Abstract
Numerous studies have found an association between shorter sleep duration and higher body mass index (BMI) in adults. Most previous studies have been cross-sectional and relied on self-reported sleep duration, which may not be very accurate. In the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study (2000–2006), the authors examine whether objectively measured sleep is associated with BMI and change in BMI. They use several nights of wrist actigraphy to measure sleep among participants in an ongoing cohort of middle-aged adults. By use of linear regression, the authors examine whether average sleep duration or fragmentation is associated with BMI and 5-year change in BMI, adjusting for confounders. Among 612 participants, sleep duration averaged 6.1 hours and was grouped into 4 categories. Both shorter sleep and greater fragmentation were strongly associated with higher BMI in unadjusted cross-sectional analysis. After adjustment, BMI decreased by 0.78 kg/m2 (95% confidence interval: −1.6, −0.002) for each increasing sleep category. The association was very strong in persons who reported snoring and weak in those who did not. There were no longitudinal associations between sleep measurements and change in BMI. The authors confirmed a cross-sectional association between sleep duration and BMI using objective sleep measures, but they did not find that sleep predicted change in BMI. The mechanism underlying the cross-sectional association is not clear.
Keywords: body mass index, cohort studies, sleep, snoring
Numerous studies have found a cross-sectional association between sleep duration and body mass index (BMI) in adults: Those reporting shorter sleep are more likely to have higher BMI (1–3). The possibility that this is a causal association and that declining sleep hours could be contributing to the obesity epidemic has heightened interest among researchers and the public (4), but there are critical gaps in the epidemiologic evidence (5). Cross-sectional studies present ambiguous evidence concerning the causal direction. In this case, both directions are plausible. Persons who weigh more may sleep less, perhaps because of comorbidities or reduced sleep quality, or persons who sleep less may gain weight, perhaps because of increased hunger or late-night eating. A third possibility is that the association is not causal in either direction but due to as yet unidentified confounding. Some studies find a U-shaped association with increased risk of obesity for both short and long sleepers (6–8).
Several established cohorts have sleep information and have examined this association longitudinally, with mixed findings (6, 9–12). Most previous studies have used a survey question to assess sleep duration, such as “How many hours of sleep do you usually get at night (or when you usually sleep)?” (10). Relying on subjective reports is a well-recognized limitation (3); validation studies of self-reported habitual sleep suggest that self-reports may be inaccurate and biased (13, 14).
In this project, we examine the cross-sectional and longitudinal associations between sleep duration and BMI using an objective measure of sleep—several nights of wrist actigraphy—in a community-based study of adults in early middle age.
MATERIALS AND METHODS
Study overview
This is a 5-year ancillary study nested within a larger ongoing multicenter cohort, the Coronary Artery Risk Development in Young Adults (CARDIA) Study. When the CARDIA Study began in 1985–1986, participants were aged 18–30 years and balanced by sex, race (black and white), and education (15). The sleep ancillary study includes participants from 1 of 4 CARDIA Study sites (Chicago, Illinois). All nonpregnant participants in the year 15 examination in Chicago (in 2000–2001) were invited to take part in the sleep study in 2003–2004; 670 of 814 (82%) consented. Participants and nonparticipants gave similar answers to questions about sleep duration and trouble falling asleep in year 15 (16).
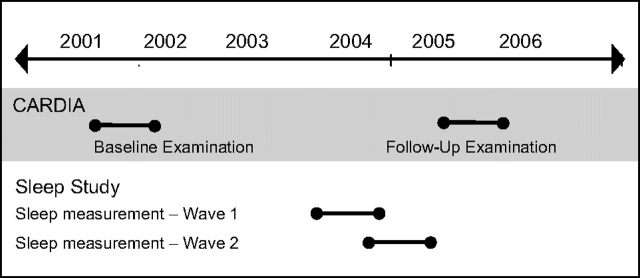
Sleep data collection took place in 2 waves between the year 15 and year 20 regular clinical examinations (Figure 1). We refer to the year 15 and year 20 examinations as the “baseline” and “follow-up” examinations throughout this paper. The follow-up examination began after final sleep data collection in 2005. All participants gave informed written consent; the institutional review boards of Northwestern University and the University of Chicago approved the study.
Figure 1.
Timeline for the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study, 2000–2006.
Body mass index measurements
Weight and height were measured during each clinical examination.
Sleep
Participants were asked to wear a wrist activity monitor (Actiwatch-16; Mini-Mitter, Inc., Bend, Oregon) Wednesday through Saturday in each wave, 6 total nights, as previously described (17). Actigraphs look like wristwatches with blank faces; they contain highly sensitive omnidirectional accelerometers that digitally count wrist movements. For each night, participants recorded when they began trying to sleep using an event marker on the actigraph (which does not affect motion recording) and a sleep log (which asked the exact time that they began trying to fall asleep and when they arose in the morning). The log is a backup when participants forgot to press the event marker. The software examines this specified period for sleep. Each 30-second epoch was scored as sleep based on the activity count during the epoch and 8 adjacent epochs. This activity score is the sum of the epoch's own activity count plus one-fifth of the activity count for the 4 epochs within 1 minute before and after and one-twenty-fifth of the epoch count for the 4 epochs between 1 and 2 minutes before and after. If the total score is below a prespecified threshold, the epoch is scored as sleep. In this study, we used the medium threshold set by the manufacturer (40 counts). Sleep periods are summed for total sleep duration.
Our sleep duration variable is the average from all available nights of actigraphy, 6 for most participants. We previously found high year-to-year consistency in sleep duration for the 2 waves (17), suggesting that our 2 time points characterize a longer window of behavior. To examine whether there was a U-shaped association between sleep duration and BMI, we categorize duration into 4 groups: <4.5 hours, 4.5–<6 hours, 6–<7.5 hours, and ≥7.5 hours. Because there was no evidence of a nonlinear association in cross-sectional analysis, we model sleep as a single ordinal variable. Analyses were repeated with sleep duration as a continuous variable and different cutpoints; all produced similar associations.
Sleep fragmentation is a measure of sleep quality determined from actigraphy. It is the sum of the percentage of time spent sleeping when the subject is moving and the percentage of immobile periods that last a minute or less. Because there are no intuitive units for fragmentation, we divide it by its standard deviation.
Actigraphy does not yield information about sleep-disordered breathing or apnea; apnea is estimated to occur in about 9% of women and 24% of men aged 40–49 years (18). Obesity is a powerful risk factor for apnea, which is usually marked by snoring and daytime sleepiness. We use the Berlin questionnaire, an apnea risk instrument of high sensitivity of 0.86 and moderate specificity of 0.77 (19). The questionnaire defines high apnea risk as the presence of any 2 of 3 components: 1) persistent snoring symptoms, 2) persistent daytime sleepiness, and 3) obesity or hypertension. Because BMI was our outcome, we could not use an apnea indicator that included obesity as part of its definition. Instead we entered the sleepiness and snoring components separately into regression models. Persistent snoring was defined as 2 of the following: snored 3 or more nights per week; snoring was louder than talking or very loud; or breathing pauses 3 or more nights per week. Persistent daytime sleepiness was defined as 2 of the following: tired 3 or more days per week after sleeping; tired during wake time 3 or more days per week; or fallen asleep while driving. Snoring is a stronger correlate of apnea than daytime sleepiness (20).
Covariates
The sociodemographic variables collected during each examination included race (black or white), sex, age, and educational attainment. We combine sex and race to form 4 race-sex groups. Education is a 5-level ordinal variable: less than high school degree, high school degree or equivalent, some college, college graduate, and postgraduate. We include 2 additional variables likely to influence BMI: current smoking and a physical activity score, assessed in each examination using the CARDIA Study Physical Activity History (21).
Statistical analysis
Typically, projects that include cross-sectional and longitudinal analyses use baseline data for the cross-sectional association. However, the primary exposure (sleep) was measured between the baseline and follow-up examinations. Because sleep data were closer temporally to the follow-up, our primary cross-sectional analysis examines the association of sleep with BMI and covariates measured at follow-up. We duplicate the cross-sectional analysis with baseline BMI and covariate data. Similar sleep-BMI associations in these 2 analyses would strongly suggest that cross-sectional results would be similar had sleep data been collected at baseline.
We estimate the unadjusted cross-sectional association between sleep duration and BMI using linear regression. We then add covariates: age, race-sex group, education, smoking, physical activity score, tiredness, and snoring. Using interaction terms, we test whether the sleep-BMI association varies by race-sex group or by snoring. To fully exploit the repeated measures structure of the BMI data, a longitudinal model is then fitted by adding baseline BMI, smoking, and physical activity to the cross-sectional model. (Note that modeling follow-up BMI and including baseline BMI as a covariate are exactly the same as modeling 5-year change in BMI, adjusting for baseline BMI.)
We repeat these models with sleep fragmentation instead of sleep duration. Because there is a strong, highly significant inverse correlation between fragmentation and duration (ρ = −0.43; P < 0.001), we do not include both in the same model.
Robust standard errors are used to calculate confidence intervals and significance tests allowing for heteroskadasticity in BMI. Analyses were carried out using Stata, version 10.0, software (StataCorp LP, College Station, Texas).
RESULTS
Of 670 sleep study participants, 667 had usable actigraphy data. Forty-eight did not participate in the follow-up examination, and 7 reported bariatric surgery, leaving a final sample of 612. There was no difference in the sleep duration distribution across 4 sleep categories between the excluded 55 participants and the 612 included. Thirty-six provided only 1 wave of sleep data.
The average measured sleep duration was 6.1 (standard deviation (SD), 1.05) hours. Table 1 presents descriptive characteristics by sleep duration. Race-sex, education, and smoking all had significant trends across sleep categories. Only 2 white women were in the shortest sleep category, and no black men were in the longest. Shorter sleep was more common in lower educational categories. Shorter sleepers were more likely to report snoring but not more likely to be tired. The average fragmentation score was 19.2 (SD, 8.0), and there was a highly significant inverse trend by sleep duration.
Table 1.
Study Sample Characteristics for Those With Actigraphy, Complete BMI Data, and No History of Bariatric Surgery (n = 612), the CARDIA Sleep Study, 2000–2006
| Total | Average Sleep Duration, hours |
Ptrend | ||||
| <4.5 | 4.5–<6 | 6–<7.5 | ≥7.5 | |||
| No. (%) | ||||||
| 612 | 51 (8.3) | 211 (34.5) | 306 (50.0) | 44 (7.2) | ||
| Mean (SD) | ||||||
| Age, years | 45.2 (3.6) | 44.5 (3.6) | 45.4 (3.7) | 45.2 (3.6) | 45.2 (3.4) | 0.66 |
| Physical activity, exercise units | 334 (264) | 306 (209) | 323 (277) | 350 (258) | 309 (300) | 0.39 |
| Physical activity 5 years prior, exercise units | 365 (296) | 402 (319) | 350 (309) | 375 (285) | 323 (282) | 0.66 |
| Sleep fragmentation, score | 19.2 (8.0) | 28.1 (11.6) | 21.0 (7.6) | 17.0 (6.2) | 15.2 (5.5) | <0.001 |
| Frequencies, % | ||||||
| Race-sex groupsa | <0.001b | |||||
| White women | 29.7 | 1.1 | 17.0 | 65.4 | 16.5 | |
| Black women | 27.8 | 7.7 | 48.2 | 40.0 | 4.1 | |
| White men | 26.8 | 7.3 | 29.9 | 58.5 | 4.3 | |
| Black men | 15.7 | 25.0 | 51.0 | 24.0 | 0 | |
| Educationa | <0.001b | |||||
| Less than high school | 5.1 | 16.1 | 61.3 | 19.4 | 3.2 | |
| High school | 16.5 | 13.9 | 34.7 | 44.6 | 6.9 | |
| Some college | 25.7 | 12.7 | 41.4 | 27.6 | 8.3 | |
| College degree | 24.5 | 4.0 | 28.0 | 60.0 | 8.7 | |
| Postgraduate | 28.3 | 3.5 | 28.9 | 61.9 | 5.8 | |
| Smoking groups | ||||||
| Smoker | 16.3 | 15.0 | 44.0 | 30.0 | 11.0 | <0.001 |
| Smoker 5 years prior | 19.8 | 16.5 | 41.3 | 35.5 | 6.6 | 0.01 |
| Apnea symptoms | ||||||
| Snoring | 12.9 | 13.9 | 31.7 | 48.1 | 6.3 | 0.24 |
| Tiredness | 31.7 | 9.3 | 34.0 | 48.5 | 8.3 | 0.93 |
Abbreviations: BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; SD, standard deviation.
Column 1 displays the frequency distribution across race-sex and education groups, and columns 2–5 display sleep distribution for race-sex or education group.
P values for race-sex groups and education are not trend tests but global tests of association.
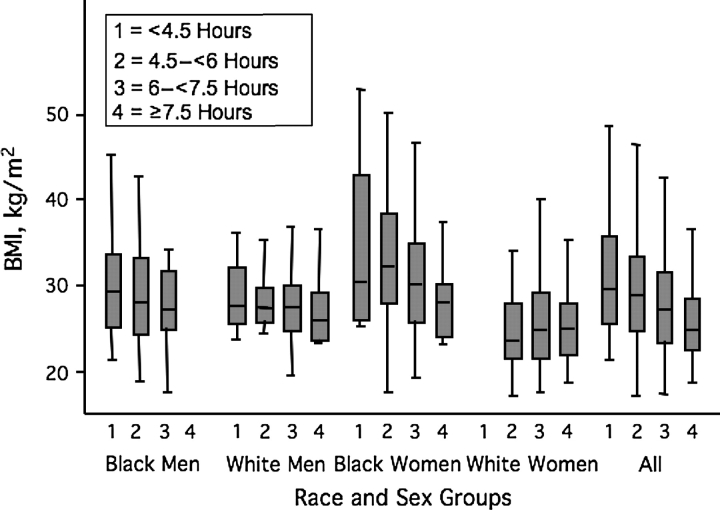
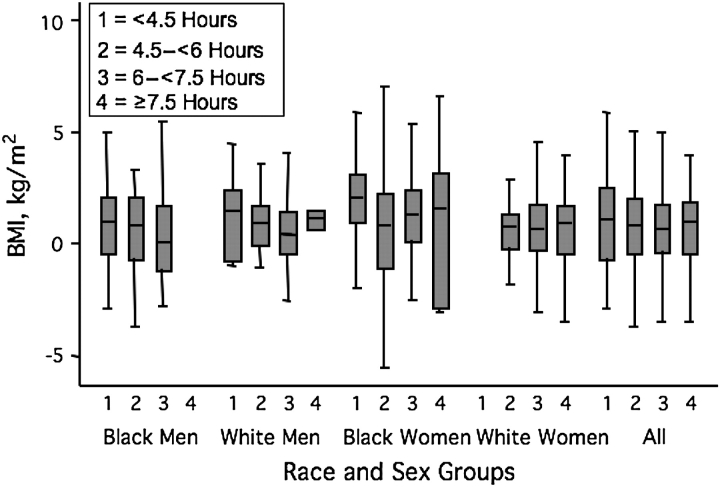
Figure 2 shows the unadjusted cross-sectional association between sleep duration and BMI. There appears to be a monotonic trend for the whole sample, but the trend is not apparent within all the race-sex subgroups. Figure 3 shows the unadjusted association between sleep categories and 5-year change in BMI. The average weight gain was 4.5 (SD, 17.4) kg. Change in BMI does not appear to vary across sleep categories.
Figure 2.
BMI distribution by average actigraph-measured sleep duration, the CARDIA Sleep Study, 2000–2006. The gray boxes show the 25th, 50th, and 75th percentiles, and the tails show the largest and smallest values, excluding outliers. White women in the shortest sleep category (n = 2) and black men in the longest sleep category (n = 0) are not represented. BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults.
Figure 3.
Distribution of 5-year change in BMI by average actigraph-measured sleep duration, the CARDIA Sleep Study, 2000–2006. The gray boxes show the 25th, 50th, and 75th percentiles, and the tails show the largest and smallest values, excluding outliers. White women in the shortest sleep category (n = 2) and black men in the longest sleep category (n = 0) are not represented. BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults.
The unadjusted cross-sectional association between sleep and BMI is highly significant, −1.61 kg/m2 per sleep category, with lower BMI in the longer sleep categories (Table 2). Adjustment for covariates attenuates the effect to −0.78 kg/m2 per sleep category, which is marginally significant. The confounding is primarily due to education and race (data not shown). All of the covariates except age (which varies little) are significantly associated with BMI. Repeating the adjusted cross-sectional model with baseline BMI and covariates yields a similar association: −0.81 kg/m2 per sleep level (95% confidence interval (CI): −1.58, −0.05; P = 0.04) (results not shown).
Table 2.
Cross-sectional Linear Multiple Regression Model of BMI (kg/m2) Regressed on Actigraph Sleep Categories (4-Level Ordinal Variable) and Longitudinal Regression Model of BMI Regressed on Actigraph Sleep Categories and BMI 5 Years Prior, the CARDIA Sleep Study, 2000–2006
| Model 1—Cross-sectional |
Model 2—Longitudinal |
|||||
| Coefficient of BMI (kg/m2) | 95% Confidence Interval | P Value | Coefficient of BMI (kg/m2) | 95% Confidence Interval | P Value | |
| Unadjusted | ||||||
| Sleep (per level) | −1.61 | −2.37, −0.86 | <0.001 | −0.02 | −0.30, 0.25 | 0.86 |
| BMI 5 years prior, kg/m2 | 1.01 | 0.96, 1.05 | <0.001 | |||
| Adjusteda | ||||||
| Sleep (per level) | −0.78 | −1.55, −0.002 | 0.05 | 0.02 | −0.27, 0.30 | 0.91 |
| Age (per year) | 0.01 | −0.14, 0.16 | 0.92 | −0.01 | −0.06, 0.05 | 0.82 |
| Race-sex groups | ||||||
| White women | Referent | Referent | ||||
| White men | 1.80 | 0.70, 2.91 | 0.001 | −0.30 | −0.72, 0.13 | 0.17 |
| Black women | 4.06 | 2.38, 5.74 | <0.001 | −0.21 | −0.78, 0.37 | 0.48 |
| Black men | 1.16 | −0.64, 2.95 | 0.21 | −0.50 | −1.15, 0.16 | 0.14 |
| Education (per level) | −0.97 | −1.51, −0.43 | <0.001 | −0.29 | −0.48, −0.10 | 0.003 |
| Smoking groups | ||||||
| Nonsmoker | Referent | Referent | ||||
| Smoker | −1.64 | −3.11, −0.17 | 0.029 | −0.13 | −0.74, 0.49 | 0.69 |
| Former smokerb | 0.44 | −0.30, 1.17 | 0.24 | |||
| New smokerb | −3.10 | −5.86, −0.33 | 0.03 | |||
| Physical activity, exercise units | ||||||
| Current (per 100 units) | −0.30 | −0.49, −0.11 | 0.002 | −0.05 | −0.14, 0.04 | 0.25 |
| 5 years prior (per 100 units) | 0.03 | −0.05, 0.12 | 0.43 | |||
| Apnea risk | ||||||
| Snoring | 3.70 | 1.93, 5.45 | <0.001 | 0.78 | 0.13, 1.43 | 0.02 |
| Tiredness | 0.70 | −0.47, 1.87 | 0.24 | −0.17 | −0.58, 0.23 | 0.40 |
| BMI 5 years prior, kg/m2 | 1.00 | 0.95, 1.04 | <0.001 | |||
Abbreviations: BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults.
Includes all listed covariates.
Changed smoking status between baseline and follow-up.
The association between sleep duration and BMI does not vary significantly by race-sex groups (F = 1.13 on 3 df; P = 0.34). However, it is significantly different for those who do versus those who do not report snoring (term, Pinteraction = 0.04), with a much stronger association among those who report snoring. In adjusted models stratified by snoring, there is little association between sleep duration and BMI for those who do not report snoring (per sleep level: 0.35 kg/m2, 95% CI: −0.44, 1.15; P = 0.39), but there is a strong association for those who report snoring (per sleep level: 3.66 kg/m2, 95% CI: 1.01, 6.31; P = 0.01).
In the longitudinal model (Table 2), there is no evidence of an association between sleep and change in BMI in either unadjusted or adjusted models; the sleep coefficient is close to zero. Other variables do predict BMI change. Baseline BMI is highly predictive of follow-up BMI: Each additional BMI unit 5 years prior is associated with exactly 1 unit higher BMI after adjusting for other factors. Persons with less education and persons who report snoring gained more weight. Persons who began smoking after baseline gained less weight. The association between sleep and change in BMI did not vary by race-sex group (F = 1.06 on 3 df; P = 0.36) or by snoring (P = 0.22).
Fragmentation is strongly associated with BMI in cross-sectional analyses; there is weak evidence of a longitudinal effect (Table 3).
Table 3.
Cross-sectional Linear Multiple Regression Model of BMI (kg/m2) Regressed on Actigraph Sleep Fragmentation (per Standard Deviation) and Longitudinal Regression Model of BMI Regressed on Actigraph Sleep Fragmentation and BMI 5 Years Prior, the CARDIA Sleep Study, 2000–2006
| Model 1—Cross-sectional |
Model 2—Longitudinal |
|||||
| Coefficient of BMI (kg/m2) | 95% Confidence Interval | P Value | Coefficient of BMI (kg/m2) | 95% Confidence Interval | P Value | |
| Unadjusted | ||||||
| Fragmentation, score (per SD) | 1.20 | 0.53, 1.88 | <0.001 | 0.17 | −0.03, 0.38 | 0.09 |
| BMI 5 years prior, kg/m2 | 1.00 | 0.96, 1.04 | <0.001 | |||
| Adjusteda | ||||||
| Fragmentation, score (per SD) | 0.58 | −0.03, 1.19 | 0.06 | 0.12 | −0.08, 0.31 | 0.25 |
| Age (per year) | 0.02 | −0.13, 0.17 | 0.82 | −0.005 | −0.06, 0.05 | 0.84 |
| Race-sex groups | ||||||
| White women | Referent | Referent | ||||
| White men | 1.86 | 0.74, 2.97 | 0.001 | −0.35 | −0.78, 0.08 | 0.11 |
| Black women | 4.35 | 2.66, 6.04 | <0.001 | −0.24 | −0.83, 0.35 | 0.43 |
| Black men | 1.50 | −0.24, 3.23 | 0.09 | −0.59 | −1.21, 0.4 | 0.07 |
| Education (per level) | −0.91 | −1.45, −0.37 | 0.001 | −0.27 | −0.46, −0.09 | 0.004 |
| Smoking | ||||||
| Nonsmoker | Referent | Referent | ||||
| Smoker | −1.76 | −3.23, −0.30 | 0.018 | −0.17 | −0.78, 0.43 | 0.57 |
| Former smokerb | 0.36 | −0.36, 1.09 | 0.33 | |||
| New smokerb | −3.10 | −5.87, −0.34 | 0.03 | |||
| Physical activity, exercise units | ||||||
| Current (per 100 units) | −0.29 | −0.48, −0.10 | 0.003 | −0.05 | −0.14, 0.04 | 0.28 |
| 5 years prior (per 100 units) | 0.03 | −0.05, 0.12 | 0.42 | |||
| Apnea risk | ||||||
| Snoring | 3.53 | 1.80, 5.27 | <0.001 | 0.76 | 0.12, 1.40 | 0.02 |
| Tiredness | 0.73 | −0.43, 1.90 | 0.22 | −0.17 | −0.58, 0.23 | 0.40 |
| BMI 5 years prior, kg/m2 | 1.00 | 0.95, 1.04 | <0.001 | |||
Abbreviations: BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; SD, standard deviation.
Includes all listed covariates.
Changed smoking status between baseline and follow-up.
DISCUSSION
Using actigraphy, an objective sleep measurement, we confirmed the cross-sectional association between shorter sleep duration and higher BMI that has been reported in previous studies relying on self-reported sleep duration. However, we find no evidence that sleep duration influences weight change over 5 years. Our findings advance the evidence about sleep and weight in several additional ways: 1) Markers of socioeconomic status (race and education) strongly confound the cross-sectional association. 2) Sleep fragmentation, an index of restlessness, is also associated with BMI in the cross-sectional analysis. Few prior studies have considered measures of sleep quality. Because fragmentation and duration, both estimated from actigraphy, are strongly inversely correlated, their effects cannot be untangled. 3) Snoring is an effect modifier of the cross-sectional association. The sleep duration-BMI association observed across the entire sample is mostly due to the strong association among the subset who report snoring.
Despite substantial evidence of a cross-sectional correlation between BMI and sleep duration, such data do not prove causality. Far fewer studies have investigated the prospective association, which would provide more compelling evidence. The paucity of studies is primarily because so few cohorts have sleep information. In the First National Health and Nutrition Examination Survey (NHANES I), there was a cross-sectional association, but only among those aged 32–49 years (10). Sleep duration did not significantly predict change in BMI over 8–10 years (n = 3,208). The effect was in the hypothesized direction but was not adjusted for baseline BMI. In the Nurses’ Health Study, women aged 39–65 years (n = 68,183) were followed up to 16 years or to the age of 65 years (8). After adjustment for baseline BMI and confounders, self-reported sleep durations of less than 7 hours were significantly associated with slightly greater weight gain: Those reporting ≤5 hours and 6 hours gained, on average, 0.73 kg and 0.26 kg more than those reporting 7 hours. Although all participants were registered nurses, there may have been residual socioeconomic confounding, because education and household income do vary among nurses (22). In the Whitehall II cohort of British civil servants aged 35–55 years at baseline (n = 4,378), there was a significant cross-sectional association but no longitudinal association for changes in BMI, waist circumference, or incident obesity (12), similar to results from our study. These studies did not examine effect modification by snoring or apnea.
Two smaller studies with self-reported sleep hours were longitudinal. In a study of 496 adults oversampling persons with psychological problems, information on sleep hours, height, and weight was collected at ages 27, 29, 34, and 40 years (11). The primary analysis focused on testing a durable cross-sectional association rather than a longitudinal trajectory: Sleep duration was associated with concurrent, previous, and later obesity. The data do not clarify the causal direction: The odds ratios between sleep duration and previous obesity are slightly higher than those with later obesity. In a study of 276 adults aged 21–64 years, from families oversampled for obesity, short sleepers (5–6 hours, 15% of the sample) and long sleepers (9–10 hours, 13% of the sample) gained more weight over 6 years than did average sleepers (6). In an adjusted model, the greater weight gain was 1.8 kg for short sleepers and 1.5 kg for long sleepers relative to average sleepers.
One prior study among adults examined the cross-sectional association with actigraphy among 983 persons aged 57–97 years in the Netherlands (8). In contrast to our younger US sample, 28% of the Dutch population had 8 hours or more of actigraph-estimated sleep. Duration and fragmentation were estimated with an average of 6 nights of actigraphy. There was a quadratic association between sleep duration and BMI, but the higher BMI at the low end of sleep duration was not significant when adjusted for fragmentation.
Interest in sleep duration and BMI has been heightened by the discovery of a potential biologic mechanism—sensitivity of the appetite-regulating hormones leptin and ghrelin to sleep duration. Laboratory studies find that restricting sleep to 4 hours suppresses leptin and increases ghrelin; it also increases perceptions of hunger (23, 24). The Wisconsin Sleep Cohort Study also found that ghrelin levels were responsive to a single night in a sleep laboratory, while leptin levels correlated with self-reported habitual sleep (7). However, in the Rancho Bernardo cohort, leptin and ghrelin levels did not predict weight change (25). In an exercise intervention for overweight women, there was no baseline association between self-reported sleep and ghrelin or leptin. Those who reported improved sleep quality actually had relatively higher ghrelin and lower leptin levels (26). If leptin and ghrelin explain the effect of sleep on BMI, increased caloric consumption should be on the causal pathway. Sleep studies that have included questions about diet (or physical activity) have not found this (2, 9, 11); however, diet and physical activity are difficult to measure.
The opposite causal direction is also plausible, that obesity reduces sleep duration or quality. Obesity is the major risk factor for apnea, which interrupts sleep with arousals prompted by oxygen desaturation (27). However, obese persons without apnea also have disturbed sleep and report greater daytime sleepiness (28). Poor sleep quality tends to cause persons to report shorter sleep than persons with better sleep quality who have the same amount of measured sleep (14, 29), presumably because their sleepiness influences their estimate of sleep duration. The 2 causal directions are not necessarily mutually exclusive.
While our objective sleep measurement is an improvement on most prior studies, there is no measurement method that is both perfectly accurate and nondisruptive. The “gold standard” is polysomnography, but it requires a technician and multiple sensors, both of which may affect routine behavior. Actigraphy's advantage is low respondent burden that does not alter sleep behavior, as there is no “first night effect” (30–33). Many prior studies have compared actigraphy with concurrent polysomnography in a sleep laboratory. In a comprehensive review, correlations for sleep duration were over 0.9 in healthy adults (30). Some of the discrepancy is because the 2 methods key into different points in the sleep onset process. Nor is polysomnography perfectly reliable, particularly for identifying shallow stage 1 sleep (34). Almost all previous epidemiologic studies have relied on self-reported habitual sleep, which does not correlate highly with either polysomnography or actigraphy. In the Sleep Heart Health Study, the correlation between a single night of home polysomnography and previously reported habitual sleep was only 0.18 (13). In the CARDIA Sleep Study, we used a measurement-error model to evaluate the correlation between self-reported habitual sleep and 3 nights of actigraphy and found a correlation of 0.47 (14). We also found that obese persons systematically reported less sleep than the nonobese at the same level of measured sleep.
There were challenges in actigraphy data collection. Most participants forgot to press the event marker at least once, but almost all provided backup data in their sleep log. When both were missing (fewer than 5% of bedtimes or waketimes), we used a discernible decline or increase in activity to mark the beginning or end of the period scanned for sleep. A few “actiwatches” were not mailed back (even after reminders); this occurred more often in the second wave, perhaps because the personal sleep report was less of an inducement the second time. Some participants did not wear the actiwatch the exact days requested. We included 2 weeknights and 1 weekend night in each wave, but we did not know participants’ actual work schedules. We designed our study with 2 waves of data collection, allowing us to determine that day-to-day variability was high but year-to-year variability was low. Based on this data collection experience, we would collect a single 7-day period in future studies.
Another limitation is our reliance on self-reported snoring in lieu of a clinical apnea diagnosis. Snoring is a highly sensitive, but not very specific, marker of obstructive sleep apnea, resulting in moderate positive predictive value (35). Some persons are unaware that they snore. It is unclear whether the snoring effect modification would be stronger or weaker if we knew apnea status. We are unaware of previous studies that have found that snoring predicts weight gain, although snoring ascertained at the end of the study period was associated with greater weight gain over 10 years in the Nurses’ Health Study (36). This association needs confirmation in longitudinal studies with snoring ascertained at baseline.
Our measurement of sleep after baseline is another limitation. However, the cross-sectional associations were very similar whether we used follow-up or baseline BMI, suggesting that baseline sleep measurement would have yielded similar cross-sectional and longitudinal estimates. In separate analyses, our sleep duration measurement has predicted other longitudinal clinical outcomes (e.g., incident coronary artery calcification) (37). The lack of a longitudinal sleep effect on BMI does not seem to be due to a lack of power to detect any significant predictors of weight change, since other variables are associated with weight change.
Prior evidence that shorter sleep leads to weight gain among adults has not been consistent. Differences in the ages of study populations may contribute to apparent heterogeneity, as may variations across populations in the accuracy of self-reported sleep. Future studies are needed to confirm our finding that the cross-sectional association between sleep duration and BMI is due to the powerful association among the subset who snore. The mechanism underlying the cross-sectional association is not clear; this study does not provide evidence that persons who sleep less are more likely to gain weight.
Acknowledgments
Author affiliations: Department of Health Studies, University of Chicago, Chicago, Illinois (Diane S. Lauderdale, Paul J. Rathouz); Department of Medicine, University of Chicago, Chicago, Illinois (Kristen L. Knutson); Department of Preventive Medicine, Northwestern University, Chicago, Illinois (Lijing L. Yan, Kiang Liu); Research and Development Division, the George Institute for International Health, Beijing, China (Lijing L. Yan); Peking University Clinical Research Institute and Health Economics and Management Institute, Beijing, China (Lijing L. Yan); and Department of Epidemiology and Biostatistics, University of California at San Francisco, San Francisco, California (Stephen B. Hulley).
Research for this study was supported by grant AG 11412 from the National Institute on Aging. The CARDIA Study is supported by US Public Health Service contracts NO1-HC-48047, NO1-HC-48048, NO1-HC-48049, NO1-HC-48050, and NO1-HC-95095 from the National Heart, Lung, and Blood Institute.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- SD
standard deviation
References
- 1.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knutson KL, Spiegel K, Penev P, et al. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson H. Medicine: sleep it off. Nature. 2006;443(7109):261–263. doi: 10.1038/443261a. [DOI] [PubMed] [Google Scholar]
- 5.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12(4):289–298. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Chaput JP, Després JP, Bouchard C, et al. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007;15(1):253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 7.Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index [electronic article] PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Berg JF, Knvistingh Neven A, Tulen JH, et al. Actigraphic sleep duration and fragmentation are related to obesity in the elderly: the Rotterdam Study. Int J Obes (Lond) 2008;32(7):1083–1090. doi: 10.1038/ijo.2008.57. [DOI] [PubMed] [Google Scholar]
- 9.Patel SR, Malhotra A, White DP, et al. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164(10):947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangwisch JE, Malaspina D, Boden-Albala B, et al. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28(10):1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 11.Hasler G, Buysse DJ, Klaghofer R, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27(4):661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 12.Stranges S, Cappuccio FP, Kandala NB, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol. 2008;167(3):321–329. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva GE, Goodwin JL, Sherrill DL, et al. Relationship between reported and measured sleep times: the Sleep Heart Health Study (SHHS) J Clin Sleep Med. 2007;3(6):622–630. [PMC free article] [PubMed] [Google Scholar]
- 14.Lauderdale DS, Knutson KL, Yan LL, et al. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 16.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early middle-aged adults: the CARDIA Study. Am J Epidemiol. 2006;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 17.Knutson KL, Rathouz PJ, Yan LL, et al. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA Study. Sleep. 2007;30(6):793–796. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 19.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Tsai WH, Remmers JE, Brant R, et al. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167(10):1427–1432. doi: 10.1164/rccm.200112-110OC. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Jacobs DR, Jr, Sidney S, et al. Physical activity in young black and white women. The CARDIA Study. Ann Epidemiol. 1993;3(6):636–644. doi: 10.1016/1047-2797(93)90087-k. [DOI] [PubMed] [Google Scholar]
- 22.Lauderdale DS. Adiposity and physical activity as predictors of mortality [letter] N Engl J Med. 2005;352(13):1381–1384. [PubMed] [Google Scholar]
- 23.Spiegel K, Tasali E, Penev P, et al. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 24.Spiegel K, Leproult R, L'hermite-Balériaux M, et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 25.Langenberg C, Bergstrom J, Laughlin GA, et al. Ghrelin, adiponectin, and leptin do not predict long-term changes in weight and body mass index in older adults: longitudinal analysis of the Rancho Bernardo cohort. Am J Epidemiol. 2005;162(12):1189–1197. doi: 10.1093/aje/kwi338. [DOI] [PubMed] [Google Scholar]
- 26.Littman AJ, Vitiello MV, Foster-Schubert K, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007;31(3):466–475. doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 27.Patil SP, Schneider H, Schwartz AR, et al. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132(1):325–337. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci. 2006;1083(1):329–344. doi: 10.1196/annals.1367.023. [DOI] [PubMed] [Google Scholar]
- 29.Regestein QR, Friebely J, Shifren JL, et al. Self-reported sleep in postmenopausal women. Menopause. 2004;11(2):198–207. doi: 10.1097/01.gme.0000097741.18446.3e. [DOI] [PubMed] [Google Scholar]
- 30.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 31.Jean-Louis G, von Gizycki H, Zizi F, et al. The actigraph data analysis software. I. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills. 1997;85(1):207–216. doi: 10.2466/pms.1997.85.1.207. [DOI] [PubMed] [Google Scholar]
- 32.Mendels J, Hawkins DR. Sleep laboratory adaptation in normal subjects and depressed patients (“first night effect”) Electroencephalogr Clin Neurophysiol. 1967;22(6):556–558. doi: 10.1016/0013-4694(67)90063-6. [DOI] [PubMed] [Google Scholar]
- 33.Toussaint M, Luthringer R, Schaltenbrand N, et al. First-night effect in normal subjects and psychiatric inpatients. Sleep. 1995;18(6):463–469. doi: 10.1093/sleep/18.6.463. [DOI] [PubMed] [Google Scholar]
- 34.Tryon WW. Issues in the validity of actigraphic sleep assessment. Sleep. 2004;27(1):158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 35.Hoffstein V, Szalai JP. Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep. 1993;16(2):118–122. [PubMed] [Google Scholar]
- 36.Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol. 1999;150(8):806–816. doi: 10.1093/oxfordjournals.aje.a010085. [DOI] [PubMed] [Google Scholar]
- 37.King CR, Knutson KL, Rathouz PJ, et al. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–2866. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]