al. Metabolic stability, receptor binding, cAMP generation, insulin secretion and antihyperglycaemic activity of novel N-terminal Glu9-substituted analogues of glucagon-like peptide-1. Biol Chem. 2003;384:1543–1551. doi: 10.1515/BC.2003.171. [DOI] [PubMed] [Google Scholar]
- Green BD, Gault VA, Flatt PR, Harriott P, Greer B, O'Harte FPM. Comparative effects of GLP-1 and GIP on cAMP production, insulin secretion, and in vivo antidiabetic actions following substitution of Ala8/Ala2 with 2-aminobutyric acid. Arch Biochem Biophys. 2004a;428:136–143. doi: 10.1016/j.abb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Green BD, Gault VA, O'Harte FP, Flatt PR. Structurally modified analogues of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) as future antidiabetic agents. Curr Pharm Des. 2004b;10:3651–3662. doi: 10.2174/1381612043382774. [DOI] [PubMed] [Google Scholar]
- Green BD, Mooney MH, Gault VA, Irwin N, Bailey CJ, Harriott P, et al. Lys9 for Glu9 substitution in glucagon-like peptide-1(7–36) amide confers dipeptidylpeptidase IV resistance with cellular and metabolic actions similar to those of established antagonists glucagon-like peptide-1(9–36) amide and exendin(9–39) Metabolism. 2004c;53:252–259. doi: 10.1016/j.metabol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Green BD, Lavery KS, Irwin N, O'Harte FP, Harriott P, Greer B, et al. Novel GLP-1 analogue (Val8) GLP-1 results in significant improvements of glucose tolerance and pancreatic β cell function after 3 weeks daily administration in obese diabetic (ob/ob) mice. J Pharmacol Exp Ther. 2006;318:914–921. doi: 10.1124/jpet.105.097824. [DOI] [PubMed] [Google Scholar]
- Green BD, Flatt PR, Bailey CJ. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diab Vasc Dis Res. 2007;3:159–165. doi: 10.3132/dvdr.2006.024. [DOI] [PubMed] [Google Scholar]
- Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys. 2008;478:136–142. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Gribble F. RD Lawrence lecture 2008: targeting GLP-1 release as a potential strategy for the therapy of type 2 diabetes. Diabet Med. 2008;25:889–894. doi: 10.1111/j.1464-5491.2008.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, et al. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–2252. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- Gutniak MK, Linde B, Holst JJ, Efendic S. Subcutaneous injection of the incretin hormone glucagon-like peptide 1 abolishes postprandial glycemia in NIDDM. Diabetes Care. 1994;17:1039–1044. doi: 10.2337/diacare.17.9.1039. [DOI] [PubMed] [Google Scholar]
- Hargrove DM, Nardone NA, Persson LM, Parker JC, Stevenson RW. Glucose-dependent action of glucagon-like peptide-1(7–37) in vivo during short- or long-term administration. Metabolism. 1995;44:1231–1237. doi: 10.1016/0026-0495(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Heinrich G, Gros P, Lund PK, Bentley RC, Habener JF. Pre-proglucagon messenger ribonucleic acid: nucleotide and encoded amino acid sequences of the rat pancreatic complementary deoxyribonucleic acid. Endocrinology. 1984;115:2176–2181. doi: 10.1210/endo-115-6-2176. [DOI] [PubMed] [Google Scholar]
- Holscher C, Li L. New roles for insulin-like hormones in neuronal signalling and protection: new hopes for novel treatments of Alzheimer's disease? Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.023. DOI: 10.1016/j.neurobiolaging.2008.08.023 [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Holz GG. Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe-Sodmann K, McGregor GP, Bridenbaugh R, Goke R, Goke B, Thole H, et al. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7–36) amide and comparison of the substrate specificity of the enzyme for other glucagon-like peptides. Regul Pept. 1995;58:149–156. doi: 10.1016/0167-0115(95)00063-h. [DOI] [PubMed] [Google Scholar]
- Hupe-Sodmann K, Goke R, Goke B, Thole HH, Zimmermann B, Voigt K, et al. Endoproteolysis of glucagon-like peptide (GLP)-1(7–36) amide by ectopeptidases in RINm5F cells. Peptides. 1997;18:625–632. doi: 10.1016/s0196-9781(97)00123-x. [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Williams P, Witten MB, Tedesco R, Strader CD. Differential effects of intracerebroventricular glucagon-like peptide-1 on feeding and energy expenditure regulation. Peptides. 1998;19:869–875. doi: 10.1016/s0196-9781(98)00033-3. [DOI] [PubMed] [Google Scholar]
- Idris I, Patiag D, Gray S, Donnelly R. Exendin-4 increases insulin sensitivity via a PI-3-kinase-dependent mechanism: contrasting effects of GLP-1. Biochem Pharmacol. 2002;63:993–996. doi: 10.1016/s0006-2952(01)00924-8. [DOI] [PubMed] [Google Scholar]
- Isbil-Buyukcoskun N, Gulec G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul Pept. 2004;118:33–38. doi: 10.1016/j.regpep.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Juntti-Berggren L, Pigon J, Karpe F, Hamsten A, Gutniak M, Vignati L, et al. The antidiabetogenic effect of GLP-1 is maintained during a 7-day treatment period and improves diabetic dyslipoproteinemia in NIDDM patients. Diabetes Care. 1996;19:1200–1206. doi: 10.2337/diacare.19.11.1200. [DOI] [PubMed] [Google Scholar]
- Kang JH, Kim MJ, Jang HI, Koh KH, Yum KS, Rhie DJ, et al. Proximal cyclic AMP response element is essential for exendin-4 induction of rat EGR-1 gene. Am J Physiol Endocrinol Metab. 2007;292:E215–E222. doi: 10.1152/ajpendo.00181.2006. [DOI] [PubMed] [Google Scholar]
- Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- Kanse SM, Kreymann B, Ghatei MA, Bloom SR. Identification and characterization of glucagon-like peptide-1 7–36 amide-binding sites in the rat brain and lung. FEBS Lett. 1988;241:209–212. doi: 10.1016/0014-5793(88)81063-9. [DOI] [PubMed] [Google Scholar]
- Kavianipour M, Ehlers MR, Malmberg K, Ronquist G, Ryden L, Wikstrom G, et al. Glucagon-like peptide-1(7–36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides. 2003;24:569–578. doi: 10.1016/s0196-9781(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- Kervran A, Blache P, Bataille D. Distribution of oxyntomodulin and glucagon in the gastrointestinal tract and the plasma of the rat. Endocrinology. 1987;121:704–713. doi: 10.1210/endo-121-2-704. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Pridal L. Glucagon-like peptide-1-(9–36) amide is a major metabolite of glucagon-like peptide-1-(7–36) amide after in vivo administration to dogs, and it acts as an antagonist on the pancreatic receptor. Eur J Pharmacol. 1996;318:429–435. doi: 10.1016/s0014-2999(96)00795-9. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Kiel D, Teng M, Behrens C, Bhumralkar D, Kodra JT, et al. Small-molecule agonists for the glucagon-like peptide 1 receptor. Proc Natl Acad Sci USA. 2007;104:937–942. doi: 10.1073/pnas.0605701104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082–3089. doi: 10.1210/jc.2002-021545. [DOI] [PubMed] [Google Scholar]
- Larsson H, Holst JJ, Ahrén B. Glucagon-like peptide-1 reduces hepatic glucose production indirectly through insulin and glucagon in humans. Acta Physiol Scand. 1997;160:413–422. doi: 10.1046/j.1365-201X.1997.00161.x. [DOI] [PubMed] [Google Scholar]
- Lin F, Wang R. Molecular modeling of the three-dimensional structure of GLP-1R and its interactions with several agonists. J Mol Model. 2009;15:53–65. doi: 10.1007/s00894-008-0372-2. [DOI] [PubMed] [Google Scholar]
- Liu HK, Green BD, Gault VA, McCluskey JT, McClenaghan NH, O'Harte FP, et al. N-acetyl-GLP-1: a DPP IV-resistant analogue of glucagon-like peptide-1 (GLP-1) with improved effects on pancreatic [β]-cell-associated gene expression. Cell Biol Int. 2004;28:69–73. doi: 10.1016/j.cellbi.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Engelhardt S, Eschenhagen T. What Is the role of β-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- Lund PK, Goodman RH, Habener JF. Intestinal glucagon mRNA identified by hybridization to a cloned islet cDNA encoding a precursor. Biochem Biophys Res Commun. 1981;100:1659–1666. doi: 10.1016/0006-291x(81)90709-9. [DOI] [PubMed] [Google Scholar]
- Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR. Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27:1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington's disease. Diabetes. 2009;58:318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA, Wollschläger D, Werner J, Holst JJ, Orskov C, Creutzfeldt W, et al. Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7–36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–1553. doi: 10.1007/s001250050613. [DOI] [PubMed] [Google Scholar]
- Nauck M, Duran S, Kim D, Johns D, Northrup J, Festa A, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259–267. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004a;110:955–961. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004b;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Doverspike A, Hentosz T, Zourelias L, Shen YT, Elahi D, et al. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005a;312:303–308. doi: 10.1124/jpet.104.073890. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005b;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Nakabayashi H, Kawai K, Ito T, Kawakami S, Nakagawa A, et al. The hepatic vagal reception of intraportal GLP-1 is via receptor different from the pancreatic GLP-1 receptor. J Auton Nerv Syst. 2000;80:14–21. doi: 10.1016/s0165-1838(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Orskov C, Poulsen SS. Glucagon like peptide-I-(7–36)-amide receptors only in islets of Langerhans. Autoradiographic survey of extracerebral tissues in rats. Diabetes. 1991;40:1292–1296. doi: 10.2337/diab.40.10.1292. [DOI] [PubMed] [Google Scholar]
- Orskov C, Andreasen J, Holst JJ. All products of proglucagon are elevated in plasma from uremic patients. J Clin Endocrinol Metab. 1992;74:379–384. doi: 10.1210/jcem.74.2.1730817. [DOI] [PubMed] [Google Scholar]
- Orskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- Ozyazgan S, Kutluata N, Afsar S, Ozdas SB, Akkan AG. Effect of glucagon-like peptide-1(7–36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology. 2005;74:119–126. doi: 10.1159/000084277. [DOI] [PubMed] [Google Scholar]
- Perry TA, Greig NH. A new Alzheimer's disease interventive strategy: GLP-1. Curr Drug Targets. 2004;5:565–571. doi: 10.2174/1389450043345245. [DOI] [PubMed] [Google Scholar]
- Plamboeck A, Holst J, Carr R, Deacon C. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48:1882–1890. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- Poornima I, Brown S, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 (GLP-1) infusion sustains LV systolic function and prolongs survival in the spontaneously hypertensive-heart failure prone rat. Circ Heart Fail. 2008;1:153–160. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Grande C, Alarcón C, Castilla C, López Novoa JM, Villanueva-Peñacarrillo ML, Valverde I. Renal catabolism of truncated glucagon-like peptide 1. Horm Metab Res. 1993;25:612–616. doi: 10.1055/s-2007-1002190. [DOI] [PubMed] [Google Scholar]
- Sandhu H, Wiesenthal SR, MacDonald PE, McCall RH, Tchipashvili V, Rashid S, et al. Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes. 1999;48:1045–1053. doi: 10.2337/diabetes.48.5.1045. [DOI] [PubMed] [Google Scholar]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- Schirra J, Katschinski M, Weidmann C, Schäfer T, Wank U, Arnold R, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu I, Hirota M, Ohboshi C, Shima K. Identification and localization of glucagon-like peptide-1 and its receptor in rat brain. Endocrinology. 1987;121:1076–1082. doi: 10.1210/endo-121-3-1076. [DOI] [PubMed] [Google Scholar]
- Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern J, Maher TD, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007;100:824–829. doi: 10.1016/j.amjcard.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36) amide against ischemia–reperfusion injury in rat heart. Regul Pept. 2008;146:243–249. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Stoffel M, Espinosa R, III, Le Beau MM, Bell GI. Human glucagon-like peptide-1 receptor gene. Localization to chromosome band 6p21 by fluorescence in situ hybridization and linkage of a highly polymorphic simple tandem repeat DNA polymorphism to other markers on chromosome 6. Diabetes. 1993;42:1215–1218. doi: 10.2337/diab.42.8.1215. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Oba K, Igari Y, Matsumura N, Watanabe K, Futami-Suda S, et al. Colestimide lowers plasma glucose levels and increases plasma glucagon-like peptide-1(7–36) levels in patients with type 2 diabetes mellitus complicated by hypercholesterolemia. J Nippon Med Sch. 2007;74:338–343. doi: 10.1272/jnms.74.338. [DOI] [PubMed] [Google Scholar]
- The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, et al. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol Regul Integr Comp Physiol. 1997;272:R726–R730. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic β cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Waeber G. Glucagon-like peptide-I and the control of insulin secretion in the normal state and in NIDDM. Diabetes. 1993;42:1219–1225. doi: 10.2337/diab.42.9.1219. [DOI] [PubMed] [Google Scholar]
- Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin-(9–39) an antagonist of the receptor. Diabetes. 1993;42:1678–1682. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- Thrainsdottir I, Malmberg K, Olsson A, Gutniak M, Rydén L. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diab Vasc Dis Res. 2004;1:40–43. doi: 10.3132/dvdr.2004.005. [DOI] [PubMed] [Google Scholar]
- Timmers L, Henriques JP, De Kleijn DP, Devries JH, Kemperman H, Steendijk P, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Uttenthal LO, Blazquez E. Characterization of high-affinity receptors for truncated glucagon-like peptide-1 in rat gastric glands. FEBS Lett. 1990;262:139–141. doi: 10.1016/0014-5793(90)80173-g. [DOI] [PubMed] [Google Scholar]
- Vila Petroff MG, Egan JM, Wang X, Sollott SJ. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89:445–452. doi: 10.1161/hh1701.095716. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219–224. doi: 10.1016/0014-5793(94)01430-9. [DOI] [PubMed] [Google Scholar]
- White JW, Saunders GF. Structure of the human ghicagon gene. Nucleic Acids Res. 1986;14:4719–4730. doi: 10.1093/nar/14.12.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms B, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Nauck MA. Gastric emptying, glucose responses, and insulin secretion after a liquid test meal: effects of exogenous glucagon-like peptide-1 (GLP-1)-(7–36) amide in type 2 (noninsulin-dependent) diabetic patients. J Clin Endocrinol Metab. 1996;81:327–332. doi: 10.1210/jcem.81.1.8550773. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Giguere J, Parisien M, Jeng W, St-Pierre SA, Brubaker PL, et al. Biological activities of glucagon-like peptide-1 analogues in vitro and in vivo. Biochemistry. 2001;40:2860–2869. doi: 10.1021/bi0014498. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang SX, Sha WW, Zhou X, Wang WL, Han LP, et al. Effects and mechanism of glucagon-like peptide-1 on injury of rats cardiomyocytes induced by hypoxia-reoxygenation. Chin Med J. 2008;121:2134–2138. [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima IG, Shen YT, et al. The direct effects of glucagon-like peptide-1 (GLP-1) on myocardial contractility and glucose uptake in normal and post-ischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–1113. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- Zinman B, Hoogwerf BJ, Milton DR, Giaconia JM, Kim DD, Trautmann ME, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477–485. doi: 10.7326/0003-4819-146-7-200704030-00003. [DOI] [PubMed] [Google Scholar]


