Abstract
Formation of reactive nitrogen and oxygen intermediates (RNI and ROI) is an essential part of the innate immune response. Markers of systemic RNI production are increased in the setting of systemic lupus erythematosus (SLE) activity. Several lines of evidence suggest mechanisms through which the activity of inducible nitric oxide synthase (iNOS) is pathogenic in SLE, including the ability of peroxynitrite (ONOO−, a product of iNOS activity) to modify proteins, lipids, and DNA. These modifications can alter enzyme activity and may increase the immunogenicity of self antigens, leading to a break in immune tolerance. In humans, observational data suggest that overexpression of iNOS and increased production of ONOO− lead to glomerular and vascular pathology. Therapies designed to target iNOS activity or scavenge ROI and RNI are in development and may provide the means to reduce the pathogenic consequences of ROI and RNI in SLE.
Keywords: Nitric oxide, Lupus, Reactive oxygen, species, Reactive nitrogen, species, 3-Nitrotyrosine, Lipid peroxidation, Apoptosis, Autoantigens
Introduction
Systemic lupus erythematosus (SLE) is a classic autoimmune disease defined by the formation of immune complexes with autoantigens. However, the innate immune system plays an integral role in propagating inflammatory responses initiated by this acquired immune response. An important part of that innate immune response is the production of reactive nitrogen and oxygen intermediates (RNI and ROI). One of the most widely studied RNI, nitric oxide (NO), is overproduced in the setting of lupus activity. Its pathogenic potential in lupus or any other disease lies largely in the extent of its production and the proximity of its synthesis to ROI such as superoxide (SO). NO and SO react to form peroxynitrite (ONOO−), a much more reactive and potentially pathogenic molecule. There is convincing evidence in murine lupus nephritis that inducible nitric oxide synthase (iNOS) activity increases with the progression of disease and leads to glomerular, joint, and dermal pathology. In addition, ONOO−-mediated modifications of proteins and DNA may increase the immunogenicity of these self antigens, leading to a break in immune tolerance. Redox-sensitive signaling pathways can be activated by the production of ROI/RNI, leading to further transcription of inflammatory mediators. In humans, there are observational data suggesting that overexpression of iNOS and increased production of ONOO− lead to glomerular and vascular pathology. Therapies designed to target iNOS activity or scavenge ROI/RNI have not been tested in humans in part due to concerns over the specificity of many available compounds for their targets. However, several new compounds are in development that offer promise for human trials.
Biology of reactive nitrogen intermediates (RNI)
Free radicals are highly reactive molecules with unpaired electrons. They represent an important arm of host defense against a variety of pathogens [1]. Not only are reactive oxygen and nitrogen intermediates (RONI) directly toxic to invading pathogens, they activate redox-sensitive signaling pathways such as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) that in turn regulate the transcription of proinflammatory proteins such as cytokines [2]. In systemic lupus erythematosus (SLE), overproduction of free radicals in the absence of infection may lead to a break in immune tolerance, increased tissue damage, and altered enzyme function. In this review, the discussion of reactive intermediates (RI) will be confined to reactive oxygen and nitrogen free radicals. Examples of ROI include superoxide (SO), hydrogen peroxide, and hydroxyl radicals, while nitric oxide (NO) and peroxynitrite (ONOO−) are the RNI to be discussed. Reactive oxygen and nitrogen intermediates (RONI) play an important role in cellular signaling processes when produced at low levels. At higher levels, these molecules can cause direct toxicity to cells and induce modifications to lipids, amino acids, and DNA.
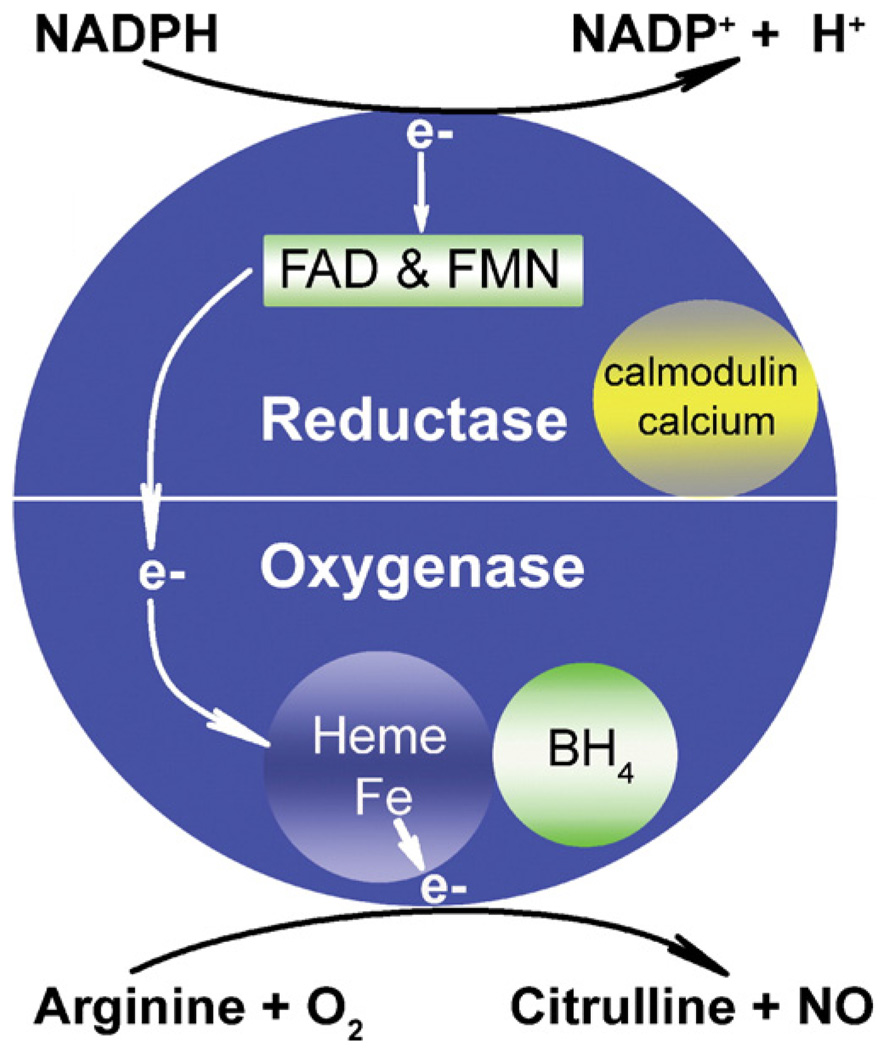
NO is a membrane-permeable free radical molecule synthesized by nitric oxide synthase (NOS) using arginine and oxygen as substrates. Three isoforms of NOS are transcribed from three separate genes. All isoforms dimerize in the presence of cofactors to become active. Each monomer contains a reductase and oxygenase domain. The reductase domain catalyzes the transfer of two electrons to heme iron in the oxygenase domain. Calmodulin, nicotinamide adenine dinucleotide phosphate (NADPH), flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) are required cofactors for the reductase domain. Electrons from the reductase domain are transferred to the oxygenase domain of the adjacent monomer, where heme and tetrahydrobiopterin (BH4) act as cofactors. Here, a reaction between O2 and l-arginine is catalyzed, resulting in formation of NO and citrulline (Fig. 1). Two isoforms (endothelial or eNOS and neuronal or nNOS) are generally constitutively expressed and are dependent on sufficient concentrations of calcium for activity. In the vascular system, NO produced by eNOS is a potent vasodilator and regulator of vascular tone in response to shear stress. Nitroglycerin mimics the activity of eNOS by acting as a donor of NO [3]. The beneficial effect of NO produced by the constitutively expressed NOS isoforms is blunted when NO is produced in an environment high in ROI as discussed later.
Figure 1.
NO synthesis from arginine by iNOS and cofactors. Electrons (e–) are donated by NADPH to FAD and FMN in the reductase domain. This step requires Ca2+ (much higher levels for eNOS and nNOS than for iNOS) and calmodulin. Two cycles of electrons are then transferred by these carriers to heme iron in the oxygenase domain of the adjacent dimer. This reaction is similar to that in P450 enzymes. The role of tetrahydrobiopterin (BH4) in this process is unclear, but it may assist in the coupling of NADPH oxidation and NO formation, thus preventing SO formation. With arginine and O2 as substrates, donated electrons then catalyze two reaction steps, the formation of Nω-hydroxy-l-arginine (NHA) followed by conversion of NHA to NO and citrulline. SO is formed when l-arginine substrate is limited, and electrons from the reductase domain react directly with oxygen [3]. This figure is reprinted from [77].
A third NOS gene (NOS2) produces an inducible isoform (iNOS) that is primarily expressed in immune cells, most notably macrophages and macrophage-derived cells. INOS is expressed in response to inflammatory stimuli that are well characterized in murine cells. Among these stimuli are several cytokines and toll-like receptor ligands such as lipopolysaccharide, interleukin-6 (IL6), interferon-γ (IFNγ), IL1β, and tumor necrosis factor-α (TNFα). In human cells, complex mixtures of cytokines are necessary for induction. In most cells, signaling pathways converge on the janus kinase/signal transducer and activator of transcription (JAK/STAT) and/or the nuclear factor-kappa B (NF-κB) pathways [4]. Nuclear hormone receptors may play a role in regulation of iNOS induction. There is evidence to support a role for estrogen as an inducer [5] and PPARγ ligands as inhibitors [6] of iNOS induction in response to IFNγ or IFNγ + LPS stimulation respectively in murine cells. iNOS is expressed during pathologic states in human endothelial cells, synovial fibroblasts, polymorphonuclear cells, lymphocytes, and natural killer cells [7]. In normal human tissue, expression is strong in myocytes, skeletal muscle, and Purkinje cells [8].
iNOS produces log-fold higher amounts of NO than the constitutively expressed isoforms. In a low arginine environment, iNOS cannot transfer nitrogen to molecular oxygen, and electrons from the reductase domain combine with oxygen to produce SO [9]. NO, when combined with SO, forms peroxynitrite (ONOO−), a more reactive and toxic molecule than NO itself. ONOO− produced by immune cells is capable of killing intracellular pathogens and tumors cells. Glutathione peroxidase, catalase, superoxide dismutase, heme oxygenase, and antioxidants serve to protect host cells during inflammatory states by reducing the total ROI burden that can contribute to ONOO− production [10,11].
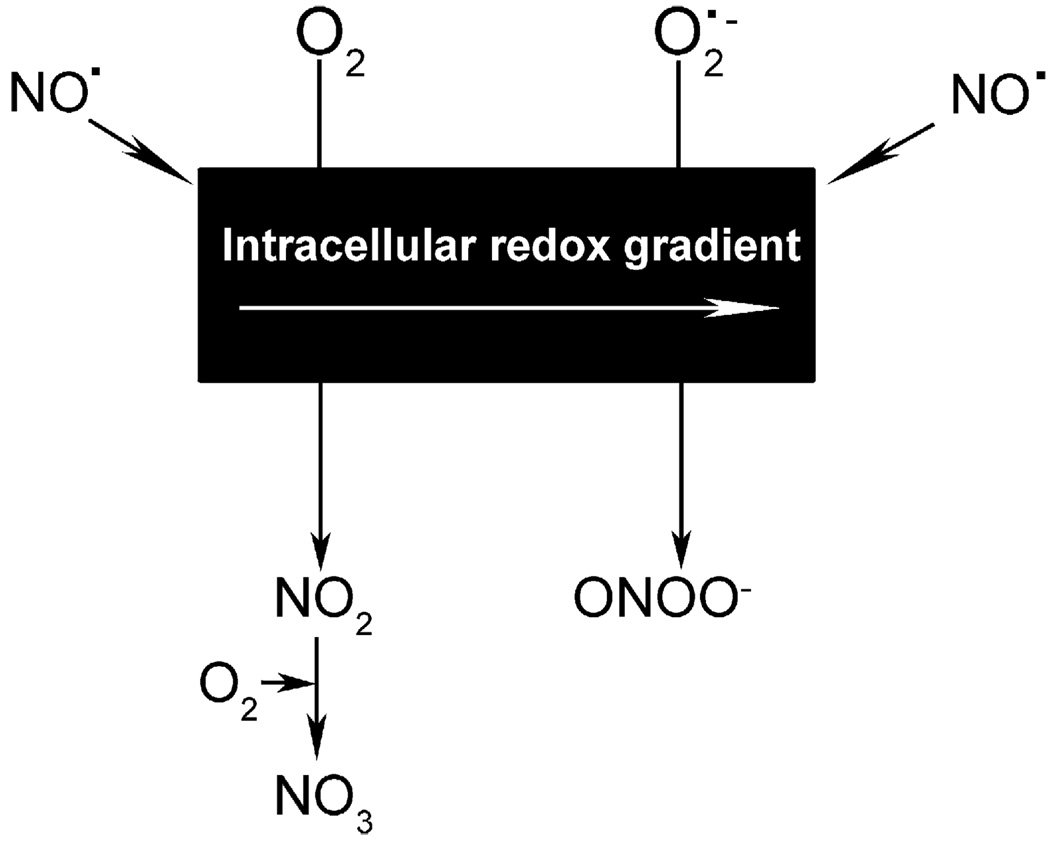
NO has the potential to induce both physiologic and pathologic effects, a dichotomy that pervades the literature. The ability of NO to induce cellular pathology is largely dependent on its conversion to more unstable nitrogen intermediates such as ONOO−. In turn, the production of ONOO− is dependent on levels of SO in the cellular microenvironment in which NO is released. The following will serve as examples of this concept. When NO is produced in proximity to mitochondria in a highly oxidative state, it can react with SO to form ONOO−. This molecule can in turn induce apoptosis of that cell via cytochrome c-mediated caspase activation [12]. Mitochondria in this state can be found in activated T cells of lupus subjects more frequently than in healthy controls [13]. Alternatively, if NO is produced in low levels in the absence of ROI, it promotes cell survival. This effect may occur as a result of nitrosation and inactivation of procaspases, which prevents caspase-mediated apoptosis [12]. Another example lies in the interaction between RNI and eicosanoid synthesis. ONOO− can act as a peroxide substrate for cyclooxygenase-2 (COX2) and increase its catalytic activity [14]. The opposite occurs with prostacyclin synthase; ONOO− reduces enzyme activity, possibly by nitrating a tyrosine residue near the heme binding site [15]. Thus, the fate of NO and its ultimate pathogenicity depends on the redox state of its immediate cellular milieu (Fig. 2).
Figure 2.
Cellular microenvironment changes the fate of NO. After synthesis by iNOS, eNOS, or nNOS, NO freely diffuses across membranes, forming a concentration gradient. Within this microenvironment also exists a redox gradient (represented by the black rectangle) formed by the presence of oxidant/reductant-coupled species. The redox state thus determines whether NO ultimately forms what are usually benign vs. pathogenic RNI. As an example, when formed in the presence of O2, NO can oxidize to NO2 and NO3. Whereas when formed in the presence of superoxide (SO or O2•−), NO oxidizes to form peroxynitrite (ONOO−) [12]. This figure is reprinted from [77].
Nitric oxide biology in murine models of lupus
Observational studies
While iNOS activity can suppress parasitemia or tumor growth, its overexpression in the setting of lupus disease activity appears to lead to organ damage and an altered immune response. Several studies involving murine models of lupus support this hypothesis. Both MRL/MpJ-Faslpr/J (MRL/lpr) and (New Zealand Black × New Zealand White)F1 (NZB/W) mice develop spontaneous proliferative lupus nephritis. MRL/lpr mice developed increasing levels of urine NO metabolites (nitrate + nitrite or NOX) in parallel with the onset of glomerulonephritis [16]. This increase in iNOS activity was associated with formation of 3-nitrotyrosine (3NTyr), a product of ONOO− and tyrosine (Tyr). Such modifications reduced the activity of catalase in the MRL/lpr kidney. Because catalase removes superoxide, its inactivation may have exposed cells to increased oxidative stress and accelerated tissue damage or modification of biologically active molecules [17].
Immune complex formation and tissue deposition do not appear to be dependent on iNOS activity in murine lupus, as iNOS inhibitor therapy, while improving renal histopathology, had no effect on glomerular immune complex deposition in MRL/lpr mice [16]. The expression of iNOS may instead be a result of downstream innate immune responses to immune complex formation with autoantigens. For example, serum 3NTyr levels were increased after implantation of human β2-glycoprotein I antibody producing hybridomas into mice with severe combined immunodeficiency syndrome [18]. A similar link between autoantibody deposition and iNOS expression/3NTyr formation has been observed in passive transfer models of anti-glomerular basement membrane (GBM) and myeloperoxidase (MPO) antibody glomerulonephritis. Expression of iNOS protein and/or formation of 3NTyr in glomerular tissue followed passive transfer of antibodies [19–21]. While immune complex deposition is not dependent on iNOS activity, ONOO− can increase the immunogenicity of autoantigens by forming neoepitopes as discussed below.
Manipulation of iNOS in murine lupus
Several studies utilizing competitive inhibitors of iNOS suggest that iNOS activity is pathogenic in murine lupus. Inhibiting iNOS activity in MRL/lpr mice before disease onset with the nonspecific arginine analog l-NG-monomethyl-l-arginine (l-NMMA) reduced 3NTyr formation in the kidney, partially restored renal catalase activity, and inhibited cellular proliferation and necrosis within the glomerulus [16,17,22]. This effect occurred in the absence of a change in immunoglobulin or complement deposition in the glomerulus, suggesting that increased iNOS expression occurred downstream of immune complex deposition and complement activation [16]. The partially selective iNOS inhibitor l-N6-(1-iminoethyl)lysine (l-NIL) had a similar effect when used to treat these mice prior to disease onset. In this study, the l-NIL-treated mice exhibited significant improvements in glomerular histopathology compared to controls and slight improvements compared to l-NMMA-treated mice. However, proteinuria was only partially inhibited in the l-NIL-treated mice, whereas l-NMMA-treated mice developed no significant proteinuria [22]. l-NMMA therapy in NZB/W mice that were already expressing clinical nephritis had a similar but less profound effect on proteinuria and renal histopathology than did preventative therapy. However, l-NMMA as monotherapy for the treatment of active disease was less effective in the rapidly progressive MRL/lpr model [23].
Some interventions that do not directly inhibit iNOS enzyme activity may derive additional benefit by their ability to reduce expression of iNOS. For instance, chemical induction of heme oxygenase-1 and oral administration of mycophenolate mofetil were both effective therapies for treating glomerulonephritis in MRL/lpr mice, and both reduced iNOS expression in the kidney [24—26]. The histone deacetylase inhibitor Trichostatin A, which attenuated renal disease in MRL/lpr mice, also inhibited NO production in cultured mesangial cells from the same murine model of lupus [27].
In contrast to the effectiveness of pharmacologic iNOS inhibition in murine lupus is the observation that iNOS−/−MRL/lpr mice, while having reduced signs of vasculitis and IgG rheumatoid factor production, had similar glomerular pathology to their MRL/lpr wild-type littermates [28]. One possibility is that inhibition of iNOS with arginine analogs may reduce pathology through non-iNOS-mediated mechanisms. The mechanisms behind the disparate effects of pharmacologic and genetic blockade of iNOS are still under investigation.
Potential mechanisms for pathogenicity of RNI suggested by studies in murine models of lupus
The mechanisms through which iNOS activity may be pathogenic in SLE has been studied in animal models and in vitro (Table 1). As mentioned above, ONOO−, a byproduct of iNOS activity, can nitrate protein amino acids and change the catalytic activity of enzymes. One such enzyme, catalase, serves to protect host tissues from free radical attack [17]. In vascular tissue, prostacyclin synthase [29] and eNOS [30] are inactivated by ONOO−, leading to vasoconstriction. These observations suggest that one mechanism through which iNOS activity is pathogenic is via deactivation of tissue protective enzymes.
Table 1.
Pathogenic effects of ONOO− on cellular molecules
|
Increasing attention is focusing on the manner in which immune tolerance is broken by presentation of autoantigens in a novel manner. Two such processes are noteworthy: (1) presentation of nuclear antigens in the proinflammatory context of late apoptotic blebs and (2) post-translational modification of self antigens to form novel epitopes or neoepitopes. Because nuclear antigens are presented in late apoptotic blebs [31], regulation of apoptosis and clearance of apoptotic cells are important areas of investigation. NO and ONOO− are both integral in regulating non-receptor-mediated apoptosis in many cellular systems [12]. To investigate the role of iNOS activity in apoptosis, MRL/lpr mice with active disease were treated with l-NMMA, an iNOS inhibitor. Compared to controls, treated mice exhibited reduced levels of splenocyte apoptosis. Treatment of cultured splenocytes isolated from mice with active disease with a NO donor resulted in increased levels of apoptosis [32]. NO or other RNI appeared to increase non-receptor-mediated apoptosis despite the well-described defect in receptor-mediated apoptosis in this murine model of lupus [33].
Another mechanism for inducing autoimmunity is via formation of neoepitopes in autoantigens. ONOO− can nitrate self antigens in a manner that leads to a break in immune tolerance. For instance, normal mice immunized with nitrated IgG produced anti-nitrotyrosine antibodies that cross reacted with single stranded DNA (ssDNA) [34]. Human native DNA modified with ONOO− induced greater immunogenicity in experimental animals than native DNA without modifications [35,36].
The literature is rife with reports of the seemingly antithetic properties of NO. As discussed above, many of the pathologic consequences of NO production arise from its synthesis in the setting of high reactive oxygen stress. NO can diffuse freely across membranes due to its uncharged nature but has a half-life of only approximately 30 s in biological systems [7]. Thus, iNOS activity can lead to ONOO− production only if it occurs within or in close proximity to a cell with high reactive oxygen content. One mechanism for production of SO and NO in close proximity is through the parallel production of SO by the reductase domain of iNOS itself. This process has been observed in murine macrophages [9]. Support of this mechanism in lupus comes from experiments involving pharmacologic inhibition of iNOS in the MRL/lpr and NZB/W models. Mice given l-NIL or l-NMMA demonstrated significant reductions in markers of systemic oxidant stress (urine F2-isoprostanes) compared to mice treated with distilled water [37]. This observation raises the possibility that some of the pathogenic effects of iNOS activity in SLE arise from its ability to produce ROI in proximity to NO.
Reactive nitrogen intermediate (RNI) biology in human SLE
Observational studies
While there is compelling evidence for aberrant reactive nitrogen production in the pathogenesis of murine lupus nephritis, the lack of appropriately selective iNOS inhibitors for human use limits human studies to observation. Increased expression of the iNOS enzyme has been reported in multiple tissues among SLE subjects. Several laboratories have described increased expression in the glomeruli of subjects with proliferative lupus nephritis [38—40]. In one study, glomerular iNOS staining colocalized with markers of apoptosis and staining for p53, a proapoptotic signaling molecule [39]. These data suggest that one mechanism for iNOS-mediated glomerular damage is increased signaling for apoptosis.
The skin often reflects disease activity in SLE, and iNOS expression in this organ appears to parallel that activity. Immunostaining for iNOS protein and mRNA was elevated in 33% of epidermal tissue samples from cutaneous lupus subjects before exposure to ultraviolet B irradiation but all samples after exposure [41]. Among subjects with systemic disease, skin biopsy specimens from the buttocks revealed higher iNOS expression in endothelial cells and keratinocytes than in controls. Endothelial expression correlated with lupus disease activity. The presence of iNOS in unaffected skin endothelial cells suggests systemic expression [42], while its induction with UV exposure offers one mechanism for increased expression during disease activity.
Studies of iNOS tissue expression are generally limited by practical concerns to organs that are frequently or easily biopsied. Therefore, serum and urine markers of systemic NO production have been studied in larger SLE populations. In humans, the use of these surrogate markers is complicated by the genetic and dietary heterogeneity of the population and concurrent diseases. Several studies have reported increased serum levels of NOX in lupus patients in association with disease activity [40,43—46]. Diets high in NOX can dramatically influence the ability to accurately measure systemic NO production through measures of serum or urine NOX [47]. One study, in which a low NOX diet was used to reduce dietary sources of NOX as a confounding factor, also reported a correlation between NOX and SLE disease activity [40].
Because ONOO− has more pathogenic potential than NO itself, assays for 3-nitrotyrosine (3NTyr) were developed to measure the effect of ONOO− production on serum proteins containing tyrosine (Tyr). In an Australian lupus cohort composed primarily of Caucasian and Asian subjects, serum 3NTyr levels were elevated in comparison to controls, and levels correlated with disease activity. Protein-bound carbonyls, markers of systemic oxidation, were also elevated during disease activity in this population [48]. Serum 3NTyr levels correlated with disease activity, particularly renal disease activity, in African-American but not Caucasian SLE subjects in one largely African-American cohort [40]. One possible mechanism for the unfavorable outcomes observed in some African-Americans with lupus is an increased predisposition towards RNI and ROI production in response to the inflammatory stimuli associated with lupus disease activity [40]. This predisposition may be inherited. In a study of two NOS2 polymorphisms in African-American female SLE and control subjects, a significantly increased prevalence of these polymorphisms was observed in those with SLE [49]. Supporting a functional role for the polymorphisms described are studies reporting increased markers of systemic NO production and improved malaria survival in some African populations with these polymorphisms [50—52].
One mechanism through which ONOO− can be pathogenic in the setting of SLE is through the creation of neoepitopes on self antigens. Serum from lupus patients exhibited increased binding to NO- and ONOO−-modified plasmid DNA when compared to calf thymus and native plasmid DNA [36,53]. The serum from SLE subjects also had greater binding to nitrated poly-l-tyrosine than unmodified poly-l-tyrosine [54]. These combined studies suggest that ONOO− modifications of self antigens can create neoepitopes with increased binding affinity over native antigens. Whether binding to these epitopes later leads to epitope spreading to unmodified epitopes has not been investigated.
ONOO− can modify lipids as well. Peroxidation of arachidonate by ONOO− can lead to formation of isoprostanes that can stimulate monocyte adhesion to endothelial cells [55] and induce vasoconstriction in smooth muscles [56]. ONOO− can also oxidize LDL. Circulating complexes of anti-phospholipid antibodies and oxidized LDL were found in increased amounts in SLE subjects with secondary anti-phospholipid syndrome [57]. Some phospholipids within oxidized LDL have platelet activating factor-like activity and can stimulate growth of smooth muscle cells [58]. Not all lipid peroxidation products of ONOO− are pathogenic, however. Nitro-linoleate, a product of ONOO− and linoleic acid, can have anti-inflammatory properties in neutrophils [59] and inhibit platelet activation [60]. Thus, the complete clinical effect of ONOO− formation and lipid peroxidation on lupus disease phenotype and cardiovascular disease associated with SLE is unknown. Studies using ONOO− scavenging agents in SLE may shed light on this issue.
Translation of current knowledge into human therapies
Expression of iNOS is an important arm of the innate immune response when it occurs in the setting of infectious stimuli. In the setting of lupus, its expression occurs outside of this context with additional expression in non-immune cells such as endothelial cells and keratinocytes [42]. It is generally accepted that ONOO− is one of the more pathogenic and abundant of the RNI derived from iNOS activity. Both eNOS and nNOS-derived NO can combine with SO produced in close proximity to produce ONOO; however, because iNOS produces log-fold higher amounts of NO and is a known source of SO production, it is the most logical isoform target for prevention of ONOO− production [3]. Pharmacologic inhibition of iNOS has been performed in murine models of lupus using a number of competitive inhibitors of the l-arginine substrate. For an inhibitor to be highly selective, it must have 50- to 100-fold more selectivity for iNOS than eNOS and nNOS. This is important for development of drugs in humans as inhibition of eNOS can lead to hypertension and reduced glomerular filtration rate [61], while inhibition of nNOS can lead to reduced cognitive function [62]. l-NMMA, l-NIL, and aminoguanidine, all effective in treating murine lupus [16,22,63], do not have the necessary specificity for iNOS over eNOS or nNOS [3]. However, newer compounds such as GW273629 and GW274150 have selectivities for iNOS that are 125 and 330 times greater than for eNOS. Their selectivity for iNOS over nNOS is 1.5 and 100 times greater. Given its superior overall selectivity, GW274150 offers the most hope for use in humans and is being developed by Glaxo-Smith-Kline for the treatment of rheumatoid arthritis, asthma [64], and migraine headaches [65].
Another approach to inhibiting iNOS activity is to prevent dimerization of monomers to form the active homodimer. Using combinatorial chemistry, a pyrimidine imidazole compound ((N-[(1,3-benzodioxol-5-yl)methyl]-1-[2-(1H-imidazol-1-yl)pyrimidin-4-yl]-4-(methoxycarbonyl)-piperazine-2-acetamide or BBS2) is a selective inhibitor of iNOS activity that acts by binding to the surface of the oxygenase domain and preventing homodimer formation. Its IC50 is approximately 1 nM in cell-based assays, and its selectivity for inhibiting iNOS is >1000-fold greater than for eNOS. However, its selectivity for iNOS versus nNOS is only five-fold [66]. It has been reported to be effective in preventing endotoxemic shock [67] and smoke inhalation injury in animal models [68]. The effect of its low selectivity for nNOS after chronic administration in humans is not known.
Conclusion
Production of NO from constitutive NOS signals for vasodilation and neurotransmission under physiologic circumstances. Increased expression of iNOS in response to infection or malignancy is an important arm of the innate immune response. In such circumstances, ONOO− is often produced. However, increased expression of iNOS in response to inflammatory stimuli present in SLE may lead to increased tissue damage, altered enzyme activity, and increased expression of neoepitopes in self antigens. There is compelling evidence that pharmacologic inhibition of iNOS leads to reduced disease activity and damage in murine models of lupus. Observational studies in humans indicate that RNI are overproduced during lupus disease activity and that expression of iNOS occurs in tissues damaged during such activity. Studies of therapies designed to inhibit iNOS or scavenge pathogenic ROI and RNI have not been performed in humans with lupus. Several compounds designed to inhibit iNOS activity, prevent dimerization of iNOS, or scavenge ROI and RNI are in development and offer hope that such studies will occur in the next few years.
Table 2.
Selectivity of various compounds for iNOS and mechanisms of action
| Compound | IC50 for iNOS (µM) | Selectivity in vitro (fold) | Mechanism of action | |
|---|---|---|---|---|
| iNOS vs. eNOS | iNOS vs. nNOS | |||
| l-NMMAa | 6.6 | 0.5 | 0.7 | Competitive inhibition |
| l-NILa | 1.6 | 49 | 23 | Competitive inhibition |
| Aminoguanidinea | 31 | 11 | 5.5 | Competitive inhibitionc |
| GW274150a | 1.4 | 333 | 104 | Competitive inhibition |
| 1400Wa | 0.23 | >4000 | 32 | Competitive inhibition |
| BBS2b | 0.028 | 1000 | 5 | Prevents dimerization |
| AEOL-10113d | e | e | e | Scavenges ONOO− |
These are the results of in vitro assays of purified enzyme [3,66]. Reported is the fold selectivity of various compounds for iNOS vs. other isoforms and the IC50 of each compound for iNOS. Generalized mechanisms of action are stated. l-NMMA = NG-monomethyl-l-arginine. l-NIL = l-N6-(1-iminoethyl)lysine.
Results are from Boyd et al. [12].
Results are from Blasko et al. [66].
Aminoguanidine has NO-independent anti-inflammatory activities [78].
Results from [69].
Does not inhibit iNOS activity. This table is adapted from [77].
References
- 1.Zahrt TC, Deretic V. Reactive nitrogen and oxygen intermediates and bacterial defenses: unusual adaptations in Mycobacterium tuberculosis. Antioxid. Redox Signal. 2002;4:141–159. doi: 10.1089/152308602753625924. [DOI] [PubMed] [Google Scholar]
- 2.Rahman I, Yang SR, Biswas SK. Current concepts of redox signaling in the lungs. Antioxid. Redox Signal. 2006;8:681–689. doi: 10.1089/ars.2006.8.681. [DOI] [PubMed] [Google Scholar]
- 3.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinert H, Pautz A, Linker K, et al. Regulation of the expression of inducible nitric oxide synthase. Eur. J. Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Karpuzoglu E, Ahmed SA. Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide. doi: 10.1016/j.niox.2006.03.009. (in press) (Electronic publication ahead of print). [DOI] [PubMed] [Google Scholar]
- 6.Reilly CM, Oates JC, Sudian J, et al. Prostaglandin J(2) inhibition of mesangial cell iNOS expression. Clin. Immunol. 2001;98:337–345. doi: 10.1006/clim.2000.4985. [DOI] [PubMed] [Google Scholar]
- 7.Lincoln J, Hoyle CHV, Burnstock G. Nitric Oxide in Health and Disease. New York, NY: Cambridge Univ. Press; 1997. [Google Scholar]
- 8.Human Proteome Resource Program. NOS2A in normal tissues. [accessed May 19, 2006]; Available from: http://www.hpr.se/tissue_profile.php?antibody_id=2014.
- 9.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. U. S. A. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaertner SA, Janssen U, Ostendorf T, et al. Glomerular oxidative and antioxidative systems in experimental mesangio-proliferative glomerulonephritis. J. Am. Soc. Nephrol. 2002;13:2930–2937. doi: 10.1097/01.asn.0000034908.43113.5d. [DOI] [PubMed] [Google Scholar]
- 11.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular—renal system. Free Radical Biol. Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Boyd CS, Cadenas E. Nitric oxide and cell signaling pathways in mitochondrial-dependent apoptosis. Biol. Chem. 2002;383:411–423. doi: 10.1515/BC.2002.045. [DOI] [PubMed] [Google Scholar]
- 13.Perl A, Gergely P, Jr, Nagy G, et al. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollace V, Muscoli C, Masini E, et al. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt P, Youhnovski N, Daiber A, et al. Specific nitration at tyrosine 430 revealed by high resolution mass spectrometry as basis for redox regulation of bovine prostacyclin synthase. J. Biol. Chem. 2003;278:12813–12819. doi: 10.1074/jbc.M208080200. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg JB, Granger DL, Pisetsky DS, et al. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-l-arginine. J. Exp. Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keng T, Privalle CT, Gilkeson GS, et al. Peroxynitrite formation and decreased catalase activity in autoimmune MRL-lpr/lpr mice. Mol. Med. 2000;6:779–792. [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado Alves J, Mason LJ, Ames PR, et al. Antipho-spholipid antibodies are associated with enhanced oxidative stress, decreased plasma nitric oxide and paraoxonase activity in an experimental mouse model. Rheumatology (Oxford) 2005;44:1238–1244. doi: 10.1093/rheumatology/keh722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bremer V, Tojo A, Kimura K, et al. Role of nitric oxide in rat nephrotoxic nephritis: comparison between inducible and constitutive nitric oxide synthase. J. Am. Soc. Nephrol. 1997;8:1712–1721. doi: 10.1681/ASN.V8111712. [DOI] [PubMed] [Google Scholar]
- 20.Heeringa P, van Goor H, Moshage H, et al. Expression of iNOS, eNOS, and peroxynitrite-modified proteins in experimental anti-myeloperoxidase associated crescentic glomerulonephritis. Kidney Int. 1998;53:382–393. doi: 10.1046/j.1523-1755.1998.00780.x. [DOI] [PubMed] [Google Scholar]
- 21.Ohse T, Ota T, Kieran N, et al. Modulation of interferon-induced genes by lipoxin analogue in anti-glomerular basement membrane nephritis. J. Am. Soc. Nephrol. 2004;15:919–927. doi: 10.1097/01.asn.0000119962.69573.cc. [DOI] [PubMed] [Google Scholar]
- 22.Reilly CM, Farrelly LW, Viti D, et al. Modulation of renal disease in MRL/lpr mice by pharmacologic inhibition of inducible nitric oxide synthase. Kidney Int. 2002;61:839–846. doi: 10.1046/j.1523-1755.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- 23.Oates JC, Ruiz P, Alexander A, et al. Effect of late modulation of nitric oxide production on murine lupus. Clin. Immunol. Immunopathol. 1997;83:86–92. doi: 10.1006/clin.1997.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda Y, Takeno M, Iwasaki M, et al. Chemical induction of HO-1 suppresses lupus nephritis by reducing local iNOS expression and synthesis of anti-dsDNA antibody. Clin. Exp. Immunol. 2004;138:237–244. doi: 10.1111/j.1365-2249.2004.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lui SL, Tsang R, Wong D, et al. Effect of mycophenolate mofetil on severity of nephritis and nitric oxide production in lupus-prone MRL/lpr mice. Lupus. 2002;11:411–418. doi: 10.1191/0961203302lu214oa. [DOI] [PubMed] [Google Scholar]
- 26.Yu CC, Yang CW, Wu MS, et al. Mycophenolate mofetil reduces renal cortical inducible nitric oxide synthase mRNA expression and diminishes glomerulosclerosis in MRL/lpr mice. J. Lab. Clin. Med. 2001;138:69–77. doi: 10.1067/mlc.2001.115647. [DOI] [PubMed] [Google Scholar]
- 27.Mishra N, Reilly CM, Brown DR, et al. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J. Clin. Invest. 2003;111:539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilkeson GS, Mudgett JS, Seldin MF, et al. Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2. J. Exp. Med. 1997;186:365–373. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 30.Zou MH, Shi C, Cohen RA. Oxidation of the zinc—thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Invest. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oates JC, Gilkeson GS. Nitric oxide induces apoptosis in spleen lymphocytes from MRL/lpr mice. J. Investig. Med. 2004;52:62–71. doi: 10.1136/jim-52-01-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh AK. Lupus in the Fas lane? J. R. Coll. Physicians Lond. 1995;29:475–478. [PMC free article] [PubMed] [Google Scholar]
- 34.Ohmori H, Oka M, Nishikawa Y, et al. Immunogenicity of autologous IgG bearing the inflammation-associated marker 3-nitrotyrosine. Immunol. Lett. 2005;96:47–54. doi: 10.1016/j.imlet.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Habib Moinuddin S, Ali R. Peroxynitrite modified DNA: a better antigen for systemic lupus erythematosus anti-DNA autoantibodies. Biotechnol, Appl. Biochem. 2005 doi: 10.1042/BA20050156. [DOI] [PubMed] [Google Scholar]
- 36.Dixit K, Ali R. Role of nitric oxide modified DNA in the etiopathogenesis of systemic lupus erythematosus. Lupus. 2004;13:95–100. doi: 10.1191/0961203304lu492oa. [DOI] [PubMed] [Google Scholar]
- 37.Njoku CJ, Patrick KS, Ruiz P, Jr, et al. Inducible nitric oxide synthase inhibitors reduce urinary markers of systemic oxidant stress in murine proliferative lupus nephritis. J. Investig. Med. 2005;53:347–352. doi: 10.2310/6650.2005.53705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furusu A, Miyazaki M, Abe K, et al. Expression of endothelial and inducible nitric oxide synthase in human glomerulonephritis. Kidney Int. 1998;53:1760–1768. doi: 10.1046/j.1523-1755.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang JS, Tseng HH, Shih DF, et al. Expression of inducible nitric oxide synthase and apoptosis in human lupus nephritis. Nephron. 1997;77:404–411. doi: 10.1159/000190316. [DOI] [PubMed] [Google Scholar]
- 40.Oates JC, Christensen EF, Reilly CM, et al. Prospective measure of serum 3-nitrotyrosine levels in systemic lupus erythematosus: correlation with disease activity. Proc. Assoc. Am. Physicians. 1999;111:611–621. doi: 10.1046/j.1525-1381.1999.99110.x. [DOI] [PubMed] [Google Scholar]
- 41.Kuhn A, Fehsel K, Lehmann P, et al. Aberrant timing in epidermal expression of inducible nitric oxide synthase after UV irradiation in cutaneous lupus erythematosus. J. Invest. Dermatol. 1998;111:149–153. doi: 10.1046/j.1523-1747.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 42.Belmont HM, Levartovsky D, Goel A, et al. Increased nitric oxide production accompanied by the up-regulation of inducible nitric oxide synthase in vascular endothelium from patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:1810–1816. doi: 10.1002/art.1780401013. [DOI] [PubMed] [Google Scholar]
- 43.Wanchu A, Khullar M, Deodhar SD, et al. Nitric oxide synthesis is increased in patients with systemic lupus erythematosus. Rheumatol. Int. 1998;18:41–43. doi: 10.1007/s002960050055. [DOI] [PubMed] [Google Scholar]
- 44.Gilkeson G, Cannon C, Oates J, et al. Correlation of serum measures of nitric oxide production with lupus disease activity. J. Rheumatol. 1999;26:318–324. [PubMed] [Google Scholar]
- 45.Andersen GN, Caidahl K, Kazzam E, et al. Correlation between increased nitric oxide production and markers of endothelial activation in systemic sclerosis–findings with the soluble adhesion molecules E-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1. Arthritis Rheum. 2000;43:1085–1093. doi: 10.1002/1529-0131(200005)43:5<1085::AID-ANR19>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 46.Ho CY, Wong CK, Li EK, et al. Elevated plasma concentrations of nitric oxide, soluble thrombomodulin and soluble vascular cell adhesion molecule-1 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2003;42:117–122. doi: 10.1093/rheumatology/keg045. [DOI] [PubMed] [Google Scholar]
- 47.Jungersten L, Edlund A, Petersson AS, et al. Plasma nitrate as an index of nitric oxide formation in man: analyses of kinetics and confounding factors. Clin. Physiol. 1996;16:369–379. doi: 10.1111/j.1475-097x.1996.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 48.Morgan PE, Sturgess AD, Davies MJ. Increased levels of serum protein oxidation and correlation with disease activity in systemic lupus erythematosus. Arthritis Rheum. 2005;52:2069–2079. doi: 10.1002/art.21130. [DOI] [PubMed] [Google Scholar]
- 49.Oates JC, Levesque MC, Hobbs MR, et al. Nitric oxide synthase 2 promoter polymorphisms and systemic lupus erythematosus in African-Americans. J. Rheumatol. 2003;30:60–67. [PubMed] [Google Scholar]
- 50.Hobbs MR, Udhayakumar V, Levesque MC, et al. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet. 2002;360:1468–1475. doi: 10.1016/S0140-6736(02)11474-7. [DOI] [PubMed] [Google Scholar]
- 51.Xu W, Humphries S, Tomita M, et al. Survey of the allelic frequency of a NOS2A promoter microsatellite in human populations: assessment of the NOS2A gene and predisposition to infectious disease. Nitric Oxide. 2000;4:379–383. doi: 10.1006/niox.2000.0290. [DOI] [PubMed] [Google Scholar]
- 52.Kun JF, Mordmuller B, Perkins DJ, et al. Nitric oxide synthase 2 (G-954C), increased nitric oxide production, and protection against malaria. J. Infect. Dis. 2001;184:330–336. doi: 10.1086/322037. [DOI] [PubMed] [Google Scholar]
- 53.Habib Moinuddin S, Ali R. Peroxynitrite-modified DNA: a better antigen for systemic lupus erythematosus anti-DNA autoantibodies. Biotechnol. Appl. Biochem. 2006;43:65–70. doi: 10.1042/BA20050156. [DOI] [PubMed] [Google Scholar]
- 54.Khan F, Ali R. Antibodies against nitric oxide damaged poly l-tyrosine and 3-nitrotyrosine levels in systemic lupus erythematosus. J. Biochem. Mol. Biol. 2006;39:189–196. doi: 10.5483/bmbrep.2006.39.2.189. [DOI] [PubMed] [Google Scholar]
- 55.Huber J, Bochkov VN, Binder BR, et al. The isoprostane 8-iso-PGE2 stimulates endothelial cells to bind monocytes via cyclic AMP- and p38 MAP kinase-dependent signaling pathways. Antioxid. Redox Signal. 2003;5:163–169. doi: 10.1089/152308603764816523. [DOI] [PubMed] [Google Scholar]
- 56.Fukunaga M, Makita N, Roberts LJd, et al. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am. J. Physiol. 1993;264:C1619–C1624. doi: 10.1152/ajpcell.1993.264.6.C1619. [DOI] [PubMed] [Google Scholar]
- 57.Lopez LR, Simpson DF, Hurley BL, et al. OxLDL/β2GPI complexes, autoantibodies in patients with systemic lupus erythematosus, systemic sclerosis, and antiphospholipid syndrome: pathogenic implications for vascular involvement. Ann. N. Y. Acad. Sci. 2005;1051:313–322. doi: 10.1196/annals.1361.073. [DOI] [PubMed] [Google Scholar]
- 58.Heery JM, Kozak M, Stafforini DM, et al. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J. Clin. Invest. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coles B, Bloodsworth A, Clark SR, et al. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ. Res. 2002;91:375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 60.Coles B, Bloodsworth A, Eiserich JP, et al. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phos-phoprotein through elevation of cAMP. J. Biol. Chem. 2002;277:5832–5840. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 61.Delles C, Jacobi J, Schlaich MP, et al. Assessment of endothelial function of the renal vasculature in human subjects. Am. J. Hypertens. 2002;15:3–9. doi: 10.1016/s0895-7061(01)02242-7. [DOI] [PubMed] [Google Scholar]
- 62.Kirchner L, Weitzdoerfer R, Hoeger H, et al. Impaired cognitive performance in neuronal nitric oxide synthase knockout mice is associated with hippocampal protein derangements. Nitric Oxide. 2004;11:316–330. doi: 10.1016/j.niox.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Yang CW, Yu CC, Ko YC, et al. Aminoguanidine reduces glomerular inducible nitric oxide synthase (iNOS) and transforming growth factor-beta 1 (TGF-β1) mRNA expression and diminishes glomerulosclerosis in NZB/W F1 mice. Clin. Exp. Immunol. 1998;113:258–264. doi: 10.1046/j.1365-2249.1998.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glaxo-Smith-Kline. Product Development Pipeline. [accessed May 19, 2006];2006 February; Available from: http://www.gsk.com/financial/product_pipeline/docs/pipeline.pdf.
- 65.National Library of Medicine. ClinicalTrials.gov. [accessed May 19, 2006]; Available from: http://www.ClinicalTrials.gov.
- 66.Blasko E, Glaser CB, Devlin JJ, et al. Mechanistic studies with potent and selective inducible nitric-oxide synthase dimerization inhibitors. J. Biol. Chem. 2002;277:295–302. doi: 10.1074/jbc.M105691200. [DOI] [PubMed] [Google Scholar]
- 67.Ichinose F, Hataishi R, Wu JC, et al. A selective inducible NOS dimerization inhibitor prevents systemic, cardiac, and pulmonary hemodynamic dysfunction in endotoxemic mice. Am. J. Physiol.: Heart Circ. Physiol. 2003;285:H2524–H2530. doi: 10.1152/ajpheart.00530.2003. [DOI] [PubMed] [Google Scholar]
- 68.Enkhbaatar P, Murakami K, Shimoda K, et al. Inducible nitric oxide synthase dimerization inhibitor prevents cardiovascular and renal morbidity in sheep with combined burn and smoke inhalation injury. Am. J. Physiol.: Heart Circ. Physiol. 2003;285:H2430–H2436. doi: 10.1152/ajpheart.00055.2003. [DOI] [PubMed] [Google Scholar]
- 69.Piganelli JD, Flores SC, Cruz C, et al. A metalloporphyrin-based superoxide dismutase mimic inhibits adoptive transfer of autoimmune diabetes by a diabetogenic T-cell clone. Diabetes. 2002;51:347–355. doi: 10.2337/diabetes.51.2.347. [DOI] [PubMed] [Google Scholar]
- 70.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation–reduction reactions in innate immunity. Free Radical Biol. Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 71.Crow JP, Calingasan NY, Chen J, et al. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann. Neurol. 2005;58:258–265. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- 72.Ohmori H, Kanayama N. Immunogenicity of an inflammation-associated product, tyrosine nitrated self-proteins. Autoimmun. Rev. 2005;4:224–229. doi: 10.1016/j.autrev.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 73.Habib, Moinuddin S, Ali R. Acquired antigenicity of DNA after modification with peroxynitrite. Int. J. Biol. Macromol. 2005;35:221–225. doi: 10.1016/j.ijbiomac.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog. Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 75.Frostegard J, Svenungsson E, Wu R, et al. Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum. 2005;52:192–200. doi: 10.1002/art.20780. [DOI] [PubMed] [Google Scholar]
- 76.Botti H, Trostchansky A, Batthyany C, et al. Reactivity of peroxynitrite and nitric oxide with LDL. IUBMB Life. 2005;57:407–412. doi: 10.1080/15216540500137701. [DOI] [PubMed] [Google Scholar]
- 77.Oates JC, Gilkeson GS. Nitric Oxide in SLE. In: Tsokos GC, Gordon C, Smolen JS, editors. Systemic Lupus Erythematosus: A Companion to Rheumatology. Elsevier; 2006. (in press) (ISBN: 0323044344ISBN-13: 9780323044349). [Google Scholar]
- 78.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]