Abstract
While the prognosis of acute childhood leukemia has improved, long-term survivors are increasingly experiencing late effects of the treatment. Cranially irradiated survivors are predisposed to the development of CNS tumors. Our aim was to describe the incidence of secondary brain tumors and to define the significance of treatment-related risk factors and host characteristics in a cohort of childhood leukemia survivors. Our cohort consisted of 60 consecutive cranially irradiated adult survivors of childhood leukemia treated in Oulu University Hospital (Oulu, Finland); MRI of the brain was performed on 49. The sites of the tumors, their histology, and details of the leukemia treatment were determined. Of the 49 patients, 11 (22%) 1–8 years of age at the time of diagnosis developed meningioma later in life, while no other brain tumors were seen. In this cohort, the development of meningioma seemed to show undisputable linkage with long latency periods (mean, 25 years; range, 14–34 years) and an increasing incidence 20 years after the treatment (47%). Three patients had multiple meningiomas, two had recurrent disease, and one had an atypical meningioma. Age at the time of irradiation, gender, or cumulative doses of chemotherapeutic agents showed no significant association with the development of meningiomas. The high incidence of meningiomas in this study was associated with long follow-up periods. Although the cohort is small, it seems probable that the increasing incidence of meningioma will shadow the future of cranially irradiated leukemia survivors. Systematic brain imaging after the treatment is therefore justifiable.
Keywords: acute leukemia, brain tumor, cranial radiation therapy, meningioma, second neoplasm
Treatment of childhood leukemia is considered one of the true success stories in the field of medicine. The 5-year event-free survival of acute lymphoblastic leukemia (ALL) patients is almost 80%, while the prognosis of acute myeloblastic leukemia remains less favorable.1 Even though the number of survivors is increasing, the late effects of treatment have become a rising concern. Several investigations have shown that survivors of leukemia have an increased risk of developing CNS tumors, which are closely associated with previous radiotherapy.2–11
Meningiomas and gliomas appear to be the most common latent brain tumors in leukemia survivors. Meningiomas seem to be associated with long latency periods, whereas gliomas tend to occur within 5 years after the treatment. Unlike gliomas, meningiomas do not show any plateau; their incidence continues to increase as a function of time.4,10 Meningiomas have long been recognized as possible late sequelae of irradiation. The first patient with a meningioma presumed to have developed as a result of high-dose irradiation was reported by Mann et al. in 1953.12 Traditionally, meningiomas have been considered rare complications of leukemia treatment.2,4,5,13,14 Only recent reports have suggested they might be far more common than earlier believed.
The exact incidence of meningiomas in leukemia patients who have received cranial irradiation for their disease in childhood still remains unclear. Consequently, the magnitude of the risk of developing meningioma is not well defined. In this study, our primary goal was to describe the incidence of meningiomas and other brain tumors in survivors of childhood leukemia treated with cranial irradiation at Oulu University Hospital (Oulu, Finland) during 1971–1997. We assessed the significance of risk factors predisposing these patients to the development of radiation-induced meningioma. For this purpose, we gathered detailed information on the radiation therapy received by the subjects. We also assessed the role of chemotherapeutic agents and personal characteristics such as age at diagnosis and gender.
Subjects and Methods
This study is a substudy of a larger research project on the late effects of childhood leukemia treatment being conducted at the Department of Pediatrics in Oulu University Hospital during the years 2002–2008. As a part of different research projects, the patients have been followed with brain MRI since 1991, although the follow-up has not been systematic. Two patients whose meningioma cases were published in 1994 and monitored by MRI afterward are included in this study. The following eligibility criteria were applied: prophylactic cranial irradiation during the leukemia treatment, minimum remission of 10 years, and older than 18 years at the time of invitation. A total of 60 patients fulfilled the criteria.
Informed consent was obtained from the patients. The research protocol was approved by the ethical committee of Oulu University Hospital.
We gathered information on the chemotherapy administered to each patient at different time points. We recorded the date of initiation and the duration of cranial irradiation therapy. In addition, age at the time of irradiation, the cumulative dosage schedule, fractionation, and the X-ray tube used were determined. We also identified the indication of radiotherapy and checked whether it had been administered as part of the primary treatment protocol or after a relapse.
MRI was performed by using a 1.5-T magnet. The protocol included T2-weighted and fluid-attenuated inversion recovery (FLAIR) images in the axial plane together with T1-weighted sagittal and axial or coronal images. Contrast-enhanced T1-weighted images were obtained in the coronal and/or axial planes. The images were analyzed by one radiologist paying special attention to tumors and abnormal enhancement.
If the patient had received surgery, the histological samples were reviewed by our pathologist. Meningiomas were classified according to the WHO classification system for their histological type and grade by using hematoxylin and eosin and ethidium monoazide staining and by immunohistochemistry against epithelial membrane antigen (EMA, Dako 1:500), progesterone receptor (Dako 1:50), cell cycle–associated proliferation antigen Ki-67 (Dako 1:50) and CD34 (Novocastra 1:500).
Statistical Analysis
Statistical analysis was accomplished using SPSS software, version 13 (SPSS Inc., Chicago, IL, USA). The methods used were independent samples t-test and chi-square test for continuous and discrete variables, respectively. The cumulative survival without meningiomas during the period between cranial irradiation and the latest MRI was evaluated with Kaplan-Meier survival analysis. The same method was used to analyze the significance of radiation dose and follow-up time in the development of meningiomas. p-Values < 0.05 were considered statistically significant.
Results
Of the entire cohort of 60 patients, 49 patients (82%) were evaluated based on MRI of the brain between 2002 and 2008. This patient group consisted of 30 females and 19 males. Of the 11 patients who did not participate in the study, four were lost to follow-up, five refused to participate in the study, one was unable to participate for family reasons, and one was severely ill with nononcological disease. The patients in the nonimaged group were significantly older at diagnosis than those in the imaged group (10.1 years vs. 4.9 years, p > 0.01), but their follow-up periods were comparable.
MRI was performed in 49 patients. Contrast-enhanced T1-weighted images were obtained in 39 patients. Routine MRI does not include the use of intravenous contrast, and 10 patients with normal MR images did not receive it as a part of their MRI. For some of the patients, the imaging was performed in the local hospitals where the radiologist had decided not to use intravenous contrast.
Incidence of Brain Tumors
Eleven patients developed meningioma, which gives a cumulative incidence of 22.4% (11 of 49). Our results are shown summarized in Table 1. Four patients presented with neurological symptoms: one with generalized seizures, and the others with headache and visual disturbances. Three patients had multiple meningiomas, and two experienced recurrence of their disease. The meningiomas were significantly larger in diameter if diagnosed at time of symptomatology (mean, 51 mm vs. 23 mm; p = 0.001). None of the patients have died yet, and except for three patients, the postoperative morbidity has been low. Two patients (one symptomatic, one asymptomatic at diagnosis of meningioma) had epileptic seizures following surgery, one of whom also had oculomotor paresis and postoperative meningitis. A third patient found in routine surveillance suffered from temporary aphasia during the first postoperative days.
Table 1.
Characteristics of patients treated with cranial irradiation for acute lymphoblastic leukemia who developed meningiomas
| Gender | Age at Diagnosis (Years) | Dose (Gy) | Latency (Years) | Symptoms | Site | Size of Tumor (mm) | Resection | Extent of Resection | Histology | WHO Grade | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 2 | 24 | 33 | No | Left frontotemporal | 32 × 35 × 12 | Yes | Complete | Meningio-epithelial | I | No |
| F | 5 | 25 | 32 | No | Left frontal | 12 × 12 × 5 | No | — | — | — | No |
| F | 2 | 21 | 18 | Yes | Several (seven meningiomas) | 65 × 51 × 55 (occipital) | Yes | Complete (occipital) | Atypical and meningio-epithelial | II, I | Yes (several meningiomas) |
| F | 3 | 25 | 34 | No | Right temporal and pons | 20 × 15 × 10 5 × 5 × 5 |
No | — | — | — | No |
| F | 4 | 25 | 30 | No | Right convexity and sphenoid | 17 × 8 × 11 12 × 11 × 6 |
Yes | Complete (convex) Incomplete (sphenoid) |
Fibrotic | I | No |
| M | 3 | 46 | 28 | Yes | Left parietal | 41 × 50 × 42 | Yes | Complete | Transitional | I | No |
| F | 5 | 25 | 14 | No | Parasagittal | 30 × 30 × 15 | Yes | Complete | Meningio-epithelial | I | Yes |
| F | 1 | 21 | 26 | No | Right frontal | 9 × 8 × 13 | No | — | — | — | No |
| F | 3 | 25 | 21 | Yes | Sphenoid | 50 × 50 × 38 | Yes | Complete | Fibrotic | I | No |
| M | 8 | 24 | 24 | Yes | Right frontal | 33 × 36 × 40 | Yes | Complete | Meningio-epithelial | I | No |
| F | 1 | 22 | 16 | No | Anterior cranial fossa | 31 × 23 × 16 | Yes | Complete | Transitional | I | No |
Incidence of Other Secondary Neoplasms
Five other secondary neoplasms were diagnosed in four patients. Each of these patients also developed meningioma. One patient developed spinal root schwannoma, one squamous carcinoma grade I of the tongue, one low-grade papillary urothelial carcinoma in the bladder, and one patient had three secondary neoplasms (grade II clear-cell carcinoma of the kidney after 22 years, basal cell carcinoma in the face after 24 years, and infiltrating urothelial carcinoma in the bladder after 27 years).
Histology
Histology was available for the eight meningiomas that had been resected. Seven meningiomas corresponded to WHO grade I: four were meningioepithelial, two fibrous, and one transitional. The histology of the eighth meningioma showed increased mitoses and “geographic” necrosis. This meningioma developed in a female patient who has had multiple grade I recurrences every 3 years since operation. Her current problem is a widely infiltrating meningioma originating from the right orbit.
Risk Factors
The patients with meningioma were younger at diagnosis, although no statistical significance was found (Table 2). However, if the 11 nonimaged patients are included in the nonmeningioma group, young age at diagnosis becomes a significant risk factor (meningioma group vs. nonmeningioma group: mean, 3.7 vs. 6.3 years; median, 3.3 vs. 5.0 years; range, 1.5–8.6 vs. 0.8–15.4 years; p = 0.005). Despite the slight female predominance, there appeared to be no significant association between gender and the development of meningioma (p = 0.492, Fisher). The presence of CNS relapse (p = 0.812) also did not indicate an increased risk of meningioma. The significance of risk factors is summarized in Table 2.
Table 2.
Statistical significance of the main risk factors for developing meningioma
| Risk Factor | Meningioma | No Meningioma | p-Value |
|---|---|---|---|
| Age at diagnosis (years) | |||
| Mean | 3.8 | 5.3 | 0.176 |
| Median | 3.7 | 4.5 | |
| Range | 1.5–8.6 | 0.8–15.0 | |
| Gender (F/M) | 8/3 | 22/16 | 0.492 |
| Time since cranial irradiation (years) | |||
| Mean | 25.0 | 18.7 | 0.01 |
| Median | 26.0 | 15.6 | |
| Range | 13.9–33.8 | 9.5–32.0 | |
| Relapse (%) | 3 (6%) | 9 (18%) | 0.807 |
| Maintenance (years) | 3.2 | 2.8 | 0.473 |
| Dose (Gy) | 0.017 | ||
| 18 | 0 | 13 | |
| 21–25 | 10 | 20 | |
| 30–46 | 1 | 5 | |
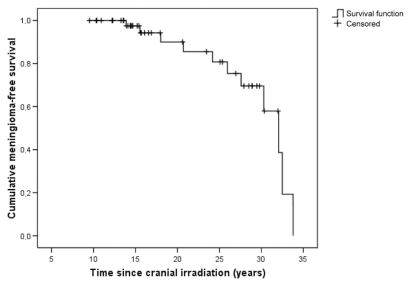
Meningiomas occurred 14–34 years (mean, 25 years) after cranial radiation therapy. Their incidence was calculated for two periods of cranial irradiation practice in the treatment of leukemia: earlier, and later than 1980 (Table 3). The patients treated by 1980 had a cumulative incidence of 47%. The pattern of increasing risk for meningiomas is shown in a Kaplan-Meier survival analysis (Fig. 1).
Table 3.
Distribution of cumulative doses of cranial irradiation and meningiomas in patients diagnosed by 1980 and after 1980
| Number of Patients (Number of Meningiomas) |
|||
|---|---|---|---|
| Dose | 1971–1980 | 1981–1995 | Total |
| 18 Gy | 0 (0) | 13 (0) | 13 (0) |
| 21–25 Gy | 16 (8) | 14 (2) | 30 (10) |
| 30–46 Gy | 3 (1) | 3 (0) | 6 (1) |
| Total | 19 (9) | 30 (2) | 49 (11) |
Fig. 1.
Cumulative survival without meningiomas in 49 cranially irradiated leukemia patients.
Influence of Chemotherapeutic Agents and Hormone Treatment
During 1974–1981, treatment was characterized by remission induction with prednisolone, vincristine, pulsed cyclophosphamide, and intrathecal methotrexate, followed by prophylactic cranial irradiation (21–25 Gy) and up to 3 years of maintenance with oral antimetabolite treatment (methotrexate and 6-mercaptopurine) and monthly reinductions with vincristine and prednisolone. In a risk-directed protocol introduced in 1976, the treatment of high-risk patients was intensified with adriamycin for induction and low-dose cytarabine for consolidation. Maintenance was augmented by weekly oral cyclophosphamide.
In 1981, high-risk patients were treated according to the BFM81 protocol, which incorporated even more intensive induction into the treatment program. The Nordic protocols for non–high-risk patients were introduced in 1986. They typically included repeated high-dose methotrexate infusions. In the Nordic protocol of 1992, cranial irradiation was given only to high-risk patients older than 5 years. All high-risk patients received high-dose methotrexate and high-dose cytarabine as CNS treatment. The Nordic treatment protocols have been described in detail elsewhere.15
The analysis failed to show the significance of any of the cytotoxic agents in the development of meningioma. In the early treatment eras, patients were treated with high cumulative doses of cyclophosphamide, but the mean doses did not differ statistically significantly between patients with or without meningioma (21,360 and 6,099 mg/m2, respectively; p = 0.112). Nor did the mean doses of adriamycin and 6-mercaptopurine or the length of antimetabolite treatment differ between the meningioma and nonmeningioma groups. The respective doses were 170 and 230 mg/m2 for adriamycin (p = 0.560) and 83,200 and 64,500 mg/m2 for 6-mercaptopurine (p = 0.143). The mean length of treatment was 3.2 and 2.8 years, respectively. None of the patients with meningioma were treated with growth hormone after cranial irradiation.
Influence of Radiation Therapy
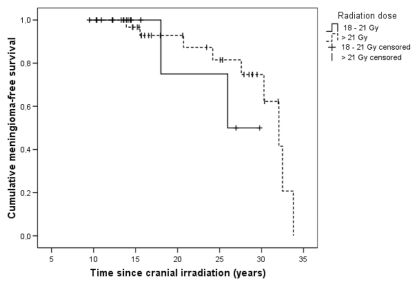
We observed a radiation dose–response relationship (p = 0.017) for meningiomas between groups treated with 18 Gy, 21–25 Gy, or >25 Gy (Table 3). This significance no longer exists if time since irradiation is taken into account (Fig. 2). All the patients diagnosed by 1980 were treated with a dose minimum of 21 Gy (Table 3). The typical fractionation schedule was 1.4–2.0 Gy given in 9–17 sessions. The source of irradiation was a cobalt unit with 1.25-MeV gamma rays for 22 leukemia patients, until the 6-MV linear accelerator was introduced in 1985. Ten patients with meningioma were irradiated with the cobalt unit.
Fig. 2.
Cumulative survival without meningiomas in 49 leukemia patients treated with a radiation dose ≤ 21 Gy (n = 17) or >21 Gy (n = 32).
Discussion
Meningiomas occurred in more than one-fifth (22%) of the 49 cranially irradiated long-term survivors of childhood leukemia who had been followed for a median of 21 years. No other brain tumors were detected. We observed an exceptionally long latency period (median, 26 years). In survivors diagnosed by 1980, the incidence increased up to 47%. Ten patients did not receive intravenous contrast in T1-weighted images, so the presence of small meningiomas in these patients cannot be excluded. Compared with the incidence for sporadic meningiomas, that for radiation-induced meningiomas seems to be substantially higher. According to the Finnish Cancer Registry, the overall meningioma incidence rates were 1.6 for men and 5.5 for women per 100,000, and only 17% of meningiomas occurred in patients younger than 50 years of age.16
All cohort members had an opportunity for MRI of the brain. Participation in our study was relatively good at 82%. Until recently, the estimates for the incidence of brain tumors have varied between 1% at 20 years4,6 and 4% at 30 years.14 With the increasing number of survivors of leukemia and the increasingly long follow-up periods, the old assumption of the rarity of secondary brain tumors should now be reconsidered. The accumulation of meningiomas among patients treated in the early era of leukemia treatment could imply an increasing incidence in the future. Goshen et al.17 reported an incidence of meningiomas in survivors of leukemia almost similar to ours: their cumulative incidence rates were 17% in irradiated patients. To our knowledge, these two studies are unique in their application of MRI of the brain regardless of symptoms.
Survivors of leukemia show an independent association with brain tumors, with a greater than 20-fold excess risk of developing a brain neoplasm subsequent to the treatment of childhood leukemia.2,5,14,18,19 The assumption of irradiation being the most important risk factor for almost all secondary malignancies has been widely accepted.7 Unlike meningiomas, which are characterized by a long latency period, gliomas tend to occur within 5 years after the treatment.10 Relling et al.20 reported a relatively high incidence of malignant brain tumors (13% at 8 years) in leukemia patients following cranial irradiation and intensive antimetabolite treatment. Neglia et al.21 noted that 22 of their 4,581 leukemia patients developed malignant brain tumors, accounting for a majority (64%) of all brain tumors observed in childhood cancer patients. The lack of malignant brain tumors in our study could be explained by the relatively small cohort size.
While the risk for malignant brain tumors seems to decline dramatically at 5 years after treatment,10 the risk of developing meningiomas shows no signs of reaching a plateau. This observation is supported by some previous studies.2,14,22,23 A long latency period seems to be typical for radiation-induced meningiomas, and studies with follow-up periods of less than 10 years have reported low incidence rates of meningioma.4,6 For our patients, the survival curve shows an abrupt decline with a prolonged latency period (Fig. 1). The small patient number definitely has an effect on the slope of the curve, although it has been proposed that irradiation confers a lifetime risk for tumor development.22
The incidence of brain tumors with respect to the total dose of cranial irradiation has remained controversial.5,9,13,19,20 In our cohort, meningiomas occurred only in patients treated with radiation doses of 21 Gy or more. Patients treated with lower doses had notably shorter follow-up periods. When the time since irradiation is taken into account, both patient groups (18–21 Gy vs. >21 Gy) seemed to be at equal risk for developing meningioma. Neglia et al.10 reported high relative risks for all brain tumors at doses exceeding 30 Gy, and the risk for meningioma was on the order of 50–100.10 Moreover, it has been shown that low-dose irradiation from either treatment4 or atomic explosions24,25 is a significant risk factor for low-grade brain tumors. Shorter latency periods and more malignant phenotypes have been reported to follow from high total doses of therapy,3,22,26 while aggressive and malignant meningiomas were uncommon in atomic bomb survivors.27 It is believed that ionizing irradiation, directly proportional to the irradiation dose, causes mutations in the genome either directly or indirectly by forming free radicals. The higher doses therefore elicit a more rapid loss of cellular control mechanisms and a failure of the DNA repair system, leading eventually to tumor formation.28
Although 10 affected patients had undergone therapy with a cobalt unit, it is hardly an independent risk factor. The accumulation of meningiomas in the early era of treatment is probably primarily explained by the time factor or differences in other treatment characteristics rather than the source of irradiation used.
The high sensitivity of arachnoidal tissue to irradiation is especially crucial in the meninges of children, which are more vulnerable to oncogenic stimulation.29 This finding is supported by several previous studies of CNS tumors,2,5,19,21,30–32 as well as of other secondary malignant neoplasms (SMNs).13 It has been reported that radiation at a young age leads to the development of meningioma within a shorter time.22 Only a few studies have found discrepancies with this result. Walter et al.4 did not find any association between age and low-grade brain tumors, and some studies dealing with SMNs in general reported no statistically significant correlations.5,14 In our study, very young age at diagnosis did not differ statistically significantly in meningioma and nonmeningioma groups. This could probably be explained by the small cohort size. Especially when the analysis is performed with inclusion of the nonimaged patients in the nonmeningioma group, young age becomes a statistically significant risk factor.
Female predominance is a generally acknowledged risk factor of spontaneous meningiomas. The female-to-male ratio in radiation-induced meningiomas has remained controversial. In our study, gender did not significantly affect the distribution of meningiomas, which is in agreement with some previous studies.22 Nor has gender proven relevant for other brain tumors4,6 or other SMNs in ALL patients.13 Neglia et al.21 and Robison et al.32 reported an independent association between female sex and SMNs of any type in childhood cancer patients. Löning et al.5 reported a slightly elevated, non-significant risk for brain tumors in male ALL patients.
The history of leukemia relapse did not predict the development of meningioma, which has also been shown by Walter et al.4 for brain tumors. The role of chemotherapeutic agents has not been elucidated in detail. To our knowledge, this is one of the first studies where the exact cumulative doses and the duration of treatment were carefully determined. Here, even high cumulative doses of DNA-damaging alkylating agents or antimetabolites failed to show statistical significance for increased risk for meningioma. The mean dose of cyclophosphamide in meningioma patients was higher, although not significantly, and could prove significant in larger studies. The duration of antimetabolite treatment was not related to tumor development. In recent studies, treatment with growth hormone has been associated with a 2-fold risk of developing meningioma.33 In our study, none of the leukemia patients with meningioma had received growth hormone.
In our series, one patient developed meningiomatosis (seven meningiomas), including one atypical meningioma and a neurinoma. There are literature reports of an association between high doses of irradiation and multiplicity and malignancy of meningiomas,3,22,26 as well as a tendency to recur.22,26
Although there are strongly predisposing factors such as radiation therapy, the significance of individual characteristics and genetic factors should not be ignored. The fact that meningiomas and the other SMNs developed in the same patients suggests a genetic susceptibility of these patients to developing cancer. No genetic testing was performed in this study. However, ongoing research on the role of mutations has failed to show a significant association between mutations and the development of radiation-induced meningiomas. Mutations in the NF2, TP53, and PTEN genes seem to be more frequent in sporadic meningiomas.23 One of our patients developed squamous cell carcinoma of the tongue, which is rare in young people but has been reported in patients treated with chemotherapeutic agents for hematological malignancies.13 The association between squamous cell carcinoma and irradiation has also been discussed, although a genetic etiology cannot be excluded.30
In summary, the meningiomas arose after a very long latency period in a large proportion of our cranially irradiated patients. In this small cohort, the other treatment characteristics were not proven to be significant in statistical analysis. Many current leukemia protocols no longer include cranial irradiation in the primary treatment of acute leukemia in children. This will significantly decrease the number of survivors at risk for radiation-induced meningiomas in the future. Consequently, the importance of this late effect will decrease in the younger cohorts of patients. In any case, the high incidence of these treacherous brain tumors cannot be ignored. Thousands of previously treated young adults might be at constant risk of developing meningioma. In our series, meningiomas found by screening were significantly smaller in size than the symptomatic ones. In most cases, smaller meningiomas are technically easier to operate, which supports systematic evaluation of leukemia survivors by brain MRI. For our patients, we now recommend MRI surveillance in 5-year intervals starting from 10 years after the treatment. However, this is one of the first studies reporting such a high incidence of meningiomas. Our results should stimulate large, multi-institutional studies to address the benefit of systematic screening and the true extent of this problem.
Acknowledgments
This article was supported by grants from the Cancer Society of Northern Finland, the Finnish Cancer Society, and the Nona and Kullervo Väre Foundation, Finland. It was presented at the 24th annual meeting of the Nordic Society for Paediatric Haematology and Oncology, May 6–9, 2006, in Tampere, Finland, and at the 40th Congress of the International Society of Pediatric Oncology, October 2–6, 2008, in Berlin, Germany.
References
- 1.Pui CH. Childhood leukemias. N Engl J Med. 1995;332:1618–1630. doi: 10.1056/NEJM199506153322407. [DOI] [PubMed] [Google Scholar]
- 2.Neglia JP, Meadows AT, Robison LL, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;325:1330–1336. doi: 10.1056/NEJM199111073251902. [DOI] [PubMed] [Google Scholar]
- 3.Musa BS, Pople IK, Cummins BH. Intracranial meningiomas following irradiation: a growing problem? . Br J Neurosurg. 1995;9:629–637. doi: 10.1080/02688699550040918. [DOI] [PubMed] [Google Scholar]
- 4.Walter AW, Hancock ML, Pui CH, et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol. 1998;16:3761–3767. doi: 10.1200/JCO.1998.16.12.3761. [DOI] [PubMed] [Google Scholar]
- 5.Löning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Münster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000;95:2770–2775. [PubMed] [Google Scholar]
- 6.Bhatia S, Sather HN, Pabustan OB, Trigg ME, Gaynon PS, Robison LL. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood. 2002;99:4257–4264. doi: 10.1182/blood.v99.12.4257. [DOI] [PubMed] [Google Scholar]
- 7.Pui CH, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 8.Haddy TB, Mosher RB, Dinndorf PA, Reaman GH. Second neoplasms in survivors of childhood and adolescent cancer are often treatable. J Adolesc Health. 2004;34:324–329. doi: 10.1016/j.jadohealth.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Phillips LE, Frankenfeld CL, Drangsholt M, Koepsell TD, Van BG, Longstreth WT., Jr Intracranial meningioma and ionizing radiation in medical and occupational settings. Neurology. 2005;64:350–352. doi: 10.1212/01.WNL.0000149766.65843.19. [DOI] [PubMed] [Google Scholar]
- 10.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 11.Paakko E, Talvensaari K, Pyhtinen J, Lanning M. Late cranial MRI after cranial irradiation in survivors of childhood cancer. Neuroradiology. 1994;36:652–655. doi: 10.1007/BF00600433. [DOI] [PubMed] [Google Scholar]
- 12.Mann I, Yates PC, Ainslie JP. Unusual case of double primary orbital tumour. Br J Ophthalmol. 1953;37:758–762. doi: 10.1136/bjo.37.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball DV, Gelber RD, Li F, Donnelly MJ, Tarbell NJ, Sallan SE. Second malignancies in patients treated for childhood acute lymphoblastic leukemia. J Clin Oncol. 1998;16:2848–2853. doi: 10.1200/JCO.1998.16.8.2848. [DOI] [PubMed] [Google Scholar]
- 14.Gold DG, Neglia JP, Dusenbery KE. Second neoplasms after megavoltage radiation for pediatric tumors. Cancer. 2003;97:2588–2596. doi: 10.1002/cncr.11356. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson G, Schmiegelow K, Forestier E, et al. Improving outcome through two decades in childhood ALL in the Nordic countries: the impact of high-dose methotrexate in the reduction of CNS irradiation. Nordic Society of Pediatric Haematology and Oncology (NOPHO) Leukemia. 2000;14:2267–2275. doi: 10.1038/sj.leu.2401961. [DOI] [PubMed] [Google Scholar]
- 16.Larjavaara S, Haapasalo H, Sankila R, Helèn P, Auvinen A. Is the incidence of meningeomas underestimated? A regional survey. Br J Cancer. 2008;99:182–184. doi: 10.1038/sj.bjc.6604438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goshen Y, Stark B, Kornreich L, Michowiz S, Feinmesser M, Yaniv I. High incidence of meningioma in cranial irradiated survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:294–297. doi: 10.1002/pbc.21153. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins MM, Draper GJ, Kingston JE. Incidence of second primary tumours among childhood cancer survivors. Br J Cancer. 1987;56:339–347. doi: 10.1038/bjc.1987.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nygaard R, Garwicz S, Haldorsen T, et al. Second malignant neoplasms in patients treated for childhood leukemia. A population-based cohort study from the Nordic countries. The Nordic Society of Pediatric Oncology and Hematology (NOPHO) Acta Paediatr Scand. 1991;80:1220–1228. doi: 10.1111/j.1651-2227.1991.tb11812.x. [DOI] [PubMed] [Google Scholar]
- 20.Relling MV, Rubnitz JE, Rivera GK, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999;354:34–39. doi: 10.1016/S0140-6736(98)11079-6. [DOI] [PubMed] [Google Scholar]
- 21.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 22.Strojan P, Popovic M, Jereb B. Secondary intracranial meningiomas after high-dose cranial irradiation: report of five cases and review of the literature. Int J Radiat Oncol Biol Phys. 2000;48:65–73. doi: 10.1016/s0360-3016(00)00609-x. [DOI] [PubMed] [Google Scholar]
- 23.Joachim T, Ram Z, Rappaport ZH, et al. Comparative analysis of the NF2, TP53, PTEN, KRAS, NRAS and HRAS genes in sporadic and radiation-induced human meningiomas. Int J Cancer. 2001;94:218–221. doi: 10.1002/ijc.1467. [DOI] [PubMed] [Google Scholar]
- 24.Sadamori N, Shibata S, Mine M, et al. Incidence of intracranial meningiomas in Nagasaki atomic-bomb survivors. Int J Cancer. 1996;67:318–322. doi: 10.1002/(SICI)1097-0215(19960729)67:3<318::AID-IJC2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Shintani T, Hayakawa N, Kamada N. High incidence of meningioma in survivors of Hiroshima. Lancet. 1997;349:1369. doi: 10.1016/S0140-6736(05)63205-9. [DOI] [PubMed] [Google Scholar]
- 26.Yousaf I, Byrnes DP, Choudhari KA. Meningiomas induced by high dose cranial irradiation. Br J Neurosurg. 2003;17:219–225. doi: 10.1080/0268869031000153080. [DOI] [PubMed] [Google Scholar]
- 27.Shintani T, Hayakawa N, Hoshi M, et al. High incidence of meningioma among Hiroshima atomic bomb survivors. J Radiat Res (Tokyo) 1999;40:49–57. doi: 10.1269/jrr.40.49. [DOI] [PubMed] [Google Scholar]
- 28.Shenoy SN, Munish KG, Raja A. High dose radiation induced meningioma. Br J Neurosurg. 2004;18:617–621. doi: 10.1080/02688690400022789. [DOI] [PubMed] [Google Scholar]
- 29.Cantini R, Giorgetti W, Valleriani AM, Burchianti M, Amodeo C. Radiation-induced cerebral lesions in childhood. Childs Nerv Syst. 1989;5:135–139. doi: 10.1007/BF00272113. [DOI] [PubMed] [Google Scholar]
- 30.Boice JD. Cancer following medical irradiation. Cancer. 1981;47:1081–1090. doi: 10.1002/1097-0142(19810301)47:5+<1081::aid-cncr2820471305>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Ghim TT, Seo JJ, O’Brien M, Meacham L, Crocker I, Krawiecki N. Childhood intracranial meningiomas after high-dose irradiation. Cancer. 1993;71:4091–4095. doi: 10.1002/1097-0142(19930615)71:12<4091::aid-cncr2820711247>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 32.Robison LL, Green DM, Hudson M, et al. Long-term outcomes of adult survivors of childhood cancer. Cancer. 2005;104:2557–2564. doi: 10.1002/cncr.21249. [DOI] [PubMed] [Google Scholar]
- 33.Rutter MM, Rose SR. Long-term endocrine sequelae of childhood cancer. Curr Opin Pediatr. 2007;19:480–487. doi: 10.1097/MOP.0b013e3282058b56. [DOI] [PubMed] [Google Scholar]