Abstract
Insulin-like growth factor binding protein 2 (IGFBP-2) is a malignancy-associated protein measurable in tumors and blood. Increased IGFBP-2 is associated with shortened survival of advanced glioma patients. Thus, we examined plasma IGFBP-2 levels in glioma patients and healthy controls to evaluate its value as a plasma bio-marker for glioma. Plasma IGFBP-2 levels in 196 patients with newly diagnosed glioma and 55 healthy controls were analyzed using an IGFBP-2 ELISA kit. Blood was collected before surgery, after two-cycle adjuvant chemotherapy, and at recurrence. Plasma IGFBP-2 levels were correlated with disease-free survival (DFS) using Cox regression analyses. We found that preoperative plasma IGFBP-2 levels were significantly higher in high-grade glioma patients (n = 43 for grade III glioma; n = 72 for glioblastoma multiforme [GBM]) than in healthy controls (n = 55; p < 0.001) and low-grade (grade II) glioma patients (n = 81; p < 0.001). No significant differences in preoperative plasma IGFBP-2 levels were observed between grade III glioma and GBM patients or between grade II glioma patients and healthy controls. After recurrence, plasma IGFBP-2 levels were significantly increased in GBM patients (n = 26; p < 0.001). Preoperative plasma IGFBP-2 levels were significantly correlated with DFS in GBM patients (hazard ratio, 1.404; 95% confidence interval, 1.078–1.828; p = 0.012). We conclude that preoperative plasma IGFBP-2 levels are significantly higher in high-grade glioma patients than in low-grade glioma patients and healthy subjects, and are significantly correlated with recurrence and DFS in patients with GBM. Longitudinal studies with a larger study population are needed to confirm these findings.
Keywords: glioma, IGFBP-2, plasma surrogate bio-marker, prognosis, recurrence
Glioma is the most common primary brain tumor. High-grade gliomas (WHO grade III and grade IV [glioblastoma multiforme (GBM)]) defined by the current pathologic classification encompass a heterogeneous population of tumors with highly variable prognoses.1 Standard clinicopathologic factors—such as age, tumor grade, KPS, and extent of tumor resection—are commonly used to assess prognosis of patients with glioma, but these predictions are often inaccurate for individual patients. Combined 1p and 19q loss is used as an indicator for chemotherapy and radiotherapy sensitivities and survival for patients with anaplastic oligodendrogliomas (AOs).2,3 For anaplastic astrocytomas (AAs) and anaplastic oligoastrocytomas (AOAs), there is no molecular marker for diagnosis, treatment evaluation, and prognosis. Decreased expression of the gene O6-methylguanine-DNA methyltransferase by methylation of its promoter was recently reported to confer a favorable response to temozolomide chemotherapy and longer survival in GBM patients,4 but this analysis requires tumor tissues that are often unavailable.
Identification of molecular markers assayed in accessible specimens such as blood would be of considerable value in tumor diagnosis, response assessment, and patient prognosis. For prostate and ovarian cancer, serum prostate-specific antigen and cancer antigen 125 markers are often used clinically for diagnosis.5,6 No such blood markers have been identified for glioma patients, although a few candidate proteins (cathepsin D, low-molecular-weight caldesmon, YKL-40, matrix metalloproteinase-9, and glial fibrillary acidic protein) have been recently reported.7–10 In this study, we tested the potential use of measuring plasma insulin-like growth factor binding protein 2 (IGFBP-2) levels in the setting of glioma.
IGFBP-2 is a secreted protein measurable in tissues and blood. It was initially found through genomic studies to be overexpressed in high-grade gliomas.11–13 Later studies using a tissue microarray found that IGFBP-2 was overexpressed in 80% of glioblastomas and in 20% of AAs and AOAs.14 IGFBP-2 was also reported to be overexpressed in other tumors, including prostate cancer,15,16 ovarian cancer,17 adrenocortical cancer,18 breast cancer,19 colorectal carcinomas,20 and leukemia,21 as well as in drug-resistant tumors.22 Previous studies have shown that increased IGFBP-2 expression in a tumor promotes glioma cell migration and invasion through activation of the matrix metalloproteinase 2 protein and integrin pathway 23–27 and that this increased expression is associated with glioma malignancy and poor survival of glioma patients.12,13,16,18,28–33 Attenuation of IGFBP-2 by the tumor suppressor protein IIp45 or using small interfering RNA resulted in decreased cell motility and invasion.23,26 Recently, IGFBP-2 has been found to be a “driver” oncogene for glioma development and progression.28 IGFBP-2 overexpression has been linked to PTEN (phosphatase and tensin homolog) deletion and results in Akt activation.27,28
Studies of serum IGFBP-2 levels in patients with prostate cancer have shown that plasma IGFBP-2 levels are significantly associated with tumor progression, invasion, and metastasis.34,35 An elevated serum IGFBP-2 level predicts a high risk of relapse after hematopoietic stem cell transplantation in childhood acute myeloid leukemia.36
We examined the possibility that plasma IGFBP-2 could serve as a potential surrogate biomarker for glioma patients. We measured the plasma IGFBP-2 levels in glioma patients and healthy control subjects and analyzed the association between plasma IGFBP-2 levels and various clinicopathologic variables.
Materials and Methods
Study Population
We retrospectively examined blood samples and patient records for 196 patients who had been newly diagnosed with glioma (81 with grade II glioma, 43 with grade III glioma, and 72 with GBM) and enrolled in the Glioma Therapy Center of Beijing Tiantan Hospital from January 2006 through March 2007. Our screening criteria for patients in the study were the following: (1) Patients must have had no other cancers or diseases such as acute infection, diabetes, and ischemia and must have received no other previous radiotherapy, chemotherapy, or corticosteroid therapy. (2) Patients must have received similar treatments: surgical resection performed by neurosurgeons who used similar operational techniques and principles and tumor pathologic evaluation performed by two neuropathologists separately using the WHO 2000 classification of CNS tumors. Every patient in the study received the same radiotherapy regimen. One month after the surgery, all patients received 60-Gy radiotherapy for 6 weeks. Patients with high-grade glioma (grade III and GBM) also received chemotherapy after radio-therapy. Patients received routine contrast-enhanced MRI examinations before and after surgical operation, before chemotherapy, and after two, four, and six cycles of chemotherapy. (3) Patients gave informed consent. Patients who died from non-glioma-related causes were not included for the study. The criteria ensured that the patient group was uniform, which strengthens the analysis. The Glioma Therapy Center accepts patients from all over China; most of them are younger than 60 years. Fifty-five healthy volunteers matched for age and sex were also included in the study. The study was approved by the Beijing Tiantan Hospital Research Ethics Board.
The information on tumor resection for all patients examined was taken from medical charts. Tumor resection categories were as follows: (1) gross total resection, (2) partial removal with residual tumor ≤30%, and (3) residual tumor >30% or biopsy. Tumor volume was calculated using the following formula: longest diameter × widest diameter × thickness (section thickness × the number of layers) × 1/2, based on MRI scans.
Clinicopathologic variables were retrospectively collected from medical records through March 2008. Disease-free survival (DFS) was defined from the date of surgery to the first MRI-confirmed recurrence or death, whichever occurred first.
Plasma Sample Collection
Stored plasma samples had been obtained from blood taken before surgery, after two cycles of chemotherapy, and at recurrence of the disease. Blood was drawn into tubes with EDTA anticoagulant and then centrifuged at 3,500 rpm for 10 min to recover plasma. The upper-layer plasma was collected and aliquoted into 1.5-ml Eppendorf tubes and then recentrifuged at 10,000 rpm for 3 min at 4°C to remove any white cells and debris. The plasma was transferred to fresh tubes and stored at −80°C until analysis. Twenty patients had plasma taken before surgery and after two cycles of adjuvant chemotherapy without recurrence, 12 patients before surgery and after recurrence, 15 patients after two cycles of adjuvant chemotherapy without recurrence and after recurrence, and 10 patients at all three time points.
ELISA Assays
We measured IGFBP-2 levels in plasma using the IGFBP-2 ELISA kit (purchased from RapidBio Lab, Calabasas, CA, USA) according to the manufacturer’s instructions. Each sample was repeated three times, and an averaged concentration of IGFBP-2 was used for further statistical analysis.
Immunohistochemical Analysis
IGFBP-2 immunostains were done using formalin-fixed, paraffin-embedded tissues. Four-micrometer-thick sections were cut from each paraffin block, dewaxed in xylene, rinsed in graded ethanol, and rehydrated in double-distilled water. The sections were then treated with 3% H2O2 for 5 min at room temperature to block endogenous peroxidase activity. For antigen retrieval, slides were pretreated by steaming in sodium citrate buffer (10 mM sodium citrate, pH 6.0) for 15 min at 100°C. After washing with phosphate-buffered saline for 3 min, the sections were manually immunostained with an anti-human IGFBP-2 mouse monoclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 1:400 dilution with standard avidin-biotin-peroxidase. Staining for IGFBP-2 was scored manually for the percentage of positive cells. We chose the five most heavily stained high-resolution fields under ×200 magnification, determined the percentage of the positive cells, and calculated the average of the percentages. Five categories were used to assess the staining intensity: 0, no positive staining cells; 1, 1%–10% positive staining cells; 2, 11%–30%; 3, 31%–60%; 4, >60% positive staining cells.
Statistical Analysis
SPSS statistical software for Windows 13.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Differences in plasma IGFBP-2 levels between control subjects and glioma patients and among glioma patients were assessed using a two-sample t-test. Correlations between plasma IGFBP-2 levels and tumor size and between immunostaining and preoperative plasma IGFBP-2 levels analyzed case by case were assessed by Pearson correlation analysis. When immunostaining and preoperative plasma IGFBP-2 levels were analyzed by grading group, an independent-sample t-test was used for difference comparison. Cox regression was used to correlate plasma IGFBP-2 with DFS while adjusting for clinicopathologic variables. Receiver-operator characteristic (ROC) analysis was used to determine specificity and sensitivity of plasma IGFBP-2 levels for prediction of survival. DFS in the two groups was compared using the Kaplan-Meier plotting method. Significance of differences in DFS between the two groups was calculated using the log-rank test. We defined p ≤ 0.05 as significant. All tests were two-sided.
Results
Elevated Preoperative Plasma IGFBP-2 Levels in High-Grade Gliomas
Characteristics of the study population (55 healthy subjects and 196 patients) and clinicopathologic variables of the patients are summarized in Table 1. We found that the preoperative plasma IGFBP-2 levels did not vary significantly with age and sex in both the control and the patient groups, as reported previously,37 nor did it vary significantly with preoperative KPS score for glioma patients.
Table 1.
Characteristics of the study population and clinicopathologic variables of glioma patients
| Subject Group |
||||
|---|---|---|---|---|
| Characteristic | Normal | Grade II | Grade III | Grade IV |
| n | 55 | 81 | 43 | 72 |
| Male/female ratio | 1.39 | 1.38 | 1.75 | 1.69 |
| Age (years), mean ± SD | 42.10 ± 8.80 | 40.93 ± 10.58 | 42.89 ± 11.76 | 44.84 ± 12.20 |
| Preoperative KPS score, mean ± SD | 80.78 ± 11.22 | 79.55 ± 10.34 | 76.55 ± 9.79 | |
| Extent of resection, mean ± SDa | 1.7 ± 0.4 | 1.6 ± 0.5 | 1.5 ± 0.5 | |
| Tumor size (cm3), mean ± SD (% patients with imaging data) | 80.7 ± 72.5 (9.8%) | 39.3 ± 51.0 (14%) | 55.0 ± 41.5 (44%) | |
| Patients with follow-up, n (%) | 50 (61.7%) | 33 (76.7%) | 52 (72%) | |
| Median follow-up, weeks | 67.3 | 83.3 | 84.5 | |
| Deaths, n (%) | 4 (8%) | 7 (21.2%) | 19 (36.5%) | |
| Recurrences, n (%) | 2 (4%) | 8 (24.2%) | 32 (61.5%) | |
| Disease-free survival (weeks), mean ± SD | 53.28 ± 24.66 | 49.2 ± 24.81 | ||
Tumor resection categories: 1, gross total resection; 2, partial removal with residual tumor ≤30%; 3, residual tumor >30% or biopsy.
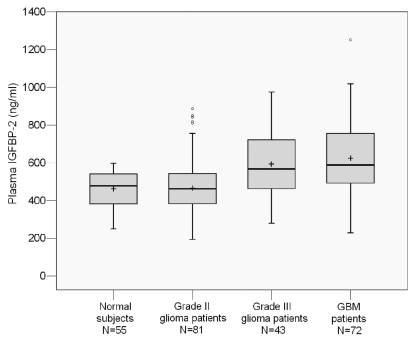
As shown in Fig. 1, we found that preoperative IGFBP-2 levels in plasma were significantly higher in patients with high-grade glioma (mean ± SD: grade III, 586.7 ± 174.0 ng/ml; GBM, 622.3 ± 201.6 ng/ml) than in healthy controls (458.68 ± 91.41 ng/ml; p < 0.001). Eighty-one percent (58 of 72) of GBM patients had IGFBP-2 levels above the mean level of healthy controls, and 61% (44 of 72) of GBM patients had IGFBP-2 levels higher than the upper limit of control levels. We also found a significant increase in preoperative plasma IGFBP-2 levels in patients with high-grade versus low-grade glioma (p < 0.001), consistent with previous reports of higher IGFBP-2 levels in advanced tumor tissues compared with low-grade gliomas.12,13 In contrast, we observed no significant differences in pre-operative plasma IGFBP-2 levels between patients with low-grade (grade II) glioma (mean, 478.13 ng/ml) and healthy controls (p = 0.369) or between patients with grade III glioma and GBM (p = 0.314; Fig. 1). The findings suggest that the preoperative plasma IGFBP-2 level can distinguish between high-grade glioma patients and low-grade glioma patients and healthy subjects.
Fig. 1.
Levels of plasma insulin-like growth factor binding protein 2 (IGFBP-2) in 55 healthy control subjects and 196 patients with gliomas. In these box plots, the box indicates interquartile range; the center bar, median; +, mean; whiskers, data range; and dots, outliers.
Correlation between Plasma IGFBP-2 Levels and GBM Recurrence
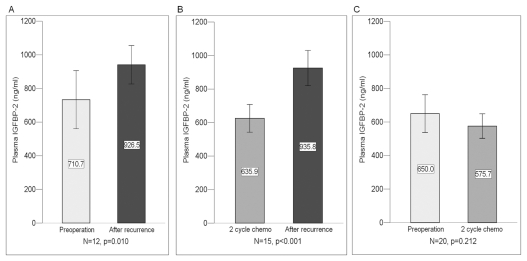
We further assessed any change in plasma IGFBP-2 levels before and after GBM recurrence. We found that the plasma IGFBP-2 levels increased significantly after recurrence compared with the levels before surgery (n = 12; p = 0.010) and after two-cycle adjuvant chemotherapy without recurrence (n = 15; p < 0.001). Plasma IGFBP-2 levels after surgery followed by two-cycle chemotherapy without recurrence were slightly lower than levels before surgery (n = 20; p = 0.212; Fig. 2A–C). The change in plasma IGFBP-2 levels before surgery and after recurrence was significantly correlated with GBM recurrence (p = 0.002), suggesting that the preoperative plasma IGFBP-2 level could be predictive of GBM recurrence after surgery.
Fig. 2.
Comparison of levels of plasma insulin-like growth factor binding protein 2 (IGFBP-2) before and after recurrence in 27 patients with glioblastoma multiforme. (A) Before surgery (mean ± SD, 710.7 ± 257.1 ng/ml) and after recurrence (926.5 ± 169.8 ng/ml). (B) After two-cycle adjuvant chemotherapy without recurrence (635.9 ± 154.4 ng/ml) and after recurrence (935.8 ± 200.0 ng/ml). (C) Before surgery (650.0 ± 240.4 ng/ml) and after two-cycle adjuvant chemotherapy without recurrence (575.7 ± 156.3 ng/ml).
Prediction of Preoperative Plasma IGFBP-2 Levels for Prognosis
To determine the prognostic value of the plasma IGFBP-2 levels for GBM patients, we assessed 52 GBM cases using univariate Cox regression analysis. We found that the preoperative IGFBP-2 levels in plasma were correlated with DFS in patients with GBM (p = 0.007). Taking into account the impact of standard clinical prognostic factors—such as age, gender, preoperative KPS, and extent of surgery—on DFS, we conducted both univariate and multivariate Cox regression analyses. As shown in Table 2, the multivariate analysis revealed that the preoperative plasma IGFBP-2 level was a significant prognostic factor in GBM patients, independent of the conventional clinical variables (hazard ratio [HR], 1.404; 95% confidence interval [CI], 1.078–1.828; p = 0.012). Age, gender, preoperative KPS, and extent of surgery were not associated with DFS in GBM in this study.
Table 2.
Predictors for disease-free survival in 52 GBM patients
| Variable | Value | Univariate Analysis p-Value | Multivariate Analysis Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 44.32 ± 12.54 | 0.781 | 0.996 (0.950–1.043) | 0.850 |
| Gender, n (%) | ||||
| Male | 36 (69.2%) | 0.463 | 0.638 (0.185–2.194) | 0.476 |
| Female | 16 (30.8%) | |||
| KPS | 78.00 ± 8.93 | 0.099 | 0.411 (0.149–1.131) | 0.085 |
| Extent of resection, mean ± SDa | 1.41 ± 0.50 | 0.858 | 1.456 (0.497–4.269) | 0.494 |
| Plasma IGFBP-2, mean ± SD | 647.18 ± 214.15 | 0.007 | 1.404 (1.078–1.828) | 0.012 |
Tumor resection categories: 1, gross total resection; 2, partial removal with residual tumor ≤30%; 3, residual tumor >30% or biopsy.
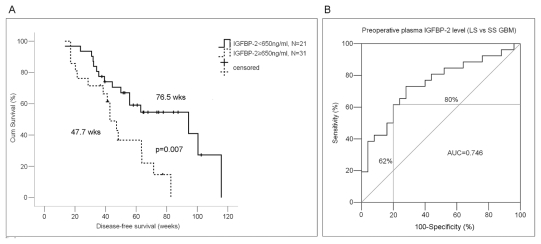
We thus assessed the discriminative value of preoperative plasma IGFBP-2 levels for survival of patients with GBM using Kaplan-Meier analysis. The mean IGFBP-2 level in 52 GBM patients analyzed was 647.2 ng/ml, so we used a level of 650 ng/ml as the cutoff point to divide the GBM patients into two subgroups. We found that the preoperative plasma IGFBP-2 level could significantly discriminate the survival of the two GBM subgroups, with a mean DFS of 47.7 weeks (median DFS, 42.9 weeks; 95% CI, 35.4–50.3 weeks) versus a mean of 76.5 weeks (median DFS, 94.3 weeks; 95% CI, 36.9–151.7 weeks; HR, 1.48; p = 0.007; Fig. 3A).
Fig. 3.
Discriminative assessment of levels of preoperative plasma insulin-like growth factor binding protein 2 (IGFBP-2) for disease-free survival (DFS) in glioblastoma multiforme (GBM) patients. (A) Relationship between preoperative plasma IGFBP-2 level and DFS. Kaplan-Meier survival analysis and a log-rank test showed that GBM patients with high preoperative plasma IGFBP-2 (≥650 ng/ml) had a significantly worse DFS than did GBM patients with low preoperative plasma IGFBP-2 (<650 ng/ml; p = 0.012). (B) Receiver-operator characteristic curve analysis of preoperative plasma IGFBP-2 level. In 52 GBM patients, at the mean level (650 ng/ml) of preoperative plasma IGFBP-2, the discriminative power reached 62% sensitivity and 80% specificity for short-survival cases (<1 year) versus long-survival cases (≥1 year). AUC, area under the curve.
We then used all 52 GBM cases to construct the ROC curve to evaluate prognostic performance of the preoperative plasma IGFBP-2 level for GBM patients. The mean DFS of the 52 cases was 367 days (no patient died between 360 and 367 days), so we used 360 days (1 year) as a time horizon. Patients with DFS longer than 1 year were assigned as long-survival cases, and those with DFS shorter than 1 year as short-survival cases. For patients who did not have recurrent tumors, DFS was calculated from the date of surgery to the last follow-up date. Based on the ROC curve, we found that at the mean preoperative plasma IGFBP-2 level (650 ng/ml), the discriminative power reached 80% specificity and 62% sensitivity (Fig. 3B). Patients with elevated preoperative plasma IGFBP-2 levels (≥650 ng/ml) had significantly poorer DFS compared with patients with lower IGFBP-2 levels.
Correlation between Plasma IGFBP-2 Levels and Tumor IGFBP-2 Expression
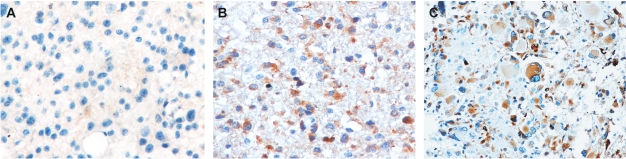
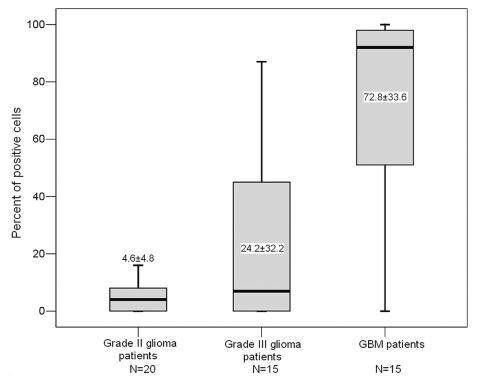
To examine the consistency of IGFBP-2 levels in tumor and plasma, we measured IGFBP-2 levels in tumor specimens from 50 patients (20 with grade II glioma, 15 with grade III glioma, and 15 with GBM) using immunohistochemical analysis and compared them with their preoperative plasma IGFBP-2 levels. Representative immunohistochemical staining of IGFBP-2 in gliomas is illustrated in Fig. 4. We did not observe a strict correlation between immunostaining and preoperative plasma IGFBP-2 levels when conducted on a case-by-case analysis, but when levels were analyzed by grading group, we found significantly higher immunostained and preoperative plasma IGFBP-2 levels in high-grade gliomas than in low-grade gliomas (p < 0.001). Immunostained IGFBP-2 levels were lower in grade II than in grade III gliomas (p = 0.028) and lower in grade III than in GBM specimens (p = 0.001; Fig. 5). However, no significant difference in preoperative plasma IGFBP-2 levels was observed between grade III glioma and GBM, as described previously (p = 0.314).
Fig. 4.
Photographs of immunohistochemical staining of insulin-like growth factor binding protein 2 in different grades of gliomas. Positive cells are stained brown. (A) Diffuse astrocytoma (WHO grade II). (B) Anaplastic astrocytoma (WHO grade III). (C) Glioblastoma multiforme (WHO grade IV). Magnification, ×200.
Fig. 5.
Tissue insulin-like growth factor binding protein 2 (IGFBP-2) expression levels in glioma tumors. Significant differences were observed between grade II and grade III gliomas (p = 0.028) and between grade III gliomas and glioblastoma multiforme (p < 0.001).
Stability of Plasma IGFBP-2 Levels during the Day
The time of blood collection could introduce a variation in plasma IGFBP-2 levels. We thus measured the IGFBP-2 levels in a series of plasma samples from healthy volunteers collected before and after breakfast, after lunch, and after dinner. We found a negligible variability in plasma IGFBP-2 levels (445.6–459.9 ng/ml) during the day, which was consistent with a previous report that plasma IGFBP-2 levels are stable during the day.38
Association between Plasma IGFBP-2 Levels and Tumor Volume
We also examined whether plasma IGFBP-2 levels were associated with tumor volume. We measured tumor volume on MRI scans of 32 patients with high-grade gliomas and found no significant association between preoperative plasma IGFBP-2 levels and tumor volume (p = 0.503). In this patient series, 11 cases had tumors larger than 5 cm.
Discussion
To our knowledge, our results present the first evidence that preoperative plasma IGFBP-2 levels differ between high-grade glioma patients and low-grade glioma patients or healthy subjects and that higher levels are statistically correlated with recurrence and DFS in GBM. However, we did not find that preoperative plasma IGFBP-2 levels differed between patients with low-grade glioma and healthy subjects, which is consistent with previous reports of IGFBP-2 expression in normal brain and glioma tumor tissues.11,12 IGFBP-2 levels have been reported to be higher in GBM than in grade III glioma tissues,11,12,29 but we observed no significant difference in plasma IGFBP-2 levels between grade III glioma and GBM. The lack of significant difference might be due to the heterogeneous origins of the grade III gliomas, which consist of three subtypes: AAs, AOs, and AOAs. These subtypes may exhibit differential expression of IGFBP-2. Thus, we performed a subset analysis of these subtypes of grade III glioma compared with GBM, but we still did not observe any significant difference in plasma IGFBP-2 levels between each grade III glioma subtype and GBM. One possible scenario for the difference in levels is that IGFBP-2 protein is overexpressed and secreted into blood in both grade III gliomas and GBM but that, in GBM, IGFBP-2 is also retained in the cells and tissues because of its binding to proteins such as integrin and other extracellular matrix proteins. Another possibility is that grade III gliomas and GBM cause some other systemic response, such as inflammatory reaction, that may affect the plasma IGFBP-2 level.
We also found a significant increase in plasma IGFBP-2 levels after GBM recurrence compared with levels before surgery and with levels after two-cycle adjuvant chemotherapy without recurrence, and a correlation between recurrence and the change in plasma levels of IGFBP-2 before and after recurrence. These observations suggest that the preoperative IGFBP-2 level in plasma can be a predictor for GBM recurrence and that the change in plasma IGFBP-2 levels can be used to monitor tumor progression after surgery. As described above, we did not observe the correlation between the preoperative plasma IGFBP-2 level and the quantitative immunostaining of IGFBP-2 in a case-by-case analysis, but the preoperative plasma IGFBP-2 levels could predict recurrence, suggesting that the secreted form of IGFBP-2 more strongly affects the tumor recurrence than does the nonsecreted one.
In this study population, we found no effects of age, sex, or preoperative KPS on preoperative plasma IGFBP-2 levels. Of our patients, 94% were younger than 60 years (range, 14–66 years); 15 subjects were older than 60 years, and 8 were younger than 20 years. It has been reported that circulating IGFBP-2 levels do not vary significantly in persons younger than 60 years,38 which is consistent with our finding. Furthermore, because we assessed a relatively young population, the majority of the patients (79% with grade II, 68% with grade III, and 63% with GBM) had KPS scores greater than 80, which raised the question of whether the lack of effect of preoperative KPS on the preoperative plasma IGFBP-2 levels was due to good KPS scores or because plasma IGFBP-2 levels are indeed independent of patient KPS. Therefore, a larger population with a broader age range is needed to confirm these findings.
We also found that preoperative plasma IGFBP-2 levels were correlated with DFS of GBM patients, independent of common clinical prognostic factors such as age, sex, preoperative KPS, and extent of tumor resection, and that levels discriminated the GBM patients into distinctive prognostic subgroups, suggesting that a molecular surrogate marker such as the plasma IGFBP-2 level can supplement current WHO histologic grading of GBM tumors, which cause highly variable survival times, for a more accurate prediction of survival. We did not find any correlation of age, KPS, and extent of tumor resection and DFS of GBM patients in this study. Among the 52 GBM patients we included in the multivariate Cox analysis, only 27 patients had both imaging and DFS data available, so we could not include the variable of tumor size for the multivariate analysis. Based on the 32 GBM cases we used for the correlation analysis between preoperative plasma IGFBP-2 level and tumor size, we did not find any correlation between the preoperative plasma IGFBP-2 level and tumor size of GBM. However, in the future, if we collect more cases with both imaging and DFS data available, we should conduct the multivariate analysis to assess whether tumor size affects the prognostic value of the preoperative plasma IGFBP-2 level.
In summary, plasma IGFBP-2 levels have the potential to be used as a surrogate plasma biomarker for follow-up of progression and survival in high-grade gliomas. A larger and broader population with an extended follow-up will be needed to validate these findings.
Acknowledgments
We thank Yuling Yang and Na Liu for blood and tissue sample collection and clinical data retrieval. We thank Virginia Mohlere, Department of Scientific Publications, University of Texas M.D. Anderson Cancer Center, for editing the manuscript. The study was partially supported by the National Natural Science Foundation of China (grant 30772238).
References
- 1.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 2.Ino Y, Berwnsky RA, Zlatescu MC, et al. Molecular subtypes of ana-plastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res. 2001;7:839–845. [PubMed] [Google Scholar]
- 3.Bauman GS, Ino Y, Ueki K, et al. Allelic loss of chromosome 1p and radiotherapy plus chemotherapy in patients with oligodendrogliomas. Int J Radiat Oncol Biol Phys. 2000;48:825–830. doi: 10.1016/s0360-3016(00)00703-3. [DOI] [PubMed] [Google Scholar]
- 4.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalgo ML, Carter HB. Update on PSA testing. J Natl Compr Canc Netw. 5:737–742. doi: 10.6004/jnccn.2007.0065. [DOI] [PubMed] [Google Scholar]
- 6.Goonewardene TI, Hall MR, Rustin GJ. Management of asymptomatic patients on follow-up for ovarian cancer with rising CA-125 concentrations. Lancet Oncol. 2007;8:813–821. doi: 10.1016/S1470-2045(07)70273-5. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda ME, Iwadate Y, Machida T, et al. Cathepsin D is a potential serum marker for poor prognosis in glioma patients. Cancer Res. 2005;65:5190–5194. doi: 10.1158/0008-5472.CAN-04-4134. [DOI] [PubMed] [Google Scholar]
- 8.Zheng P-P, Hop WC, Sillevis Smitt PAE, et al. Low-molecular weight caldesmon as a potential serum marker for glioma. Clin Cancer Res. 2005;11:4388–4392. doi: 10.1158/1078-0432.CCR-04-2512. [DOI] [PubMed] [Google Scholar]
- 9.Hormigo A, Gu B, Karimi S, et al. YKL-40 and matrix metalloproteinase-9 as potential serum biomarkers for patients with high-grade gliomas. Clin Cancer Res. 2006;12:5698–5704. doi: 10.1158/1078-0432.CCR-06-0181. [DOI] [PubMed] [Google Scholar]
- 10.Jung CS, Foerch C, Schänzer A, et al. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain. 2007;130(pt 12):3336–3341. doi: 10.1093/brain/awm263. [DOI] [PubMed] [Google Scholar]
- 11.Fuller GN, Rhee CH, Hess KR, et al. Reactivation of insulin-like growth factor binding protein 2 expression in glioblastoma multiforme: a revelation by parallel gene expression profiling. Cancer Res. 1999;59:4228–4232. [PubMed] [Google Scholar]
- 12.Sallinen SL, Sallinen PK, Haapasalo HK, et al. Identification of differentially expressed genes in human gliomas by DNA microarray and tissue chip techniques. Cancer Res. 2000;60:6617–6622. [PubMed] [Google Scholar]
- 13.Elmlinger MW, Deininger MH, Schuett BS, et al. In vivo expression of insulin-like growth factor-binding protein-2 in human gliomas increases with the tumor grade. Endocrinology. 2001;142:1652–1658. doi: 10.1210/endo.142.4.8084. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanety H, Madjar Y, Dagan Y, et al. Serum insulin-like growth factor-binding protein-2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum prostate-specific antigen. J Clin Endocrinol Metab. 1993;77:229–233. doi: 10.1210/jcem.77.1.7686915. [DOI] [PubMed] [Google Scholar]
- 16.Bubendorf L, Kolmer M, Kononen J, et al. Hormone therapy failure in human prostate cancer: analysis by complementary DNA and tissue microarrays. J Natl Cancer Inst. 1999;91:1758–1764. doi: 10.1093/jnci/91.20.1758. [DOI] [PubMed] [Google Scholar]
- 17.Flyvbjerg A, Mogensen O, Mogensen B, et al. Elevated serum insulin- like growth factor-binding protein 2 (IGFBP-2) and decreased IGFBP-3 in epithelial ovarian cancer: correlation with cancer antigen 125 and tumor-associated trypsin inhibitor. J Clin Endocrinol Metab. 1997;82:2308–2313. doi: 10.1210/jcem.82.7.4085. [DOI] [PubMed] [Google Scholar]
- 18.Boulle N, Logie A, Gicquel C, et al. Increased levels of insulin-like growth factor II (IGF-II) and IGF-binding protein-2 are associated with malignancy in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1998;83:1713–1720. doi: 10.1210/jcem.83.5.4816. [DOI] [PubMed] [Google Scholar]
- 19.Busund LT, Richardsen E, Busund R, et al. Significant expression of IGFBP2 in breast cancer compared with benign lesions. J Clin Pathol. 2005;58:361–366. doi: 10.1136/jcp.2004.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.el Atiq F, Garrouste F, Remacle-Bonnet M, et al. Alterations in serum levels of insulin-like growth factors and insulin-like growth-factor-binding proteins in patients with colorectal cancer. Int J Cancer. 1994;57:491–497. doi: 10.1002/ijc.2910570409. [DOI] [PubMed] [Google Scholar]
- 21.Mohnike KL, Kluba U, Mittler U, et al. Serum levels of insulin-like growth factor-I, -II and insulin-like growth factor binding proteins -2 and -3 in children with acute lymphoblastic leukaemia. Eur J Pediatr. 1996;155:81–86. doi: 10.1007/BF02075755. [DOI] [PubMed] [Google Scholar]
- 22.Juncker-Jensen A, Lykkesfeldt AE, Worm J, et al. Insulin-like growth factor binding protein 2 is a marker for antiestrogen resistant human breast cancer cell lines but is not a major growth regulator. Growth Horm IGF Res. 2006;16:224–239. doi: 10.1016/j.ghir.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Song SW, Fuller GN, Khan A, et al. IIp45, a novel IGFBP-2 binding protein, antagonizes IGFBP-2 stimulation of glioma cell invasion. Proc Natl Acad Sci USA. 2003;100:13970–13975. doi: 10.1073/pnas.2332186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang GK, Hu L, Fuller GN, Zhang W. An interaction between insulin-like growth factor-binding protein 2 (IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell mobility. J Biol Chem. 2006;281:14085–14091. doi: 10.1074/jbc.M513686200. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Wang H, Shen W, et al. Insulin-like growth factor binding protein 2 enhances glioblastoma invasion by activating invasion-enhancing genes. Cancer Res. 2003;63:4315–4321. [PubMed] [Google Scholar]
- 26.Fukushima T, Tezuka T, Shimomura T, et al. Silencing of insulin-like growth factor-binding protein-2 in human glioblastoma cells reduces both invasiveness and expression of progression-associated gene CD24. J Biol Chem. 2007;282:18634–18644. doi: 10.1074/jbc.M609567200. [DOI] [PubMed] [Google Scholar]
- 27.Mehrian-Shai R, Chen CD, Shi T, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci U S A. 2007;104:5563–5568. doi: 10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunlap SM, Celestino J, Wang H, et al. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc Natl Acad Sci U S A. 2007;104:11736–11741. doi: 10.1073/pnas.0703145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald KL, O’Sullivan MG, Parkinson JF, et al. IQGAP1 and IGFBP2: valuable biomarkers for determining prognosis in glioma patients. J Neuropathol Exp Neurol. 2007;66:405–417. doi: 10.1097/nen.0b013e31804567d7. [DOI] [PubMed] [Google Scholar]
- 30.Freije WA, Castro-Vargas FE, Fang Z, et al. Gene expression profiling of glioma strongly predicts survival. Cancer Res. 2004;64:6503–6510. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- 31.Nigro JM, Misra A, Zhang L, et al. Integrated array-comparative genomic hybridization and expression array profiles identify clinically relevant molecular subtypes of glioblastoma. Cancer Res. 2005;65:1678–1686. doi: 10.1158/0008-5472.CAN-04-2921. [DOI] [PubMed] [Google Scholar]
- 32.Philips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resembles stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Scrideli CA, Carlotti CG, Jr, Mata JF, et al. Prognostic significance of co-overexpression of the EGFR/IGFBP-2/HIF-2A genes in astrocytomas. J Neurooncol. 2007;83:233–239. doi: 10.1007/s11060-007-9328-0. [DOI] [PubMed] [Google Scholar]
- 34.Shariat SF, Lamb DJ, Kattan MW, et al. Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and -3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20:833–841. doi: 10.1200/JCO.2002.20.3.833. [DOI] [PubMed] [Google Scholar]
- 35.Bensalah K, Lotan Y, Karam JA, Shariat SF. New circulating biomarkers for prostate cancer. Prostate Cancer Prostatic Dis. 2008;11:112–120. doi: 10.1038/sj.pcan.4501026. [DOI] [PubMed] [Google Scholar]
- 36.Dawczynski K, Kauf E, Schlenvoigt D, et al. Elevated serum insulin-like growth factor binding protein-2 is associated with a high relapse risk after hematopoietic stem cell transplantation in childhood AML. Bone Marrow Transplant. 2006;37:589–594. doi: 10.1038/sj.bmt.1705281. [DOI] [PubMed] [Google Scholar]
- 37.Ho PJ, Baxter RC. Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin Endocrinol. 1997;46:333–342. [PubMed] [Google Scholar]
- 38.Rechler M. Insulin-like growth factor binding proteins. Vit Horm. 1993;47:100–114. doi: 10.1016/s0083-6729(08)60444-6. [DOI] [PubMed] [Google Scholar]