Abstract
In a 2002–2004 prospective cohort study of deliveries of infants at <28 weeks at 14 US centers, the authors sought the antecedents of white matter damage evident in newborn cranial ultrasound scans (ventriculomegaly and an echolucent lesion) and of cerebral palsy diagnoses at age 2 years. Of the 1,455 infants enrolled, those whose mothers received an antenatal steroid tended to have lower risks of ventriculomegaly and an echolucent lesion than their peers (10% vs. 23%, P < 0.001 and 7% vs. 11%, P = 0.06, respectively). Risk of ventriculomegaly was increased for infants delivered because of preterm labor (adjusted odds ratio (OR) = 2.3, 95% confidence interval (CI): 1.1, 4.9), preterm premature rupture of fetal membranes (OR = 3.6, 95% CI: 1.5, 8.7), and cervical insufficiency (OR = 2.8, 95% CI: 1.4, 5.5) when compared with infants delivered because of preeclampsia. Risk of an echolucent lesion was increased for infants delivered because of preterm labor (OR = 2.7, 95% CI: 1.2, 5.7) and intrauterine growth retardation (OR = 3.3, 95% CI: 1.2, 9.4). The doubling of diparesis risk associated with preterm labor and with preterm premature rupture of fetal membranes did not achieve statistical significance, nor did the doubling of quadriparesis risk and the tripling of diparesis risk associated with cervical insufficiency.
Keywords: cerebral palsy; cervix uteri; fetal membranes, premature rupture; infant, premature; leukomalacia, periventricular; obstetric labor, preterm; pre-eclampsia; steroids
Babies born at extremely low gestational ages (<28 weeks) are at increased risk of neonatal white matter damage and later motor, cognitive, and behavioral impairments (1). One explanation for the link between extremely low gestational age and cerebral white matter damage is that the same disorders that lead to preterm birth can also damage the developing brain (2). With this explanation, the pregnancy disorder and its correlates are the potential focus of attention (3–6).
Cranial ultrasound lesions evident when the infant is in the intensive care nursery predict cerebral palsy diagnoses years later (7). However, explanations for the link between white matter damage and cerebral palsy need not invoke any pregnancy or prenatal characteristic. Rather, they view lesions indicative of white matter damage as the earliest expression of the very disorder that will become clinically evident years later as movement dysfunctions (8, 9). Ventriculomegaly and an echolucent lesion are considered indicators for different aspects of white matter damage, with ventriculomegaly a marker of diffuse and echolucent lesion a marker of focal white matter damage (10).
Our large sample of infants born before the 28th week enabled us to assess how well pregnancy disorders and their clinical correlates predict early postnatal cerebral white matter damage and the later cerebral palsy diagnoses of quadriparesis, diparesis, and hemiparesis. Because these 3 cerebral palsy diagnoses differ in their correlates (11, 12), we sought the antecedents of each cerebral palsy diagnosis separately.
MATERIALS AND METHODS
The ELGAN study
The Extremely Low Gestational Age Newborns (ELGAN) study was designed to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in extremely low gestational age newborns. During 2002–2004, women delivering before 28 weeks of gestation at one of 14 participating institutions in 11 cities in 5 US states were asked to enroll in the study. At each site, the enrollment and consent processes were approved by the local institutional review board. Participating centers and ELGAN study investigators are listed in the Appendix.
Mothers were approached for consent either upon antenatal admission or shortly after delivery, depending on the clinical circumstance and institutional preference. A total of 1,249 mothers of 1,506 infants consented. Infants with birth defects and/or aneuploidy were excluded. Approximately 260 women were either missed or did not consent to participate.
Protocol scans
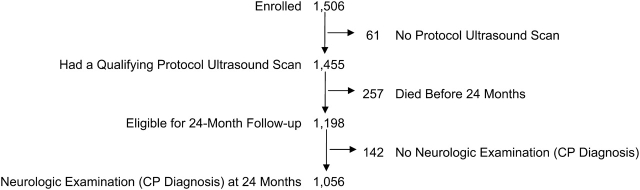
Technicians at all of the hospitals performed routine scans using high-frequency transducers (7.5 MHz and 10 MHz). Ultrasound studies always included the 6 standard quasicoronal views and 5 sagittal views, using the anterior fontanel as the sonographic window (13). The first of 3 sets of protocol scans was obtained between the first and fourth days (n = 1,123). The second protocol scans were obtained between the fifth and 14th days (n = 1,302), and the third protocol scans were obtained between the 15th day and the 40th week (n = 1,268). Of the 1,506 infants enrolled, 61 (4%) failed to have at least one of the protocol scans. We therefore had a final sample of 1,455 infants for this analysis (Figure 1).
Figure 1.
Description of the sample of children analyzed regarding maternal antenatal complications and risk of neonatal cerebral white matter damage and later cerebral palsy (CP), United States, 2002–2004.
Reading procedures
All ultrasound scans were read independently by 2 readers who were unaware of clinical information. When the 2 readers differed in their recognition of a lesion, the films were sent to a third (tie-breaking) reader who was unaware of the opinions of the first 2 readers. Details about the procedure and efforts to minimize observer variability are presented elsewhere (14).
Demographic and pregnancy variables
After delivery, a trained research nurse interviewed each mother in her native language using a structured questionnaire and following protocols detailed in the study manual. The mother's report of her own characteristics and exposures, as well as the sequence of events leading to preterm delivery, were preferentially weighted over those in her medical record. The only exception occurred when the mother's recollection of her last menstrual period was uncertain (discussed below). The research nurse reviewed the mother's chart using a second structured data collection form. We relied on the medical record for events following admission.
The clinical circumstances that led to each maternal admission and ultimately to each preterm delivery were operationally defined by using both data from the maternal interview and data abstracted from the medical record (15). Preterm labor was defined as progressive cervical dilation with regular contractions and intact membranes. Preterm premature rupture of fetal membranes (pPROM) was defined as the presence of vaginal pooling with either documented nitrazine-positive testing or ferning prior to regular uterine activity. Preeclampsia was defined as new-onset hypertension and proteinuria of sufficient severity to warrant delivery for either a maternal or fetal indication. For a diagnosis of cervical insufficiency, a woman had to present with cervical dilation of more than 2 cm in the absence of membrane rupture and detected or perceived uterine activity. Placental abruption was defined as presentation with a significant amount of vaginal bleeding (documented either in the medical record or by a postpartum hematocrit of <24%) and a clinical diagnosis of placental abruption in the absence of cervical change. Presentations included under the category of fetal indication/intrauterine growth retardation included nonreassuring fetal testing, oligohydramnious, Doppler abnormalities of umbilical cord blood flow, or severe intrauterine growth retardation based on antepartum ultrasound examination. For the purposes of this study, the initial complication that caused presentation to medical attention was the focus of analysis.
The gestational age estimates were based on a hierarchy of the quality of information available. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or ultrasound before the 14th week of gestation (62%). When these measures were not available, we relied sequentially on an ultrasound at 14 weeks or more (29%), last menstrual period without ultrasound confirmation (7%), and gestational age recorded in the log of the neonatal intensive care unit (1%). The birth weight z score is the number of standard deviations the infant's birth weight is above or below the median weight of infants at the same gestational age in a standard data set. We chose the standard offered by Yudkin et al. (16).
24-Month neurologic examination
Families were invited to bring their child for a developmental assessment close to the time when he or she would be 24-months corrected gestational age. A total of 1,056 (88% of the eligible 1,198) children had a neurologic examination then (Figure 1). Of these children, 77% were assessed within the range of 23.5–27.9 months.
The neurologic evaluation was performed by examiners using a structured data collection form. Procedures to standardize the neurologic examination and minimize examiner variability are presented elsewhere (17). The topographic diagnosis of cerebral palsy (quadriparesis, diparesis, or hemiparesis) was based on an algorithm that used these data (12).
Data analysis
The newborn, rather than the mother, was the focus of these analyses. Women who delivered multiple gestations are therefore represented multiple times. We included multiple gestations with singletons after we documented a lack of cerebral palsy concordance among twins and triplets. Multifetal gestations did not have a higher risk of any cerebral palsy diagnosis. In addition, only 3 of 112 complete sets of twins were concordant for having a cerebral palsy diagnosis, whereas 2 would be expected by chance.
For descriptive Tables 1 and 2, we used the standard epidemiologic approach in cohort analysis. We compared the frequency of each ultrasound lesion and each cerebral palsy diagnosis among infants in strata defined by the characteristics and exposures of their mother, pregnancy, and delivery.
Table 1.
Risk of Cranial Ultrasound Lesions and Cerebral Palsy Diagnoses in Relation to Delivery Characteristics (Row Percentages), United States, 2002−2004
| Delivery Characteristic | Ultrasound Lesion |
Cerebral Palsy Diagnosis |
|||||
| Ventriculomegaly | Echolucent Lesion | No. | Quadriparesis | Diparesis | Hemiparesis | No. | |
| Maternal antibiotics | |||||||
| Yes | 11 | 7 | 426 | 9 | 4 | 1 | 313 |
| No | 12 | 8 | 950 | 5 | 3 | 2 | 712 |
| Antenatal steroid | |||||||
| Complete | 10 | 8 | 906 | 6 | 4 | 2 | 681 |
| Partial | 12 | 6 | 385 | 4 | 3 | 3 | 260 |
| Any | 10* | 7 | 1,296 | 6 | 3 | 2 | 943 |
| None | 23 | 11 | 159 | 8 | 4 | 0 | 113 |
| Pregnancy complication | |||||||
| Preterm labor | 14** | 10** | 642 | 6 | 4 | 3 | 474 |
| pPROM | 11 | 7 | 307 | 6 | 4 | 2 | 231 |
| Preeclampsia | 5 | 4 | 197 | 5 | 1 | 1 | 139 |
| Abruption | 9 | 2 | 152 | 3 | 3 | 2 | 114 |
| Cervical insufficiency | 16 | 9 | 87 | 15 | 5 | 0 | 55 |
| Fetal indication | 10 | 11 | 70 | 9 | 2 | 0 | 43 |
| Duration of labor, hours | |||||||
| 0 | 7** | 4** | 375 | 4 | 2 | 0.4** | 256 |
| >0 to ≤12 | 14 | 10 | 334 | 5 | 2 | 1 | 233 |
| >12 | 13 | 8 | 746 | 7 | 5 | 3 | 567 |
| Duration of membrane rupture, hours | |||||||
| <1 | 12 | 8 | 863 | 6 | 3 | 1 | 616 |
| 1 to 24 | 13 | 10 | 240 | 9 | 4 | 4 | 170 |
| >24 to 72 | 14 | 7 | 104 | 4 | 3 | 5 | 78 |
| >72 | 10 | 6 | 248 | 6 | 4 | 1 | 192 |
| Magnesium | |||||||
| Tocolysis | 12*** | 8 | 781 | 5 | 3 | 2 | 571 |
| Seizure prophylaxis | 5 | 4 | 182 | 5 | 3 | 1 | 135 |
| None | 14 | 9 | 480 | 8 | 5 | 1 | 341 |
| Vaginal delivery | |||||||
| Yes | 15** | 11* | 511 | 7 | 6 | 3** | 354 |
| No | 10 | 6 | 944 | 6 | 2 | 1 | 702 |
| Type of gestation | |||||||
| Singleton | 12 | 8 | 987 | 6 | 4 | 2 | 700 |
| Multiple | 12 | 7 | 468 | 7 | 3 | 2 | 356 |
| Row percentage | 12 | 8 | 6 | 4 | 2 | ||
| Maximum no. of infants | 172 | 113 | 1,455 | 64 | 37 | 19 | 1,056 |
Abbreviation: pPROM, preterm premature rupture of fetal membranes.
* P ≤ 0.001; **P ≤ 0.01; ***P ≤ 0.05; all values were obtained by Pearson's χ2 or Fisher's exact test.
Table 2.
Risk of Cranial Ultrasound Lesions and Cerebral Palsy Diagnoses in Relation to Neonatal and Gestational Age Characteristics (Row Percentages), United States, 2002−2004
| Infant Characteristic | Ultrasound Lesion |
Cerebral Palsy Diagnosis |
|||||
| Ventriculomegaly | Echolucent Lesion | No. | Quadriparesis | Diparesis | Hemiparesis | No. | |
| Sex | |||||||
| Female | 10*** | 7 | 681 | 5 | 3 | 1*** | 505 |
| Male | 14 | 9 | 774 | 7 | 4 | 3 | 551 |
| Gestational age, weeks | |||||||
| 23 | 21* | 11 | 109 | 15 | 8 | 4* | 48 |
| 24 | 17 | 10 | 268 | 12 | 8 | 2 | 164 |
| 25 | 12 | 8 | 296 | 6 | 2 | 2 | 214 |
| 26 | 12 | 8 | 349 | 4 | 2 | 2 | 273 |
| 27 | 6 | 5 | 433 | 3 | 3 | 1 | 357 |
| Birth weight, g | |||||||
| ≤750 | 14 | 8 | 621 | 9 | 5 | 3 | 385 |
| 751 to 1,000 | 10 | 7 | 579 | 4 | 2 | 2 | 467 |
| ≥1,001 | 11 | 7 | 232 | 5 | 4 | 1 | 184 |
| >1,250 | 13 | 17 | 23 | 15 | 0 | 0 | 20 |
| Birth weight z scorea | |||||||
| <−2 | 8 | 4 | 104 | 2 | 0 | 5** | 56 |
| <−1 | 10 | 5 | 206 | 6 | 3 | 1 | 142 |
| −1 to 1 | 12 | 8 | 992 | 6 | 4 | 2 | 736 |
| >1 | 13 | 10 | 131 | 7 | 5 | 1 | 102 |
| >2 | 18 | 18 | 22 | 20 | 0 | 0 | 20 |
| Birth head circumference z scorea | |||||||
| <−2 | 8 | 4 | 134 | 5 | 0 | 4 | 82 |
| <−1 | 10 | 7 | 308 | 6 | 5 | 1 | 235 |
| −1 to 1 | 13 | 8 | 757 | 6 | 4 | 2 | 550 |
| >1 | 14 | 10 | 162 | 9 | 3 | 2 | 118 |
| >2 | 16 | 9 | 43 | 11 | 0 | 3 | 35 |
| Row percentage | 12 | 8 | 6 | 4 | 2 | ||
| Maximum no. of infants | 172 | 113 | 1,455 | 64 | 37 | 19 | 1,056 |
* P ≤ 0.001; **P ≤ 0.01; ***P ≤ 0.05; all values were obtained by Pearson's χ2 or Fisher's exact test.
Yudkin et al. (16) standard.
Ultrasound characteristics of the brain tended to occur together in our sample. Therefore, we compared infants who had each lesion with the 975 infants whose scans showed no abnormality.
The generalized form of the null hypothesis is that the occurrence of each brain ultrasound image and cerebral palsy diagnosis does not vary with each maternal/pregnancy/delivery characteristic or exposure.
The multivariable analyses focused on the contribution of the pregnancy disorder that preceded preterm delivery. We adjusted for gestational age and receipt of any antenatal steroids. In all analyses, the referent group consisted of infants delivered because of preeclampsia, the subgroup with the lowest prevalence of ventriculomegaly and echolucent lesion in our cohort. Because differences in population characteristics and obstetric management are likely to exist between institutions within our network, we accounted for potential nonindependence of babies within different institutions by using conditional logistic regression that specifies a term for each delivering center.
In early sets of analyses, we adjusted for gestational age in 2 ways, by both week of gestation (23, 24, 25, 26, 27) and groups of weeks (23–24, 25–26, 27). Because the results were almost identical, we present only those with the groups-of-weeks adjustment in this paper.
RESULTS
Pregnancy and antenatal management characteristics
Delivery and antenatal management characteristics are given in Table 1. The risk of white matter damage or a cerebral palsy diagnosis did not differ with the administration of maternal antenatal antibiotics. Infants whose mothers did not receive an antenatal corticosteroid had an increased rate of cerebral white matter damage compared with those exposed to an antenatal steroid. Incomplete and complete courses appeared to be equally protective against cranial ultrasound lesions, although they seemed to have a minimal effect on the occurrence of a cerebral palsy diagnosis.
The risk of ventriculomegaly was lowest for neonates delivered of pregnancies complicated by preeclampsia, whereas the risk of echolucent lesion was lowest in pregnancies complicated by both preeclampsia and placental abruption. Cranial ultrasound lesions were most prevalent among infants delivered of pregnancies complicated by preterm labor or cervical insufficiency. The risk of a cerebral palsy diagnosis was highest in pregnancies complicated by cervical insufficiency.
Compared with babies not exposed to labor, those who experienced labor were at increased risk of both ventriculomegaly and echolucent lesion, as well as all 3 cerebral palsy diagnoses. Vaginal delivery was associated with a significantly increased frequency of a cranial ultrasound lesion and an increased frequency of 2 of the 3 forms of cerebral palsy (diparesis and hemiparesis). Multiple gestations were not at increased risk of either ultrasound lesion or any cerebral palsy diagnosis. A total of 248 infants in the ultrasound sample and 192 in the cerebral palsy sample were delivered more than 72 hours after membrane rupture. They were not at a higher risk of a cranial ultrasound lesion or a cerebral palsy diagnosis than infants born after a shorter latency interval between membrane rupture and delivery.
Magnesium sulfate was administered as a seizure prophylactic or a tocolytic to a subset of this population. Infants exposed for the indication of maternal tocolysis were not at reduced risk of a cranial ultrasound lesion and were at a minimally reduced risk of quadriparesis and diparesis. Among those exposed to magnesium for seizure prophylaxis, however, the risk of ventriculomegaly was reduced, but the risks of a cerebral palsy diagnosis were not.
Neonatal and gestational age characteristics
Neonatal and gestational age characteristics are shown in Table 2. Compared with males, female infants had a lower risk of both ventriculomegaly and hemiparesis. As gestational age advanced, the risk of both ultrasound lesions and a later cerebral palsy diagnosis declined, especially the risks of both ventriculomegaly and hemiparesis. Unlike the trends seen with gestational age, no trends were found with birth weights or birth weight z scores.
Multivariable analyses
We created conditional logistic regression models that compared each of the pregnancy complications with preeclampsia and adjusted for gestational age and receipt of an antenatal steroid. These models also enabled us to account for the potential nonindependence of babies within each hospital (Table 3). Preterm labor, pPROM, and cervical insufficiency were associated with a more than doubling of the risk of ventriculomegaly, whereas delivery because of preterm labor was associated with a more than doubling of the risk of echolucent lesion.
Table 3.
Risk Ratios (Point Estimates and 95% Confidence Intervals) for Each Cranial Ultrasound Lesion Associated With 6 Pregnancy Disorders, United States, 2002−2004a
| Pregnancy Complication | Ultrasound Lesion |
|||||
| Ventriculomegaly |
Echolucent Lesion |
Either |
||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Preterm labor | 2.6 | 1.3, 5.1 | 2.5 | 1.2, 5.4 | 2.5 | 1.4, 4.5 |
| pPROM | 2.2 | 1.04, 4.5 | 1.6 | 0.7, 3.7 | 2.2 | 1.2, 4.0 |
| Abruption | 1.4 | 0.6, 3.3 | 0.4 | 0.1, 1.6 | 1.0 | 0.5, 2.3 |
| Cervical insufficiency | 3.0 | 1.2, 7.2 | 2.3 | 0.8, 6.6 | 2.5 | 1.1, 5.4 |
| Fetal indication | 1.8 | 0.7, 5.1 | 2.7 | 0.9, 7.7 | 2.6 | 1.1, 5.8 |
| Preeclampsia | 1.0 | 1.0 | 1.0 | |||
Abbreviations: CI, confidence interval; pPROM, preterm premature rupture of membranes; RR, risk ratio.
Conditional logistic regression was used to account for potential nonindependence of babies born in the same hospital while allowing adjustment for gestational age and for receipt of any antenatal steroid.
Children born following preterm labor and pPROM were at twice the risk of diplegia as infants delivered because of a maternal indication (Table 4). Cervical insufficiency was associated with a doubling of quadriplegia risk and a tripling of diplegia risk. However, none of the pregnancy disorders was a statistically significant predictor of quadriparesis, diparesis, or hemiparesis. Results of a multinomial model of cerebral palsy diagnosis were similar to those obtained with 3 individual conditional logistic regression models, each comparing children who had a specific cerebral palsy diagnosis with children not given any cerebral palsy diagnosis (data not shown).
Table 4.
Risk Ratios (Point Estimates and 95% Confidence Intervals) for Each Cerebral Palsy Diagnosis Associated With 6 Pregnancy Disorders, United States, 2002−2004
| Pregnancy Complication | Cerebral Palsy Diagnosis |
|||||||
| Quadriparesis |
Diparesis |
Hemiparesis |
Any Cerebral Palsy |
|||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
| Preterm labor | 1.0 | 0.4, 4.4 | 2.1 | 0.5, 9.4 | 3.2 | 0.4, 25 | 1.4 | 0.7, 2.9 |
| pPROM | 0.9 | 0.4, 2.4 | 2.2 | 0.5, 11 | 2.2 | 0.2, 20 | 1.3 | 0.6, 2.9 |
| Abruption | 0.3 | 0.1, 1.4 | 1.2 | 0.2, 8.0 | 2.2 | 0.2, 26 | 0.7 | 0.2, 1.8 |
| Cervical insufficiency | 2.4 | 0.8, 7.4 | 3.4 | 0.5, 23 | —b | 2.4 | 0.9, 6.4 | |
| Fetal indication | 2.0 | 0.5, 7.4 | 2.1 | 0.2, 24 | —b | 1.8 | 0.6, 5.9 | |
| Preeclampsia | 1.0 | 1.0 | 1.0 | 1.0 | ||||
Abbreviations: CI, confidence interval; pPROM, preterm premature rupture of membranes; RR, risk ratio.
a Conditional logistic regression was used to account for potential nonindependence of babies born in the same hospital while allowing adjustment for gestational age and for receipt of any antenatal steroid.
No child with hemiparesis was delivered because of cervical insufficiency or a fetal indication, so there were zero cells.
DISCUSSION
Overview
To our knowledge, this study is the largest to date of the relation between antenatal characteristics and the risks of cerebral white matter damage and later diagnosis of cerebral palsy among children born before the 28th week of gestation. Our major findings are discussed below.
The risk of ventriculomegaly was elevated for infants whose mothers presented with preterm labor, prelabor, premature rupture of membranes, or cervical insufficiency, whereas the risk of an echolucent lesion was elevated for infants born to women who presented with preterm labor. The lowest gestational age infants had the highest risk of ultrasound-defined cerebral white matter damage and of all 3 cerebral palsy diagnoses. A partial course of antenatal corticosteroids was as protective as a full course against white matter damage. Compared with singletons, twins and triplets were not at higher risk of white matter damage. The lowest risk of a cerebral palsy diagnosis was found for children born to women who presented with preeclampsia. Antenatal corticosteroids did not reduce the risk of a cerebral palsy diagnosis. The higher risks of a cerebral palsy diagnosis associated with other disorders leading to delivery before the 28th week of gestation did not reach statistical significance.
Relation between white matter damage and cerebral palsy diagnoses
In our sample, ultrasound lesions indicative of white matter damage predict cerebral palsy diagnoses (7). We chose to study ultrasound-defined brain lesions because they are the indicator of brain damage outcomes more “proximal” to antenatal events than the more “distal” diagnoses of cerebral palsy, which might be influenced by occurrences, exposures, and interventions in the neonatal intensive care unit and in early childhood. Thus, associations between antenatal characteristics and cerebral neonatal cranial ultrasound lesions can be expected to be stronger than associations between antenatal characteristics and a later cerebral palsy diagnosis, which was in fact what we observed.
We expected to find that the pregnancy and delivery antecedents of cerebral white matter damage were the antecedents of cerebral palsy diagnoses. We did see some similarities, but the power of this study precluded our identifying with confidence associations between pregnancy and delivery characteristics and the risk of cerebral palsy diagnoses.
Disorders that lead to preterm delivery
Infants delivered preterm because of the indication of preeclampsia have been reported to be at reduced risk of white matter damage (18, 19) and cerebral palsy (20–22). We, too, observed that infants born to preeclamptic women were at reduced risk of a cranial ultrasound abnormality and of a cerebral palsy diagnosis. One explanation for this apparent protection is that preeclampsia tends not to be characterized by intrauterine inflammation (15). Fetal indication/intrauterine growth retardation also tends not to be associated with intrauterine inflammation (15), but infants delivered because of these indications are not at such a reduced risk of white matter damage. We are not sure why this should be, but the possibility exists that preeclampsia is characterized by production of maturation-promoting or neuroprotective substances (23) but that intrauterine growth retardation unaccompanied by preeclampsia is not.
Preterm labor, frequently accompanied by indicators and correlates of inflammation (24–26), is often, but not invariably (27), associated with an increased risk of neonatal cranial ultrasound lesions. Consistent with this observation, we found that infants born to women who presented with preterm labor were at increased risk of ventriculomegaly and echolucent lesion and of a statistically insignificant doubling of the risk of diparetic cerebral palsy and a tripling of the risk of hemiparetic cerebral palsy.
Consistent with another report (28), we also found that infants whose mothers had cervical insufficiency were at increased risk of ventriculomegaly and echolucent lesion. We also observed an apparent, but statistically insignificant doubling of quadriparetic cerebral palsy risk and a tripling of diplegic cerebral palsy risk. Observations that intrauterine infection can accompany cervical insufficiency (29, 30) lead to the possibility that the cervical insufficiency associations with brain damage reflects the influences of circulating products of inflammation (31).
Corticosteroids
The utility of antenatal corticosteroids in reducing cranial ultrasound lesions has been well documented (32) and incorporated into contemporary perinatal practice (33). Clinical practice has been to allow 48 hours to elapse between the initial dose and the assumption of maximal fetal maturation. However, this interval has been based on observations of pulmonary and not central nervous system maturation. Our observations suggest that the brain protection benefit is achieved before 48 hours.
Unlike recent investigators (22), we did not observe antenatal corticosteroid-associated reductions in the risk of the cerebral palsy diagnoses. Our results, however, are similar to those of another group of investigators, who noted a reduced frequency of white matter disease after antenatal steroid exposure but did not observe a reduction in the occurrence of cerebral palsy (34).
Magnesium
Infants exposed in utero to magnesium prescribed for maternal seizure prophylaxis were at reduced risk of white matter damage, whereas infants exposed to magnesium given as a tocolytic were not. These findings support the impression that magnesium does not reduce the risk of any form of sonographically defined brain damage (35). These observations also suggest that the indication (preeclampsia) and not the therapy/prophylaxis (magnesium) is most closely associated with reduced risk of cerebral white matter damage.
Randomized clinical trials of magnesium given to women in labor have shown a reduced occurrence of cerebral palsy in the offspring (36, 37). We found that maternal receipt of magnesium for tocolysis was associated with a small reduction in the risk of quadriparetic and diparetic cerebral palsy. Because ours was an observational study, which might suffer from confounding by indication, we are reluctant to offer our findings as support equivalent to that found with randomized clinical trials.
Duration of membrane rupture
Clinically apparent intrauterine inflammation appears to increase the risk of brain damage and neurodevelopmental disability (38–40). Since pPROM has been associated with inflammatory phenomena (24), one would expect, as some have observed (22, 41), that infants born after pPROM are at increased risk of white matter damage and a later cerebral palsy diagnosis. We found a statistically significantly increased risk of ventriculomegaly but not of echolucent lesion. We also found nonsignificantly increased risks of diparesis and hemiparesis.
Some studies support the hypothesis that the longer the interval between membrane rupture and delivery, the greater the likelihood of intrauterine infection/inflammation and clinical chorioamnionitis (42, 43); another study does not (44). We confirmed previous reports (45–48) that the risk of cranial ultrasound lesions is unrelated to the latency interval between membrane rupture and delivery. Additionally, prolonged latency was not associated with an increased risk of any cerebral palsy diagnosis.
Our results are consistent with the possibility that patients with pPROM can be managed to maximize latency without increasing the potential for white matter damage or cerebral palsy. Nevertheless, we urge caution in acting on our findings. Infants of pregnancies most susceptible to clinical chorioamnionitis following membrane rupture are potentially removed very early in the latency interval from the pool of infants at risk of brain damage following prolonged membrane rupture. Thus, our inference of no brain adversities following prolonged membrane rupture might reflect informative censoring.
Methodological issues
Although some might view cerebral palsy as a homogeneous entity, we do not (11, 12, 49). In this sample, each of the clinically defined cerebral palsy diagnoses (quadriparesis, diparesis, and hemiparesis) differs considerably from the others in severity of impairment, frequency of comorbid conditions, and risk profile. Thus, although our combining all of the cerebral palsy diagnoses under one umbrella to achieve an outcome frequency of 12% is attractive for achieving power, it is not attractive for achieving homogeneity. Nevertheless, for those who prefer to view cerebral palsy as a single entity, we have included a column in Table 4 for “any cerebral palsy.”
Twins born at term appear to be at increased risk of cerebral palsy (50). In our study, however, compared with their singleton peers, twins and triplets did not have a higher risk of a cerebral palsy diagnosis, and we found little evidence of cerebral palsy concordance among the multiple gestations. Consequently, nonindependence among the multiples is unlikely to have altered our findings.
Our study has several major strengths, including a large number of infants delivered at extremely low gestational ages. These infants are at the highest risk of cranial ultrasound abnormalities and cerebral palsy. Prospective in design, our study enrolled mothers and infants before ascertainment of any outcome, and we collected high-quality data in a uniform manner. We standardized interviews of the mothers, minimized observer variability in ultrasound scan interpretation (14) as well as in assessment of the neurologic examinations (17), and standardized cerebral palsy diagnoses with a diagnostic and classification algorithm (12).
Perhaps the main limitation of this study is its low power for cerebral palsy. We designed our study to have power to discern a doubling of the risk of an echolucency from 8% to 16% associated with an exposure that occurred in a quarter of the sample. Each of the later cerebral palsy diagnoses, however, occurred less frequently than 8% of the time (e.g., quadriparesis: 6%; diparesis: 4%; hemiparesis: 2%). Also contributing to the low power was the low frequency of some of the more important exposures. For example, cervical insufficiency, which was associated with a doubling of quadriparetic cerebral palsy risk and a tripling of diparetic cerebral palsy risk, occurred in less than 5% (n = 47) of mothers. Consequently, our study was underpowered to identify with statistical significance any of the associations between a pregnancy disorder and a cerebral palsy diagnosis. Additionally, because unmeasured variables might have contributed to our findings, we recommend caution when interpreting these findings.
In summary, in this sample of infants born more than 12 weeks before term, some pregnancy disorders were associated with increased risks of ultrasound-defined cerebral white matter damage. Most of these disorders are themselves associated with inflammation, raising the possibility that their association with brain damage reflects inflammatory influences. The increased risks of cerebral palsy diagnoses for children born to women who presented with these inflammation-associated disorders did not achieve statistical significance. The very low risks of cerebral white matter damage and cerebral palsy diagnoses for the offspring of women who presented with preeclampsia were lower than those for children delivered because of fetal indications. This finding raises the possibility that preeclampsia is potentially brain protective.
Acknowledgments
Author affiliations: Division of Maternal-Fetal Medicine, Brigham and Women's Hospital, Boston, Massachusetts (Thomas F. McElrath); Neuroepidemiology Unit, Children's Hospital of Boston, Boston, Massachusetts (Elizabeth N. Allred, Alan Leviton); Department of Obstetrics/Gynecology, University of North Carolina, Chapel Hill, North Carolina (Kim A. Boggess); Division of Pediatric Neurology, Boston Medical Center, Boston, Massachusetts (Karl Kuban); Division of Neonatology, Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina (T. Michael O'Shea); and Department of Epidemiology, Michigan State University, East Lansing, Michigan (Nigel Paneth).
The ELGAN project was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (U01 NS 400069-01).
Dr. McElrath was supported by the Women's Reproductive Health Research program, National Institute of Child Health and Development (K12 HDO1255).
Conflict of interest: none declared.
Glossary
Abbreviations
- ELGAN
Extremely Low Gestational Age Newborns
- pPROM
preterm premature rupture of fetal membranes
APPENDIX
Participating Centers (Site Principal Investigator, Pathologist, Perinatologist)
Baystate Medical Center, Springfield, Massachusetts (Bhavesh Shah, Solveg Pflueger, Glenn Markenson)
Beth Israel Deaconess Medical Center, Boston, Massachusetts (Camilia R. Martin, Jonathon L. Hecht, Bruce Cohen)
Brigham and Women's Hospital, Boston, Massachusetts (Linda J. Van Marter, Harvey Kliman,* Thomas F. McElrath)
Children's Hospital, Boston, Massachusetts (Alan Leviton, Site Principal Investigator)
Massachusetts General Hospital, Boston, Massachusetts (Robert Insoft, Drucilla Roberts, Laura Riley)
Tufts New England Medical Center, Boston, Massachusetts (Cynthia Cole/John Fiascone, Ina Bhan, Sabrina Craigo/Theresa Marino)
UMass Memorial Medical Center, Worcester, Massachusetts (Francis Bednarek, Gamze Ayata, Ellen Delpapa)
Yale-New Haven Hospital, New Haven, Connecticut (Richard Ehrenkranz, Miguel Reyes-Múgica/Eduardo Zambrano, Keith P. Williams)
Forsyth Hospital, Baptist Medical Center, Winston-Salem, North Carolina (T. Michael O'Shea, Dennis W. Ross, Maggie Harper)
University Health Systems of Eastern Carolina, Greenville, North Carolina (Stephen Engelke, John Christie, Hamid Hadi)
North Carolina Children's Hospital, Chapel Hill, North Carolina (Carl Bose, Chad Livasy, Kim Boggess)
DeVos Children's Hospital, Grand Rapids, Michigan (Mariel Portenga, Barbara Doss, Curtis Cook)
Sparrow Hospital, Lansing, Michigan (Padmani Karna, Gabriel Chamyan, Steve Roth)
University of Chicago Hospital, Chicago, Illinois (Michael D. Schreiber, Aliya Husain, Mahmoud Ismail)
William Beaumont Hospital, Royal Oak, Michigan (Daniel Batton, Chung-Ho Chang, Robert Lorenz)
* Dr. Kliman, who reviewed the Brigham and Women's Hospital slides, is an Obstetrics Department faculty member at Yale-New Haven Hospital, New Haven, Connecticut.
References
- 1.Behrman RE, Butler AS, editors. Preterm Birth; Causes, Consequences, and Prevention. Washington, DC: The National Academies Press; 2007. [PubMed] [Google Scholar]
- 2.Leviton A. Preterm birth and cerebral palsy: is tumor necrosis factor the missing link? Dev Med Child Neurol. 1993;35(6):549–558. doi: 10.1111/j.1469-8749.1993.tb11688.x. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsson B. Infectious and inflammatory mechanisms in preterm birth and cerebral palsy. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):159–160. doi: 10.1016/j.ejogrb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284(11):1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 5.Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17(7):357–365. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol. 2005;32(3):523–559. doi: 10.1016/j.clp.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuban KC, Allred EN, O'Shea TM, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24(1):63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Bilsen JH, van Dongen H, Lard LR, et al. Functional regulatory immune responses against human cartilage glycoprotein-39 in health vs. proinflammatory responses in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2004;101(49):17180–17185. doi: 10.1073/pnas.0407704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson-Costello D, Borawski E, Friedman H, et al. Perinatal correlates of cerebral palsy and other neurologic impairment among very low birth weight children. Pediatrics. 1998;102(2 pt 1):315–322. doi: 10.1542/peds.102.2.315. [DOI] [PubMed] [Google Scholar]
- 10.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1996;42(1):1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Dammann O, Kuban K. The definition and classification of cerebral palsy. Cerebral palsy—rejected, refined, recovered. Dev Med Child Neurol. 2007;49(s109):17–18. doi: 10.1111/j.1469-8749.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuban KC, Allred EN, O'Shea M, et al. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153(4):466–472. doi: 10.1016/j.jpeds.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teele R, Share J. Ultrasonography of Infants and Children. Philadelphia, PA: WB Saunders; 1991. [Google Scholar]
- 14.Kuban K, Adler I, Allred EN, et al. Observer variability assessing US scans of the preterm brain: the ELGAN study. Pediatr Radiol. 2007;37(12):1201–1208. doi: 10.1007/s00247-007-0605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yudkin PL, Aboualfa M, Eyre JA, et al. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15(1):45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 17.Kuban KC, O'Shea M, Allred E, et al. Video and CD-ROM as a training tool for performing neurologic examinations of 1-year-old children in a multicenter epidemiologic study. J Child Neurol. 2005;20(10):829–831. doi: 10.1177/08830738050200101001. [DOI] [PubMed] [Google Scholar]
- 18.Leviton A, Kuban KC, Pagano M, et al. Maternal toxemia and neonatal germinal matrix hemorrhage in intubated infants less than 1751 g. Obstet Gynecol. 1988;72(4):571–576. [PubMed] [Google Scholar]
- 19.van de Bor M, Verloove-Vanhorick SP, Brand R, et al. Incidence and prediction of periventricular-intraventricular hemorrhage in very preterm infants. J Perinat Med. 1987;15(4):333–339. doi: 10.1515/jpme.1987.15.4.333. [DOI] [PubMed] [Google Scholar]
- 20.Skogstrand K, Thorsen P, Nørgaard-Pedersen B, et al. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51(10):1854–1866. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 21.Nelson KB, Grether KJ. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics. 1995;95(2):263–269. [PubMed] [Google Scholar]
- 22.Jacobsson B, Hagberg G, Hagberg B, et al. Cerebral palsy in preterm infants: a population-based case-control study of antenatal and intrapartal risk factors. Acta Paediatr. 2002;91(8):946–951. doi: 10.1080/080352502760148685. [DOI] [PubMed] [Google Scholar]
- 23.Collins M, Paneth N. Preeclampsia and cerebral palsy: are they related? Dev Med Child Neurol. 1998;40(3):207–211. doi: 10.1111/j.1469-8749.1998.tb15449.x. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Gotsch F, Pineles B, et al. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(12 pt 2):S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 25.Kaukola T, Herva R, Perhomaa M, et al. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res. 2006;59(3):478–483. doi: 10.1203/01.pdr.0000182596.66175.ee. [DOI] [PubMed] [Google Scholar]
- 26.Skrablin S, Lovric H, Banovic V, et al. Maternal plasma interleukin-6, interleukin-1beta and C-reactive protein as indicators of tocolysis failure and neonatal outcome after preterm delivery. J Matern Fetal Neonatal Med. 2007;20(4):335–341. doi: 10.1080/14767050701227877. [DOI] [PubMed] [Google Scholar]
- 27.Richardson BS, Wakim E, daSilva O, et al. Preterm histologic chorioamnionitis: impact on cord gas and pH values and neonatal outcome. Am J Obstet Gynecol. 2006;195(5):1357–1365. doi: 10.1016/j.ajog.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 28.Mitani M, Matsuda Y, Ono E, et al. Prognosis in cervical insufficiency at less than 32 weeks of gestation. Eur J Obstet Gynecol Reprod Biol. 2006;125(1):34–37. doi: 10.1016/j.ejogrb.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Salafia CM, Athanassiadis AP, et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol. 1992;166(5):1382–1388. doi: 10.1016/0002-9378(92)91609-e. [DOI] [PubMed] [Google Scholar]
- 30.Lee SE, Romero R, Park CW, et al. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency [electronic article] Am J Obstet Gynecol. 2008;198(6):633.e1–633.e8. doi: 10.1016/j.ajog.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 31.Dammann O, O'Shea TM. Cytokine and perinatal brain damage. Clin Perinatol. 2008;35(4):643–663. doi: 10.1016/j.clp.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baud O, Foix-L'Helias L, Kaminski M, et al. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341(16):1190–1196. doi: 10.1056/NEJM199910143411604. [DOI] [PubMed] [Google Scholar]
- 33.Committee on Obstetric Practice. ACOG Committee opinion: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2002;99(5 pt 1):871–873. doi: 10.1016/s0029-7844(02)02023-9. [DOI] [PubMed] [Google Scholar]
- 34.Figueras-Aloy J, Serrano MM, Rodríguez JP, et al. Antenatal glucocorticoid treatment decreases mortality and chronic lung disease in survivors among 23- to 28-week gestational age preterm infants. Am J Perinatol. 2005;22(8):441–448. doi: 10.1055/s-2005-916332. [DOI] [PubMed] [Google Scholar]
- 35.Dammann O, Leviton A. The role of perinatal brain damage in developmental disabilities: an epidemiologic perspective. Ment Retard Dev Disabil Res Rev. 1997;3:13–21. [Google Scholar]
- 36.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359(9):895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle LW, Crowther CA, Middleton P, et al. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus [electronic article] Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD004661.pub2. CD004661. [DOI] [PubMed] [Google Scholar]
- 38.Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 2005;18(2):117–123. doi: 10.1097/01.wco.0000162851.44897.8f. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Rousset CI, Hagberg H, et al. Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonatal Med. 2006;11(5):343–353. doi: 10.1016/j.siny.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Hansen-Pupp I, Hallin AL, Hellström-Westas L, et al. Inflammation at birth is associated with subnormal development in very preterm infants. Pediatr Res. 2008;64(2):183–188. doi: 10.1203/PDR.0b013e318176144d. [DOI] [PubMed] [Google Scholar]
- 41.Nelson KB, Ellenberg JH. Predictors of low and very low birth weight and the relation of these to cerebral palsy. JAMA. 1985;254(11):1473–1479. [PubMed] [Google Scholar]
- 42.Ustün C, Kökçü A, Cil E, et al. Relationship between endomyometritis and the duration of premature membrane rupture. J Matern Fetal Med. 1998;7(5):243–246. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<243::AID-MFM7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 43.Dale PO, Tanbo T, Bendvold E, et al. Duration of the latency period in preterm premature rupture of the membranes. Maternal and neonatal consequences of expectant management. Eur J Obstet Gynecol Reprod Biol. 1989;30(3):257–262. doi: 10.1016/0028-2243(89)90010-5. [DOI] [PubMed] [Google Scholar]
- 44.Ghidini A, Salafia CM, Minior VK. Lack of relationship between histologic chorioamnionitis and duration of latency period in preterm rupture of membranes. J Matern Fetal Med. 1998;7(5):238–242. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<238::AID-MFM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 45.McElrath TF, Allred EN, Leviton A. Prolonged latency after preterm premature rupture of membranes: an evaluation of histologic condition and intracranial ultrasonic abnormality in the neonate born at <28 weeks of gestation. Am J Obstet Gynecol. 2003;189(3):794–798. doi: 10.1067/s0002-9378(03)00814-7. [DOI] [PubMed] [Google Scholar]
- 46.Locatelli A, Vergani P, Ghidini A, et al. Duration of labor and risk of cerebral white-matter damage in very preterm infants who are delivered with intrauterine infection. Am J Obstet Gynecol. 2005;193(3 pt 2):928–932. doi: 10.1016/j.ajog.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 47.Park SH, Kim HJ, Yang JH, et al. Neonatal brain damage following prolonged latency after preterm premature rupture of membranes. J Korean Med Sci. 2006;21(3):485–489. doi: 10.3346/jkms.2006.21.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McElrath TF, Norwitz ER, Lieberman ES, et al. Perinatal outcome after preterm premature rupture of membranes with in situ cervical cerclage. Am J Obstet Gynecol. 2002;187(5):1147–1152. doi: 10.1067/mob.2002.127721. [DOI] [PubMed] [Google Scholar]
- 49.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 50.Pharoah PO. Risk of cerebral palsy in multiple pregnancies. Clin Perinatol. 2006;33(2):301–313. doi: 10.1016/j.clp.2006.03.017. [DOI] [PubMed] [Google Scholar]