Abstract
We studied the feasibility, efficacy, and mechanisms of dendritic cell (DC) immunotherapy against murine malignant glioma in the experimental GL261 intracranial (IC) tumor model. When administered prophylactically, mature DCs (DCm) ex vivo loaded with GL261 RNA (DCm-GL261-RNA) protected half of the vaccinated mice against IC glioma, whereas treatment with mock-loaded DCm or DCm loaded with irrelevant antigens did not result in tumor protection. In DCm-GL261-RNA–vaccinated mice, a tumor-specific cellular immune response was observed ex vivo in the spleen and tumor-draining lymph node cells. Specificity was also shown in vivo on the level of tumor challenge. Depletion of CD8+ T-cells by anti-CD8 treatment at the time of tumor challenge demonstrated their essential role in vaccine- mediated antitumor immunity. Depletion of CD25+ regulatory T-cells (Tregs) by anti-CD25 (aCD25) treatment strongly enhanced the efficacy of DC vaccination and was itself also protective, independently of DC vaccination. However, DC vaccination was essential to protect the animals from IC tumor rechallenge. No long-term protection was observed in animals that initially received aCD25 treatment only. In mice that received DC and/or aCD25 treatment, we retrieved tumor-specific brain-infiltrating cytotoxic T-lymphocytes. These data clearly demonstrate the effectiveness of DC vaccination for the induction of long-lasting immunological protection against IC glioma. They also show the beneficial effect of Treg depletion in this kind of glioma immunotherapy, even combined with DC vaccination.
Keywords: anti-CD8, anti-CD25, DC immunotherapy, glioma, regulatory T-cells
Escape from immunosurveillance is nowadays considered one of the hallmarks of malignant cell growth, and several mechanisms leading to immune suppression or immune escape have been described.1 On the other hand, it is generally accepted that a patient’s immune system can be instructed to recognize and attack several types of malignant lesions more efficiently.2,3 Hence, immunotherapy based on immunization with tumor antigen (Ag)-loaded dendritic cells (DCs) as professional Ag-presenting cells (APCs) represents a promising strategy in the multimodal treatment for different types of cancer,4,5 including malignant glioma.6–15 Immunotherapeutic approaches for malignant glioma are currently under investigation by our group14–16 and others.17,18 Results of in vitro experiments19,20 and animal studies,21 together with pilot data from clinical trials,14–16 are very promising, although it is still too early to draw definitive conclusions.22
Orthotopic rodent glioma models are highly useful to address fundamental questions regarding the balance between pro- and antiimmunogenic cellular mechanisms.23–25 In this study, we explore the prophylactic effect of immunotherapy with RNA-loaded DCs in vitro and in vivo in the well-established GL261 mouse glioma model in C57BL/6 mice. The concept of loading tumor Ags on DCs in the form of RNA molecules has been well established.26
The GL261 glioma model has been characterized in depth by others and has been widely used to test experimental treatment strategies.27–32 We recently published an extensive validation study on the use of in vivo bioluminescence (BLI) for the monitoring of DC immunotherapy in this model.21 Since GL261 tumor cells are considered moderately immunogenic, they can be used for immunotherapeutic studies. Moreover, it has been demonstrated that growing GL261 tumors damage the blood–brain barrier, which allows the infiltration of lymphocytes into the tumor and surrounding areas of the brain.33 Together with the finding of drainage of interstitial fluid from the brain into the deep cervical lymph nodes and the presence of microglial cells with APC capacities, this calls into question the assumption of strict immunological privilege in the brain.6
Regulatory T-cells (Tregs), a subpopulation of CD4+ T-cells that constitutively express the transcription factor FoxP3, the high-affinity interleukin-2 (IL-2) receptor CD25, and the B7 ligand cytotoxic T-lymphocyte–associated Ag 4 (CTLA-4 or CD154), are required for the maintenance of tolerance throughout the lifetime of an organism and are believed to represent key players in tumor immunology as well.34–36 It has been shown by others that CD4+CD25+FoxP3+ Tregs accumulate in murine and human gliomas during tumor growth, and these cells are potent suppressors of antiglioma immune responses in vivo.37 Hence, Tregs are becoming an important target in cancer immunotherapy. Since no unique surface marker has been determined yet for Tregs, in vivo depletion of this cell population in murine models is mainly based on rather nonspecific interventions with monoclonal antibodies (mAbs) targeting CD25, which can also be expressed on activated lymphocytes. This concept is not new; in 1999 Onizuka et al.38 described the rejection of leukemia, myeloma, and fibrosarcoma in vivo by the administration of anti-CD25 (aCD25) mAbs. Other reports have shown an antitumor effect of Treg depletion on murine neuroblastoma and glioma models.37,39
In this study, we aimed to further dissect the mechanisms between immunogenic and immune-suppressive cellular processes and the impact of DC vaccination and/or Treg elimination by depletion of T-cells expressing CD25 with the PC61 mAb. In particular, we questioned whether DC vaccination and/or Treg depletion could lead to persistent immunological protection against intracranial (IC) glioma. To explore whether DC vaccination could lead to immunological memory, long-term surviving mice from primary tumor challenge were IC rechallenged with GL261 cells. Besides data on clinical outcome in the different experimental conditions, we aimed to characterize the brain-infiltrating cells in these mice in depth and address their functionality ex vivo.
Materials and Methods
Animals, Cell Lines, and Culture Media
Female adult (10 weeks old) C57BL/6J mice were purchased from Harlan (Horst, The Netherlands). The animals were housed in filter-top cages and bedded with sawdust and had free access to food and water. All animal experiments were approved by the bioethics committee of the Katholieke Universiteit Leuven, which follows international guidelines.
Methylcholanthrene-induced murine C57BL/6J syngeneic GL261 glioma cells were kindly provided by Dr. Eyupoglu from the University of Erlangen (Erlangen, Germany). GL261 tumor cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml gentamycin sulfate (all from Lonza, Verviers, Belgium). MC17-51 fibrosarcoma (American Type Culture Collection [ATCC] clone CRL-2799; ATCC, Manassas, VA, USA) and Lewis lung carcinoma (LLC) tumor cells were maintained in RPMI with the same supplements as described for the GL261 cells.
Splenocytes and lymph node cells were cultured in RPMI supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 × nonessential amino acids (Lonza), and 50 μM β-mercaptoethanol (Sigma-Aldrich, Bornem, Belgium). DC medium consisted of RMPI-1640 supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM β-mercaptoethanol. Digestion medium consisted of RPMI 1640 with 10% heat-inactivated serum, 2.5 mg/ml collagenase D (Roche, Basel, Switzerland), and 5 U/ml DNase I (Invitrogen, Carlsbad, CA, USA). Cells were cultured in a humidified atmosphere containing 5% CO2 at 37°C.
Generation of Murine DC and Loading with Tumor Ags
DCs were derived from bone marrow progenitor cells as described.40,41 Recombinant murine granulocyte/macrophage colony-stimulating factor (GM-CSF) was kindly provided by Prof. Dr. Kris Thielemans (Vrije Universiteit, Brussels, Belgium). In short, bone marrow progenitor cells were cultured in GM-CSF at 20 ng/ml for 7 days. Medium was refreshed on days 3 and 5 of culture. Immature DCs (DCi) were harvested on day 7 of culture by vigorous pipetting and washed with Dulbecco’s phosphate-buffered saline (DPBS; Lonza).
For RNA-loading, DCi were loaded with total GL261 RNA (unless mentioned otherwise) through exponential decay electroporation (300 V and 150 μF) with a GenePulser electroporator (Bio-Rad, Nazareth, Belgium). RNA extraction was performed with the RNeasy Midi Kit (Qiagen, Venlo, The Netherlands) and quality controlled. RNA load was 15 μg of GL261 RNA per million DCi. Cells were resuspended in OptiMEM medium (Gibco, Invitrogen, Merelbeke, Belgium) at 20 × 106 cells/ml, and 4 × 106 DCi were used per electroporation cuvette.
For lysate loading, DCi were coincubated with GL261 lysate at 100 μg per million DCi cells/ml OptiMEM medium for 30 min at 37°C. Lysates were generated by exposing GL261 cells to six consecutive freeze/thaw cycles (3 min in liquid nitrogen and 3 min on 56°C, respectively).
Immediately after loading, DCi were cultured in DC medium with GM-CSF, and 0.5 μg/ml E. coli lipopolysaccharide (Sigma-Aldrich) was added to induce maturation. After 24 h, mature DCs (DCm) were harvested, counted and resuspended at suitable concentration for further application. Maturation was assessed by flow cytometry as previously described.21
Murine Brain Tumor Model
For the orthotopic IC model, GL261 cells were harvested, washed, counted, and adjusted to 5 × 105 living cells in 10 μl culture medium. Mice were anesthetized intraperitoneally (IP) with 6 μl/g body weight of a mixture of 18.75 mg/ml ketamine (Pfizer, Puurs, Belgium) and 0.125% xylazine hydrochloride (Bayer, Brussels, Belgium). After their skulls were shaved, mice were fixed in a stereotactic frame (Kopf Instruments, Tujunga, CA, USA) and 2% lidocaine hydrochloride (AstraZeneca, Brussels, Belgium) was applied locally for 1 min. A 1.5-cm (longitudinal) incision was made, and a burr hole was drilled through the skull at 1.0 mm lateral and 1.5 mm posterior from the bregma.
Tumor cells were injected over 1.5 min at a depth of 3 mm below the dura mater with a 26-gauge syringe (Hamilton, Bonaduz, Switzerland). After injection, the syringe was left in place for an additional 2 min and then slowly retracted. The site of the burr hole was rinsed with saline, and sterile bone wax was used to seal off the burr hole. The incision was closed with stitches, and 2% sodium fusidate (Leo Pharma, Wilrijk, Belgium) was applied. Stereotactic challenge was performed under sterile conditions. Three times per week, mice were weighed and clinical symptoms were scored with a neurological deficit scale adapted from an experimental autoimmune encephalomyelitis model, with grade 0 for healthy mice, grade 1 for slight unilateral paralysis, grade 2 for moderate unilateral paralysis and/or beginning hunchback, grade 3 for severe unilateral or bilateral paralysis and pronounced hunchback, and grade 4 for moribund mice.42 Unless otherwise mentioned, mice were sacrificed by cervical dislocation when they showed grade 4 symptoms, and brain was prelevated for histological analysis. Mice with a survival longer than 60 days (i.e., 3-fold the median survival of untreated animals) were considered long-term survivors. Rechallenge was performed between day 80 and day 90, and each time, naive mice of approximately the same age were challenged as controls.
Murine Subcutaneous Tumor Model
For subcutaneous (SC) tumor challenge, GL261 or MC17-51 tumor cells were resuspended at 1 × 105 in 50 μl culture medium. Mice were anesthetized as mentioned above, the skin of the right hind limb was shaved, and cells were injected SC over 1 min with an insulin syringe. After injection, the syringe was left in place for 1 additional min and then slowly retracted. Long (a) and short (b) perpendicular tumor diameters of the tumor were measured three times per week with a caliper. Approximation of the tumor volume was calculated using the formula V = (a × b2)/2.43
Treatment with RNA-Loaded DCs
Mice were vaccinated with one million CD11c+ DCm on days –14 and –7 before tumor challenge. IP vaccinations were given in a volume of 200 μl DPBS. Before each vaccination, flow cytometric quality control of the DCm was performed.
Functional Immune Monitoring
Spleen cells and pooled lymph node cells from the inguinal and axillary lymph nodes were used for ex vivo restimulation experiments; 2 × 105 cells were restimulated with 2 × 104 DCm pulsed with GL261 lysate (DCm-GL261-L). Phytohemagglutinin (PHA) was used as positive control.
Mouse interferon-γ ELISPOT
Ethanol-activated polyvinyl difluoride 96-well plates (Millipore, Billerica, MA, USA) were coated the AN18 mAb (15 μg/ml; MABTECH, Stockholm, Sweden). Cells were plated in triplicate with the respective stimuli and incubated for 36 h. For spot detection, the R4-6A2–biotin mAb (1 μg/ml, MABTECH) was used together with streptavidin:ALP (alkaline phosphatase labeled strepatvidin; 1:1,000, MABTECH). Finally, AP-conjugated (alkaline phosphatase) substrate (Bio-Rad) was added until spots emerged.
In Vivo Depletion of Specific Lymphocyte Subpopulations
For depletion of CD25+ cells, mice received a single bolus injection (250 μg) of the PC61 aCD25 mAb 21 days before tumor challenge. CD8a+ cells were depleted using YTS169 aCD8a mAb at 200 μg 1 day before and 100 μg 1 day after tumor challenge. mAbs were diluted in sterile phosphate-buffered saline (PBS), and all injections were given IP. Polyclonal rat immunoglobulin (Rockland, Gilbertsville, PA, USA) was used as control.
Isolation of Brain-Infiltrating Cells
Mice were anesthetized by IP injection of 100 μl sodium pentobarbital (Ceva Sante Animale, Brussels, Belgium), both femoral arteries were opened, and the animals were perfused through the left cardiac ventricle with 50 ml cold PBS. Brains were prelevated and cut in small pieces with a scalpel in a 50-ml tube in 1 ml digestion medium. Next, 2 ml digestion medium was added, and the tissue was incubated at 37°C for 30 min. After incubation, the digested tissue was passed through a cell strainer (BD; Becton Dickinson Pharmingen, Erembodegem, Belgium) and thoroughly washed. The suspension was centrifuged (400 g, 5 min), and the pellet was resuspended in 10 ml 40% Percoll (Sigma-Aldrich, Bornem, Belgium). This suspension was carefully brought on top of 4 ml 70% Percoll in a 15-ml tube and centrifuged for 25 min at 800g. After gradient centrifugation, the top myelin and debris layer was removed, and the mononuclear cell inter-phase was recovered and washed two times in DPBS. For further functional assays, cells were separated based on the expression of CD11b using CD11b MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, cells were counted and centrifuged at 300g for 10 min. Cells were resuspended in buffer (DPBS with 0.5% fetal calf serum and 2 mM EDTA) according to manufacturer guidelines. For 107 cells, 10 μl CD11b MicroBeads were added, mixed, and incubated for 15 min at 4°C. Cells were washed by adding 2 ml buffer per 107 cells and centrifuged at 300g for 10 min. Cells were resuspended in 500 μl buffer, and magnetic separation was performed with MS or LS columns, depending on the cell number (Miltenyi Biotec, Bergisch Gladbach, Germany). Both the unlabeled CD11b− fraction and the magnetically labeled CD11b+ cells were collected and washed with DPBS. Flow cytometric quality control was performed prior to further use.
Flow Cytometric Analysis
Murine DC were stained for H-2Kb, I-A/I-E, CD80, CD86, CD40, and CD11c. Lysed whole blood (obtained through retroorbital bleeding), splenocytes, draining lymph node cells (dLN; from either the inguinal and axillary lymph nodes for SC-challenged mice) or the cervical and brachial lymph nodes for IC-challenged mice, and brain-infiltrating cells were analyzed for expression of CD4, CD8a, CD25, CD62L, and CD11b. For each staining, appropriate isotype stainings were used. For intracellular detection of FoxP3, the protocol guidelines of the FoxP3 staining kit (eBioscience, San Diego, CA, USA) were followed. Expression of CD8a on DCm was also assessed by indirect staining with aCD8 mAb YTS169 followed by staining with fluorescein isothiocyanate (FITC)-labeled goat antirat (GAR) immunoglobulin (Serotec, Düsseldorf, Germany). Analysis was performed using the Cellquest software on a FACSort flow cytometer (BD). Absolute cell numbers of each specific cell population were calculated by multiplying the relative cell numbers (as percent of total) with the total number of viable cells (FSC-SSC gate [forward scatter; side scatter]).
In Vitro Tumor Cell Viability Assay
The viability assay has been performed as previously described.20 In brief, target tumor cells (5 × 103) were cocultured with effector cells (5 × 104) in a total volume of 200 μl in a flat-bottomed 96-well cell culture plate (TPP, St. Louis, MO, USA). After 2 days of coculture, medium and nonadherent cells were removed, the wells were carefully rinsed with PBS, and 100 μl of a 0.5 mg/ml 5-diphenyltetrazolium bromide (MTT; Sigma) solution in culture medium was added. The plates were wrapped in aluminum foil to protect them from light and incubated for 2 h. After incubation, the MTT solution was removed from the wells, and 100 μl dimethyl sulfoxide (Merck, Darmstadt, Germany) was added. After gentle shaking of the plates (400 rpm, 5 min), optical density (OD) was measured at 570 and 620 nm (OD570–620nm) using an enzyme-linked immunosorbent assay reader (Thermo Labsystems, Franklin, MA, USA). The OD570–620nm value was used as a measure of cell viability.
Histology
Prelevated brains from sacrificed animals were long-term fixed in 6% paraformaldehyde. Fixed brain samples were embedded in paraffin, and 10-μm-thick serial coronal sections were prepared and mounted on glass slides. All slides were stained with hematoxylin and eosin.
Statistical Analysis
All data are represented as mean ± SEM. Survival analysis was performed using the log-rank test. For comparing multiple groups, one-way analysis of variance (ANOVA) was used. For comparison of two groups, Student t-test was performed. Statistics were calculated with Prism software (version 4.0a; Graphpad Software Inc., San Diego, CA, USA).
Results
Prophylactic Vaccination with RNA-Loaded DCs Induces a Protective Antitumor Immune Response In Vivo
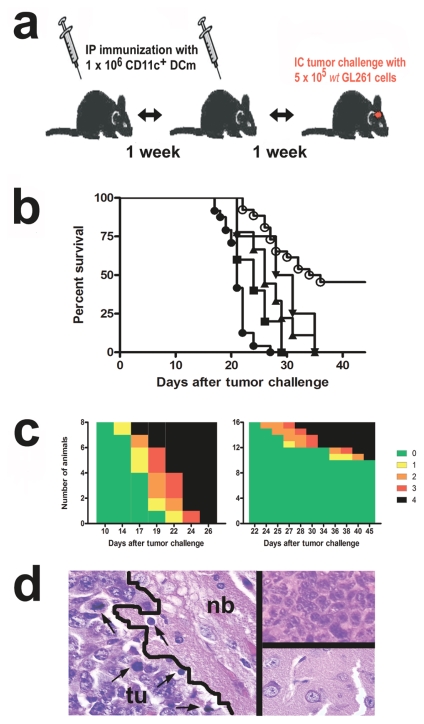
We implemented RNA-loaded DC immunotherapy in an established IC mouse glioma model.27,28 Using a preventive treatment strategy consisting of two vaccinations with DCm ex vivo loaded with GL261 RNA (DCm-GL261-RNA) before tumor challenge (Fig. 1a), we observed a significant (p < 0.001) increase in median survival (Fig. 1b) compared to untreated animals (21 vs. 35 days, respectively). Interestingly, vaccination with mock-loaded DCm (DCm-mock; 26 days, p < 0.001), DCm-LLC-RNA (29.5 days, p < 0.001), and splenocyte RNA-loaded DCs (DCm-splenocyte-RNA; 24 days, p = 0.04) also shifted median survival significantly compared to untreated animals. However, compared to DCm-mock, only GL261-RNA loading of DCs yielded a significantly (p < 0.01) better survival and protected 12 of 26 animals (46.2%) from tumor development. No protection was observed in untreated animals and animals treated with DCm-mock, DCm-LLC-RNA, or DCm-splenocyte-RNA. In some experiments, mice responding to DCm-GL261-RNA treatment were followed up to 5 months after tumor challenge and no relapse was noted. Consistent with overall survival, mapping of the tumor-induced neurological deficits (Fig. 1c) revealed not only a more pronounced clinical manifestation but also an earlier (p < 0.01) onset of symptoms in untreated animals (18.0 ± 0.82 days) compared to DCm-GL261-RNA–treated animals (29.2 ± 2.21 days). Loss of body weight less than 80% of the initial weight coincided with the onset of neurological deficit (data not shown). Histological analysis on day 14 after tumor challenge showed immune cells infiltrating the tumor bed in animals that received DCm-GL261-RNA treatment (Fig. 1d, left), whereas this phenomenon was absent or much less pronounced in untreated mice. Comparison of brain slides of untreated moribund (grade 4) mice and animals responding to DCm-GL261-RNA treatment (grade 0), sacrificed on day 21 after tumor challenge, showed the massive presence and total absence of tumor cell, respectively (Fig. 1d, right).
Fig. 1.
Vaccination with RNA-loaded dendritic cells (DCs) is partially protective against subsequent intracranial glioma challenge. (a) Overview of the DC vaccination model. Mature DCs (DCm) ex vivo loaded with GL261 RNA (DCm-GL261-RNA) were intra-peritoneally (IP) injected on day 14 and day 7 before tumor challenge. Tumor challenge consisted of intracranial (IC) implantation of 5 × 105 GL261 tumor cells under stereotaxic guidance. (b) Data represented as Kaplan-Meier graph of pooled experiments: survival curves of animals vaccinated with DCm-GL261-RNA (○, n = 25), DCm-mock (▴, n = 9), DCm Lewis lung carcinoma RNA (▾, n = 4), DCm splenocyte RNA (▪, n = 5), and untreated animals (●, n = 24). Overall log-rank p < 0.0001. (c) The tumor-induced neurological deficit is displayed graphically over time by color-coding symptom severity, both for untreated mice (left, n = 8) and DCm-GL261-RNA–treated mice (right, n = 16): grade 0 (green), healthy mice; grade 1 (yellow), slight unilateral paralysis; grade 2 (orange), moderate unilateral paralysis and/or beginning hunchback; grade 3 (red), severe unilateral or bilateral paralysis and pronounced hunchback; grade 5 (black), moribund and/or dead mice. (d) Histological analysis of hematoxylin and eosin–stained brain slides: normal brain parenchyma (nb) adjacent to the tumor bed (tu) with infiltration of immune cells (arrows) on day 14 after tumor challenge from a DCm-GL261-RNA–treated animal (left), untreated mouse with progressive disease (upper right), and mouse responding to DCm-GL261-RNA treatment (lower right). Representative pictures are shown from tissue slides obtained 21 days after tumor challenge.
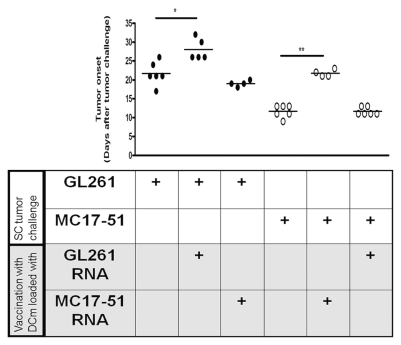
Specificity of the induced immune response was assessed in vivo by SC challenge of mice with either GL261 glioma or MC17-51 fibrosarcoma tumor cells in mice that were treated with DCs loaded with either GL261 or MC17-51 RNA (Fig. 2). In GL261-challenged mice, a delay in onset of SC tumor growth was noted in DCm-GL261-RNA–treated (28.0 ± 1.27 days) but not DCm-MC17-51-RNA–treated (19.0 ± 0.41 days) animals compared to untreated mice (21.7 ± 1.26 days). Reciprocally, tumor onset in mice that were challenged with MC17-51 fibrosarcoma cells was delayed compared to untreated mice (11.7 ± 0.67 days) if mice were treated with DCm-MC17-51-RNA (21.7 ± 0.48 days) but not if treated with DCm-GL261-RNA (11.7 ± 0.42 days).
Fig. 2.
Treatment with mature dendritic cells (DCm) loaded with GL261 RNA (DCm-GL261-RNA) results in a GL261-tumor–specific immune response in vivo. To address specificity in vivo, mice were either left untreated or prophylactically treated with DCm-GL261-RNA or DCm loaded with MC17-51 RNA and subsequently subcutaneously (SC) challenged with GL261 glioma (●) or MC17-51 fibrosarcoma (○) cells. The growth of SC tumors was measured with a caliper. For each group, the onset (in days after tumor challenge) of a detectable tumor mass is depicted.
Vaccination with DCs Loaded with Total GL261 RNA Induces a Splenic and dLN Cell Population That Is Responsive to GL261 Ags
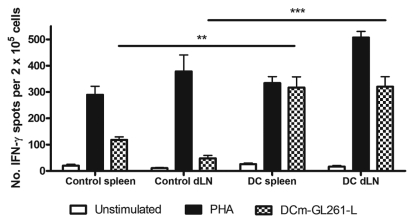
To study the immune status of animals that received DC treatment, we investigated whether cells responsive to GL261 Ags could be found within the splenocyte and/or tumor dLN cell pools. Therefore, the number of interferon-γ (IFN-γ)–producing cells upon specific in vitro restimulation with DCm-GL261-L was measured in an ELISPOT assay. Baseline values obtained prior to treatment did not reveal a significant number of IFN-γ–producing cells (data not shown). Compared to untreated animals, DC-treated mice displayed a significantly higher number of both IFN-γ–producing splenocytes (317 ± 40.9 vs. 119 ± 10.9, p < 0.01) and dLN cells (320 ± 38.3 vs. 47.7 ± 11.2, p < 0.001) when assessed 14 days after SC tumor challenge (Fig. 3). Moreover, this observation was independent of the tumor (either GL261 glioma or MC17-51 fibrosarcoma) cells used for challenge.
Fig. 3.
Ex vivo assessment of specific immunization by treatment with mature dendritic cells (DCm) loaded with GL261 RNA (DCm-GL261-RNA). Fourteen days after tumor challenge, splenocytes and draining lymph node cells (dLN) from either DCm-GL261-RNA–vaccinated (DC; n = 8) or untreated (control; n = 8) mice were ex vivo restimulated for 36 h with DCm pulsed with GL261 lysate (DCm-GL261-L) or phytohemagglutinin (PHA). Cells that were left unstimulated were used as a negative control. The production of interferon-γ (IFN-γ) was measured with ELISPOT. Data are represented as mean ± SEM number of IFN-γ spots per 2 × 105 cells. Two asterisks indicate p < 0.01; three asterisks indicate p < 0.001.
CD8+ T-Cells Are Essential for Endogenous and Vaccine-Induced Antitumor Immune Response In Vivo
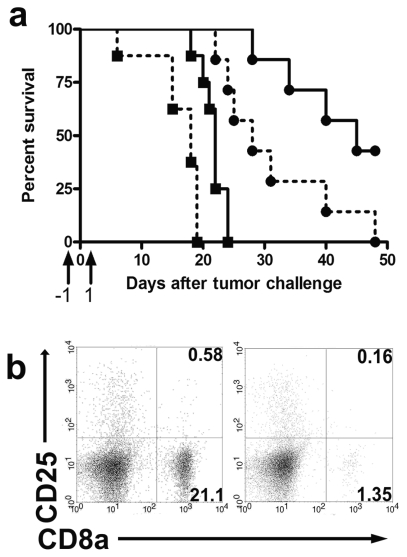
Because RNA-loaded DCs are considered to act primarily through priming of CD8+ T-cells, prophylactic DCm-GL261-RNA treatment was combined with depletion of CD8a+ T-cells to study their in vivo role in our model. Survival (Fig. 4a) was significantly shortened (median survival of 18 days, p < 0.001) in mice in which CD8a+ T-cells were depleted compared to mice that received tumor challenge only (median survival of 22 days). Treatment with DCm-GL261-RNA induced immunological protection and prolonged median survival to 45 days (p < 0.001 compared to untreated mice), whereas CD8a+ T-cell depletion in DCm-GL261-RNA–vaccinated mice shortened median survival (28 days; p = 0.02 compared to DCm-GL261-RNA–vaccinated mice). The median survival of DCm-GL261-RNA–treated CD8-depleted mice was significantly longer as compared to untreated CD8-depleted mice (28 days vs. 18 days, p < 0.001). Interestingly, whereas DCm-GL261-RNA treatment was sufficient to protect more than 40% of animals from glioma development, the combination of DCm-GL261-RNA treatment with depletion of CD8a+ T-cells resulted in 100% mortality. Efficiency of depletion was monitored over time and exceeded 90% when measured in cervical dLNs 8 days after depletion (Fig. 4b), and CD8 cell numbers returned to normal values by day 15 (data not shown). To exclude a major impact of CD8a+ cell depletion on the prophylactically administered DC, the expression of CD8a on ex vivo–generated DCs was measured by indirect staining with unlabeled aCD8 mAb YTS169 followed by GAR:FITC. The expression of CD8a on DCs was 9.02% ± 1.25% (data not shown).
Fig. 4.
Involvement of CD8+ T-cells in the endogenous and vaccine-mediated antitumor immune response. Mice were left untreated or received prophylactic treatment with mature dendritic cells (DCm) loaded with GL261 RNA. At time of tumor challenge, CD8a+ T-cells were depleted in both groups by intraperitoneal injection of the YTS169 aCD8a monoclonal antibody (mAb). Animals that were not CD8a depleted were used as controls. (a) Survival data from pooled experiments are shown for untreated mice (▪, solid line, n = 8), CD8a-depleted mice (▪, dashed line, n = 8), vaccinated mice (●, solid line, n = 7), and mice that received vaccination and were CD8a depleted (●, dashed line, n = 7). Overall log-rank p < 0.0001. Arrows on the x-axis indicate timing of administration of the aCD8a mAb. (b) Draining lymph node cells were analyzed for CD8a and CD25 expression 7 days after tumor challenge in nondepleted (left) and CD8a-depleted mice (right). Numbers on dot plots indicate relative cell fractions in respective quadrants. A lymphocyte gate was set for the analysis.
aCD25 Treatment Is Dominant to Treatment with DC Vaccination In Vivo
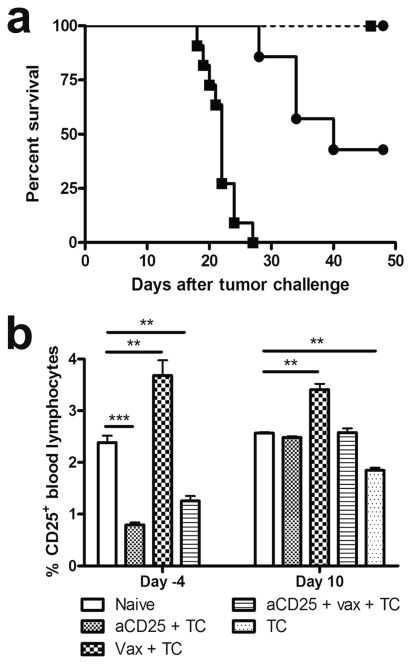
It has been well documented in experimental rodent models that malignancy recruits and expands Treg to dampen endogenous antitumor responses. In our model, FoxP3− expression on CD4+ splenocytes was assessed to investigate the influence of DC treatment on Treg. After two rounds of immunization with DCm-GL261-RNA but prior to tumor challenge, no differences in percentage of CD25+FoxP3+ cells within the CD4+ splenocyte population were noted compared to naive animals. In contrast, 14 days after tumor challenge, prophylactic DCm-GL261-RNA treatment resulted in a significant increase in splenic Treg compared to naive animals (11.9% ± 0.32% vs. 7.35% ± 0.19%, p < 0.01, n = 5). In untreated mice, an increase in Treg upon tumor challenge was also noted compared to naive mice, although to a lesser extent (9.54% ± 0.31%, p < 0.05, n = 5). Hence, these data represented the rationale to perform a series of experiments in which Treg were depleted in vivo prior to DCm-GL261-RNA treatment. We observed that independent of treatment with DCm-GL261-RNA, the injection of aCD25 (Fig. 5a) was able to rescue all tumor-challenged animals (p < 0.001). Depletion efficiency was monitored (Fig. 5b) in peripheral blood 4 days prior to and 10 days after tumor challenge (17 and 31 days, respectively, after CD25 depletion). Whereas CD25+ cells were still significantly downregulated 4 days before tumor challenge (2.38% ± 0.13% for naive mice vs. 0.80% ± 0.04% for aCD25-treated mice, p < 0.001), a normalization of the percentage of CD25-expressing lymphocytes was noted by day 10 after tumor challenge (2.57% ± 0.02% for naive mice vs. 2.48% ± 0.03% for aCD25-treated mice). Strikingly, whereas the percentage of splenic CD25-expressing CD4+ lymphocytes was 9.37% ± 0.38% in naive littermates, it was still significantly decreased in aCD25-treated mice (0.80% ± 0.08%, p < 0.001, n = 5) 14 days after tumor challenge. However, DCm-GL261-RNA vaccination after CD25 depletion nearly completely rescued the CD25-expressing CD4+ cells (8.09% ± 0.13%, p < 0.001, n = 5) compared to aCD25 treatment only. Moreover, a substantial fraction (3.35% ± 0.72%) of the restored CD25+CD4+ splenic T-cells by combined treatment expressed the Treg transcription factor FoxP3 compared to 0.65% ± 0.14% for mice that received aCD25 treatment only (p < 0.001). The total number of splenic CD4+ T-cells was not significantly different between groups (data not shown). The GL261 glioma cells did not express CD25 themselves (data not shown).
Fig. 5.
In vivo depletion of CD25+ cells is protective against subsequent tumor challenge and is dominant to dendritic cell vaccination. In order to eliminate CD25+ Treg in vivo, mice received a single intraperitoneal administration of the aCD25 monoclonal antibody PC61, 1 week before treatment with mature dendritic cells (DCm) loaded with GL261 RNA (DCm-GL261-RNA). (a) Kaplan-Meier graph from pooled experiments depicting survival of untreated mice (▪, solid line, n = 11), CD25-depleted mice (▪, dashed line, n = 7), DCm-GL261-RNA–vaccinated mice (●, solid line, n = 7), and mice treated with CD25 depletion and DCm-GL261-RNA vaccination (●, dashed line, n = 6). Overall log-rank p < 0.001. (b) Monitoring of CD25 expression was performed on blood samples obtained 4 days before and 10 days after tumor challenge. Results are shown as mean ± SEM (n = 5, for each experimental group). Percent CD25 expression was calculated by gating on lymphocytes. Abbreviations: aCD25, anti-CD25 monoclonal antibody; DC, dendritic cells; TC, T-cells. Two asterisks indicate p < 0.01; three asterisks indicate p < 0.001.
DC Vaccination but Not Prophylactic Depletion of CD25+ Lymphocytes Induces Long-Lasting Antitumor Immunity
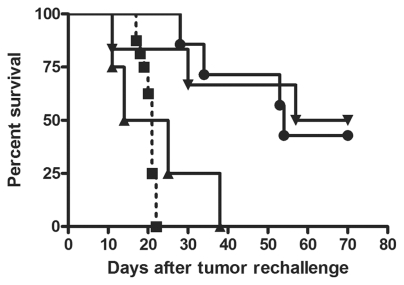
IC rechallenge of long-term survivors after a first tumor challenge revealed that initial depletion of CD25+ T-cells was not sufficient to maintain immunological protection since the median survival of 19.5 days after rechallenge in these mice was comparable with median survival of untreated animals upon first challenge, and since all of these animals also died (Fig. 6). On the other hand, aCD25 treatment with DCm-GL261-RNA vaccination resulted in protection of 50% of the animals (3 of 6), and median survival was significantly prolonged (63.5 days vs. 21 days for untreated animals, p < 0.01). Similarly, three of seven long-term surviving mice that received only DCm-GL261-RNA vaccination before primary challenge were also protected (median survival of 54 days, p < 0.001 compared to untreated mice).
Fig. 6.
Dendritic cell vaccination but not anti-CD25 monoclonal antibody (aCD25) treatment induces persistent immunological protection against intracranial glioma challenge. Long-term surviving mice after a primary tumor challenge were rechallenged orthotopically with GL261 glioma cells. Kaplan-Meier curve depicts survival of animals that were initially (before primary tumor challenge) treated with aCD25 alone (▴, n = 4), vaccination alone (●, n = 7), or aCD25 combined with vaccination (▾, n = 6). Untreated control mice are also depicted (▪, dashed line, n = 16). Overall log-rank p < 0.001.
Lymphocyte Infiltration in the Brain of DC- and/or aCD25-Treated Mice and Induction of CD8+ and CD4+ Memory T-Cells
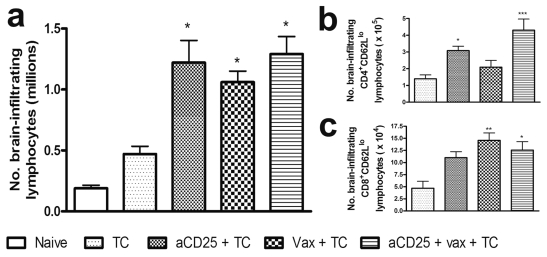
Brain-infiltrating lymphocytes were analyzed by flow cytometry 14 days after tumor challenge. Total lymphocytes (Fig. 7a) were significantly increased by aCD25 treatment, vaccination, and combined treatment compared to untreated animals. Detailed analysis of CD4+ and CD8+ lymphocyte subpopulations was performed (Table 1). Tumor-bearing mice exhibited increased numbers of CD4+ effector, Treg, and CD8+ lymphocytes compared to naive animals, although this was not significant. Separate treatment with either aCD25 or DCm-GL261-RNA vaccination alone further boosted the influx of all above-mentioned subpopulations (except for Treg in aCD25-treated mice) with a significant increase in CD25+CD8+ T-cells. Combination therapy resulted in a significant upregulation of both CD4+ effector and Treg cells, but not CD8+ T-cells, compared to untreated animals. In comparison with aCD25 treatment only, combined treatment yielded a significantly higher influx of CD4+CD25+Foxp3− and Treg cells. Compared to vaccination only, only the CD4+CD25+Foxp3− subpopulation was significantly increased in the combined treatment group.
Fig. 7.
Treatment with dendritic cells and/or anti-CD25 monoclonal antibody (aCD25) leads to infiltration of lymphocytes into the brain and the induction of immunological memory in CD8+ and CD4+ T-cells, respectively. Fourteen days after tumor challenge, mice were sacrificed, and brain-infiltrating lymphocytes were isolated as described. Mice were either left untreated or received treatment with either aCD25 (aCD25 + TC), mature dendritic cells (DCm) loaded with GL261 RNA (DCm-GL261-RNA; Vax + TC), or combined treatment (aCD25 + Vax + TC). As a control, naive littermates were analyzed. Data are from pooled experiments and are represented as mean absolute cell numbers ± SEM. For statistical analysis, treatment groups were compared to untreated animals. (a) Absolute numbers of total brain-infiltrating lymphocytes (million cells per mouse). CD62Llo cells were considered as memory T-lymphocytes. The expression of CD62L was monitored on both CD4+ (b) and CD8+ lymphocytes (c). Overall ANOVA p < 0.01 for both CD4+ and CD8+ T-cells. One asterisk indicates p < 0.05; two asterisks indicate p < 0.01; three asterisks indicate p < 0.001.
Table 1.
Characterization of brain-infiltrating lymphocytes
| Treatment | CD4+CD25− ( × 104) | CD4+CD25+Foxp3− ( × 104) | CD4+CD25+Foxp3+ ( × 103) | CD8+CD25− ( × 104) | CD8+CD25+ ( × 103) |
|---|---|---|---|---|---|
| Naive (n = 6) | 9.47 ± 1.21 | 0.04 ± 0.01 | 0.22 ± 0.11 | 10.5 ± 0.97 | 0.19 ± 0.05 |
| Untreated (n = 8) | 19.5 ± 2.37 | 2.39 ± 0.70 | 19.9 ± 7.10 | 15.4 ± 3.72 | 5.78 ± 1.32 |
| aCD25 (n = 10) | 53.4 ± 14.9 | 6.10 ± 0.92 | 11.2 ± 2.26 | 47.6 ± 16.6 | 18.0 ± 4.52* |
| DC (n = 10) | 38.5 ± 4.76 | 7.02 ± 1.45 | 28.2 ± 5.66 | 35.4 ± 5.09 | 18.2 ± 1.29* |
| aCD25 + DC (n = 9) | 61.9 ± 5.22* | 18.2 ± 4.71* | 43.9 ± 5.38* | 50.0 ± 10.5 | 12.2 ± 2.09 |
Abbreviation: aCD25, anti-CD25 monoclonal antibody; DC, dendritic cells. Brain-infiltrating lymphocytes were isolated from naive, untreated, aCD25-treated, vaccinated, and vaccinated + aCD25-treated mice 14 days after tumor challenge. Within the CD4+ population, CD25 and CD25+FoxP3− effector cells and CD25+Foxp3+ Treg were assessed. For CD8+ T-cells, CD25− and CD25+ cells were discriminated. Pooled data are represented as mean absolute cell numbers ± SEM.
Significant difference between experimental groups and untreated animals. Significant differences between combined treatment and aCD25 treatment or vaccination only are highlighted by italic and boldface numbers, respectively.
Analysis of the expression of CD62L (l-selectin) as memory T-cell marker on brain-infiltrating lymphocytes (Fig. 7b,c) revealed that DCm-GL261-RNA vaccination in combination with aCD25 treatment resulted in a significant increase of CD4+CD62Llo cells (4.30 ± 0.66 × 105) compared to untreated mice (1.39 ± 0.23 × 105, p < 0.001) and mice that received vaccination only (2.09 ± 0.41 × 105, p < 0.01). Depletion of CD25+ cells only resulted in a higher influx of CD4+CD62Llo cells (3.08 ± 0.26 × 105, p < 0.05) compared to mice that were left untreated. When CD8+CD62Llo cells were considered, a significant increase was noted in mice that received vaccination alone (1.46 ± 0.15 × 105, p < 0.01) and combined treatment (1.26 ± 0.17 × 105, p < 0.05) compared to untreated mice (4.69 ± 1.43 × 104).
Prophylactic In Vivo Depletion of Treg Allows Local Influx of Potent and Specific Antitumor Cytotoxic T-Cells into the Brain
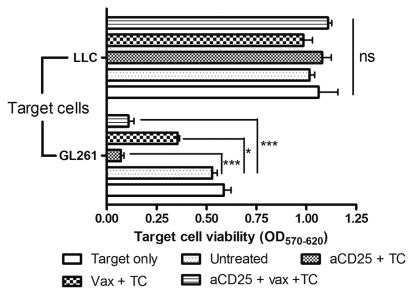
The functionality of the brain-infiltrating cells was addressed ex vivo by coculturing sorted CD11b+ myeloid cells or CD11b− lymphoid cells as effector cells with target tumor cells. After 2 days of coculture, GL261 target cell viability (Fig. 8) was reduced by coculture with CD11b− effector cells from aCD25-treated mice (OD 0.07 ± 0.02, p < 0.001), vaccinated mice (OD 0.36 ± 0.01, p < 0.05), and mice that received combined treatment (OD 0.11 ± 0.03, p < 0.001) compared to untreated animals (OD 0.59 ± 0.04). No effect on LLC target cell viability was noted. When CD11b+ effector cells were used, no significant cytotoxicity was noted against the GL261 or LLC target cells (data not shown).
Fig. 8.
Specific antitumor cytotoxicity of brain-infiltrating lymphocytes by prophylactic dendritic cell (DC) vaccination and/or in vivo depletion of regulatory T-lymphocytes (TC). Sorted CD11b− brain-infiltrating cells were used as effector cells in an in vitro tumor cell cytotoxicity assay. Effector and target cells were cocultured for 48 h at an effector-to-target ratio of 10:1. As target cells, either GL262 glioma or Lewis lung carcinoma (LLC) cells were used. Optical density (OD) is depicted as measurement of target cell viability. Data are from repeated measurements in one assay on pooled samples (n = 6 for each group). Abbreviations: aCD25, anti-CD25 mAbs; NS, not significant. One asterisk indicates p < 0.05; two asterisks indicate p < 0.01; three asterisks indicate p < 0.001.
Discussion
In this study, we validated in vivo the concept of tumor immunotherapy with ex vivo total RNA-loaded DCs in the context of malignant glioma. Moreover, the inevitable link between active immunotherapy and counteracting immune suppression was also investigated.
This immunotherapeutic approach was implemented in the well-established murine GL261 glioma model. We opted for prophylactic treatment since others reported the very aggressive nature of the GL261 model, necessitating additional intervention in curative settings.44,45 Moreover, this model reflects more the clinical therapeutic setting in which DC vaccination is also given at a stage of minimal residual disease after (sub)total resection and not at the time of bulky tumor disease.14–16 Vaccination with DCm-GL261-RNA prolonged survival and protected nearly half of the treated animals against subsequent tumor challenge. Mapping of weight loss and tumor-induced neurological deficit clearly underscored our survival data.21 Finally, histological analysis revealed DCm-GL261-RNA vaccination resulted in infiltration of lymphocytes and nonlymphoid cells, especially at the interface of the tumor mass with the normal brain parenchyma.
The specificity of any induced immune response represents a major issue when dealing with cellular cancer immunotherapy. The specificity of the immune response induced by RNA-loaded DCs in this model was demonstrated in vivo by challenging DCm-GL261-RNA–treated mice with an immunogenic fibrosarcoma cell line that is embryologically unrelated to glial tumors, or challenging DCm-MC17-51-RNA–treated mice with GL261 tumor cells. In both cases, the DC-mediated immunity against the target tumor resulted in a delay of growth of the target tumor, in contrast to the unchanged growth rate of the nontargeted tumor. Specificity was also confirmed at the level of ex vivo cytotoxic activity of in vivo stimulated brain-infiltrating lymphocytes, which clearly depicted tumor-suppressive activity against the GL261 tumor cells but not against the LLC cell line. However, we noticed that alongside immunization, treatment with DCm-LLC-RNA and DCm-splenocyte-RNA resulted in a prolonged median survival compared to untreated mice. A similar finding was observed in the group of mice treated with mock-loaded DCm. We postulate that the induction of a minor immune response upon tumor challenge itself might be boosted in a nonspecific manner by administration of activated DCs. In none of these control conditions, however, did tumor-challenged mice survive, pointing to the specific immunological protective effect of DCm-GL261-RNA treatment.
The boosted status of the immune system of DCm-GL261-RNA–vaccinated animals was shown by specific ex vivo restimulation of splenocytes and dLN cells. Splenocytes and dLN cells responding to GL261 Ags were retrieved in DCm-GL261-RNA–treated animals and only to a significantly lower extent in untreated mice.
Only half of the treated mice could be protected. With the help of online monitoring of tumor load through in vivo BLI, we recently defined two different subcategories within mice that were not protected by DCm-GL261-RNA treatment.21 One group of animals displayed in vivo flux values similar to those of untreated mice and are hence considered genuine nonresponding mice. On the other hand, we observed a fraction of partially responding mice that exhibited an initial tendency toward tumor rejection but later showed progressive disease. To our view, this is suggestive of a delicate balance between immunogenic antitumor and counteracting tolerogenic and/or suppressive mechanisms involved in the antiglioma immune response. This delicate balance, moreover, might be prone to interanimal variability. We therefore tried to unravel the balance between endogenous and/or vaccine-induced antitumor mechanisms and endogenous and/or tumor-induced tolerogenic or suppressive processes. This was accomplished by in vivo depletion of both CD8+ effector T-cells and CD25+ Treg with aCD8 and aCD25 mAb, respectively.
In accordance with recently published data by Grauer et al.,37 we have shown that CD8+ T-cells are involved both in an endogenous immune response upon tumor challenge and a vaccine-mediated protective antitumor immune response. When CD8+ T-cells were depleted at the time of tumor challenge, immunological protection was completely abolished, and all animals died. However, the median survival of DCm-GL261-RNA–treated CD8-depleted mice was still significantly longer than that of the untreated CD8-depleted mice. This indicates that DC immunotherapy is not solely acting through CD8+ T-cells. Other key players such as CD4+ helper T-cells, natural killer (NK) cells, and NK/T-cells might be likewise affected by this kind of DC treatment.46–48 In our hands, a minor fraction of the ex vivo differentiated DCs from bone marrow progenitor cells that were used for immunization showed low expression of CD8a. Hence, this CD8a+ DC subpopulation is prone to in vivo elimination by the injection of aCD8a mAb 1 week after the second vaccination. The functional consequences of this phenomenon are not clear. The CD8aa homodimer has been regarded as a cell lineage marker rather than a molecule that could contribute to functional differences between (CD8a+) lymphoid and (CD8a−) myeloid DC.49 However, Hong et al.50 recently reported that expression of CD8a on bone-marrow–derived DCs may play a functional role in enhancement of T-cell activation.
We noted that a single injection of a CD25-depleting mAb creates a temporal decrease in CD4+CD25+FoxP3+ Treg activity that is sufficient to completely protect animals from subsequent (early) IC tumor challenge. This attributes a dominant role to Treg in our experimental glioma model. A direct effect of aCD25 on GL261 cells was excluded, which agrees with recent findings of Curtin et al.,34 who also demonstrated that the efficiency of Treg depletion in a curative setting is dependent on the tumor burden since systemic administration of PC61 has a beneficial effect on survival if applied 15 days after tumor challenge but not when given 24 days after tumor challenge. Other investigators have found that curative DC-based immunotherapy in the GL261 model is efficient only if CD25-expressing Tregs are first depleted.39,51
The crucial role of Treg in glioma and other types of cancer and the impact on immunotherapy has been documented extensively both in humans and in experimental rodent models by many groups.52–55 For an excellent review on this subject, we refer to the work of W. Zou.56 Recent evidence has arisen that aCD25 treatment with the PC61 mAb does not result in a genuine depletion of Tregs but rather a functional inactivation of naive CD69lo Tregs with rapid internalization and shedding of the IL-2 receptor α unit.57 Considerable caution should be taken into account when CD25 expression on CD4+ T-cells is used to monitor Treg kinetics. Hence, in this study, only FoxP3+ cells were considered the true Treg population. In our experiments, restoration of CD25 expression in CD4+ splenocytes by vaccination after initial CD25 depletion was concomitant with an increase in FoxP3 expression. This suggests that DC treatment is capable of inducing Tregs besides its beneficial influence on the effector arm of cellular immunity. It remains an open question whether these FoxP3-expressing cells are true functional Tregs with in vivo suppressive capacity, since all animals that received combined treatment were long-term survivors from primary tumor challenge.
From rechallenge experiments, we concluded that treatment with DCm-GL261-RNA was able to induce immunological memory against the tumor, since animals that initially received combined treatment (consisting of CD25 depletion and DCm-GL261-RNA vaccination) or survived after treatment with DCm-GL261-RNA alone displayed prolonged survival and were again partially protected against tumor challenge. To our view, the observed early and also late immunological protection against glioma challenge clearly demonstrates the efficiency of immunization with DCs. On the other hand, mice that received aCD25 treatment only were not protected upon rechallenge, so either the vaccine-induced FoxP3-expressing CD4+CD25+ cells are nonfunctional Tregs, which is unlikely, or the balance between immunogenicity and tolerance is again sufficiently tilted toward the former at a later time point (day 60). The local inflammatory environment might also downregulate Treg functionality, and this can be partially mediated by IL-6–producing DCs.58,59 Jouanneau et al.60 reported recently that lysate-loaded DCs are essential for the priming but inefficient for maintaining antitumor immune responses in the GL261 model since late tumor relapses were observed, finally resulting in the cure of only 20% of treated mice. However, we did not observe late tumor relapses. Van Meirvenne et al.61 showed elegantly that in vivo depletion of Tregs enhanced both the primary and memory cytotoxic T-lymphocyte response elicited by mRNA-loaded DCs in an ovalbumin-specific tumor model. In our hands, DCm-GL261-RNA vaccination alone was sufficient for the induction of immunological protection and memory in about half of the mice, while all of the aCD25-treated mice survived but were not protected upon rechallenge. Hence, in the aforementioned setting, our data support a combined immunotherapeutic treatment consisting of Treg depletion and DCm-GL261-RNA vaccination for the induction of an optimal antitumor immune response.
Since systemic monitoring of immune reactions within the brain represents an artificial readout that can only partially reflect local events, we opted for genuine in situ investigation of brain-infiltrating cells. Taken together, we observed that both CD25 depletion and DCm-GL261-RNA vaccination, as well as combined treatment, before challenge with GL261 tumor cells allowed a massive lymphocyte infiltration into the brain. Effector lymphocytes, encompassing both CD4+CD25− and CD4+CD25+FoxP3− and CD8+ T-cells, were upregulated by both aCD25 treatment and vaccination, as well as combined treatment. Combined treatment clearly expanded the Treg population, which is in concordance with the above-mentioned systemic monitoring data. In this regard, time kinetics could provide useful information regarding the expansion and/or reduction in infiltrating lymphocytes, but this was beyond the scope of the present report. Here, we provide evidence that the CD11b− lymphocyte fraction of the brain-infiltrating cells, in particular from animals in which CD25+ cells were prophylactically eliminated, contained the real cytotoxic effector cells, whereas the CD11b+ myeloid cells had little or no cytotoxic effect.
Mice that were treated with either DCm-GL261-RNA alone or DCm-GL261-RNA together with aCD25 clearly displayed a higher number of CD8+CD62Llo lymphocytes, whereas CD4+CD62Llo lymphocytes were mainly increased by aCD25 and combined treatment. This shift toward induction of immunological memory correlates with the initially vaccinated mice being partially protected against IC rechallenge, even without CD25 depletion. The absolute increase in infiltrating CD8+ lymphocytes by DCm-GL261-RNA treatment and the concomitant memorylike phenotype of these cells further illustrate their involvement in the vaccine-mediated antitumor immune response, as already evidenced by previously mentioned in vivo CD8 codepletion experiments.
Overall, our primary goal is to improve glioma immunotherapy both by generating a more effective cellular-mediated antitumor response and by counteracting immune-suppressive mechanisms such as endogenous or tumor-induced Tregs. With the data presented here, we demonstrate the feasibility and efficiency of DC loading with total tumor RNA in vitro as well as in vivo. Moreover, we have partially unraveled the involvement of CD8+ effector T-cells and CD25+ Tregs in the experimental GL261 murine glioma model. Elimination of Tregs by aCD25 treatment or other interventions seems to represent a powerful weapon in the fight against cancer, but unfortunately it is not the ultimate tool in cancer immunotherapy after all.62–65 One should keep in mind that prolonged depletion of Tregs is not feasible due to the risk of eliciting autoimmune disease. Translated to the human system, pilot data on the use of Ontak (denileukin diftitox) and cyclophosphamide in cancer immunotherapy seemed very promising in downregulating Tregs, although conflicting data are arising.66–68 Moreover, not only are Tregs eliminated by aCD25 treatment, but also IL-2–dependent CD4+ helper T-cells and/or proliferating CD8+ lymphocytes can be affected.34 CTLA-4, a negative regulator of endogenous and vaccine-induced antitumor immunity, represents another good selective target in cancer immunotherapy. It has been shown in metastatic melanoma patients that sequential infusions of anti-CTLA-4 mAbs after DC vaccination generate a clinically meaningful antitumor immunity without grade 3 or grade 4 toxicity.69
The prognosis for patients diagnosed with high-grade malignant brain tumors and glioblastoma multiforme (GBM) in particular remains dismal, with a median survival of only 14 months for the latter subcategory. In general, patients respond poorly to the current state-of-the-art treatment strategies, consisting of maximal safe surgical resection, radiotherapy, and chemotherapy.70 Due to spreading of the tumor cells into surrounding areas of the brain, all GBM patients display tumor relapse and die within 18 months.71 Hence, there is an urgent need for new treatment modalities that are able to specifically eradicate or at least suppress the residual glioma cells without serious adverse effects and with acceptable quality of life. In this perspective, autologous DC therapy (in which monocyte-derived DC are loaded with total lysate from the patients’ own tumors) is under investigation by our research group in two phase I/II clinical trials for newly diagnosed and relapsed GBM. So far, postoperative adjuvant DC vaccination in more than 100 patients with relapsed high-grade glioma yields interesting long-term results, with about 25% 2-year survivors after vaccination, which compares favorably to any large study in recurrent high-grade glioma thus far.15 For newly diagnosed GBM, we are currently assessing the integration of DC vaccination into the conventional postoperative radiochemotherapy regimen.
The main objective of the work presented in this study was better insight into the mechanisms governing the induced immune response by DCm-GL261-RNA against malignant glioma. This work opens perspectives for a further detailed study of the cytokine expression patterns both locally in the tumor microenvironment and systemically in the different experimental treatment groups described here. In conclusion, we postulate from the in vivo data presented here that the combined immunotherapeutic approach targeting both Tregs and effector T-cells leads to optimal immunological protection against malignant glioma. The ultimate goal is to refine immunotherapy directed against this type of malignancy, thereby improving the outcome of patients diagnosed with high-grade glial tumors.
Acknowledgments
W.M. is supported by the Olivia Hendrickx Research Fund. B.V. is the recipient of a Ph.D. fellowship from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT Vlaanderen). S.D.V. is supported by the Clinical Research Fund (Klinisch Onderzoeksfonds) from the University Hospital Leuven. S.W.V.G. is a senior clinical investigator of the Fund for Scientific Research-Flanders (FWO Vlaanderen). This work was supported by the Olivia Hendrickx Research Fund (http://www.olivia.be), by Kiwanis divisie Vlaams-Brabant, and by individual donors.
We thank Lieve Coorevits for excellent technical assistance with the intracellular FoxP3 staining, Kathleen De Swert for help with the in vivo tumor challenge, and Lieve Ophalvens for histological analysis.
References
- 1.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305:197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- 4.Figdor CG, De Vries IJM, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 6.De Vleeschouwer S, Van Gool SW, Van Calenbergh F. Immunotherapy for malignant gliomas: emphasis on strategies of active specific immunotherapy using autologous dendritic cells. Childs Nerv Syst. 2005;21:7–18. doi: 10.1007/s00381-004-0994-3. [DOI] [PubMed] [Google Scholar]
- 7.Pellegatta S, Finocchiaro G. Cell therapies in neuro-oncology. Neurol Sci. 2005;26(suppl 1):43–45. doi: 10.1007/s10072-005-0405-x. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler CJ, Black KL. Dendritic cell vaccines and obstacles to beneficial immunity in glioma patients. Curr Opin Mol Ther. 2005;7:35–47. [PubMed] [Google Scholar]
- 9.Parajuli P, Sloan AE. Dendritic cell-based immunotherapy of malignant gliomas. Cancer Invest. 2004;22:405–416. doi: 10.1081/cnv-200034909. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka R. Novel immunotherapeutic approaches to glioma. Curr Opin Mol Ther. 2006;8:46–51. [PubMed] [Google Scholar]
- 11.Gabrilovich DI. Dendritic cell vaccines for cancer treatment. Curr Opin Mol Ther. 2002;4:452–458. [PubMed] [Google Scholar]
- 12.Sikorski CW, Lesniak MS. Immunotherapy for malignant glioma: current approaches and future directions. Neurol Res. 2005;27:703–716. doi: 10.1179/016164105X49481. [DOI] [PubMed] [Google Scholar]
- 13.Tuyaerts S, Aerts JL, Corthals J, et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother. 2007;56:1513–1537. doi: 10.1007/s00262-007-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vleeschouwer S, Rapp M, Sorg RV, et al. Dendritic cell vaccination in patients with malignant gliomas: current status and future directions. Neurosurgery. 2006;59:988–999. doi: 10.1227/01.NEU.0000245595.38957.3E. [DOI] [PubMed] [Google Scholar]
- 15.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Clinical experience of postoperative adjuvant dendritic cell-based immunotherapy in a large group of patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14:3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 16.De Vleeschouwer S, Van Calenbergh F, Demaerel P, et al. Transient local response and persistent tumor control of recurrent malignant glioma treated with combination therapy including dendritic cell therapy. J Neurosurg Spine. 2004;100:492–497. doi: 10.3171/ped.2004.100.5.0492. [DOI] [PubMed] [Google Scholar]
- 17.Ehtesham M, Black KL, Yu JS. Recent progress in immunotherapy for malignant glioma: treatment strategies and results from clinical trials. Cancer Control. 2004;11:192–207. doi: 10.1177/107327480401100307. [DOI] [PubMed] [Google Scholar]
- 18.Fecci PE, Mitchell DA, Archer GE, et al. The history, evolution, and clinical use of dendritic cell-based immunisation strategies in the therapy of brain tumors. J Neurooncol. 2003;64:161–176. doi: 10.1007/BF02700031. [DOI] [PubMed] [Google Scholar]
- 19.De Vleeschouwer S, Arredouani M, Adé M, et al. Uptake and presentation of malignant glioma tumor cell lysates by monocyte-derived dendritic cells. Cancer Immunol Immunother. 2005;54:372–382. doi: 10.1007/s00262-004-0615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vleeschouwer S, Spencer I, Ceuppens JL, Van Gool SW. Persistent IL-10 production is required for glioma growth suppressive activity by Th1-directed effector cells after stimulation with tumor lysate-loaded dendritic cells. J Neurooncol. 2007;84:131–140. doi: 10.1007/s11060-007-9362-y. [DOI] [PubMed] [Google Scholar]
- 21.Maes W, Deroose C, Reumers V, et al. In vivo bioluminescence imaging in an experimental mouse model for dendritic cell based immunotherapy against malignant glioma. J Neurooncol. 2008;91:127–139. doi: 10.1007/s11060-008-9691-5. [DOI] [PubMed] [Google Scholar]
- 22.Weiner LM. Cancer immunotherapy—the endgame begins. N Engl J Med. 2008;358:2664–2665. doi: 10.1056/NEJMp0803663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Andaloussi A, Sonabend AM, Han Y, Lesniak MS. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia. 2006;54:526–535. doi: 10.1002/glia.20401. [DOI] [PubMed] [Google Scholar]
- 24.Fomchenko EI, Holland E C. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–5297. doi: 10.1158/1078-0432.CCR-06-0438. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb EW, Demaria S, Lukyanov Y, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res. 2006;12:4730–4737. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell DA, Nair SK. RNA-transfected dendritic cells in cancer immunotherapy. J Clin Invest. 2000;106:1065–1069. doi: 10.1172/JCI11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyupoglu IY, Hahnen E, Heckel A, et al. Malignant glioma-induced neuronal cell death in an organotypic glioma invasion model. J Neurosurg. 2005;102:738–744. doi: 10.3171/jns.2005.102.4.0738. [DOI] [PubMed] [Google Scholar]
- 28.Szatmari T, Lumniczky K, Desaknai S, et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97:546–553. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newcomb EW, Lukyanov Y, Alonso-Basanta M, et al. Antiangiogenic effects of noscapine enhance radioresponse for GL261 tumors. Int J Radiat Oncol Biol Phys. 2008;71:1477–1484. doi: 10.1016/j.ijrobp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita M, Zhu X, Sasaki K, et al. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180:2089–2098. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Luo D, Streit WJ, Harrison JK. CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. J Neuroimmunol. 2008;198:98–105. doi: 10.1016/j.jneuroim.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonabend AM, Ulasov IV, Tyler MA, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 33.Szatmari T, Huszty G, Desaknai S, et al. Adenoviral vector transduction of the human deoxycytidine kinase gene enhances the cytotoxic and radiosensitizing effect of gemcitabine on experimental gliomas. Cancer Gene Ther. 2008;15:154–164. doi: 10.1038/sj.cgt.7701115. [DOI] [PubMed] [Google Scholar]
- 34.Curtin JF, Candolfi M, Fakhouri TM, et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One. 2008;3:e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei WZ, Morris GP, Kong YC. Anti-tumor immunity and autoimmunity: a balancing act of regulatory T cells. Cancer Immunol Immunother. 2004;53:73–78. doi: 10.1007/s00262-003-0444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grauer OM, Nierkens S, Bennink E, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121:95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 38.Onizuka S, Tawara I, Shimizu J, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 39.El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105:430–437. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- 40.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutz MB, Kukutsch N, Ogilvie ALJ, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 42.Mustafa M, Diener P, Sun JB, Link H, Olsson T. Immunopharmacologic modulation of experimental allergic encephalomyelitis: low-dose cyclosporin-A treatment causes disease relapse and increased systemic T and B cell-mediated myelin-directed autoimmunity. Scand J Immunol. 1993;38:499–507. doi: 10.1111/j.1365-3083.1993.tb03232.x. [DOI] [PubMed] [Google Scholar]
- 43.Saito R, Mizuno M, Nakahara N, et al. Vaccination with tumor cell lysate-pulsed dendritic cells augments the effect of IFN-beta gene therapy for malignant glioma in an experimental mouse intracranial glioma. Int J Cancer. 2004;111:777–782. doi: 10.1002/ijc.20331. [DOI] [PubMed] [Google Scholar]
- 44.Lumniczky K, Desaknai S, Mangel L, et al. Local tumor irradiation augments the antitumor effect of cytokine-producing autologous cancer cell vaccines in a murine glioma model. Cancer Gene Ther. 2002;9:44–52. doi: 10.1038/sj.cgt.7700398. [DOI] [PubMed] [Google Scholar]
- 45.Fecci PE, Sweeney AE, Grossi PM, et al. Systemic anti-CD25 mono-clonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12:4294–4305. doi: 10.1158/1078-0432.CCR-06-0053. [DOI] [PubMed] [Google Scholar]
- 46.Kalinski P, Giermasz A, Nakamura Y, et al. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 47.Munz C, Dao T, Ferlazzo G, et al. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105:266–273. doi: 10.1182/blood-2004-06-2492. [DOI] [PubMed] [Google Scholar]
- 48.Smyth MJ, Wallace ME, Nutt SL, et al. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 2005;201:1973–1985. doi: 10.1084/jem.20042280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traver D, Akashi K, Manz M, et al. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–2154. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- 50.Hong L, Webb TJ, Wilkes DS. Dendritic cell-T cell interactions: CD8 alpha alpha expressed on dendritic cells regulates T cell proliferation. Immunol Lett. 2007;108:174–178. doi: 10.1016/j.imlet.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grauer OM, Sutmuller RP, van Maren W, et al. E limination of regulatory T cells is essential for an effective vaccination with tumor lysate-pulsed dendritic cells in a murine glioma model. Int J Cancer. 2008;122:1794–1802. doi: 10.1002/ijc.23284. [DOI] [PubMed] [Google Scholar]
- 52.Gallimore A, Godkin A. Regulatory T cells and tumour immunity—observations in mice and men. Immunology. 2008;123:157–163. doi: 10.1111/j.1365-2567.2007.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imai H, Saio M, Nonaka K, et al. Depletion of CD4+CD25+ regulatory T cells enhances interleukin-2-induced antitumor immunity in a mouse model of colon adenocarcinoma. Cancer Sci. 2007;98:416–423. doi: 10.1111/j.1349-7006.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leon K, Garcia K, Carneiro J, Lage A. How regulatory CD25+CD4+ T cells impinge on tumor immunobiology: the differential response of tumors to therapies. J Immunol. 2007;179:5659–5668. doi: 10.4049/jimmunol.179.9.5659. [DOI] [PubMed] [Google Scholar]
- 55.Strauss L, Bergmann C, Szczepanski M, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 56.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 57.McNeill A, Spittle E, Backstrom BT. Partial depletion of CD69low-expressing natural regulatory T cells with the anti-CD25 monoclonal antibody PC61. Scand J Immunol. 2007;65:63–69. doi: 10.1111/j.1365-3083.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- 58.Fehervari Z, Sakaguchi S. Control of FoxP3+CD25+CD4+ regulatory cell activation and function by dendritic cells. Int Immunol. 2004;12:1769–1780. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- 59.Pasare C, Medzhitov R. Toll-pathway dependent blockade of CD4+CD25+ T-cell mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 60.Jouanneau E, Poujol D, Gulia S, et al. Dendritic cells are essential for priming but inefficient for boosting antitumour immune response in an orthotopic murine glioma model. Cancer Immunol Immunother. 2005;55:254–267. doi: 10.1007/s00262-005-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Meirvenne S, Dullaers M, Heirman C, et al. In vivo depletion of CD4+CD25+ regulatory T cells enhances the antigen-specific primary and memory CTL response elicited by mature mRNA-electroporated dendritic cells. Mol Ther. 2005;12:922–932. doi: 10.1016/j.ymthe.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 62.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155–168. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 63.Matsushita N, Pilon-Thomas SA, Martin LM, Riker AI. Comparative methodologies of regulatory T cell depletion in a murine melanoma model. J Immunol Methods. 2008;333:167–179. doi: 10.1016/j.jim.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 65.Comes A, Rosso O, Orengo AM, et al. CD25+ regulatory T cell depletion augments immunotherapy of micrometastases by an IL-21- secreting cellular vaccine. J Immunol. 2006;176:1750–1758. doi: 10.4049/jimmunol.176.3.1750. [DOI] [PubMed] [Google Scholar]
- 66.Morse MA, Hobeika AC, Osada T, et al. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112:610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rasku MA, Clem AL, Telang S, et al. Transient T cell depletion causes regression of melanoma metastases. J Transl Med. 2008;6:12. doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Audia S, Nicolas A, Cathelin D, et al. Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol. 2007;150:523–530. doi: 10.1111/j.1365-2249.2007.03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 71.Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas. Cancer Treat Rev. 2000;26:397–409. doi: 10.1053/ctrv.2000.0191. [DOI] [PubMed] [Google Scholar]