Abstract
Background and purpose:
Tissue deposits of the anti-arrhythmic drug amiodarone are a major source of side effects (skin discoloration, etc.). We addressed the mechanism of the concentration of amiodarone in cells, and characterized the resulting vacuolar cytopathology and its evolution towards macroautophagy.
Experimental approach:
Sequestration of amiodarone in human cells (macrophages, smooth muscle cells, HEK 293a cells) was evaluated using its violet fluorescence and cytopathology using GFP-conjugated subcellular markers. Autophagic signalling was probed by immunoblotting for the effector protein LC3. A patient biopsy of amiodarone-induced blue-gray skin discoloration was investigated for the presence of macroautophagy (immunofluorescence for LC3).
Key results:
Most of the amiodarone (1–20 µM, 4–24 h) captured by cultured cells (macrophages were most avid) was present in enlarged vacuoles. The specific vacuolar ATPase (V-ATPase) inhibitors, bafilomycin A1 or FR167356, prevented vacuolization and drug uptake. Vacuoles in HEK 293a cells were positive for markers of late endosomes and lysosomes (GFP-Rab7, -CD63) and for an effector of macroautophagy, GFP-LC3. The vacuoles accumulated endogenous LC3 and filled with lipids (Nile red staining) following longer amiodarone treatments (≥24 h). The electrophoretic mobility of both GFP-LC3 and endogenous LC3 changed, showing activation in response to amiodarone. Paraffin tissue sections of the pigmented skin exhibited granular LC3 accumulation in superficial dermis macrophages.
Conclusion and implications:
Vacuolar sequestration of amiodarone occurs at concentrations close to therapeutic levels, is mediated by V-ATPase and evolves towards persistent macroautophagy and phospholipidosis. This cytopathology is not cell type specific, but tissue macrophages appear to be particularly susceptible.
Keywords: amiodarone, macroautophagy, phospholipidosis, V-ATPase, macrophage
Introduction
Cationic drugs frequently exhibit large apparent volumes of distribution, consistent with various forms of sequestration by cells. Amiodarone is considered the most effective anti-arrhythmic drug and is widely prescribed (Vassallo and Trohman, 2007; Zimetbaum, 2007). It exhibits class III action (K channel blockade that prolongs the QT interval and myocardial repolarization), but is a complex drug also blocking Na channels and interfering with β-adrenoceptors and Ca currents (class I, II and IV effects respectively). It is currently used mainly in patients with atrial fibrillation and left ventricular dysfunction. It has a large volume of distribution (66 L·kg–1), consistent with extensive uptake by tissues, delayed onset of action and long elimination half-life (up to 6 months). The major hepatic metabolite of amiodarone, N-desethylamiodarone, is a secondary amine nearly equivalent to the parent compound, if its properties as a weak base are considered. The therapeutic plasma range for amiodarone and its metabolite is 0.7–3.7 µM (Vassallo and Trohman, 2007), but it is widely acknowledged that the tissues accumulate the drug during chronic dosing. Amiodarone produces a constellation of side effects, many of which may be related to drug-induced phospholipidosis and/or vacuolar sequestration: concentric inclusions in peripheral blood leukocytes (Adams et al., 1986; Somani et al., 1986); corneal microdeposits (>90% incidence); blue-gray skin discoloration with photosensitivity (4–9%); non-alcoholic steatohepatitis that can lead to cirrhosis (<3%); and a serious pulmonary toxicity that includes interstitial pneumonia, fibrosis and ‘foamy macrophages’ that are vacuolar cells containing the typical multilamellar bodies (Myers et al., 1987; Vassallo and Trohman, 2007; Zimetbaum, 2007; Ammoury et al., 2008; Diaz-Guzman et al., 2008; Ruangchira-Urai et al., 2008). Similarly, intracytoplasmic inclusions (termed primary lipidosis) have been observed in the optic nerve axons of patients with slow-onset amiodarone-induced optic neuropathy (Li et al., 2008). The skin discoloration parallels the actual presence of amiodarone in enlarged vacuoles in various cell types of the upper dermis (mostly macrophages, but also fibroblasts, endothelial cells, etc.), and is a storage disorder secondary to drug deposition (Ammoury et al., 2008).
We have recently exploited the anti-arrhythmic drug procainamide (class Ia, logP 1.1) as a model molecule for vacuolar (V)-ATPase-mediated drug uptake by cells (Morissette et al., 2004; 2005; 2008b;), a form of pseudotransport with millimolar affinity associated with a vacuolar cytopathology. V-ATPase is the proton pump that acidifies the trans-Golgi and derived organelles (lysosomes, endosomes, secretory granules); it is postulated that amine drugs diffuse into such organelles, become protonated at low pH and that their retro-diffusion is inefficient in their charged form (ion trapping). Another anti-arrhythmic agent amiodarone, also a substituted triethylamine (Figure 1), is much more lipophilic (logP 7.9) than procainamide. Upon chronic dosing, more than 50 drugs in various therapeutic classes induce phospholipidosis, an intracellular accumulation of phospholipids within lamellar bodies (variously called myeloid, onionoid, etc.), lysosomal structures that contain multiple concentric layers of undegraded lipids (Reasor and Kacew, 2001; Anderson and Borlak, 2006) visible using electron microscopy, but also with liposoluble fluorescent dyes such as Nile red (Casartelli et al., 2003; Nioi et al., 2007). Lipophilic cationic drugs are essentially involved in this reaction (Halliwell, 1997) that affects various cell types in lung, liver, brain, kidney, cornea and other tissues. Induction of phospholipidosis by amiodarone is extensively described clinically. Although procainamide is relatively hydrophillic and not clinically prone to induce phospholipidosis, this reaction can be observed in cultured cells exposed for more than 24 h to supratherapeutic drug levels, and we have proposed a mechanism for it (multiple cycles of macroautophagic envelopment of the enlarged vacuoles; Morissette et al., 2008b).
Figure 1.
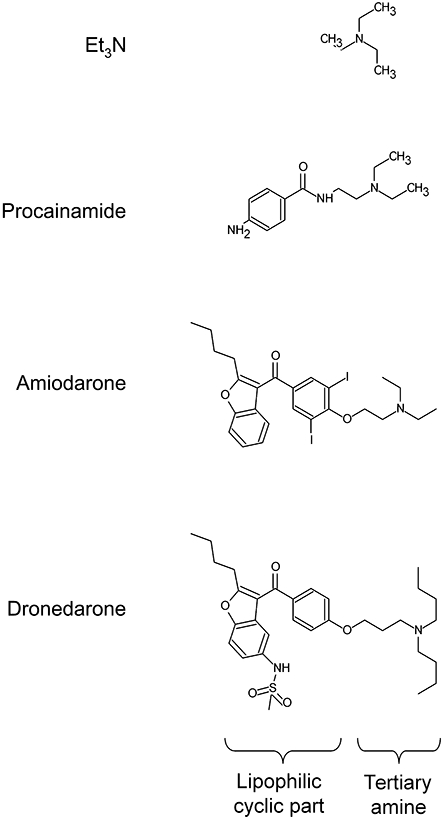
Structure of some anti-arrhythmic drugs compared to that of triethylamine (Et3N).
Here, we investigated whether amiodarone could produce a vacuolar cytopathology mediated by V-ATPase-driven ion trapping into acidic organelles at micromolar concentrations, whether this reaction evolves towards persistent macroautophagy and phospholipidosis and the presence of macroautophagy in clinically relevant material, the pigmented skin of a patient receiving this drug chronically (Ammoury et al., 2008).
Methods
Human cells and transfection
The institutional research ethics board approved the anonymous use of human umbilical cord segments obtained after elective caesarean deliveries, and the use of peripheral blood from healthy adult volunteers. Primary cultures of smooth muscle cells were obtained from explants of umbilical arteries and propagated as described (Morissette et al., 2008b). Suspensions of peripheral blood mononuclear leukocytes (PBMLs) from heparinized venous blood of six healthy donors were prepared according to Böyum (1968). PBMLs were then enriched in their monocyte fraction by adherence to plastic Petri dishes (5 × 106 cells·mL–1, 37°C, 90 min). The non-adherent fraction was removed by repeated washes; the adherent monocyte fraction was detached with the low protease containing Accutase reagent (Innovative Cell Technologies, San Diego, CA, USA), counted and plated at a density of 0.5–1 × 106 cells per 35 mm Petri dish. The adherent mononuclear cells were cultured for 4 days in α-MEM supplemented with 10% fetal bovine serum (FBS), 25 ng·mL–1 M-CSF (R&D Systems, Minneapolis, MN, USA) and antibiotics (penicillin–streptomycin). Purity was 90–92% monocytes, as assessed by Wright–Giemsa and non-specific esterase staining. These cells are further designated as macrophages, as the culturing of monocytes increases their differentiation and their contents in acidic vesicles (Basta et al., 1999). A subclone of HEK 293 cells (dubbed HEK 293a) was obtained from Sigma-Aldrich (St. Louis, MO, USA). These cells, efficiently transfectable, were grown and maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS, l-glutamine and antibiotics (Invitrogen, Burlington, Canada). Expression vectors were transiently transfected for 24 or 48 h using the polyethylenimine transfection reagent protocol (Morissette et al., 2008a). Expression vectors for GFP-Rab7 were previously described (Hunyady et al., 2002). GFP-LC3 labels autophagosomes in mammalian cells (Kabeya et al., 2000); the pEGFP-LC3 expression vector for this chimerical protein was a generous gift from Dr T. Yoshimori (Osaka University, Osaka, Japan). The GFP-CD63 construct has been previously described (Morissette et al., 2008b).
Microscopic techniques
Standard phase contrast microscopy or epifluorescence studies were performed using intact macrophages, smooth muscle or HEK 293a cells maintained in their respective serum-containing culture media and treated with the cationic drugs and/or in cells previously (24 h) transfected with a vector coding for a fluorescent protein (Olympus BX51 microscope, Markham, Canada coupled to a CoolSnap HQ digital camera, Roper Scientific, Tucson, AZ, USA). Observation of the intrinsic violet fluorescence of amiodarone (UV excitation) allowed evaluating drug uptake. Indirect immunofluorescence for endogenous LC3 was performed in fixed and permeabilized human smooth muscle cells or in paraffin tissue sections of human skin (anti-LC3B rabbit polyclonal antibodies, Novus, Biologicals, Littleton, CO, USA; dilution 1:100–1:200) revealed with Alexa fluor-488 or-594 conjugated goat anti-rabbit IgG antibodies (1:200, Invitrogen).
A local ethics committee approved the study of a skin biopsy from the face of a Caucasian patient chronically treated with amiodarone (Ammoury et al., 2008), and from that of a healthy Caucasian male. Both subjects signed an informed consent form. The first subject, a 64-year-old man with a history of myocardial infarction, ventricular arrhythmia and heart failure, had taken amiodarone 400 mg daily 5 days per week during 4.5 years (cumulative dose 277 g) until a bluish skin coloration developed in photo-exposed skin areas; he received concurrent medication as described elsewhere (acebutol, lisinopril, simvastatin and lysine acetylsalicylate; Ammoury et al. (2008). The control subject, a 33-year-old man, had no medical history and took no drug. Anti-CD68 (monoclonal, Dako 1:50, Mississauga, Canada) was used in tissue sections to identify macrophages and was revealed with Alexa fluor-594 goat anti-mouse IgG antibodies. Smooth muscle cells are suitable for detecting drug-induced phospholipidosis-like reaction using Nile red staining, which was applied as described (Morissette et al., 2008b) in cells treated for various time periods with amiodarone.
LC3 immunoblot
Anti-human LC3B rabbit polyclonal antibodies (Novus; dilution 1:3000) were used to reveal the cytosolic form (LC3-I) and the processed form (LC3-II) in total HEK 293a cell extracts run on 15% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to PVDF membranes. The same investigation was applied to cells expressing recombinant GFP-LC3 (9% SDS–PAGE, protein detection using the JL8 anti-GFP monoclonal antibodies; Invitrogen).
Data analyis
Numerical values that represent cell fluorescence, determined as the median pixel intensity inside the manually drawn cell perimeter (Photoshop software), are reported as means ± SEM, and were found to be non-normally distributed. Therefore, non-parametric analysis of variance (Kruskall–Wallis test) followed by Dunn's multiple comparison test were used to compare sets of multiple values, and the Mann–Whitney test to compare pairs of values.
Materials
Bafilomycin A1 was purchased from LC Laboratories (Woburn, MA, USA). Another V-ATPase inhibitor FR167356 (2,6-dichloro-N-[3-(1-hydroxy-1-methylethyl)-2-methyl-7-benzofuranyl]benzamide) (Niikura et al., 2004) was a gift from Astellas Pharma, Osaka, Japan. All other drugs were obtained from Sigma-Aldrich. Amiodarone HCl, FR167356 and bafilomycin A1 were dissolved in DMSO (final concentration always less than 0.1% v/v), and control cells were treated with the DMSO vehicle.
Results
Preliminary tests showed that aqueous solutions of amiodarone have a violet fluorescence (maximum at 400 nm under 353 nm excitation). This was exploited to measure semi-quantitatively the uptake of the drug by cultured cells. Human macrophages and smooth muscle cells were compared with identical fluorescence settings and image treatment in the experiments summarized in Figures 2 and 3C. Amiodarone-associated fluorescence, mostly present in the form of coarse granules, was significantly greater than background in both cell types, but with a different time course and intensity. Uptake of the drug was already evident for a 5 µM concentration after a 4 h incubation with macrophages, and it increased as a function of concentration (10 µM) in these cells. However, at 10 µM, uptake of the drug was not greater at 24 h than at 4 h. Only the 10 µM amiodarone treatment of 24 h duration produced cell fluorescence statistically higher than background in smooth muscle cells (Figures 2 and 3). In both cell types, co-treatment with the V-ATPase inhibitor bafilomycin A1 significantly reduced the uptake of amiodarone (10 µM). The inhibition was not complete in macrophages for either incubation period, and the residual cellular staining was rather diffuse than granular (Figure 2). Cells treated with bafilomycin A1 alone exhibited no violet fluorescence or vacuolar appearance (data not shown). Figure 3A is a close-up view of a macrophage treated with amiodarone (10 µM, 24 h) that shows the vacuolar but polymorphic structures in which the drug concentrated. Phase contrast views are not as discriminative, because control macrophages were heavily granular. However, a modest vacuolization of the smooth muscle cells was observed in parallel to the accumulation of the drug over 24 h (Figure 2). In the most avid cell type, the macrophage, we have extended the uptake studies to a therapeutic concentration range of amiodarone (1–2.5 µM) and found a small but significant and concentration-dependent drug uptake, based on the analysis of cell fluorescence (Figure 3B); again, the uptake plateaued at 4 h.
Figure 2.
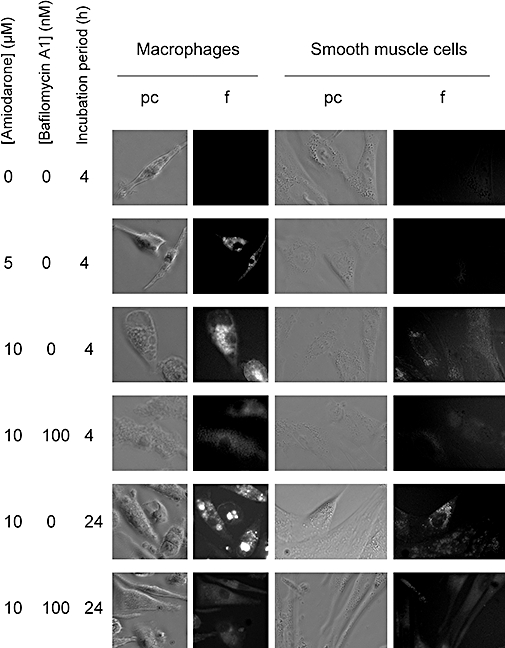
Morphological alterations associated with the treatment of human macrophages or smooth muscle cells with amiodarone (added at the indicated concentration for 4 or 24 h), bafilomycin A1 (100 nM) or both. Each field shows intact, unstained cells in phase contrast (pc) and violet fluorescence (f; UV excitation). Microscope setting and image treatment identical for both cell types. Original magnification 400×. All results were verified in leukocytes from at least two donors. Results based on smooth muscle cells were from a single donor, but are representative from those of two donors.
Figure 3.
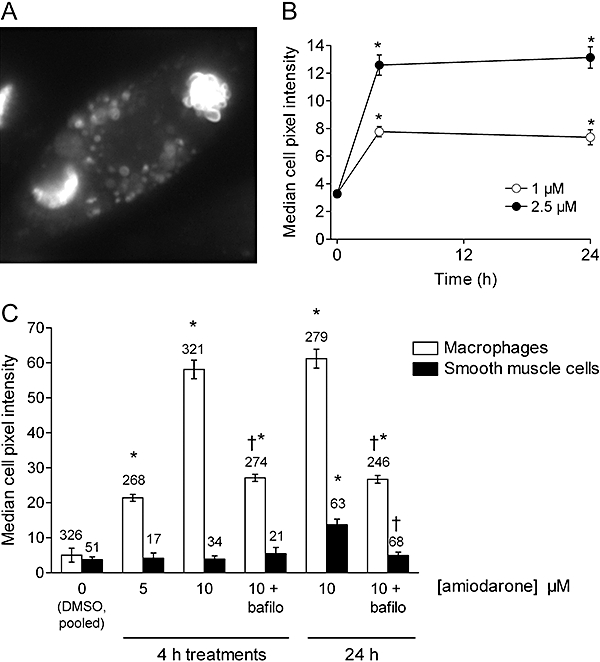
(A) Close-up view of a macrophage treated with amiodarone (10 µM, 24 h) (violet epifluorescence, 600×). (B) Quantification of the cellular violet fluorescence in macrophages treated with amiodarone (1 or 2.5 µM) for 0–24 h, as indicated. Values are means ± SEM of 67–255 cells from two or one donor, for the 1 or 2.5 µM drug levels, respectively. (C) Quantification of the cellular violet fluorescence in macrophages and smooth muscle cells treated with amiodarone, bafilomycin A1 (bafilo; 100 nM) or both (sample photographic record in Figure 2). Treatment duration and amiodarone concentration were as indicated. In (B) and (C), fluorescence intensity for each cell was determined as the median color intensity inside the manually drawn cell perimeter (Photoshop software). Then, the average value of the control cells was subtracted, and the values were compared with non-parametric tests. Values are means ± SEM of the number of determination indicated on or above each bar. In (B) and (C), the Kruskal–Wallis test indicated that the values for each cell type were significantly heterogenous (P < 10–4). Values different from those of vehicle-treated cells according to Dunn's multiple comparison test: *P < 0.001; in (C), effect of bafilomycin addition relative to the appropriate treatment: †P < 0.001 (Dunn's test).
HEK 293a cells, cultured in the presence of 10% FBS, were treated with amiodarone (10 or 20 µM), the V-ATPase inhibitor bafilomycin A1 (100 nM) or both (Figure 4, statistical analysis of quantified cell fluorescence in Figure 5). Amiodarone induced a vacuolar morphology at both concentrations and treatment durations. The size of the vacuoles was variable, but generally small relative to vacuoles induced by millimolar procainamide in this or other cell types (Morissette et al., 2005; 2008b; data not shown). The vacuolar morphology was prevented by co-treatment with bafilomycin A1, which by itself did not change the cell appearance. The violet fluorescence of amiodarone in treated cells was both granular, coinciding with vacuoles, and diffuse in the cytosol (especially at 20 µM; Figure 4). Only the first vacuolar component of amiodarone uptake was inhibited by bafilomycin co-treatment (Figure 4), accounting for a significant but partial inhibition of total cell fluorescence (Figure 5B,C) in cells treated with 20 µM amiodarone and bafilomycin A1 at the time-points 4 or 24 h.
Figure 4.

Morphological alterations associated with the treatment of HEK 293a cells with amiodarone (added at the indicated concentration for 4 or 24 h), bafilomycin A1 (100 nM) or both. Each field shows intact, unstained cells in phase contrast and violet fluorescence (UV excitation). Original magnification 600×.
Figure 5.
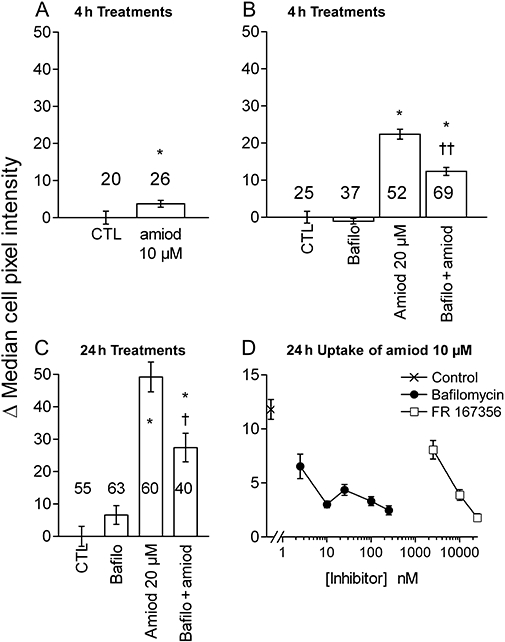
(A–C). Quantification of the cellular violet fluorescence in HEK 293a cells treated with amiodarone (amiod), bafilomycin A1 (bafilo; 100 nM) or both (sample photographic record in Figure 4). Treatment duration and amiodarone concentration as shown. Fluorescence intensity for each cell was determined as the median color intensity in the manually drawn cell perimeter (Photoshop software). Then, the average value of the control cells was subtracted, and the values were compared with non-parametric tests. Values are means ± SEM of the number of determination indicated on or above each bar. In (A), * indicates a significant difference (P < 0.05, Mann–Whitney test). The Kruskal–Wallis test indicated that the multiple values reported in (B) and (C) are significantly heterogenous (P < 10−4). Values different from their respective control according to Dunn's multiple comparison test: *P < 0.001; effect of bafilomycin addition relative to the appropriate treatment: †P < 0.05; ††P < 0.001 (Dunn's test). (D) Comparative effect of co-treatment with variable concentrations of bafilomycin A1 or FR167356 on the uptake of amiodarone (10 µM, 24 h) by HEK 293a cells (47–189 cells evaluated per experimental point). The Kruskal–Wallis test indicated that the values were significantly heterogenous (P < 10–4). All values from inhibitor-treated cells are different (P < 0.05) from that vehicle-treated cells except for FR167365, 2.5 µM (Dunn's multiple comparison test).
Bafilomycin A1 is a highly specific inhibitor of V-ATPase (Bowman et al., 2006); to study the specificity of its action as an inhibitor of amiodarone uptake in HEK 293a cells, we have used a range of bafilomycin concentrations in cells co-treated with the anti-arrhythmic drug (10 µM for 24 h, Figure 5D). Bafilomycin A1 (2.5–250 nM) suppressed amiodarone uptake in a concentration-dependent manner and maximally at 10 nM, congruent with a reported IC50 value of 10 nM in semi-purified fungal V-ATPase (Bowman et al., 2006) and similar to concentrations active in intact mammalian cells (Tapper and Sundler, 1995). A structurally unrelated synthetic inhibitor of V-ATPase, FR167356, was most active at 10–25 µM against amiodarone uptake, congruent with reported potency in assays that involve lysosomal (intracellular) V-ATPase inhibition (Niikura et al., 2004). The effect of V-ATPase inhibition on amiodarone uptake was proportionally larger for a drug concentration of 10 µM than for a 20 µM level.
Similar to previously reported results concerning procainamide-induced vacuolization (Morissette et al., 2008b), amiodarone-induced vacuoles (20 µM, 4 or 24 h) were decorated with GFP-conjugated markers of late endosomes/lysosomes (Rab7, CD63) and by the autophagy effector (GFP-LC3) in HEK 293a cells (Figure 6). The labelling of cytoplasmic particles by the latter fusion protein was rare in control cells and increased progressively at times 4 and 24 h, with a transition from mainly hollow vacuoles at 4 h to solid ones at 24 h. Rab7- and CD63-positive fine granules existed in control cells, but these proteins generally labelled larger and hollow vesicles in amiodarone-treated cells (Figure 6).
Figure 6.
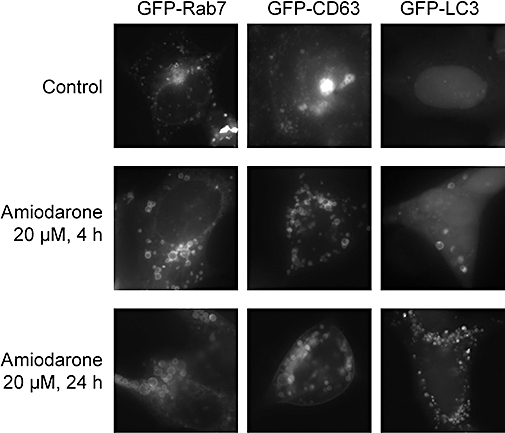
Distribution of GFP fusion proteins in transiently transfected HEK 293a cells further treated as indicated with amiodarone. Original magnification of intact, unstained cells 1000×.
LC3 is processed from a cytosolic type I, ∼18 kDa form to an autophagosome-bound, ∼16 kDa and lipidated type II. Untransfected HEK 293a cells or cells expressing recombinant GFP-LC3 expressed very little endogenous LC3-I; the second type of cells expressed a strong ∼45 kDa band reactive with the anti-GFP monoclonal antibody and corresponding to the intact fusion protein (Figure 7). Bafilomycin A1 treatment (100 nM, 24 h) induced the cleavage of LC3 I into LC3 II, but apparently not that of GFP-LC3 which was less sensitive. However, treatments that cause the vacuolar cytopathology (procainamide 2.5 mM or amiodarone 10–20 µM) caused the cleavage of both endogenous LC3 and recombinant GFP-LC3 in the same cells (Figure 7). As observed in other cell types (Morissette et al., 2008b), the total LC3 signal (I + II) was considerably stronger in cells treated with bafilomycin or with an amine causing vacuolization, consistent with an up-regulation or stabilization of the protein.
Figure 7.
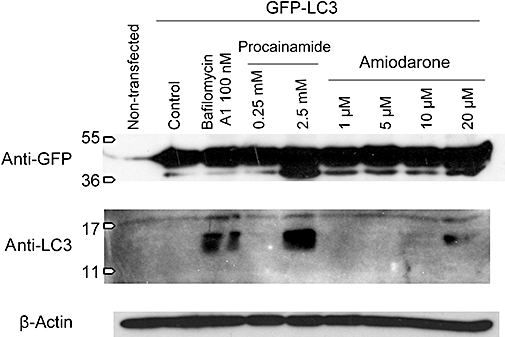
Processing of recombinant GFP-LC3 or endogenous LC3 in HEK 293a transfected with the GFP-LC3 expression vector and subsequently subjected to the indicated treatments for 24 h. Immunoblots for GFP or LC3 were applied in the same total cell extract. β-actin immunoblot was also performed to demonstrate equal loading.
Primary cultures of human smooth muscle cells react to millimolar procainamide with a vacuolar, autophagic and phospholipidosis-like response (Morissette et al., 2008b). We wanted to verify that amiodarone, at a concentration that essentially determines a vacuolar accumulation (10 µM; Figure 2), can also induce phospholipidosis and macroautophagic accumulation as a function of time in the same cell type (long exposures to the drug are not well tolerated by HEK 293a cells). The cell lipids were stained with Nile red following various treatments (red and green fluorescence; Figure 8A). The staining was relatively smooth in control cells, with few and small intensely stained particles. Bafilomycin treatment (24 h) increased the density of the small lipid particles. Amiodarone induced the formation of many vacuoles, some of which were hollow in many cells after 24 h of treatment (inset, Figure 8A), but all vacuoles became lipid filled and more abundant as a function of time (48–72 h; Figure 8A). If cells treated with amiodarone for 48 h were washed and further incubated for a 24 h period, the size of the vacuoles and their total lipid content regressed. The macroautophagic accumulation induced by long amiodarone treatments was detected by the immunofluorescence for endogenous LC3 (Figure 8B). While a short treatment (4 h) with 20 µM amiodarone did not induce granular staining, the specific fluorescence related to endogenous LC3 was important and concentration dependent at 48 h (Figure 8B). LC3 immunoreactivity was minimal in control cells, consistent with the apparent up-regulation or stabilization of granular LC3 in vacuolar cells (Morissette et al., 2008b).
Figure 8.
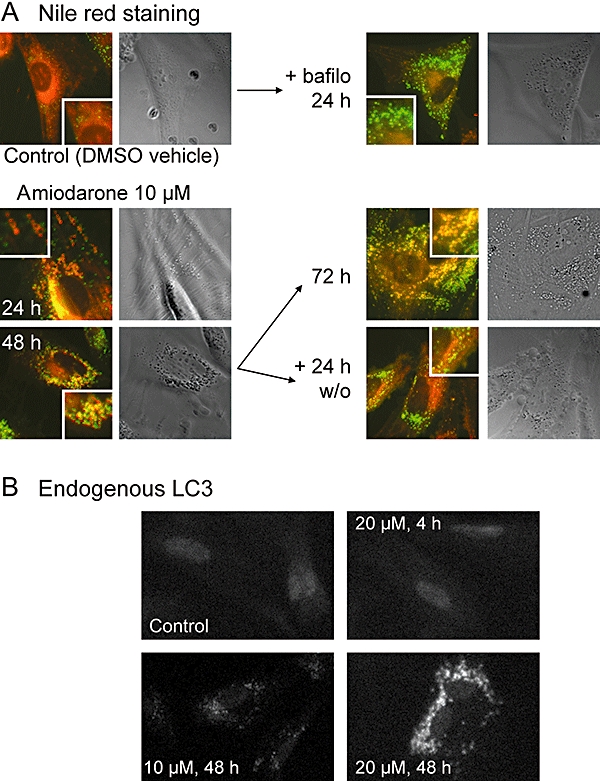
Effects of amiodarone on human smooth muscle cells. (A) Nile red staining for phospholipidosis. Typical cells treated with amiodarone (10 µM), its DMSO vehicle, bafilomycin A1 (300 nM; bafilo) or drug washout (w/o) alone or sequentially as indicated. Epifluorescence images (red and green channels merged) with enlargement in insets are shown along with the same fields in phase contrast to appreciate the vacuolar morphology. Original magnification 400×. (B) Immunofluorescence for endogenous LC3 in human smooth muscle cells treated as indicated with amiodarone, then fixed, permeabilized and stained. Original magnification 100×. Secondary antibody labelled with Alexa fluor-488.
The skin paraffin sections from the male patient with amiodarone-induced face discoloration reported by Ammoury et al. (2008) were evaluated for the presence of macroautophagy. As reported, macrophages were abundant in the superficial dermis of pigmented skin (Figure 9, top, detected as CD68-positive cells). A consecutive tissue section showed that many of these cells contained immunoreactive LC3 granules (Figure 9, top). The cells that were positive for either LC3 or CD68 were also positive for a brownish deposit already described in the skin of this and other amiodarone-treated patients (Figure 9, bottom; Delage et al., 1975; Miller and McDonald, 1984; Ammoury et al., 2008; Radman et al., 2008). This lipofuscin-like deposit has a yellowish fluorescence of its own (Miller and McDonald, 1984; Dowson et al., 1986), possibly explaining why the control tissue sections without the first antibodies exhibited a low level of fluorescence in the group of cells positive for LC3 and CD68 (Figure 9, top). The violet fluorescence of amiodarone is not expected in paraffin sections that have been exposed to multiple solvents. A biopsy from the face of a control male subject showed some small CD68-positive cells scattered at a low density in the dermis; they were not positive for LC3 (data not shown).
Figure 9.

Immunofluorescence for LC3 and CD68 in the skin of a patient with amiodarone-induced bluish skin pigmentation (paraffin sections). A mixture of phase contrast, fluorescence and transmission is shown as indicated. Secondary antibody labelled with Alexa fluor-594.
Discussion
Amiodarone caused the vacuolization of human cells (macrophages, smooth muscle cells, HEK 293a) at 1–20 µM (4 h), and the drug was present in a concentrated form in numerous cytosolic vacuoles (shown by its specific violet fluorescence under UV excitation; Figures 2, 3A and 4). Further, the V-ATPase blocker bafilomycin A1 prevented the vacuolar component of the uptake in the three tested cell types, supporting the assumption that amiodarone was subjected to the same sequestration mechanism as the one reported previously for procainamide. However, the kinetics of amiodarone uptake by HEK 293a and smooth muscle cells is slower than that of procainamide, the latter being complete after 1–2 h of incubation (Morissette et al., 2008b). A possible explanation is related to the fact that amiodarone is more tightly bound to plasma proteins (Vassallo and Trohman, 2007) and used at much lower concentrations than procainamide. Thus, albumin concentration in the culture medium with 10% FBS exceeds that of amiodarone (10–20 µM) in our experiments in HEK 293a cells; nevertheless, the avidity of the acidic organelles for the drug is sufficient to drive its cellular sequestration. This avidity is determined by the cell type, monocyte-derived macrophages possessing prominent acidic vesicles (Basta et al., 1999) that are the probable basis of the strikingly rapid and intense capture of amiodarone that clearly extends to the therapeutic concentration range in these cells (Figures 2 and 3).
In transfectable HEK 293a cells, amiodarone-induced vacuoles are partially labelled with GFP-conjugated forms of the autophagy effector protein LC3 and of the late endosome/lysosome markers Rab7 and CD63 (Figure 6). Immunoblots showed the conversion of LC3 I into LC3 II, or of its GFP-conjugate form with lesser sensitivity, at relevant amiodarone concentrations (10–20 µM, 24 h; Figure 7). The 48–72 h cell treatments with amiodarone induced the formation of lipid-filled vacuoles in human smooth muscle cells, along with the accumulation of endogenous LC3 in vacuoles (Figure 8). Thus amiodarone recapitulates the known effects of toxicological levels of procainamide (Morissette et al., 2008b), but in the micromolar concentration range, close to its therapeutic level and ∼100-fold less than active concentrations of the less lipophilic agent procainamide. The high liposolubility of amiodarone may favour the entry into cells by simple diffusion, the postulated first step of V-ATPase-mediated sequestration; the drug is also known to decrease lipid mobility in cells via hydrophobic interactions, and increase the lipid order parameter as much as cholesterol (Chatelain et al., 1986). Such a high solubility in lipids may account for the bafilomycin-resistant component of amiodarone uptake (Figures 2 and 4). In contrast, procainamide sequestration in cells was completely inhibited or reversed by bafilomycin A1 treatment in a previous study (Morissette et al., 2008b). In various cell types, bafilomycin A1 is reported to increase the lysosomal pH by two units (from about 4.5 to ∼6.5, essentially similar to cytosolic pH; Tapper and Sundler, 1995; Haggie and Verkman, 2009). Thus, the relatively small proportion of non-protonated amiodarone molecules (pKa∼9) may be essentially similar in the cytosol and in vacuoles during V-ATPase inhibition. However, this proportion becomes close to zero in vacuoles when V-ATPase is active, this forming the basis for vacuolar trapping of cationic drugs.
One of the best clinically documented forms of drug-induced phospholipidosis is that induced by amiodarone (see Introduction), and several in vivo observations related to its side effects (foamy macrophages, lamellar bodies, skin discoloration, etc.) could be initiated by V-ATPase-mediated intracellular trapping with further reorganization of vacuoles, as shown in the present results. The mechanism of drug-induced phospholipidosis is obscure, although the direct binding of amphiphilic amine drugs to phospholipids with ensuing inhibition of phospholipases has been repeatedly proposed (Reasor et al., 2006). We have previously suggested that the double envelopment of macroautophagosomes with membranes from the endoplasmic reticulum might explain the presence of lipids in the form of concentric membranes in ‘onionoid’ vesicles if multiple cycles of macroautophagy occur (Morissette et al., 2008b). This is plausible as the cell labelling with GFP-LC3, initially in the form of hollow vesicles, evolved towards filled vesicles that may have accumulated the macroautophagy effector during repeated cycles of autophagic envelopment (Figure 6). The ‘filled’ aspect of endogenous LC3 in smooth muscle cells treated for 48 h with amiodarone (Figure 8B) and the progressive ‘filling’ of the vacuoles with lipids (Figure 8A) also support this hypothesis.
Amiodarone, procainamide and other amines reach intracellular vacuoles that have a low luminal pH, become protonated, are concentrated and draw some water by an osmotic mechanism. Bafilomycin A1 inhibits the vacuolar response induced by amine drugs, but reproduces some of their effects, such as LC3 II accumulation (Figure 7) and a mild phospholipidosis-like response (Figure 8). This probably reveals a basal level of macroautophagy in all cells that resolves by lysosomal fusion with the phagosomes and their digestion. Swelled vacuoles induced by amine drugs such as amiodarone are apparently rapidly tagged for automacrophagic degradation, but the captured drugs may inhibit this process by virtue of their acid-buffering capacity, thus causing a form of persistent, but ineffective autophagy maintained by the continuous influx of amine drug, because V-ATPase of lysosomal origin is a constituent of the autophagosome. This was verified in clinically relevant material from one patient, as the dermal cells known to accumulate the drug most effectively, the macrophages, also accumulated LC3 (Figure 9). It is desirable to extend this preliminary investigation to additional patients with amiodarone-induced skin discoloration. In histiocytes of such patients, the vacuoles are electron dense probably owing to the iodinated structure of the drug (Figure 1; Miller and McDonald, 1984; Ammoury et al., 2008). Interestingly, the occurrence of the double envelopment of these structures is described in an older electron microscopy study, consistent with macroautophagy (Delage et al., 1975). The vacuoles induced by amiodarone in cultured cells regress upon drug washout (Figure 8), reminiscent of the slow regression of amiodarone side effects that are dependent on tissue deposits (corneal opacification, skin discoloration …) following discontinuation of the drug. In these situations, the autophagic digestion is likely to resume as the vacuolar accumulation of the drug is released.
Why is amiodarone-induced pigmentation maximal in exposed skin areas? (Enseleit et al., 2006; Ammoury et al., 2008). UV light exposition increases the density of neutrophils, differentiated macrophages and mononuclear cells with prominent lysosomes in skin (Cooper et al., 1993; Kabashima et al., 2007; Halliday et al., 2008). These infiltrating cells may capture amiodarone with high efficiency, especially cells of the monocyte–macrophage lineage, already known to be prominent in the physiopathology of the lung and kidney diseases induced by the drug (Myers et al., 1987; Pintavorn and Cook, 2008), and abundant in the pigmented skin of the affected patients (paraffin sections; Ammoury et al., 2008; electron microscopy, Miller and McDonald, 1984). Macrophages are rich in acidic vacuoles and concentrate comparatively large amounts of amiodarone (Figures 2 and 3). However, electron microscopy revealed that other cell types (fibroblasts, endothelial cells, etc.) possessed vacuolar inclusions in the skin of the studied patient, supporting the lack of absolute cell type specificity for V-ATPase-mediated uptake of cationic drugs. In addition, UV exposure induces inflammatory vasodilation that may facilitate drug delivery to the skin (Radman et al., 2008). We have failed to increase the sequestration of procainamide in UV-irradiated cells (data not shown), suggesting that V-ATPase activity is not directly responsive to light. On the other hand, photoreactions of amiodarone leading to toxic products cannot be ruled out, and we have not addressed this line of investigation.
Hypothyroid or hyperthyroid states induced by amiodarone may be related to its iodinated structure and interference with the metabolism of the thyroid gland; a close but non-iodinated analog in late clinical development, dronedarone (Figure 1), has been introduced to reduce this risk (Singh et al., 2007); however, it remains a tertiary amine with high logP (7.1) that will be also subjected to ion trapping and may reproduce the side effects caused by tissue deposits of drugs. The present findings may also apply to other amine drugs that cause skin discoloration, like imipramine (logP 4.4, a tertiary amine), reported to cause a vacuolar cytopathology in dermal histiocytes (Metelitsa et al., 2005).
In summary, the vacuolar sequestration of amiodarone occurs at a concentration close to therapeutic levels, is mediated by V-ATPase and evolves towards persistent macroautophagy and a phospholipidosis-like reaction. Autophagosome accumulation was verified in a clinically relevant sample. This cytopathology is not cell type specific, but tissue macrophages appear to be particularly susceptible.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (operating grant MOP-74448 to F.M., Canada Graduate Scholarships Doctoral Award to G.M.). We thank Dr Marc Pouliot for access to his microscope.
Glossary
Abbreviations:
- DMSO
dimethylsulphoxide
- PBML
peripheral blood mononuclear leukocyte
- V-ATPase
vacuolar ATPase
Conflict of interest
The authors state no conflict of interest.
References
- Adams PC, Sloan P, Morley AR, Holt DW. Peripheral neutrophil inclusion in amiodarone treated patients. Br J Pharmacol. 1986;22:736–738. doi: 10.1111/j.1365-2125.1986.tb02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoury A, Michaud S, Paul C, Prost-Squarcioni C, Alvarez F, Lamant L, et al. Photodistribution of blue-gray hyperpigmentation after amiodarone treatment: molecular characterization of amiodarone in the skin. Arch Dermatol. 2008;144:92–96. doi: 10.1001/archdermatol.2007.25. [DOI] [PubMed] [Google Scholar]
- Anderson A, Borlak J. Drug-induced phospholipidosis. FEBS Lett. 2006;580:5533–5540. doi: 10.1016/j.febslet.2006.08.061. [DOI] [PubMed] [Google Scholar]
- Basta S, Knoetig SM, Spagnuolo-Weaver M, Allan G, McCullough KC. Modulation of monocytic cell activity and virus susceptibility during differentiation into macrophages. J Immunol. 1999;162:3961–3969. [PubMed] [Google Scholar]
- Bowman BJ, McCall ME, Baertsch R, Bowman EJ. A model for the proteolipid ring and bafilomycin/concanamycin-binding site in the vacuolar ATPase of Neurospora crassa. J Biol Chem. 2006;281:31885–31893. doi: 10.1074/jbc.M605532200. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leukocytes from human blood. Scand J Clin Lab Invest. 1968;21(Suppl 9):9–29. [PubMed] [Google Scholar]
- Casartelli A, Bonato M, Cristofori P, Crivellente F, Dal Negro G, Masotto I, et al. A cell-based approach for the early assessment of the phospholipidogenic potential in pharmaceutical research and drug development. Cell Biol Toxicol. 2003;19:161–176. doi: 10.1023/a:1024778329320. [DOI] [PubMed] [Google Scholar]
- Chatelain P, Ferreira J, Laruel R, Ruysschaert JM. Amiodarone induced modifications of the phospholipid physical state. Biochem Pharmacol. 1986;35:3007–3013. doi: 10.1016/0006-2952(86)90379-5. [DOI] [PubMed] [Google Scholar]
- Cooper KD, Duraiswamy N, Hammerberg C, Allen E, Kimbrough-Green C, Dillon W, et al. Neutrophils, differentiated macrophages, and monocyte/macrophage antigen presenting cells infiltrate murine epidermis after UV injury. J Invest Dermatol. 1993;101:155–163. doi: 10.1111/1523-1747.ep12363639. [DOI] [PubMed] [Google Scholar]
- Delage C, Lagacé R, Huard J. Pseudocyanotic pigmentation of the skin induced by amiodarone: a light and electron microscopic study. Can Med Assoc J. 1975;112:1205–1208. [PMC free article] [PubMed] [Google Scholar]
- Diaz-Guzman E, Mireles-Cabodevila E, Arrosi A, Kanne JP, Budev M. Amiodarone pulmonary toxicity after lung transplantation. J Heart Lung Transplant. 2008;27:1059–1063. doi: 10.1016/j.healun.2008.05.023. [DOI] [PubMed] [Google Scholar]
- Dowson JH, Miller RA, McDonald AT. Autofluorescence emission spectra of dermal lipofuscinosis associated with amiodarone therapy. Arch Dermatol. 1986;122:244–245. [PubMed] [Google Scholar]
- Enseleit F, Wyss CA, Duru F, Noll G, Ruschitzka F. The blue man: amiodarone-induced skin discoloration. Circulation. 2006;113:e63. doi: 10.1161/CIRCULATIONAHA.105.554303. [DOI] [PubMed] [Google Scholar]
- Haggie PM, Verkman AS. Unimpaired lysosomal acidification in respiratory epithelial cells in cystic fibrosis. J Biol Chem. 2009;284:7681–7686. doi: 10.1074/jbc.M809161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Norval M, Byrne SN, Huang XX, Wolf P. The effects of sunlight on the skin. Drug Discov Today Dis Mech. 2008;5:e201–e209. [Google Scholar]
- Halliwell WH. Cationic amphiphilic drug-induced phospholipidosis. Toxicol Pathol. 1997;25:53–60. doi: 10.1177/019262339702500111. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, et al. Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol. 2002;157:1211–1222. doi: 10.1083/jcb.200111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Nagamachi M, Honda T, Nishigori C, Miyachi Y, Tokura Y, et al. Prostaglandin E2 is required for ultraviolet B-induced skin inflammation via EP2 and EP4 receptors. Lab Invest. 2007;87:49–55. doi: 10.1038/labinvest.3700491. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Safety. 2008;31:127–141. doi: 10.2165/00002018-200831020-00003. [DOI] [PubMed] [Google Scholar]
- Metelitsa AI, Nguyen GK, Lin AN. Imipramine-induced facial pigmentation: case report and literature review. J Cutan Med Surg. 2005;9:341–345. doi: 10.1007/s10227-005-0139-7. [DOI] [PubMed] [Google Scholar]
- Miller RA, McDonald AT. Dermal lipofuscinosis associated with amiodarone therapy. Report of a case. Arch Dermatol. 1984;120:646–649. [PubMed] [Google Scholar]
- Morissette G, Moreau E, C-Gaudreault R, Marceau F. Massive cell vacuolization induced by organic amines such as procainamide. J Pharmacol Exp Ther. 2004;310:395–406. doi: 10.1124/jpet.104.066084. [DOI] [PubMed] [Google Scholar]
- Morissette G, Moreau E, C-Gaudreault R, Marceau F. N-substituted 4-aminobenzamides (procainamide analogs): an assessment of multiple cellular effects concerning ion trapping. Mol Pharmacol. 2005;68:1576–1589. doi: 10.1124/mol.105.016527. [DOI] [PubMed] [Google Scholar]
- Morissette G, Couture JP, Désormeaux A, Adam A, Marceau F. Lack of direct interaction between enalaprilat and the kinin B1 receptors. Peptides. 2008a;29:606–612. doi: 10.1016/j.peptides.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Morissette G, Lodge R, Marceau F. Intense pseudotransport of a cationic drug mediated by vacuolar ATPase: procainamide-induced autophagic cell vacuolization. Toxicol Appl Pharmacol. 2008b;228:364–377. doi: 10.1016/j.taap.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Myers JL, Kennedy JI, Plumb VJ. Amiodarone lung: pathologic findings in clinically toxic patients. Hum Pathol. 1987;18:349–354. doi: 10.1016/s0046-8177(87)80164-8. [DOI] [PubMed] [Google Scholar]
- Niikura K, Takano M, Sawada M. A novel inhibitor of vacuolar ATPase, FR167356, which can dissociate between osteoclast vacuolar ATPase and lysosomal ATPase. Br J Pharmacol. 2004;142:558–566. doi: 10.1038/sj.bjp.0705812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nioi P, Perry BK, Wang EJ, Gu YZ, Snyder RD. In vitro detection of drug-induced phospholipidosis using gene expression and fluorescent phospholipid-based methodologies. Tox Sci. 2007;99:162–173. doi: 10.1093/toxsci/kfm157. [DOI] [PubMed] [Google Scholar]
- Pintavorn P, Cook WJ. Progressive renal insufficiency associated with amiodarone-induced phospholipidosis. Kidney Int. 2008;74:1354–1357. doi: 10.1038/ki.2008.229. [DOI] [PubMed] [Google Scholar]
- Radman M, Tomas D, Situm M, Solter M. What could be expected after fifteen years of amiodarone therapy? Eur J Dermatol. 2008;18:202–203. doi: 10.1684/ejd.2008.0372. [DOI] [PubMed] [Google Scholar]
- Reasor MJ, Kacew S. Drug-induced phospholipidosis: are there functional consequences? Exp Biol Med. 2001;226:825–830. doi: 10.1177/153537020122600903. [DOI] [PubMed] [Google Scholar]
- Reasor MJ, Hastings KL, Ulrich RG. Drug-induced phospholipidosis: issues and future directions. Expert Opin Drug Saf. 2006;5:567–583. doi: 10.1517/14740338.5.4.567. [DOI] [PubMed] [Google Scholar]
- Ruangchira-Urai R, Colby TV, Klein J, Nielsen GP, Kradin RL, Mark EJ. Nodular amiodarone lung disease. Am J Surg Pathol. 2008;32:1654–1660. doi: 10.1097/PAS.0b013e31816d1cbc. [DOI] [PubMed] [Google Scholar]
- Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, et al. Dronedanone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–999. doi: 10.1056/NEJMoa054686. [DOI] [PubMed] [Google Scholar]
- Somani P, Simon VA, Temesy-Armos PN, Gross SA, Didio LJ. Amiodarone-associated changes in human neutrophils. Am J Cardiol. 1986;57:666–672. doi: 10.1016/0002-9149(86)90856-8. [DOI] [PubMed] [Google Scholar]
- Tapper H, Sundler R. Bafilomycin A1 inhibits lysosomal, phagosomal and plasma membrane H+-ATPase and induces lysosomal enzyme secretion in macrophages. J Cell Physiol. 163:137–144. doi: 10.1002/jcp.1041630116. [DOI] [PubMed] [Google Scholar]
- Vassallo P, Trohman RG. Prescribing amiodarone: an evidence-based review of clinical indications. JAMA. 2007;298:1312–1322. doi: 10.1001/jama.298.11.1312. [DOI] [PubMed] [Google Scholar]
- Zimetbaum P. Amiodarone for atrial fibrillation. N Engl J Med. 2007;356:935–941. doi: 10.1056/NEJMct065916. [DOI] [PubMed] [Google Scholar]


