Abstract
Remarkable advances in our understanding of olfactory perception have been made in recent years, including the discovery of new mechanisms of olfactory signaling and new principles of olfactory processing. Here we discuss the insight that has been gained into the receptors, cells, and circuits that underlie the sense of smell.
Introduction
Animals in their natural environments are immersed in odors. These odors are rich in information, and animals have evolved sophisticated olfactory systems to detect and interpret them. The ability to encode the identity and intensity of odors can allow an animal to locate food sources, thereby permitting survival, to identify mates, promoting reproduction, and to avoid predators, averting death.
Olfactory systems have evolved great sensitivity and discriminatory power. A single molecule of a female moth pheromone is believed sufficient to elicit a response from a male antennal neuron (Kaissling and Priesner, 1970). Honeybees can distinguish between many pairs of structurally similar odorants (Laska et al., 1999), and mice can likewise distinguish between many pairs of enantiomers (compounds that are mirror images of each other) (Laska and Shepherd, 2007).
The olfactory system is like the visual and auditory systems in that it detects and discriminates a wide range of stimuli. Odors differ from light and sounds, however, in that odors can not be classified by a simple parameter such as wavelength or frequency. The complexity of odorant identity poses a challenge that has been met through the use of a large number of diverse odor receptors. The multiplicity of receptors allows detection of a vast number of odors. Discrimination depends on combinatorial coding and on circuit-level interactions at multiple steps of olfactory processing, and it can be enhanced by olfactory learning.
The functional organization of the olfactory system is remarkably similar in organisms ranging from insects to mammals, suggesting that it represents an extremely good solution to some difficult problems. Thus, principles elucidated in one experimental organism often apply to many others. In both insects and mammals, odorants bind to receptors in the cilia or dendrites of olfactory receptor neurons (ORNs), each of which expresses one or a small number of receptor types. In both kinds of animals, ORNs that express the same odor receptor send axons to the same glomeruli, spheroidal structures that consist of ORN axon terminals and the dendrites of second-order neurons. The glomeruli form the antennal lobe of the insect brain, or its mammalian equivalent, the olfactory bulb. In both of these centers the olfactory signals are processed and relayed to higher centers of the brain.
Recent discoveries have provided new understanding of how the identity and intensity of odors are first encoded in the olfactory organs, and how they are subsequently decoded in the central nervous system. Studies in the past few years have identified new odor receptor families, new signaling mechanisms, and even a new mammalian olfactory organ. Analysis of the insect antennal lobe, the mammalian olfactory bulb, and higher brain regions has led to a better understanding of how olfactory signaling is shaped by circuit-level interactions between neurons. It has also shed light on the fascinating question of how olfactory stimuli such as pheromones elicit innate behaviors.
In this review we focus on recent insight gained into the molecules, cells, and circuits that underlie olfactory perception. Particular attention is paid to mammals and insects, in which illuminating advances have recently been made. We first consider the primary molecular sensors of odorants, the odorant receptors, and the neurons in which they are expressed, with a view to understanding how the initial pattern of sensory input is generated by an olfactory stimulus. We then examine how this input is transformed at the first processing center in the brain, the antennal lobe or olfactory bulb. Next we consider the processing that occurs at higher centers in the brain and how it relates to perception. Finally we discuss olfactory perception in the context of odor space.
A multiplicity of olfactory organs
Both mammals and insects rely upon multiple olfactory organs (Figure 1). In each organ, odors partition into an aqueous fluid that bathes the cilia or dendrites of sensory neurons. However, the organs differ in location and numerical complexity, in the receptors that they express, and in the targets of their neurons within the central nervous system. To what extent do these anatomical and molecular differences underlie functional differences, either in the kinds of stimuli that the organs encode or in the behaviors that they drive?
Figure 1. Olfactory system anatomy.
(A) Sagittal view of a rodent head, showing four olfactory organs: the main olfactory epithelium (MOE), vomeronasal organ (VNO), Grueneberg ganglion (GG) and septal organ of Masera (SO). Olfactory receptor neurons (ORNs) in the MOE, GG and SO all project to the main olfactory bulb (MOB), whereas the VNO neurons project to the accessory olfactory bulb (AOB). Olfactory information is further processed in higher brain regions, such as the anterior olfactory nucleus (AON), the olfactory tubercle (OT), entorhinal cortex (ENT), piriform cortex (PIR) and cortical amygdala (CoAMG). Inset: coronal section of the brain. (B) Frontal view of a Drosophila head. There are two pairs of olfactory organs: the third antennal segments and maxillary palps. Olfactory information is first relayed to the antennal lobe, which contains multiple glomeruli. Subsequent processing takes place at the lateral horn of the protocerebrum and Kenyon cells in the mushroom body. Connectivity has been simplified for clarity.
In mammals, the main olfactory epithelium lies in the dorsal nasal cavity, and its sensory neurons send projections to glomeruli in the main olfactory bulb. A wide variety of volatile odorants partition from the air into the fluid surrounding the cilia of ORNs, where they are detected by odor receptors. The mammalian vomeronasal organ lies just below the ventral nasal cavity. Its sensory neurons express different receptors, and they project to glomeruli in the neighboring accessory olfactory bulb. Functional experiments have demonstrated that the vomeronasal organ is sensitive to a variety of pheromones, molecules that are released by an individual and that induce innate behaviors in conspecific animals (Table 1).
Table 1.
Ligands and functions for mammalian olfactory organs and receptors
| Organ | Receptors | Ligands | Origin | Proposed Functions |
|---|---|---|---|---|
| MOE | ORs | general odors | food, environment | odor recognition, discrimination, attraction/ repulsion |
| MHC class I peptides | urine, bodily secretions | social recognition of other strains | ||
| TAARs | volatile amines | urine | stress response, gender recognition, acceleration of female puberty onset |
|
| GC-D | CO2 (bicarbonate) | atmosphere | avoidance behavior | |
| peptide hormones (uroguanylin & guanylin) |
urine | salt/water homeostasis, detection of cues related to hunger, satiety, or thirst |
||
| VNO | V1Rs | volatile pheromones, sulfated steroids |
urine | conspecific recognition, male sexual behavior, maternal aggression, regulation of female estrous cycles, stress level indicator |
| V2Rs | MHC class I peptides | urine, bodily secretions | mate recognition in the context of pregnancy block (Bruce effect) |
|
| exocrine gland-secreting peptides (ESPs) |
tears from specific genders or strains |
information about gender and individual identity, conspecific recognition |
||
| major urinary protein (MUP) complex |
male urine | male aggression | ||
| sulfated steroids | female urine | indication of stress levels | ||
| Formyl Peptide Receptors |
formyl peptides | gram-negative bacteria | indication of pathogenicity or health status | |
| CRAMP, lipoxin, uPAR peptides |
immune system | |||
| GG | TAARs, V2r83 |
alarm pheromones | stressed conspecifics | avoidance of dangerous situations |
| SO | ORs | general odors | food, environment | alerting role or “mini-nose” |
Abbreviations: MOE, main olfactory epithelium; OR, olfactory receptor; GG, Grueneberg ganglion; SO, septal organ of Masera; TAARs, trace amine-associated receptors; TRPM5, transient receptor potential channel M5; GC-D, receptor guanylyl cyclase; VNO, vomeronasal organ; MHC, major histocompatibility complex
In recent years it has become clear that there is functional overlap between the main olfactory epithelium and the vomeronasal organ. Certain pheromones have been found to activate neurons in the main olfactory system, and the activity of this system has been found necessary for several sexual and social behaviors that likely depend on pheromones (Lin et al., 2004; Luo et al., 2003; Mandiyan et al., 2005; Spehr et al., 2006; Wang et al., 2006; Xu et al., 2005). Likewise, some general odorants not known to act as pheromones have been found to activate the accessory olfactory system and modulate behavior in the absence of a functional main olfactory system (Sam et al., 2001; Trinh and Storm, 2003; Xu et al., 2005).
A third mammalian organ, the septal organ of Masera (SO), also contains sensory neurons that express odor receptors (Table 1) (Kaluza et al., 2004; Tian and Ma, 2004). The SO was recently shown to respond to multiple volatile odorants that are also detected by the main olfactory epithelium (Grosmaitre et al., 2007; Ma et al., 2003). Interestingly, a subset of ORNs from both the SO and the main olfactory epithelium may respond to mechanical pressure and thus may report changes in air pressure induced by sniffing (Grosmaitre et al., 2007).
Recently another mammalian organ was found to subserve olfaction: the Grueneberg ganglion contain sensory neurons that express olfactory receptors (Fleischer et al., 2006; Fleischer et al., 2007). Moreover, these neurons are activated by volatile alarm pheromones and are required for a freezing behavior in mice, indicating a role in pheromonal signaling (Brechbuhl et al., 2008).
Insects also rely on multiple, distinct organs for olfaction (Figure 1b). Adult Drosophila contain two olfactory organs, the antenna and the maxillary palp. Both contain sensory hairs, or sensilla, that house the dendrites of up to four ORNs, but ORNs from the different organs project to glomeruli in different regions of the antennal lobe. Although these organs respond to overlapping sets of odorants, the maxillary palp lies close to the labellum, the main taste organ of the head, and there is evidence that olfactory input via the maxillary palp enhances taste-mediated behaviors (Shiraiwa, 2008). Other insect olfactory organs include the labial pits of moths, which respond to CO2 and some odorants (Bogner et al., 1986).
Further increasing the extent of anatomical diversity, insect olfactory sensilla fall into different morphological types known as basiconic, trichoid, and coeloconic sensilla (Table 2). Whereas basiconic sensilla are found on both the antenna and the maxillary palp in Drosophila, trichoid and coeloconic sensilla are located exclusively on the antenna and may serve distinct chemosensory functions. Whereas basiconic ORNs respond to general odorants, trichoid neurons respond poorly to most odorants but respond to pheromones (Clyne et al., 1997; Hallem and Carlson, 2006; van der Goes van Naters and Carlson, 2007). Recent studies have demonstrated that activation of specific trichoid neurons is both necessary and sufficient to mediate the stereotyped courtship behavior elicited by the Drosophila pheromone 11-cis-vaccenyl-acetate (cVA) (Ha and Smith, 2006; Kurtovic et al., 2007). This functional division among sensillar types appears to be evolutionarily conserved, as other insects also detect pheromones with trichoid sensilla (de Bruyne and Baker, 2008). Coeloconic ORNs express a distinct class of olfactory receptors that are likely to underlie the strong response of these neurons to a variety of amines and carboxylic acids (Benton et al., 2009; Yao et al., 2005).
Table 2.
Ligands and functions for insect olfactory organs and receptors
| Organ | Sensilla | Receptors | Ligands | Origin | Proposed Functions |
|---|---|---|---|---|---|
| Antenna | Basiconic (ab1~ ab10) |
Ors | many volatile compounds, food odors |
food, environment | odor recognition, discrimination, attraction/ repulsion |
| Gr21a & Gr63a | CO2 | atmosphere, stressed flies |
avoidance behavior | ||
| Coeloconic (ac1~ ac4) |
Or35a | many volatile compounds, food odors |
food, environment | unknown | |
| IRs | volatile amines, carboxylic acids, a few food odors |
||||
| unknown | humidity | environment | dessication avoidance | ||
| Trichoid (at1~ at4) |
Ors | cis-vaccenyl acetate | male genitalia, recently mated females |
deterrent for courting males and mated females, aggregation pheromone |
|
| cuticle extracts | male or female flies | gender and conspecific detection | |||
| Maxillary Palp |
Basiconic (pb1~ pb3) |
Ors | many volatile compounds, food odors |
food, environment | taste enhancement |
In summary, mammals and insects each receive olfactory input via multiple organs. Although there is some degree of overlap in the kinds of stimuli to which the organs of a species are sensitive, there is increasing evidence that different olfactory organs are functionally distinct and that their wiring to different targets in the brain may underlie differences in the behavioral output that they drive. An intriguing direction for future olfactory research is to define the functional roles of the different olfactory organs in a variety of odor-driven behaviors.
Odor receptors: an expanding roster of dynamic gene families
Whereas vision depends on a handful of related receptors, olfaction relies on large numbers of receptors that belong to multiple families. The dimension and diversity of the receptor repertoire have likely arisen to facilitate the detection and discrimination of the vast number of odorants that are encountered by animals in their environments. New families of odor receptors have recently been discovered in both mammals and insects, and their functional roles are currently being explored.
A variety of odor receptor families are expressed in mammalian ORNs (Table 1). Mammalian genomes typically contain ~250–1200 functional OR genes (Niimura and Nei, 2007), which are the predominant receptors of the main olfactory epithelium and the SO (Table 1). A minority of ORNs express the trace amine-associated receptors (TAARs), some of which respond to volatile amines found in urine and are likely to act in the detection of social cues (Fleischer et al., 2007; Liberles and Buck, 2006). There are ~15 TAAR genes in the mouse, and TAARs are found in all vertebrate genomes examined thus far (Hashiguchi and Nishida, 2007). The vomeronasal organ expresses receptors of the V1R family (~200 genes in the mouse, excluding pseudogenes) and the V2R family (~100) (Touhara and Vosshall, 2009). One V2R gene, and some TAAR genes, are expressed in the Grueneberg ganglion. Recently another family of vomeronasal organ receptors has been discovered: the formyl peptide receptor-like proteins (~7) (Liberles et al., 2009; Riviere et al., 2009). These receptors respond to molecules related to disease and inflammation and may identify pathogens or report the health status of the animal. All five classes of mammalian receptors are predicted to contain seven transmembrane domains and have either been shown to signal via G proteins or are likely to do so based on their sequence similarity to known G protein-coupled receptors (GPCRs).
Insect olfaction is also mediated by receptors of multiple classes (Table 2). Insect genomes contain 60–340 members of the phylogenetically distinct insect Or (Odor receptor) family (Touhara and Vosshall, 2009). In addition, a few members of the Gr (Gustatory receptor) family are expressed in olfactory organs, where some have been found to mediate response to CO2 (Jones et al., 2007; Kwon et al., 2007; Suh et al., 2004). Recently another family of ~60 receptors called IRs (Ionotropic receptors) has been identified, of which several are expressed in ORNs of coeloconic sensilla (Benton et al., 2009). Ors and Grs are predicted to contain seven transmembrane domains, whereas IRs are related to ionotropic glutamate receptors and are predicted to contain three transmembrane domains and a pore-loop.
The evolutionary dynamics of odor receptors shows a great deal of fluidity. The Or families of insects in particular show great diversity. Within an insect species, many pairs of receptors show little sequence identity, and between some species of the same order, such as Drosophila and Anopheles, it is difficult to identify orthologous pairs of receptors (Hill et al., 2002). This divergence is likely to reflect rapid evolution. There are intriguing questions concerning the mechanisms by which receptor repertoires have evolved to meet the ecological needs of the species. As a species adapts to a new environment or food source, as in the evolution of human host-seeking behavior in mosquitoes, do new clades of receptors arise via duplication and divergence to sense new odors, such as human odors in the case of mosquitoes, or do the extant receptors adapt? Now that functional assays for insect odorant receptors are available, such questions can be addressed by systematic analyses of receptor repertoires.
In mammals, it is clear that OR families have evolved in part through both expansion and pseudogenization (a process by which mutations render genes nonfunctional). For example, 15–78% of ORs are pseudogenes, and all human V2R genes have been pseudogenized (Touhara and Vosshall, 2009). The evolutionary forces leading to gene pseudogenization and expansion can be seen in mammalian ORs, which fall into two classes. Aquatic vertebrate genomes nearly exclusively contain older class I ORs that are generally tuned toward water-soluble odorants, whereas the genomes of terrestrial vertebrates contain both class I and class II ORs, which are tuned toward hydrophobic odors (Freitag et al., 1998; Saito et al., 2009). In the dolphin, an aquatic mammal, the class II receptors have been pseudogenized (Freitag et al., 1998).
There is also widespread genetic variation among mammalian OR repertoires within species, including single nucleotide polymorphisms and copy number variation. Such natural genetic variation contributes to perceptual differences within human populations. Humans vary in their perception of specific odors. An individual may be anosmic, or insensitive, to a particular odor. An odor may be perceptible, but with an altered detection threshold. In some cases an odor may acquire an altered perceptual quality. Two recent studies showed that differerences in sensitivity to androstenone, a steroid, and isovaleric acid, which has a sweaty odor, can be attributed at least in part to polymorphisms in two specific OR genes (Keller et al., 2007; Menashe et al., 2007).
The role of individual members of a receptor repertoire in perception can be examined prospectively through mutational studies in Drosophila. Loss of odor receptors has been shown to cause a reduction in behavioral or electrophysiological responses to specific odorants (Dobritsa et al., 2003; Jones et al., 2007; Kreher et al., 2008; Kurtovic et al., 2007; Semmelhack and Wang, 2009). However, the deletion of an individual Drosophila Or does not necessarily eliminate the behavioral response to odorants it detects (Elmore et al., 2003; Keller and Vosshall, 2007). A simple interpretation of the residual response is that it is mediated by other receptors of the repertoire with partially overlapping function. Consistent with this explanation, a recent analysis of the response to ethyl acetate reveals that the response to high concentrations depends primarily on one receptor, whereas the response to low concentrations depends primarily on another (Kreher et al., 2008).
The primary representation of an odor
The roles of individual receptors in olfactory perception raise interesting questions about how an odor is encoded by an entire receptor repertoire. The primary representation of an odor lies in the differential activities of the population of odor receptors. Although this representation is transformed at successive levels of olfactory circuitry, ultimately the perception and discrimination of odors is founded upon the profile of receptor activity. Insight into the nature of this primary representation has come from systematic analysis of the responses of receptor repertoires to panels of odors (Hallem and Carlson, 2006; Kreher et al., 2008; Malnic et al., 1999; Saito et al., 2009; Xia et al., 2008).
Three basic principles emerge from such analysis. First, individual odorants activate subsets of receptors. This finding supports a model of combinatorial coding, in which most odorants are identified not by the activation of a single receptor, but by the pattern of receptors that are activated. Second, individual receptors are activated by subsets of odorants. Receptors vary in their breadth of tuning: some are broadly tuned, responding to many odors, whereas others are narrowly tuned, responding to few. The receptors lie along a smooth continuum of tuning breadths. Broadly tuned receptors are most sensitive to structurally similar odorants. Third, higher concentrations of odorants elicit activity from greater numbers of receptors. Thus, odor intensity as well as odor identity is represented by the number of activated receptors.
The primary representations of odors can vary in their temporal dynamics. An individual odorant can elicit a response of short duration from some ORNs and a long-lasting response from others. Likewise, an individual ORN can give a short response to some odors and a long response from others (Hallem et al., 2004). Analysis in an in vivo expression system, in which odor receptors are misexpressed in a mutant, “empty” Drosophila neuron that lacks an endogenous receptor, suggests that the termination dynamics of a neuron are dictated primarily by the receptor, as opposed to the cellular environment in which it operates (Hallem et al., 2004).
In addition to activation, receptors exhibit another mode of response: inhibition. Odor-induced reduction of basal ORN activity has been documented in both vertebrates and invertebrates (for review, see Reisert and Restrepo, 2009). Analysis of the responses of Drosophila receptors to panels of odorants have shown that an individual odorant can activate some receptors and inhibit others, whereas an individual receptor can be activated by some odorants and inhibited by others. The existence of two response modes may add a degree of freedom to odor coding (de Brito Sanchez and Kaissling, 2005).
Perhaps the most biologically interesting form of receptor inhibition, however, is the ability of certain odorants to antagonize the response of receptors to activating odorants (Oka et al., 2004). This kind of antagonism may be essential to the coding of natural odors, which consist not of a single monomolecular species but of complex mixtures of molecules. We note that odor antagonism may also be of direct practical importance. For example, 1-hexanol has been found to inhibit the response of Drosophila CO2 receptors, and it is possible that compounds that inhibit the CO2 response of mosquitoes or other human-seeking insect pests could be useful in their control (Kwon et al., 2007; Lu et al., 2007; Turner and Ray, 2009).
The primary representations of different odors can be distinguished largely because of a key principle of mammalian and insect odor receptor expression: individual ORNs express only one or a small number of receptors. Thus the signaling of specific ORNs reflects the activity of specific odor receptors. This pattern of organization is in sharp contrast to that of the mammalian taste system, in which multiple bitter receptors are coexpressed in the same neurons (Mueller et al., 2005), impeding the discrimination of different bitter compounds.
Major questions remain concerning the initial representations of odorants. One critical direction for the field is to analyze the coding of odorant mixtures, which, although more difficult to study, is more representative of the problems that the olfactory system has evolved to solve. The temporal dynamics of odor representations need more attention; an impediment to such analysis is that airborne odors are more difficult to deliver with temporal precision than are visual or acoustic stimuli. We note that flies engineered to express only a single Or are still capable of odor discrimination, which may reflect the salience of temporal differences in the responses elicited by different odors (DasGupta and Waddell, 2008).
Translating chemical signals to electrical signals
What are the molecular events through which chemical signals—odors—are converted into electrical signals in the ORNs? The mechanisms have evolved under pressure to provide sensitivity, faithful temporal representation of odor stimuli, and a means of adaptation. Mechanisms of olfactory signal transduction have recently been reviewed in detail (Kato and Touhara, 2009; Nakagawa and Vosshall, 2009). Different types of ORNs in various olfactory organs use different signaling mechanisms. Here we will highlight several areas in which recent progress has been particularly rapid or that offer particular opportunities for progress.
The olfactory systems of terrestrial animals face a challenge: most airborne odorants are hydrophobic, but ORNs must operate in an aqueous environment. To reach the receptors, therefore, hydrophobic odorants must traverse an aqueous fluid. In both mammals and insects, this fluid contains high concentrations of odorant binding proteins (OBPs), which are believed to solubilize and transport odorants. Mammals contain a few distinct OBPs, but insects contain remarkable numbers: in Drosophila there are 51 diverse members of the OBP family, a number comparable to the number of Ors (for review, see Pelosi et al., 2006). The crystal structures of some insect OBPs have been solved (Sandler et al., 2000), and the remarkable size and diversity of the insect OBP family have attracted much interest in their functional roles in olfaction.
Do OBPs confer odor-specificity upon ORNs? When Drosophila Ors were expressed individually in the empty neuron system, the Ors conferred odor-specificities that matched those of the ORNs from which they originated, suggesting that the Or is sufficient to endow the ORN with its odor-specificity, at least in many cases (Hallem et al., 2004). Could pheromone reception be an exceptional case? A recent study found evidence that an OBP called LUSH, which binds the Drosophila pheromone cVA, interacts directly with the receptor of the cVA-sensitive ORN: a mutant LUSH protein, designed to mimic the cVA-bound conformation of LUSH, could elevate the activity of the ORN in the absence of cVA (Laughlin et al., 2008). The generality of this result is not yet clear, given that the binding protein for the silkmoth pheromone bombykol is not essential for the activation of the bombykol receptor, either in Xenopus oocytes (Nakagawa et al., 2005) or in the empty neuron system (Syed et al., 2006). However, the latter study found evidence that the presence of the bombykol OBP enhances sensitivity. Perhaps the development of an “empty sensillum” system, a mutant sensillum containing no endogenous OBPs, would provide a useful laboratory in which to examine physiologically in vivo the functions of the 51 members of the Drosophila OBP family.
After an odorant reaches a receptor, what happens? In the case of mammalian ORs, elegant structure-function analysis has provided evidence that the odorant binds to a pocket surrounded by transmembrane domains 3,5, and 6 of the receptor (Katada et al., 2005), a conclusion supported by a recent computational analysis (Saito et al., 2009). Binding is apparently mediated largely by hydrophobic and van der Waals interactions, and is looser than for many other GPCRs, which often bind their ligands via ionic or hydrogen bonds (Katada et al., 2005). The looseness of OR-odorant binding is consistent with the finding that odorant dwell times are extremely short (<1ms) (Bhandawat et al., 2005). Loose interactions are also in agreement with the broad tuning of many odorant receptors, and they enhance combinatorial coding.
Although physiological and computational analyses have been very informative, our understanding of OR-ligand interactions and olfactory transduction would be enhanced enormously by the determination of the structure of an OR. The crystal structure of rhodopsin was extremely useful in understanding the conformational change it undergoes upon activation, and a comparable structure for an OR, although an immense challenge, would provide a great advance to the field of olfaction.
The activation of a mammalian odor receptor leads to a concatenation of events: the activation of a G protein, the activation of adenylyl cyclase, the elevation of cyclic AMP (cAMP) levels, the opening of a cyclic-nucleotide gated channel, the influx of Ca2+, and the opening of a Ca2+ activated Cl− channel, recently identified as Anoctamin2 (Stephan et al., 2009). The Cl− influx provides the major amplification step in olfactory transduction, which is unique to vertebrate ORNs and is enabled by a chloride transporter that maintains a high Cl− concentration in the cilia (Reisert et al., 2005). By contrast, in phototransduction amplification occurs via the activation of many G proteins by a single activated rhodopsin molecule, which does not occur in olfaction, consistent with the short odorant dwell time (Bhandawat et al., 2005). We note that in the olfactory system, mutation of the G protein, the adenylyl cyclase, and the cyclic-nucleotide channel have all been shown to have severe effects on olfactory function, suggesting that cAMP-dependent signaling is the dominant transduction mechanism in the main olfactory epithelium (for review, see Touhara and Vosshall, 2009).
To ensure faithful temporal representation of odor stimuli, expeditious signal termination is required. Interestingly, the key termination mechanisms reported in phototransduction—phosphorylation of the receptor, arrestin binding to the receptor, and RGS protein-mediated inactivation of the G protein—have not been shown to play a major role in the rapid termination of an olfactory signal. Rather, Ca2+-mediated feedback inhibition of the cyclic-nucleotide gated channel, activation of phosphodiesterase, and inhibition of the adenylyl cyclase, along with extrusion of Ca2+, have been implicated (for review, see Bradley et al., 2005). Perhaps the receptor is not a target for signal termination in olfaction because of the short odorant dwell time.
The acuity of olfactory perception relies upon adaptation. Adaptation allows extension of the dynamic range of ORNs such that they are informative over a broader range of odorant concentrations, and it enables an animal to detect new scents above a background odor. Common mechanisms may contribute to both adaptation and termination. However, the details remain to be elucidated. For instance, although the cyclic-nucleotide-gated channel and a phosphodiesterase (PDE1C) had been believed to play a key role in adaptation and termination, respectively, recent studies have not supported these notions (Cygnar and Zhao, 2009; Song et al., 2008). Determination of the mechanisms of adaptation will require more detailed electrophysiological studies of ORNs in genetically-manipulated animals.
Insect olfactory transduction has been the focus of much recent attention. Insect odor receptors have seven transmembrane domains and have long been assumed to be GPCRs like their counterparts in mammals and in the nematode C. elegans. However, in contrast to mammals and worms, no G protein mutant has been found to suffer a severe loss of olfactory function. Moreover, the topology of the insect Ors is inverted relative to GPCRs (Benton et al., 2006; Smart et al., 2008), such that the N terminus is intracellular, and each Or appears to form a heteromultimer with one particular Or family member, Or83b (Benton et al., 2006; Neuhaus et al., 2005).
Two recent studies show that insect odor receptors can act as ionotropic receptors: a canonical Or, together with Or83b, can form a ligand-gated cation channel (Sato et al., 2008; Wicher et al., 2008). Both studies examine heterologous cells expressing an Or with Or83b and observe an odorant-induced, rapidly developing, transient inward current. Both groups find the rapid, inward transient to be independent of G protein signaling; however, one of the groups also observes a second, slower and larger component to the odorant-induced inward current (Wicher et al., 2008). This second component is slower both in onset and decay kinetics, and is sensitive to inhibition by the GDP analog GDP-βS, as if insect odor receptors can also function as metabotropic receptors that signal via a G protein-mediated pathway. Interestingly, this metabotropic pathway produces cyclic nucleotides, and Or83b is activated directly by cyclic nucleotides. The observation of this second component of the current led to the proposal of a two-step signaling model. Upon odorant-binding, the ligand-gated Or/Or83b channel complex would produce a fast, inward current, followed by a larger and slower metabotropic cyclic nucleotide-gated current.
Taken together, these results provide strong evidence that Ors can act as ligand-gated ion channels. A detailed understanding of their role in G protein signaling will require further analysis of the nature of their interactions with the G protein. Understanding of both signaling modes would benefit enormously from structural analysis of the receptor. Above all, conclusions from the studies in heterologous cell systems will need to be confirmed in fly ORNs. It will also be of interest to compare the signaling mechanism of Ors with those of IRs and of the Grs that act in carbon dioxide signaling.
Transformation of olfactory signals at the first processing center
The primary representation of an odorant is distributed among a large number of ORNs. This representation is transformed into a secondary representation at the first processing center, the olfactory bulb in mammals and the antennal lobe in insects. The secondary representation is distributed among a much smaller number of output cells, which transmit information to higher regions of the brain. The organization of the center that accomplishes this signal transformation is surprisingly similar in mammals and insects (Figure 2).
Figure 2. Olfactory bulb and antennal lobe circuitry.
Excitatory neurons are shown in orange and inhibitory neurons in blue. (A) Olfactory receptor neurons (ORNs) in the olfactory epithelium that express different olfactory receptors project axons to separate glomeruli (dashed outlines) in the olfactory bulb where they synapse on mitral and tufted (M/T) cells, whose apical dendrite is usually localized to a single glomerulus. Juxtaglomerular cells (blue) contribute to intraglomerular inhibition. In the glomerulus, ORNs form synapses on juxtaglomerular cell dendrites, which in turn inhibit ORN axon terminals. Reciprocal synapses are also found between juxtaglomerular cell and M/T cell dendrites. Reciprocal synapses are formed between the dendrites of granule cells and M/T cells. M/T cells excite granule cells, which respond by inhibiting M/T cells. Due to the lateral spread of M/T secondary dendrites, granule cells contact multiple M/T cells associated with different glomeruli, and thus can mediate both intra- and interglomerular inhibition. (B) In Drosophila, ORNs expressing the same olfactory receptors in the antenna or maxillary palp synapse on projection neurons in a single glomerulus, analogous to the olfactory bulb. GABA-releasing local neurons (LNs) pre-synaptically inhibit ORN axon terminals in multiple glomeruli, mediating interglomerular inhibition. Excitatory cholinergic LNs mediate interglomerular excitation.
In most mammals and insects examined, ORNs that express the same receptor converge upon one or two glomeruli (see also, Maresh et al., 2008; for review, see Wilson and Mainen, 2006). The convergence ratio is high, on the order of 50 ORNs per glomerulus in Drosophila and 5000 ORNs per glomerulus in rodents. In the glomerulus, the axon terminals of ORNs form synapses with the dendrites of the output neurons, which are called mitral and tufted (M/T) cells in mammals and projection neurons in insects. Most individual M/Tcells and projection neurons receive direct excitatory input from only one type of ORN, expressing one type of odor receptor. The high convergence ratios of ORNs to projection neurons may allow for the integration and amplification of weak signals; they may also allow for the averaging of stronger signals, which should lead to a higher signal-to-noise ratio in the projection neurons, that is, a higher ratio of response strength to variance.
The activities of M/T cells and projection neurons are also regulated by interneurons that allow communication within and between glomeruli. In the mammalian olfactory bulb, these interneurons lie in two layers: interneurons called juxtaglomerular cells lie in the glomerular layer, and granule cells lie in a deeper layer called the external plexiform layer (Figure 2A). Juxtaglomerular cells receive direct excitatory input from ORN axons and form inhibitory synapses onto ORN axons within the same glomerulus. Granule cells form inhibitory synapses onto M/T cells of multiple glomeruli and mediate interglomerular information transfer (for reviews, see Shepherd et al., 2007; Wilson and Mainen, 2006). In the insect antennal lobe, interneurons called local neurons connect glomeruli and are primarily inhibitory (Figure 2B) (Wilson and Mainen, 2006).
The circuitry of each of these processing centers and the computations that they perform have been analyzed through anatomical, electrophysiological, and imaging studies (for reviews, see Laurent, 2002; Wilson and Mainen, 2006). Here we will focus on the cellular basis of the transformations that occur in these centers, with special attention to recent advances made in the antennal lobe of Drosophila.
An ideal means of analyzing these transformations is to compare the response profiles of presynaptic ORNs to their postsynaptic M/T cell or projection neuron partners. This approach is conceptually simple but technically difficult. The complexity of vertebrate olfaction makes it very difficult to carry out such analysis systematically.
Drosophila is an attractive system in which to carry out such analysis because of its numerical simplicity and defined organization. In the fly there are 18 defined types of sensilla in the antenna and three in the maxillary palp, and the ORNs they contain have been functionally analyzed through single-unit electrophysiology (de Bruyne et al., 1999; de Bruyne et al., 2001; Hallem et al., 2004; van der Goes van Naters and Carlson, 2007; Yao et al., 2005) (Table 2). The odor response profiles of ~35 ORN classes have been defined in systematic studies. The odor response profiles of most of the antennal Or receptors have been analyzed in considerable detail (Hallem and Carlson, 2006). Receptor-to-neuron maps have been constructed, and a glomerular projection map has been generated (Couto et al., 2005; Fishilevich and Vosshall, 2005; Hallem et al., 2004). The glomeruli have stereotyped locations and can be genetically labeled via ORNs or PNs using the promoter-GAL4/UAS-reporter system.
Projection neurons innervating one particular glomerulus, DM2, respond to a broader range of odorants than their presynaptic ORNs in a patch-clamp analysis (Wilson et al., 2004b). Similar results obtained for six other glomeruli, suggest that this broadening of range represents a general principle of olfactory processing in the antennal lobe. Moreover, weak ORN responses, but not strong ones, are amplified in projection neurons, a process called nonlinear amplification (Bhandawat et al., 2007). However, all of these glomeruli received input from ORNs that respond to a number of general odors. Examination of a specialized glomerulus that responds to a Drosophila pheromone does not reveal a comparable broadening (Schlief and Wilson, 2007).
What mechanism underlies the broadening of receptive range observed in most glomeruli? Lateral excitatory inputs mediated by a class of cholinergic local neurons may make a minor contribution to this broadening (Olsen et al., 2007; Root et al., 2007; Shang et al., 2007). Interestingly, however, the broader tuning widths and nonlinear amplification among projection neurons are mainly due to strong ORN-projection neuron synapses (Kazama and Wilson, 2008). Weak presynaptic ORN activity is sufficient to trigger robust neurotransmitter release at this synapse and cause substantial projection neuron responses, thereby producing amplification. Strong ORN activity leads to depletion of synaptic neurotransmitter, explaining why strong ORN responses are not amplified proportionally in projection neurons. The strong synapses between ORNs and projection neurons are attributable to the presence of numerous synaptic vesicle release sites and a high release probability (Kazama and Wilson, 2008). High probabilities of vesicle release have also been found in the mammalian olfactory bulb (Murphy et al., 2004), which likely reflects the same principle of information processing.
Odor perception may be enhanced by this nonlinear amplification in interesting ways (for review, see Masse et al., 2009). The fly encounters an enormous range of odorant concentrations in its natural environment, and the ability to evaluate odor intensity may facilitate navigation. ORNs respond to odors over a range as wide as eight orders of magnitude (Hallem and Carlson, 2006), which raises questions about how such a wide range can be efficiently conveyed via the relatively narrow firing frequency ranges of ORNs and projection neurons (~two orders of magnitude). One mechanism is through gain control: by altering the relationship between the input firing rate of ORNs and the output firing rate of projection neurons, nonlinear amplification prevents saturation of projection neuron responses when ORN responses are high (Kazama and Wilson, 2008). Another interesting consequence of the physiology of the ORN-projection neuron synapse is that it may emphasize the initial phase of an ORN response, given that the first spikes produce a larger effect on projection neurons. Such emphasis may also aid navigation: moth projection neurons have been shown to respond quickly to changes in odor concentration (Vickers et al., 2001).
A second mechanism of gain control arises from lateral inhibitory communications among glomeruli, mediated by GABAergic local neurons (Olsen and Wilson, 2008). The site of inhibition is presynaptic, that is, at the ORN axon terminals. For an individual glomerulus, the strength of the lateral inhibition that it receives is proportional to the total ORN activity across the antenna. Thus, high levels of overall ORN activity downregulate projection neuron activity and prevent their saturation. The extent of downregulation, however, may vary for different glomeruli (Root et al., 2008).
The mammalian olfactory bulb also exhibits presynaptic inhibition, mediated primarily by intraglomerular connections (McGann et al., 2005). Many juxtaglomerular cells receive direct excitatory input from ORN axons and form inhibitory synapses onto ORN axons within the same glomerulus, thereby providing intraglomerular feedback inhibition that may extend the dynamic range of the glomerulus. The strength of inhibition appears independent of ORN activity (Pirez and Wachowiak, 2008), suggesting a different mechanism from that of Drosophila. Interglomerular inhibition occurs mainly on M/T cells and is mediated by granule cells in the external plexiform layer (for review, see Wilson and Mainen, 2006).
It will be interesting to determine how inhibition may contribute to other aspects of odor perception. Does the activation of one glomerulus inhibit surrounding glomeruli in such a way as to enhance odor discrimination? To resolve this question, it will be helpful to have more detailed maps of the patterns of lateral inhibition, and to have a better understanding of the functional organization of glomeruli. Many studies have provided evidence for a coarse chemotopy, in the sense that the location of glomeruli is related to the chemical nature of the odors that activate them (for review, see Johnson and Leon, 2007). However, recent large-scale studies have not found clear evidence for fine-scale chemotopic maps (Hallem and Carlson, 2006; Soucy et al., 2009), and the relationship between function and topography remains an intriguing issue.
We note finally that odor-evoked oscillations in electrical activity have been found in the olfactory bulb of mammals as well as in the antennal lobe of locusts, bees, and moths: the activities of populations of neurons are synchronized in an oscillatory pattern (for review, see Laurent, 2002). Disruption of this oscillatory network affects the ability of honeybees to discriminate similar odorants (Stopfer et al., 1997), consistent with a function for oscillatory mechanisms in enhancing olfactory acuity.
Circuitry and coding in higher brain regions
In mammals and insects, the second order neurons—M/T cells and projection neurons—innervate multiple higher brain regions. In these regions the olfactory information is integrated with information from other sensory modalities, information from past experience, and information concerning the animal’s behavioral state, to shape olfactory perception and to instruct behavior.
In mammals, M/T cells synapse directly on pyramidal neurons in the olfactory cortex. The olfactory cortex contains several distinct regions, including the piriform cortex, the olfactory tubercle, the anterior olfactory nucleus, and certain parts of the amygdala and entorhinal cortex. Unlike other sensory systems, olfactory signals are not relayed through the thalamus before reaching the cortex. Olfactory cortical neurons form dense reciprocal connections with neurons from other regions of the olfactory cortex. They also form connections with other regions such as the orbitofrontal cortex, thalamus, and hypothalamus, allowing them to act as sites of integration.
Odor coding has been analyzed in pyramidal neurons of the piriform cortex and has been found to be sparse: an odor stimulus elicits responses from only a small fraction of spatially dispersed neurons, and these responses consist of few action potentials (Figure 3a) (Illig and Haberly, 2003; Litaudon et al., 2003; Poo and Isaacson, 2009). Moreover, each neuron responds to only a limited number of odors (Litaudon et al., 2003; Poo and Isaacson, 2009). Thus, cortical pyramidal neurons appear to be much more narrowly tuned than their presynaptic neurons.
Figure 3. Transformation of odor representations.
(A) Responses of 48 second-order neurons (projection neurons, PNs) and 42 third-order neurons (Kenyon cells) in locusts to a panel of 14 odors. Black squares indicate activation of a neuron by an odor; white squares indicate lack of response or inhibitory responses. Ns respond to many odors, in contrast to Kenyon cells, which respond to only a few (sparse coding). Additionally, similar activation patterns of 2nd order neurons, e.g. odors 7 (blue) and 13 (red), result in highly divergent activation patterns of 3rd order neurons, a process termed “decorrelation”. This decorrelation is thought to make these odors easier to discriminate. Adapted from Perez-Orive et al., 2002. Reprinted with permission from AAAS. (B) A simplified illustration of how sparsening and decorrelation of responses occur in 3rd order neurons. Odors A and B each activate multiple 2nd order neurons (colored circles), with similar patterns of activated neurons. However, due to the requirement of synchronized inputs from multiple 2nd order neurons (coincidence detection), many fewer 3rd order neurons are activated (sparsening) with more distinct activation pattern (decorrelation).
What is the underlying basis of this reduction in tuning width? Unlike M/T cells, whose activity is driven primarily by a single type of odor receptor, pyramidal cortical neurons receive synapses from multiple M/T cells that carry output from multiple glomeruli and thus multiple odor receptors (Franks and Isaacson, 2006). The pyramidal neurons act as coincidence detectors: they only fire action potentials when a certain subset of M/T cells is synchronously active. The coincident activity of several presynaptic M/T cells is required to overcome widespread inhibition mediated by local interneurons, inhibition that is odor-evoked (Poo and Isaacson, 2009). Given that an individual odor is unlikely to activate the precise combination of odor receptors necessary for an individual pyramidal neuron to fire, very few pyramidal neurons fire, and hence coding is sparse. This form of processing is likely to enhance the capacity of the system to discriminate structurally similar odorants. Even if two odorants activate very similar subsets of glomeruli in the olfactory bulb, their representations in cortical regions are distinct; they are decorrelated as a result of the requirement for coincidence detection (Figure 3b).
In Drosophila and many other insects, projection neurons innervate the lateral horn of the protocerebrum and to the mushroom bodies, where they synapse on neurons known as Kenyon cells. Coding in the mushroom body is remarkably similar to that in the piriform cortex. Kenyon cells receive inputs from multiple glomeruli and are more narrowly tuned than their presynaptic projection neurons; their sparse coding also depends on coincidence detection, and on global inhibition (Lin et al., 2007; Perez-Orive et al., 2002; Tanaka et al., 2004; Turner et al., 2008; Wang et al., 2004).
An individual olfactory stimulus may elicit different percepts depending on prior experience and olfactory learning. In rodents, it is well established that neural representations of odors in higher brain regions as well as the olfactory bulb are dictated not only by odorant structure, but also by experience (Wilson et al., 2004a). Recent functional magnetic resonance imaging studies on humans have found evidence for experience-based changes in the piriform cortex (Li et al., 2008; Li et al., 2006). The anatomical basis of olfactory learning in this brain region may involve an extensive system of association fibers that connects neurons within the same and different olfactory cortical regions and whose synaptic strengths can be modified by olfactory experience (Wilson et al., 2004a). Olfactory learning can enhance odor discrimination, and may be important to the survival of many organisms. In humans, changes in cortical neuronal activity due to olfactory learning are correlated with improved discrimination of similar odorants (Li et al., 2008).
In many animals, certain odors exhibit behavioral responses that appear not to be learned, but rather to be innate. These responses are reminiscent of the innate responses to sweet and bitter substances that are driven by the gustatory system. In addition to responses to pheromones, which are innate, both insects and mammals can be innately attracted to or repelled by certain non-pheromonal odorants through a mechanism that depends on the activation of specific glomeruli or glomerular subsets (Kobayakawa et al., 2007; Semmelhack and Wang, 2009; Suh et al., 2007).
Innate responses are believed to be mediated by hard-wired circuits that link specific ORNs to specific neurons in higher centers, and evidence to support this notion comes from genetic labeling studies in Drosophila. These studies reveal that axons of projection neurons from the same glomerulus show stereotyped projections to the lateral horn (Marin et al., 2002; Wong et al., 2002). Moreover, projection neurons associated with food odors target a different region of the lateral horn than do projection neurons associated with pheromones, which may underlie the difference in innate behaviors elicited by these two classes of odorants (Jefferis et al., 2007). Interestingly, projection neurons sensitive to the pheromone cVA have sexually dimorphic projections, suggesting a mechanism by which the pheromone evokes different behaviors in males and females (Datta et al., 2008). Anatomical and physiological analysis did not find such a high level of stereotypy in the mushroom body (Marin et al., 2002; Murthy et al., 2008; Tanaka et al., 2004), consistent with the finding that the structure of the mushroom body is influenced by experience and that it plays a central role in learning (Heisenberg, 2003).
Regulation of the first processing center by higher centers
Thus far we have considered the flow of olfactory information from the receptors to the first processing center to higher brain regions, that is, the “bottom-up” pathway. However, odor perception is not a simple feedforward process in mammals: there is also a “top-down”, or centrifugal, pathway that provides feedback and other forms of regulation. Ultimately odor perception is shaped by the interaction of the two pathways.
Higher brain regions regulate olfactory bulb activity in a manner that is influenced by learning, by anticipation of an odor or a reward, and by behavioral states including hunger (for review see Rinberg and Gelperin, 2006). The centrifugal fibers that effect this regulation have been divided into two classes based on their location of origin. One class originates in cortical regions, primarily the olfactory cortex. The other class originates from brain regions containing neurons that release neuromodulators.
The centrifugal fibers that arise in areas of the olfactory cortex, including the piriform cortex and the anterior olfactory nucleus, provide feedback to the olfactory bulb. These fibers release the excitatory neurotransmitter glutamate and form synapses primarily on subsets of granule cells. Such fibers regulate the lateral inhibition of M/T cells and can undergo experience-dependent synapse strengthening (Balu et al., 2007; Gao and Strowbridge, 2009). Interestingly, whereas the targets of ORNs and M/T cells are ipsilateral, subsets of centrifugal fibers from the anterior olfactory nucleus receive input from specific glomeruli and send feedback projections to isofunctional glomeruli in the contralateral olfactory bulb (Yan et al., 2008). This pattern of organization suggests a role for these fibers in coordinating signal processing between the two brain hemispheres, a possibility that is of interest in light of recent evidence that rats use internasal comparisons to locate odor sources (Rajan et al., 2006).
Neuromodulatory centrifugal fibers originating in other regions selectively target granule, M/T, and/or juxtaglomerular cells and release norepinephrine, serotonin, or acetylcholine. Disruption of these inputs influences olfactory-driven behaviors. For example, the blocking of norephinephrine receptors prevents rat pups from learning to associate tactile stimuli with specific odors, an ethologically important form of associative olfactory learning (Sullivan et al., 1992). More recent investigations have focused on the circuit-level effects of neuromodulators. For example, the pairing of an odor with norepinephrine release leads to a long-lasting decrease in M/T cell responses specific to that odor (Shea et al., 2008), whereas serotonin release non-selectively reduces odor-evoked ORN synaptic activity due to an increase in juxtaglomerular cell-mediated presynaptic inhibition (Petzold et al., 2009). Through such effects on signal processing in the olfactory bulb, the centrifugal neuromodulatory fibers have been suggested to influence odor discrimination, to provide a means of gain control, and to generate local experience-dependent changes in the olfactory bulb. It will be particularly interesting to learn more about the mechanisms through which these fibers are activated.
In the insect antennal lobe, centrifugal inputs are largely uncharacterized. Moths provide an interesting exception: serotonergic fibers increase behavioral sensitivity of male moths to some sex pheromones by supplying centrifugal input from the protocerebrum to antennal projection neurons and local neurons (Kloppenburg and Mercer, 2008). In Drosophila, serotonin was recently shown to excite both projection and local neurons and to increase ORN presynaptic inhibition; it is proposed to suppress weak ORN responses (Dacks et al., 2009). An integrated analysis of the anatomy, physiology, and behavioral effects of centrifugal inputs in insects seems likely to be a fruitful avenue of future research.
We note finally another interesting modulator of olfactory activity in mammals: sniffing. Changes in sniffing frequency affect odor intake into the nasal cavity and thus regulate the magnitude and temporal dynamics of ORN activation. Sustained high-frequency sniffing depresses activity in ORNs responsive to the presented odor, perhaps as a result of OR adaptation (Verhagen et al., 2007). Increases in the rate of sniffing are often observed when mammals encounter novel odorants and when they anticipate odor presentation; higher sniffing rates may enhance discrimination (Kepecs et al., 2007).
Odor spaces
Olfaction can be thought of as a series of transformations. Information about the structure of an odorant is transformed into a succession of neural representations, and is ultimately transformed into a perception. A long term goal of the field is to understand the rules governing each transformation. Ultimately one would like to be able to predict each transformation. If a chemist synthesizes a new molecule, one would like to be able to predict its activity profile across an odor receptor repertoire, the activities of glomeruli and of higher centers, and whether the molecule will evoke a fruity or a musky odor, or whether it will elicit attraction or repulsion.
There are major challenges to meeting this goal. First, predictive ability is limited by the biological complexity of odor perception. In many cases the relationship between odor structure, neural representations, and perception is not strictly deterministic, owing to the role of experience and other factors. Second, prediction depends on identifying parameters that adequately describe the odorants, the neural representations, and the perception.
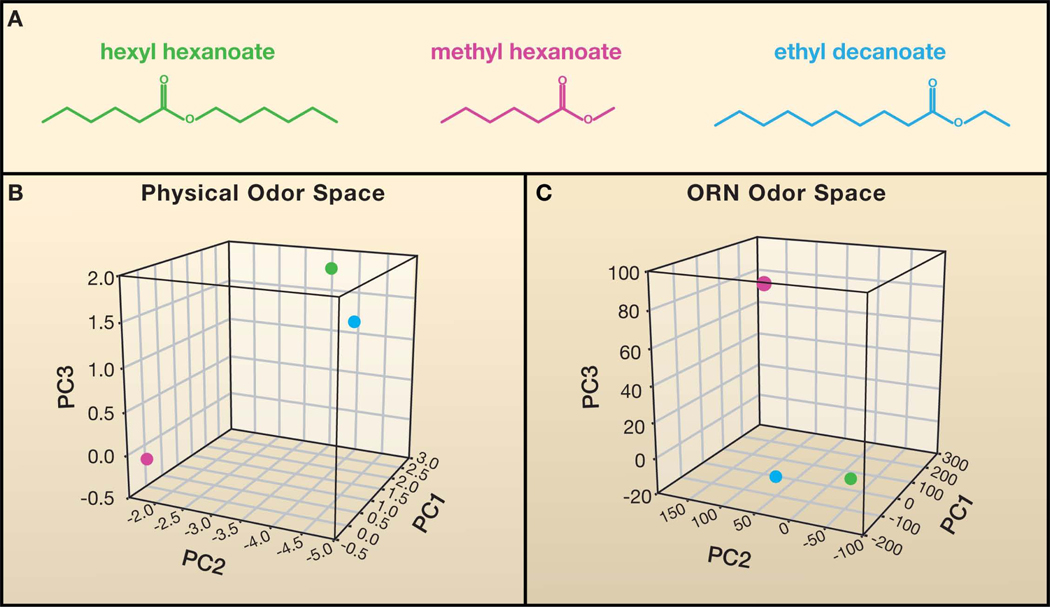
Odor structure cannot be described by a simple variable such as wavelength. It is therefore difficult to compare two odorant structures quantitatively: is the structure of hexyl hexanoate more closely related to that of methyl hexanoate or ethyl decanoate (Figure 4A)? Odorants vary in an indeterminate number of parameters, including carbon-chain length, molecular weight, and polarity. Recently a multidimensional, physicochemical odorant space was devised to describe odorant structure: 1,664 molecular descriptors for more than 1,500 odorants were used to construct a 1,664-dimensional odor space, in which each dimension represents one feature of odorant structure (Haddad et al., 2008). An odorant can be mapped to a unique location in this space according to its values for each descriptor (Figure 4B). The physicochemical relationship between two odorants can then be quantitated as the Euclidean distance between them in this space.
Figure 4. Representations of odor space.
(A) Chemical structures of three odorants. It is difficult to compare the degree of relatedness between odorants by visual inspection. (B) A physical odor space constructed using 32 optimized descriptors of odorant structure (Haddad et al., 2008), for the odor panel used in Hallem and Carlson, 2006. The Euclidean distance between hexyl hexanoate (green) and ethyl decanoate (blue) is smaller than the distance of either odorant to methyl hexanoate (magenta), indicating that hexyl hexanoate and ethyl decanoate are more structurally similar. The first three principal components (PC) are shown. Similarly, hexyl hexanoate and ethyl decanoate map closer to each other than to methyl hexanoate in a neural odor space (C), based on the functional data reported in Hallem and Carlson, 2006.
This physical odor space is useful in predicting odor perception. Principle component analysis reveals a correlation between odorant structure, as defined in the space, and its perceived pleasantness among humans (Khan et al., 2007). Further analysis led to the development of a simpler, optimized odor space based on 32 of the descriptors, chosen following analysis of the functional responses these odorants elicited in several experimental systems (Haddad et al., 2008). Interestingly, a subsequent study of odor responses elicited from a set of human and mouse ORs yields a slightly different set of optimized descriptors. The optimized descriptors may differ for ORs of different species (Saito et al., 2009). Much of the variation in OR response could be explained by a relatively small subset of descriptors.
Another kind of olfactory space is a neural odor space, which illustrates how a particular odorant is represented in the olfactory system. A neural space can be constructed from direct measurements of odor responses from neurons of the system, or in the case of ORNs, from responses of odor receptors in an expression system, provided that the expression system faithfully represents the activities of receptors in their endogenous neurons (Hallem et al., 2004). Constructing such a space is at present a major undertaking, but is more feasible in systems that are numerically simpler. For example, in an analysis of the Drosophila antennal receptor repertoire, most of the Ors expressed in the antenna could be examined. Of these, ~75% were found to yield responses and were systematically tested with 110 odorants (Hallem and Carlson, 2006). An odor space was then constructed in which each axis represents the response of one receptor. Each odorant was then mapped to a position in this space based on the response magnitude for each receptor. In such a space, two odorants will map close together if they elicit similar responses patterns across the receptor repertoire (Figure 4C). Are two odorants that are close in this neural space also close in perceptual quality? Analysis of the odor receptor repertoire of Drosophila larvae, along with an accompanying behavioral analysis, provides support for this notion (Kreher et al., 2008).
Analogous neural spaces can be constructed for each successive level of processing, and the distribution of odorants in each successive space is likely to differ from that of its predecessors. The non-linearity of the ORN-projection neuron transformation, for example, acts to generate a broader distribution of odorants in projection neuron space than in ORN space (Bhandawat et al., 2007). It will be interesting to determine whether the relative positions of odorants at successive levels of processing provide more accurate predictions of perceptual relationships, such as perceived odor similarity. In interpreting such spaces, however, it is important to consider that certain innate olfactory behaviors may be mediated by small subsets of the neurons, rather than the activation patterns of the entire set of neurons (Semmelhack and Wang, 2009). Although combinatorial coding underlies odor perception, it may not be essential for the initiation of many odor-induced innate behaviors.
An interesting problem for future research is to determine how neural spaces have evolved to meet the ecological needs of the species. Different species rely on different odorants as cues, and it seems likely that neural odor spaces have undergone changes in structure to promote the detection and discrimination of particular subsets of odorants.
Conclusion
Olfactory perception has a more diverse basis than previously appreciated—entire new families of receptor genes and a new signal transduction mechanism have recently been discovered. The molecular, cellular, and anatomical diversity of the signaling systems may reflect the immense variety of signals that are detected and encoded. It will be of great interest to gain further insight into how the distinct features of different signaling systems subserve their biological functions.
New insight has also been gained into the mechanisms by which signals are processed in the glomeruli and in higher brain regions. Despite their evolutionary distance, the parallels between insect and mammalian olfactory circuitry are striking, perhaps reflecting similar challenges in extracting critical olfactory information. Further understanding of olfactory processing will surely benefit from more detailed maps of neural circuitry. Just as the discovery of new receptor genes provides a new dimension to our knowledge of how environmental signals are transduced, the delineation of the circuitry should provide new insight into how olfactory representations are transformed into a succession of neural representations.
Ultimately, understanding of olfactory perception requires a higher-order biological perspective. Although the mechanisms by which odors are coded and decoded can be powerfully deconstructed through molecular and physiological analysis, the significance of these mechanisms must be examined in behaving animals, of a wide variety of species.
Acknowledgments
We thank C. Greer for comments on the manuscript and A. Carey for help with odor space construction. We acknowledge funding from the NIH and a Senior Scholar Award from the Ellison Medical Foundation to J.C., and a grant from the Foundation for the NIH through the Grand Challenges in Global Health Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balu R, Pressler RT, Strowbridge BW. Multiple modes of synaptic excitation of olfactory bulb granule cells. J Neurosci. 2007;27:5621–5632. doi: 10.1523/JNEUROSCI.4630-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Reisert J, Yau KW. Elementary response of olfactory receptor neurons to odorants. Science. 2005;308:1931–1934. doi: 10.1126/science.1109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner F, Boppre M, Ernst KD, Boeckh J. CO2 sensitive receptors on labial palps of Rhodogastria moths (Lepidoptera: Arctiidae): physiology, fine structure and central projection. J Comp Physiol A. 1986;158:741–749. doi: 10.1007/BF01324818. [DOI] [PubMed] [Google Scholar]
- Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol. 2005;15:343–349. doi: 10.1016/j.conb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science. 2008;321:1092–1095. doi: 10.1126/science.1160770. [DOI] [PubMed] [Google Scholar]
- Clyne P, Grant A, O'Connell R, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invert Neurosci. 1997;3:127–135. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Cygnar KD, Zhao H. Phosphodiesterase 1C is dispensable for rapid response termination of olfactory sensory neurons. Nat Neurosci. 2009;12:454–462. doi: 10.1038/nn.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacks AM, Green DS, Root CM, Nighorn AJ, Wang JW. Serotonin Modulates Olfactory Processing in the Antennal Lobe of Drosophila. J Neurogenet. 2009:1–13. doi: 10.3109/01677060903085722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta S, Waddell S. Learned odor discrimination in Drosophila without combinatorial odor maps in the antennal lobe. Curr Biol. 2008;18:1668–1674. doi: 10.1016/j.cub.2008.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- de Brito Sanchez MG, Kaissling KE. The antennal benzoic acid receptor cell of the female silk moth Bombyx mori L.: structure-activity relationship studies with halogen substitutes. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191:189–196. doi: 10.1007/s00359-004-0588-2. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Baker TC. Odor detection in insects: volatile codes. J Chem Ecol. 2008;34:882–897. doi: 10.1007/s10886-008-9485-4. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: the Drosophila maxillary palp. J Neurosci. 1999;19:4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Elmore T, Ignell R, Carlson JR, Smith DP. Targeted mutation of a Drosophila odor receptor defines receptor requirement in a novel class of sensillum. J Neurosci. 2003;23:9906–9912. doi: 10.1523/JNEUROSCI.23-30-09906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Fleischer J, Schwarzenbacher K, Besser S, Hass N, Breer H. Olfactory receptors and signalling elements in the Grueneberg ganglion. J Neurochem. 2006;98:543–554. doi: 10.1111/j.1471-4159.2006.03894.x. [DOI] [PubMed] [Google Scholar]
- Fleischer J, Schwarzenbacher K, Breer H. Expression of trace amine-associated receptors in the Grueneberg ganglion. Chem Senses. 2007;32:623–631. doi: 10.1093/chemse/bjm032. [DOI] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Strong single-fiber sensory inputs to olfactory cortex: implications for olfactory coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Freitag J, Ludwig G, Andreini I, Rossler P, Breer H. Olfactory receptors in aquatic and terrestrial vertebrates. J Comp Physiol A. 1998;183:635–650. doi: 10.1007/s003590050287. [DOI] [PubMed] [Google Scholar]
- Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat Neurosci. 2009;12:731–733. doi: 10.1038/nn.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci. 2007;10:348–354. doi: 10.1038/nn1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Khan R, Takahashi YK, Mori K, Harel D, Sobel N. A metric for odorant comparison. Nat Methods. 2008;5:425–429. doi: 10.1038/nmeth.1197. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Hashiguchi Y, Nishida M. Evolution of trace amine associated receptor (TAAR) gene family in vertebrates: lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in the olfactory epithelium. Mol Biol Evol. 2007;24:2099–2107. doi: 10.1093/molbev/msm140. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Jr, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Kaissling KE, Priesner E. Die Reichshwelle des Seidenspinners. Naturwissenschaften. 1970;57:23–28. doi: 10.1007/BF00593550. [DOI] [PubMed] [Google Scholar]
- Kaluza JF, Gussing F, Bohm S, Breer H, Strotmann J. Olfactory receptors in the mouse septal organ. J Neurosci Res. 2004;76:442–452. doi: 10.1002/jnr.20083. [DOI] [PubMed] [Google Scholar]
- Katada S, Hirokawa T, Oka Y, Suwa M, Touhara K. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Touhara K. Mammalian olfactory receptors: pharmacology, G protein coupling and desensitization. Cell Mol Life Sci. 2009 doi: 10.1007/s00018-009-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron. 2008;58:401–413. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Vosshall LB. Influence of odorant receptor repertoire on odor perception in humans and fruit flies. Proc Natl Acad Sci U S A. 2007;104:5614–5619. doi: 10.1073/pnas.0605321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 2007;98:205–213. doi: 10.1152/jn.00071.2007. [DOI] [PubMed] [Google Scholar]
- Khan RM, Luk CH, Flinker A, Aggarwal A, Lapid H, Haddad R, Sobel N. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci. 2007;27:10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg P, Mercer AR. Serotonin modulation of moth central olfactory neurons. Annu Rev Entomol. 2008;53:179–190. doi: 10.1146/annurev.ento.53.103106.093408. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska M, Galizia CG, Giurfa M, Menzel R. Olfactory discrimination ability and odor structure-activity relationships in honeybees. Chem Senses. 1999;24:429–438. doi: 10.1093/chemse/24.4.429. [DOI] [PubMed] [Google Scholar]
- Laska M, Shepherd GM. Olfactory discrimination ability of CD-1 mice for a large array of enantiomers. Neuroscience. 2007;144:295–301. doi: 10.1016/j.neuroscience.2006.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin JD, Ha TS, Jones DN, Smith DP. Activation of pheromonesensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Luxenberg E, Parrish T, Gottfried JA. Learning to smell the roses: experience-dependent neural plasticity in human piriform and orbitofrontal cortices. Neuron. 2006;52:1097–1108. doi: 10.1016/j.neuron.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, Siltberg-Liberles J, Liberles DA, Buck LB. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc Natl Acad Sci U S A. 2009;106:9842–9847. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Lai JS, Chin AL, Chen YC, Chiang AS. A map of olfactory representation in the Drosophila mushroom body. Cell. 2007;128:1205–1217. doi: 10.1016/j.cell.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Lin W, Arellano J, Slotnick B, Restrepo D. Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system. J Neurosci. 2004;24:3703–3710. doi: 10.1523/JNEUROSCI.0188-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaudon P, Amat C, Bertrand B, Vigouroux M, Buonviso N. Piriform cortex functional heterogeneity revealed by cellular responses to odours. Eur J Neurosci. 2003;17:2457–2461. doi: 10.1046/j.1460-9568.2003.02654.x. [DOI] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJ, Takken W, Carlson JR, Zwiebel LJ. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- Ma M, Grosmaitre X, Iwema CL, Baker H, Greer CA, Shepherd GM. Olfactory signal transduction in the mouse septal organ. J Neurosci. 2003;23:317–324. doi: 10.1523/JNEUROSCI.23-01-00317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Maresh A, Rodriguez Gil D, Whitman MC, Greer CA. Principles of glomerular organization in the human olfactory bulb--implications for odor processing. PLoS One. 2008;3:e2640. doi: 10.1371/journal.pone.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–R713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48:1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Menashe I, Abaffy T, Hasin Y, Goshen S, Yahalom V, Luetje CW, Lancet D. Genetic elucidation of human hyperosmia to isovaleric acid. PLoS Biol. 2007;5:e284. doi: 10.1371/journal.pbio.0050284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Glickfeld LL, Balsen Z, Isaacson JS. Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci. 2004;24:3023–3030. doi: 10.1523/JNEUROSCI.5745-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59:1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Vosshall LB. Controversy and consensus: noncanonical signaling mechanisms in the insect olfactory system. Curr Opin Neurobiol. 2009;19:284–292. doi: 10.1016/j.conb.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Stortkuhl K, Hatt H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat Neurosci. 2005;8:15–17. doi: 10.1038/nn1371. [DOI] [PubMed] [Google Scholar]
- Niimura Y, Nei M. Extensive gains and losses of olfactory receptor genes in Mammalian evolution. PLoS One. 2007;2:e708. doi: 10.1371/journal.pone.0000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Omura M, Kataoka H, Touhara K. Olfactory receptor antagonism between odorants. EMBO J. 2004;23:120–126. doi: 10.1038/sj.emboj.7600032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P, Zhou JJ, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63:1658–1676. doi: 10.1007/s00018-005-5607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009 doi: 10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- Pirez N, Wachowiak M. In vivo modulation of sensory input to the olfactory bulb by tonic and activity-dependent presynaptic inhibition of receptor neurons. J Neurosci. 2008;28:6360–6371. doi: 10.1523/JNEUROSCI.0793-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: "sparse" coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311:666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- Reisert J, Lai J, Yau KW, Bradley J. Mechanism of the excitatory Cl- response in mouse olfactory receptor neurons. Neuron. 2005;45:553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Restrepo D. Molecular Tuning of Odorant Receptors and Its Implication for Odor Signal Processing. Chem Senses. 2009 doi: 10.1093/chemse/bjp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Gelperin A. Olfactory neuronal dynamics in behaving animals. Semin Cell Dev Biol. 2006;17:454–461. doi: 10.1016/j.semcdb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proc Natl Acad Sci U S A. 2007;104:11826–11831. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a Mammalian receptor repertoire. Sci Signal. 2009;2:ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB. Neuropharmacology. Odorants may arouse instinctive behaviours. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Sandler BH, Nikonova L, Leal WS, Clardy J. Sexual attraction in the silkworm moth: structure of the pheromone-binding-protein-bombykol complex. Chem Biol. 2000;7:143–151. doi: 10.1016/s1074-5521(00)00078-8. [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10:623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci. 2008;28:10711–10719. doi: 10.1523/JNEUROSCI.3853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Willhite D, Migliore M, Greer CA. The olfactory granule cell: from classical enigma to central role in olfactory processing. Brain Res Rev. 2007;55:373–382. doi: 10.1016/j.brainresrev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Shiraiwa T. Multimodal chemosensory integration through the maxillary palp in Drosophila. PLoS One. 2008;3:e2191. doi: 10.1371/journal.pone.0002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart R, Kiely A, Beale M, Vargas E, Carraher C, Kralicek AV, Christie DL, Chen C, Newcomb RD, Warr CG. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol. 2008;38:770–780. doi: 10.1016/j.ibmb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Song Y, Cygnar KD, Sagdullaev B, Valley M, Hirsh S, Stephan A, Reisert J, Zhao H. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 2008;58:374–386. doi: 10.1016/j.neuron.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Spehr M, Kelliher KR, Li XH, Boehm T, Leinders-Zufall T, Zufall F. Essential role of the main olfactory system in social recognition of major histocompatibility complex peptide ligands. J Neurosci. 2006;26:1961–1970. doi: 10.1523/JNEUROSCI.4939-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci U S A. 2009;106:11776–11781. doi: 10.1073/pnas.0903304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- Suh GS, Ben-Tabou de Leon S, Tanimoto H, Fiala A, Benzer S, Anderson DJ. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Res Dev Brain Res. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Syed Z, Ishida Y, Taylor K, Kimbrell DA, Leal WS. Pheromone reception in fruit flies expressing a moth's odorant receptor. Proc Natl Acad Sci U S A. 2006;103:16538–16543. doi: 10.1073/pnas.0607874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka NK, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Tian H, Ma M. Molecular organization of the olfactory septal organ. J Neurosci. 2004;24:8383–8390. doi: 10.1523/JNEUROSCI.2222-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6:519–525. doi: 10.1038/nn1039. [DOI] [PubMed] [Google Scholar]
- Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2008;99:734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- Turner SL, Ray A. Modification of CO(2) avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009 doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Vickers NJ, Christensen TA, Baker TC, Hildebrand JG. Odour-plume dynamics influence the brain's olfactory code. Nature. 2001;410:466–470. doi: 10.1038/35068559. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo HF, Pologruto TA, Hannan F, Hakker I, Svoboda K, Zhong Y. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J Neurosci. 2004;24:6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Best AR, Sullivan RM. Plasticity in the olfactory system: lessons for the neurobiology of memory. Neuroscientist. 2004a;10:513–524. doi: 10.1177/1073858404267048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004b;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wang G, Buscariollo D, Pitts RJ, Wenger H, Zwiebel LJ. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc Natl Acad Sci U S A. 2008;105:6433–6438. doi: 10.1073/pnas.0801007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yan Z, Tan J, Qin C, Lu Y, Ding C, Luo M. Precise circuitry links bilaterally symmetric olfactory maps. Neuron. 2008;58:613–624. doi: 10.1016/j.neuron.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Yao CA, Ignell R, Carlson JR. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci. 2005;25:8359–8367. doi: 10.1523/JNEUROSCI.2432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]