Abstract
Associations between stress hormones and preterm delivery have not been fully explored. In this study, pregnant women enrolled from 52 clinics in 5 Michigan communities (1998–2004) provided urine samples for 3 days (waking and bedtime) during midpregnancy. Urinary catecholamine levels (epinephrine, norepinephrine, and dopamine) were measured in a subcohort (247 preterm and 760 term deliveries), and a 3-day median value was calculated. Polytomous logistic regression models assessed relations between catecholamine quartiles (of the median) and a 4-level outcome variable (i.e., term (referent) and 3 preterm delivery subtypes: spontaneous; premature rupture of membranes; and medically indicated). Final models incorporated other relevant covariates (e.g., creatinine, demographic, behavior). The risk of spontaneous preterm delivery was increased in the highest versus lowest quartile of norepinephrine and dopamine: norepinephrine, waking (adjusted odds ratio (AOR) = 3.7, 95% confidence interval (CI): 1.8, 7.9) and bedtime (AOR = 2.5, 95% CI: 1.3, 4.9); dopamine, waking (AOR = 2.6, 95% CI: 1.4, 5.1) and bedtime (AOR = 2.3, 95% CI: 1.2, 4.6). Adjusted odds ratios were further strengthened after removing women whose placentas showed evidence of acute infection or vascular pathology. High catecholamine levels in maternal urine may be indicative of excess stressors and/or predisposition to elevated sympathetic activation that contributes to increased risk of spontaneous preterm delivery.
Keywords: catecholamines, dopamine, epinephrine, gestational age, norepinephrine, pregnancy, pregnancy outcome, premature birth
Preterm births, that is, delivery at <37 weeks’ gestation, account for a substantial proportion of infant mortality and long-term disability in developed countries (1, 2). Over the last decade, rates of very early preterm delivery have not declined, and rates of late preterm delivery have increased among both singleton- and multiple-gestation pregnancies (3). Disparities in risk of preterm delivery continue, with higher rates among African-American women (4) and women of lower socioeconomic status (5–7).
Spontaneous and medically indicated preterm deliveries have many causes that act alone or in combination. The most frequently identified causes include infection/inflammation, premature rupture of fetal membranes, and vascular complications such as preeclampsia, gestational hypertension, bleeding/placental abruption, and decreased placental perfusion (8). These causes are identified through various forms of evidence that span clinical signs/symptoms, biomarkers, and placental pathology. The term “idiopathic” is frequently used to refer to preterm deliveries that are initiated by spontaneous labor; these represent an estimated 20%–50% of preterm delivery depending on the extent of prematurity and inclusion/exclusion criteria (9–11).
Maternal stress, variably defined, has also been hypothesized to play a role in preterm delivery. Proposed mechanisms include stress-induced alterations in immune function leading to infection, stress effects contributing to vascular complications, stress-related behaviors (e.g., poor diet, smoking, substance abuse), and hormonal pathways triggered by stress (12–14). Prospective studies testing the stress and/or anxiety-preterm delivery hypothesis have typically measured maternal stressors and stress/anxiety through self-report, and there are examples of both positive (15–17) and null (18–20) findings. Fewer studies have evaluated biomarkers of maternal stress, primarily cortisol, in relation to preterm delivery, and again results have been mixed (21–24).
In this prospective study of pregnant women, midpregnancy urine levels of catecholamines, key stress/arousal biomarkers, were assessed in relation to the risk of preterm delivery. Placental pathology was used to define 2 common preterm delivery pathways (infection, vascular complications) and to determine whether a catecholamine-preterm delivery association is observed outside these pathways.
MATERIALS AND METHODS
Study design and eligibility
The Pregnancy Outcomes and Community Health (POUCH) Study prospectively enrolled women in their 15th−27th weeks of pregnancy from 52 clinics in 5 Michigan communities from September 8, 1998, through June 15, 2004 (25). Eligibility criteria included a singleton pregnancy with no known chromosomal abnormality or birth defect, maternal serum α-fetoprotein (MSAFP) screening at 15–22 weeks, maternal age of ≥15 years, no prepregnancy diabetes mellitus, and proficiency in English. The study received institutional review board approval at Michigan State University, the Michigan Department of Community Health, and 9 community hospitals.
Sampling for the cohort and subcohort
Women who met the eligibility criteria, expressed interest in the study, and had normal MSAFP levels were stratified by race/ethnicity and randomly sampled into the cohort. In addition, all interested women with unexplained MSAFP levels of ≥2 multiples of the mean were invited to participate (7% of the cohort but typically 3%–5% of the screened population), because this biomarker had been linked to preterm delivery previously (26, 27) and was of particular interest in the POUCH Study. A total of 3,038 women were enrolled, and follow-up through delivery was completed for 3,019 (99.5%) cohort women.
A subcohort was assembled (n = 1,371) and studied in greater detail to maximize resource efficiency. The subcohort included the following: 1) all women who delivered preterm (<37 weeks); 2) all women with unexplained elevated MSAFP (≥2 multiples of the mean); and 3) a race-stratified random sample of women with normal MSAFP levels and term deliveries, with an oversampling from the African-American stratum. The sampling scheme was designed to optimize statistical power for studying at-risk subgroups within a subcohort (i.e., preterm, African Americans, and women with high MSAFP). All subcohort analyses use sampling weights that account for the cohort and subcohort sampling scheme and thereby remove any bias due to oversampling from certain strata.
Because of Health Insurance Portability and Accountability Act (HIPAA) regulations, it was not possible to determine an exact participation rate or to compare characteristics between cohort participants and nonparticipants. However, race/ethnic-specific comparisons between POUCH Study data and birth file data from the 5 communities showed that the POUCH Study sample was very similar to community mothers on most factors measured (i.e., parity, educational levels, and the proportions of women with Medicaid insurance, preterm delivery, previous stillbirth, previous preterm infant, and previous low birth weight infant).
Study protocol
At enrollment, POUCH Study cohort women met with a study nurse, signed consent forms, completed in-person interviews and self-administered questionnaires, and had biologic samples collected. Shortly after recruitment was underway in each community, the POUCH Study added an optional at-home data collection protocol (28) aimed at measuring stress biomarkers (cortisol and catecholamines) and blood pressure. As part of this protocol, women collected urine (for catecholamine levels) twice a day for 3 consecutive days immediately upon waking and just before bedtime, and they recorded waking, sleeping, and sample collection times.
Subcohort sample for catecholamine analyses
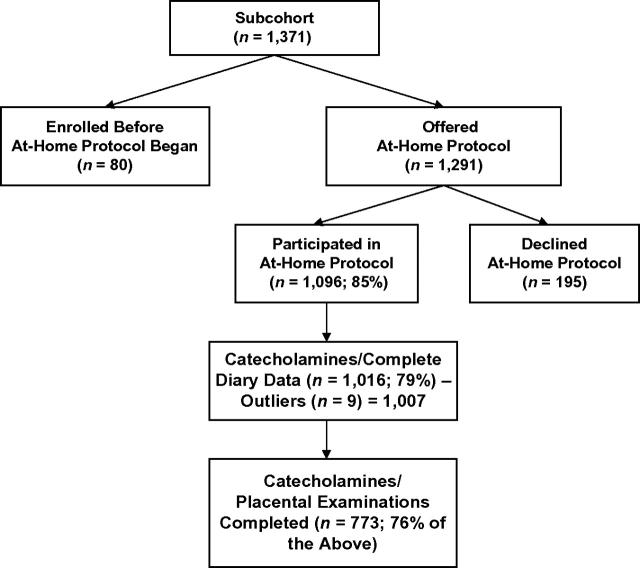
Approximately 85% of the eligible subcohort women agreed to complete the at-home protocol (Figure 1), and 79% (n = 1,016) successfully returned urine samples and diary data. An additional 9 women were excluded because their urine catecholamine levels were extreme outliers (6 standard deviations above the mean), leaving a sample of 1,007 women (247 preterm, 760 term). Of the 1,007 women, placental pathology was completed to date on 773 (176 preterm, 597 term).
Figure 1.
Flow chart of data on catecholamine levels in maternal urine at midpregnancy, Pregnancy Outcomes and Community Health Study, 1998–2004.
Measurement of catecholamines
Urine samples collected at home were frozen (−20°C) during storage and shipping. At the laboratory, frozen urine was thawed within 1 week of receipt, acidified with hydrochloric acid to a pH of 3–5, and refrozen (−20°C). Samples were later thawed and assayed for levels of epinephrine, norepinephrine, and dopamine by using high-performance liquid chromatography (29). A solvent extraction method was used with 3,4-dihydroxybenzylamine hydrobromide as an internal standard. A heptane/octanol solution was used for extraction followed by an acetic acid extraction. The mobile phase consisted of 0.175 mM decanesulfonic acid, 0.1 mM ethylenediaminetetraacetic acid, 0.15 mM sodium hydride × peroxidase, and 5% methanol (pH 5.1) for catecholamine analysis (flow rate, 1.2 mL/minute). The electrode potential was set to 850 mV versus a Ag/AgCl reference electrode. At the same time, urine creatinine was measured by using a colorimetric assay kit (Assay Designs, Ann Arbor, Michigan). Intraassay coefficients ranged from 5% to 13% and interassay coefficients ranged from 10% to 14% with epinephrine assays being at the top of the range.
Outcome measures
Preterm delivery.
Gestational age at delivery was calculated by using the date of the last menstrual period or gestational age from an early ultrasound (<22 weeks’ gestation) if the 2 estimates differed by more than 2 weeks. Two abstractors, a physician and a labor and delivery nurse, independently reviewed subcohort prenatal and labor and delivery records to identify clinical circumstances leading to preterm delivery. Disagreements were resolved through reexamination of medical records by an expert team. Preterm delivery was divided into 3 groups: 1) Spontaneous preterm delivery included women with regular contractions that led to cervical changes (≥2 cm of dilatation); 2) premature rupture of membranes preterm delivery included women with rupture of membranes before or simultaneously with onset of contractions; and 3) medically indicated preterm delivery included women induced or given cesarean sections before either preterm labor or premature rupture of membranes.
Placental pathology.
Placentas were formalin fixed and received gross examination by using standard protocols that included recording of gross hemorrhage accompanied by compression (evidence of abruption). Nine tissue samples from each placenta were embedded in paraffin blocks, sectioned, and stained with hematoxylin and eosin for microscopic assessment. Details of the sampling and microscopic evaluation have been described elsewhere (30). The study pathologist, who was blinded to gestational age at delivery, all clinical data, and gross examination findings, recorded placental findings in a structured, computer-based descriptive instrument adapted from one developed by Dr. Carolyn Salafia (Placental Analytics LLC, Larchmont, New York, and Institute for Basic Research, Staten Island, New York). Evidence of significant infection/inflammation, referred to here as “histologic chorioamnionitis,” was defined as an advanced maternal inflammatory response or fetal inflammatory response, both of which were found to be related to preterm delivery in previous POUCH Study analyses (30). A total of 29 placental vascular findings were divided into 5 conceptual groups or pathology-based latent constructs: 1) maternal vascular-obstructive (MV-O) (e.g., villous infarcts and atherosis); 2) maternal vascular-disturbance of integrity (MV-I) (e.g., evidence of retroplacental, retromembranous, and decidual hemorrhage); 3) maternal vascular-developmental (MV-D) (e.g., unaltered/abnormal decidual vessels); 4) fetal vascular-obstructive (FV-O) (e.g., thrombi and avascular villi); and 5) fetal vascular-disturbance of integrity (FV-I) (e.g., villous stromal hemorrhage and intervillous thrombi). The POUCH Study placental vascular groups were adapted from the Placental Diagnostic Coding Tool of Dr. Raymond Redline (P. K. Senagore, Michigan State University, personal communication, 1999) and have been described elsewhere in greater detail (31). For each of the 5 groups, findings were summed, and a score was assigned to each woman. The distributions of these scores among the term, normal MSAFP deliveries formed the basis for 2 cutoff values, very high (approximately the top decile) and high (approximately the top quintile), within each vascular pathology group.
Covariate data
Information on demographics, medical and reproductive history, prepregnancy weight and height, levels of substance use (i.e., cigarette smoking and use of alcohol, marijuana, or other illicit drugs) before and during pregnancy, and levels of caffeinated beverage intake during pregnancy was gathered at enrollment. Maternal prepregnancy weight and height were used to calculate a body mass index (weight (kg)/height (m)2). Data on medication use during pregnancy up through the time of enrollment were obtained through maternal interview and by abstracting prenatal/labor and delivery records. Medical records were also the source of information on maternal hypertensive complications (chronic, gestational, preeclampsia) and clinically diagnosed placental abruption.
Analytical strategy
For descriptive purposes, Spearman's rank correlation coefficients were used to measure correlations among 1) catecholamine levels across the 3 days; 2) waking and bedtime catecholamine levels on a specific day; and 3) levels of the different catecholamines within the same urine sample. Median (from the 3 days) waking and bedtime epinephrine, norepinephrine, and dopamine levels were calculated for each woman. These median values were divided into quartiles. Cutpoints for the quartiles were based on distributions in women delivering at term with normal MSAFP.
Waking and bedtime epinephrine, norepinephrine, and dopamine were each evaluated as exposure measures in separate models. Polytomous regression was used to assess relations between catecholamine quartiles and a 4-level pregnancy outcome variable (i.e., term (referent), spontaneous preterm delivery, premature rupture of membranes preterm delivery, and medically indicated preterm delivery). We also conducted a homogeneity test for equivalence to examine whether the preterm delivery clinical subtypes each had significantly different associations with the catecholamines. We compared the log-likelihoods of 2 polytomous logistic models (chi-square test statistic), one that constrained all the catecholamine odds ratios so that they were identical for each preterm delivery subtype and one that allowed the odds ratios to vary by preterm delivery subtype (32). After observing that catecholamines were specifically associated with spontaneous preterm delivery, we further subdivided this category by gestational week at delivery (i.e., <35 weeks and 35–36 weeks) to consider the relations with timing of spontaneous preterm delivery.
All regression models included urinary creatinine levels to adjust for urine concentration. In a second series of models, other potential confounders were incorporated, that is, maternal age, week of pregnancy when urine was sampled, race/ethnicity, Medicaid insurance status, parity, and body mass index. Later models also tested confounding by exposures that might alter maternal catecholamine levels, that is, maternal smoking, marijuana use, illicit drug use, caffeinated beverage intake, psychotropic medications, and tocolytics.
A final set of analyses was aimed at identifying the biologic pathways linked to elevated maternal catecholamine levels. In logistic regression models, catecholamine quartiles (independent variable) were evaluated in relation to histologic chorioamnionitis (dependent variable, yes/no) and then in relation to each of the 5 placental vascular pathology groups (dependent variable, high score vs. not high score). Null findings in these models motivated a repeat of the polytomous regression models described above to test the persistence of the catecholamine-spontaneous preterm delivery association after exclusion of women with common causes of preterm delivery, that is, histologic chorioamnionitis and a very high score (top decile) for any of the 5 vascular pathology groupings.
Weights were incorporated in all regression models to reflect oversampling of high MSAFP into the cohort and the subcohort sampling scheme. All regression analyses were conducted by using PROC SURVEYLOGISTIC procedures with SAS, version 9.1, software (33).
RESULTS
Maternal characteristics in the subcohort sample with urinary catecholamines (n = 1,007) were similar to those in the entire subcohort, with the exception that a larger percentage, 26% versus 13%, was enrolled at 25–27 weeks’ gestation (Table 1). As expected, the study sample had higher percentages of African-American women (39%) and women who delivered preterm (25%) than those in the original cohort (25% and 11%, respectively), because these groups were oversampled for the subcohort, the source of the study sample.
Table 1.
Maternal Characteristics and Pregnancy Outcome in the Study Subcohort With Urinary Catecholamines Measured at Midpregnancy (n = 1,007) and in the Entire Subcohort, Pregnancy Outcomes and Community Health Study, 1998–2004
| Maternal Characteristics | Study Sample (n = 1,007) |
Entire Subcohort (n = 1,371), | |
| No. | % | % | |
| Maternal age, years | |||
| <20 | 178 | 18 | 18 |
| 20–29 | 563 | 56 | 56 |
| ≥30 | 266 | 26 | 26 |
| Race/ethnicitya | |||
| White | 543 | 54 | 51 |
| African American | 391 | 39 | 42 |
| Others | 73 | 7 | 7 |
| Maternal education, years | |||
| <12 | 218 | 22 | 23 |
| 12 | 285 | 28 | 29 |
| >12 | 504 | 50 | 48 |
| Medicaid insured | |||
| No | 450 | 45 | 43 |
| Yes | 557 | 55 | 57 |
| Week of pregnancy at enrollment | |||
| <20 | 159 | 16 | 16 |
| 20–24 | 584 | 58 | 71 |
| 25–27 | 264 | 26 | 13 |
| Parity/preterm delivery history | |||
| No previous livebirth | 424 | 42 | 42 |
| Previous livebirth without preterm delivery | 534 | 53 | 53 |
| Previous livebirth with preterm delivery | 49 | 5 | 5 |
| Pregnancy outcomea | |||
| Term | 760 | 75 | 75 |
| Preterm medically indicated | 75 | 7 | 8 |
| Preterm PROM | 67 | 8 | 7 |
| Preterm spontaneous preterm labor | 105 | 10 | 10 |
Abbreviation: PROM, premature rupture of membranes.
Percentages reflect oversampling of African Americans and preterm into the subcohort.
Distributions of median catecholamine levels (median from across the 3 days) were skewed to the right (Table 2). The percentage of median catecholamine values below the limit of detection was considerable for waking and bedtime epinephrine (about 27%) but negligible (<3%) for the other catecholamines. For each catecholamine, day-to-day correlations and waking-to-bedtime correlations were moderate, with Spearman's rank correlation coefficients in the range of 0.44–0.59. Within a single sample of urine, the range of correlations varied for epinephrine and norepinephrine levels (waking, 0.09–0.10; bedtime, 0.14–0.22), norepinephrine and dopamine levels (waking, 0.27–0.31; bedtime, 0.30–0.37), and epinephrine and dopamine levels (waking, 0.03–0.06; bedtime, 0.08–0.15).
Table 2.
Urinary Catecholamine Levels at Midpregnancy in the Entire Study Subcohorta (n = 1,007), Pregnancy Outcomes and Community Health Study, 1998–2004
| Meanb | Medianb | SDb | % Below the Level of Detectionb | Day-to-Day Correlationc Range (Days 1–3) | Waking-to-Bedtime Correlationc Range (Days 1–3) | |
| Epinephrine, nm/dL | ||||||
| Waking | 43.5 | 21 | 66.4 | 26.2 | 0.49–0.50 | |
| Bedtime | 43.1 | 21 | 67.5 | 27.8 | 0.44–0.48 | 0.44–0.48 |
| Norepinephrine, nm/dL | ||||||
| Waking | 86.1 | 53 | 104.5 | 2.9 | 0.50–0.59 | |
| Bedtime | 105.8 | 68 | 116.2 | 2.7 | 0.51–0.55 | 0.49–0.58 |
| Dopamine, nm/dL | ||||||
| Waking | 539.9 | 439 | 355.0 | 0.2 | 0.51–0.54 | |
| Bedtime | 546.6 | 426 | 385.0 | 0.2 | 0.49–0.50 | 0.46–0.51 |
Abbreviation: SD, standard deviation.
Descriptive statistics not weighted for subcohort sampling scheme.
Based on the distribution of the median catecholamine level from the 3-day samples.
Spearman's rank correlation coefficients.
In polytomous regression models with adjustment for creatinine levels, urinary catecholamine levels were positively associated with spontaneous preterm delivery (Table 3). Top quartile adjusted odd ratios were statistically significant for waking norepinephrine (adjusted odds ratio (AOR) = 3.4, 95% confidence interval (CI): 1.6, 7.0), bedtime norepinephrine (AOR = 2.3, 95% CI: 1.2, 4.5), waking dopamine (AOR = 2.6, 95% CI: 1.3, 5.0), and bedtime dopamine (AOR = 2.4, 95% CI: 1.3, 4.6). The homogeneity test for equivalence of models showed that the model allowing catecholamine odds ratios to vary by preterm delivery subtype produced a better fit (chi-square values, P < 0.001) than the model that constrained odds ratios to be identical across preterm delivery subtypes. These results persisted for all catecholamines, waking and bedtime, and reinforced the specificity of the relation between catecholamines and spontaneous preterm delivery.
Table 3.
Associations Between Midpregnancy Urinary Catecholamine Levels and Preterm Delivery, Adjusted for Urinary Creatinine Levels (n = 1,007), Pregnancy Outcomes and Community Health Study, 1998–2004
| Term Delivery (Referent Group), no. | Preterm Delivery |
|||||||||
| Medically Indicated |
Premature Rupture of Membranes |
Spontaneous Preterm Labor |
||||||||
| No. | AOR | 95% Confidence Interval | No. | AOR | 95% Confidence Interval | No. | AOR | 95% Confidence Interval | ||
| Epinephrine | ||||||||||
| Waking | ||||||||||
| Quartile 1 (referent) | 204 | 18 | 1.0 | 19 | 1.0 | 26 | 1.0 | |||
| Quartile 2 | 187 | 17 | 1.1 | 0.5, 2.3 | 10 | 0.6 | 0.3, 1.4 | 18 | 0.8 | 0.4, 1.6 |
| Quartile 3 | 182 | 25 | 1.6 | 0.8, 3.2 | 20 | 1.0 | 0.5, 2.0 | 23 | 1.0 | 0.5, 1.8 |
| Quartile 4 | 187 | 15 | 1.0 | 0.5, 2.1 | 18 | 1.1 | 0.5, 2.2 | 38 | 1.7 | 1.0, 3.0 |
| Bedtime | ||||||||||
| Quartile 1 (referent) | 222 | 22 | 1.0 | 17 | 1.0 | 29 | 1.0 | |||
| Quartile 2 | 168 | 18 | 1.1 | 0.6, 2.2 | 14 | 1.1 | 0.5, 2.4 | 18 | 0.9 | 0.5, 1.7 |
| Quartile 3 | 183 | 17 | 0.9 | 0.5, 1.9 | 15 | 0.9 | 0.4, 2.0 | 23 | 1.0 | 0.6, 1.9 |
| Quartile 4 | 187 | 18 | 1.0 | 0.5, 1.9 | 21 | 1.5 | 0.7, 2.9 | 35 | 1.5 | 0.9, 2.7 |
| Norepinephrine | ||||||||||
| Waking | ||||||||||
| Quartile 1 (referent) | 197 | 17 | 1.0 | 23 | 1.0 | 11 | 1.0 | |||
| Quartile 2 | 187 | 24 | 1.7 | 0.8, 3.4 | 15 | 0.7 | 0.4, 1.5 | 26 | 2.7* | 1.3, 5.9 |
| Quartile 3 | 191 | 13 | 0.9 | 0.4, 1.9 | 11 | 0.5 | 0.2, 1.1 | 31 | 2.8* | 1.3, 5.9 |
| Quartile 4 | 185 | 21 | 1.4 | 0.7, 2.8 | 18 | 0.8 | 0.4, 1.6 | 37 | 3.4* | 1.6, 7.0 |
| Bedtime | ||||||||||
| Quartile 1 (referent) | 200 | 20 | 1.0 | 21 | 1.0 | 18 | 1.0 | |||
| Quartile 2 | 187 | 21 | 1.2 | 0.6, 2.4 | 18 | 1.0 | 0.5, 1.9 | 25 | 1.7 | 0.9, 3.4 |
| Quartile 3 | 191 | 20 | 1.1 | 0.5, 2.2 | 11 | 0.6 | 0.3, 1.3 | 25 | 1.7 | 0.8, 3.3 |
| Quartile 4 | 182 | 14 | 0.9 | 0.4, 1.8 | 17 | 1.0 | 0.5, 2.0 | 37 | 2.3* | 1.2, 4.5 |
| Dopamine | ||||||||||
| Waking | ||||||||||
| Quartile 1 (referent) | 201 | 33 | 1.0 | 18 | 1.0 | 15 | 1.0 | |||
| Quartile 2 | 182 | 14 | 0.5 | 0.2, 1.0 | 21 | 1.5 | 0.8, 3.0 | 17 | 1.3 | 0.6, 2.8 |
| Quartile 3 | 187 | 13 | 0.5 | 0.2, 1.0 | 17 | 1.2 | 0.6, 2.5 | 38 | 2.9* | 1.5, 5.6 |
| Quartile 4 | 190 | 15 | 0.5 | 0.3, 1.0 | 11 | 0.8 | 0.3, 1.7 | 35 | 2.6* | 1.3, 5.0 |
| Bedtime | ||||||||||
| Quartile 1 (referent) | 234 | 26 | 1.0 | 24 | 1.0 | 21 | 1.0 | |||
| Quartile 2 | 179 | 21 | 1.3 | 0.7, 2.5 | 17 | 1.0 | 0.5, 2.0 | 20 | 1.3 | 0.6, 2.6 |
| Quartile 3 | 171 | 14 | 0.8 | 0.4, 1.8 | 14 | 0.9 | 0.4, 1.9 | 30 | 2.1* | 1.1, 4.0 |
| Quartile 4 | 176 | 14 | 0.9 | 0.4, 2.0 | 12 | 0.8 | 0.4, 1.6 | 34 | 2.4* | 1.3, 4.6 |
Abbreviation: AOR, adjusted odds ratio.
P < 0.05.
The addition of other covariates (age, pregnancy week at urine sampling, race/ethnicity, Medicaid insurance status, parity, body mass index) had little impact on the association between catecholamines and preterm delivery (Table 4). After further subdivision of spontaneous preterm delivery by weeks of gestation, the upper quartiles of waking and bedtime epinephrine were associated primarily with an increased risk of spontaneous preterm delivery at <35 weeks (Table 4). Other potential confounders were added to these models one by one, that is, levels of cigarette smoking, alcohol, marijuana and other illicit drugs before and during pregnancy, levels of caffeinated beverage intake during pregnancy, psychotropic medications, and tocolytics. These covariates did not alter catecholamine-adjusted odds ratios by greater than 10% (results not shown). Analyses were repeated after exclusion of the 103 women who reported illicit substance use before or during pregnancy, and the results remained unchanged. Similar catecholamine results were observed when the data were modeled separately for Medicaid and non-Medicaid insured, women with high and low educational levels, and African Americans and whites/others.
Table 4.
Associations Between Midpregnancy Urinary Catecholamine Levels and Preterm Delivery,a Adjusted for Urinary Creatinine Levels, Maternal Age, Pregnancy Week at Sampling, Race/Ethnicity, Medicaid Insurance Status, Parity, and Body Mass Index (n = 1,007), Pregnancy Outcomes and Community Health Study, 1998–2004
| Preterm Delivery |
||||||
| Spontaneous Preterm Labor |
Spontaneous Preterm Labor |
|||||
| At 35–36 Weeks |
At <35 Weeks |
|||||
| AOR | 95% Confidence Interval | AOR | 95% Confidence Interval | AOR | 95% Confidence Interval | |
| Epinephrine | ||||||
| Waking | ||||||
| Quartile 1 (referent) | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 0.8 | 0.4, 1.5 | 0.7 | 0.3, 1.5 | 1.3 | 0.3, 6.0 |
| Quartile 3 | 1.0 | 0.5, 1.8 | 0.7 | 0.3, 1.4 | 2.6 | 0.7, 9.6 |
| Quartile 4 | 1.8* | 1.0, 3.2 | 1.3 | 0.7, 2.6 | 4.7* | 1.4, 16.3 |
| Bedtime | ||||||
| Quartile 1 (referent) | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 0.9 | 0.4, 1.7 | 0.6 | 0.3, 1.4 | 2.2 | 0.6, 8.6 |
| Quartile 3 | 1.0 | 0.5, 1.8 | 0.6 | 0.3, 1.3 | 3.3* | 1.0, 11.4 |
| Quartile 4 | 1.6 | 0.9, 2.9 | 1.4 | 0.7, 2.6 | 3.1 | 0.9, 11.1 |
| Norepinephrine | ||||||
| Waking | ||||||
| Quartile 1 (referent) | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 2.8* | 1.3, 6.0 | 2.9* | 1.2, 7.4 | 2.6 | 0.7, 9.6 |
| Quartile 3 | 2.6* | 1.2, 5.6 | 2.8* | 1.1, 6.9 | 2.2 | 0.6, 8.6 |
| Quartile 4 | 3.7* | 1.8, 7.9 | 3.6* | 1.5, 9.0 | 3.9* | 1.1, 13.7 |
| Bedtime | ||||||
| Quartile 1 (referent) | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 1.6 | 0.8, 3.2 | 1.6 | 0.7, 3.7 | 1.6 | 0.5, 5.5 |
| Quartile 3 | 1.6 | 0.8, 3.2 | 1.4 | 0.6, 3.3 | 2.1 | 0.6, 7.0 |
| Quartile 4 | 2.5* | 1.3, 4.9 | 2.6* | 1.2, 5.7 | 2.1 | 0.6, 7.4 |
| Dopamine | ||||||
| Waking | ||||||
| Quartile 1 (referent) | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 1.2 | 0.6, 2.7 | 0.9 | 0.3, 2.5 | 1.9 | 0.6, 6.1 |
| Quartile 3 | 2.8* | 1.4, 5.4 | 2.9* | 1.3, 6.5 | 2.6 | 0.9, 7.6 |
| Quartile 4 | 2.6* | 1.4, 5.1 | 3.5* | 1.6, 7.5 | 0.9 | 0.2, 3.7 |
| Bedtime | ||||||
| Quartile 1 (referent) | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 1.1 | 0.5, 2.4 | 1.4 | 0.5, 3.5 | 0.8 | 0.2, 2.7 |
| Quartile 3 | 2.1* | 1.1, 4.1 | 2.7* | 1.2, 6.3 | 1.2 | 0.4, 3.9 |
| Quartile 4 | 2.3* | 1.2, 4.6 | 3.3* | 1.5, 7.7 | 1.1 | 0.3, 3.4 |
Abbreviation: AOR, adjusted odds ratio.
P < 0.05.
The adjusted odds ratios for medically indicated preterm delivery and premature rupture of membranes preterm delivery were similar to those in Table 3 and are not displayed in this table.
Separate logistic regression models were used to evaluate quartiles of waking and bedtime epinephrine, norepinephrine, and dopamine in relation to histologic chorioamnionitis (no/yes) and each of the 5 placental vascular pathology groups (dichotomized as high/not high). There were no significant associations between any of the catecholamine measures and these placental pathology findings (data not shown). Polytomous regression models with adjustment for urinary creatinine levels (discussed above) were repeated after exclusion of all cases of histologic chorioamnionitis. The relation between spontaneous preterm delivery and both norepinephrine and dopamine persisted with fourth quartile adjusted odds ratios ranging from 2.3 to 5.5 (Table 5). Similarly, the association between high levels of catecholamines and spontaneous preterm delivery remained after exclusion of women with a vascular pathology score in the top 10th percentile among any of the 5 pathology groups (Table 5), and after exclusion of women with evidence of abruption (gross, microscopic, or clinical diagnosis) or hypertensive complications (data not shown). A final series of models excluded women with either histologic chorioamnionitis or high vascular pathology scores. Although sample sizes were markedly reduced, the exclusion of these pathways to preterm delivery further strengthened the fourth quartile adjusted odds ratios for waking norepinephrine (AOR = 8.3, 95% CI: 1.1, 60.7), bedtime norepinephrine (AOR = 3.4, 95% CI: 1.0, 12.2), and waking dopamine (AOR = 6.1, 95% CI: 1.7, 22.1) in relation to spontaneous preterm delivery.
Table 5.
Associations Between Catecholamine Levels and Spontaneous Preterm Labor and Deliverya After Exclusion of Women With Placental Pathology Related to Preterm Delivery, Pregnancy Outcomes and Community Health Study, 1998–2004
| No High HCA (n = 682) |
No Top 10% Vascular Findings (n = 572) |
No High HCA and No Top 10% Vascular Findings (n = 504) |
||||
| AORb | 95% Confidence Interval | AORb | 95% Confidence Interval | AORb | 95% Confidence Interval | |
| Epinephrine | ||||||
| Waking | ||||||
| Quartile 1 | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 0.8 | 0.3, 2.0 | 0.9 | 0.4, 2.3 | 1.2 | 0.5, 3.3 |
| Quartile 3 | 0.7 | 0.3, 1.6 | 0.6 | 0.3, 1.6 | 0.5 | 0.2, 1.5 |
| Quartile 4 | 1.6 | 0.8, 3.2 | 1.1 | 0.5, 2.5 | 1.3 | 0.6, 3.2 |
| Bedtime | ||||||
| Quartile 1 | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 1.0 | 0.4, 2.2 | 0.7 | 0.3, 1.8 | 1.0 | 0.4, 2.6 |
| Quartile 3 | 0.7 | 0.3, 1.6 | 0.3 | 0.1, 0.9 | 0.3 | 0.1, 1.1 |
| Quartile 4 | 1.2 | 0.6, 2.5 | 1.0 | 0.5, 2.1 | 1.0 | 0.5, 2.5 |
| Norepinephrine | ||||||
| Waking | ||||||
| Quartile 1 | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 5.3* | 1.5, 18.1 | 5.6* | 1.3, 25.3 | 7.2 | 1.0, 54.0 |
| Quartile 3 | 3.9* | 1.1, 13.5 | 4.2 | 0.9, 19.0 | 5.6 | 0.7, 41.8 |
| Quartile 4 | 5.5* | 1.6, 18.2 | 6.0* | 1.4, 25.9 | 8.3* | 1.1, 60.7 |
| Bedtime | ||||||
| Quartile 1 | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 2.3 | 0.9, 6.5 | 1.9 | 0.7, 5.3 | 2.3 | 0.6, 8.7 |
| Quartile 3 | 2.0 | 0.7, 5.7 | 1.6 | 0.6, 4.3 | 1.7 | 0.4, 6.4 |
| Quartile 4 | 3.7* | 1.4, 9.7 | 2.5 | 1.0, 6.3 | 3.4* | 1.0, 12.2 |
| Dopamine | ||||||
| Waking | ||||||
| Quartile 1 | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 2.0 | 0.7, 5.7 | 1.5 | 0.4, 5.1 | 3.0 | 0.7, 12.7 |
| Quartile 3 | 5.0* | 2.0, 13.3 | 3.7* | 1.3, 11.1 | 7.4* | 2.0, 27.1 |
| Quartile 4 | 3.6* | 1.4, 9.1 | 3.8* | 1.3, 10.9 | 6.1* | 1.7, 22.1 |
| Bedtime | ||||||
| Quartile 1 | 1.0 | 1.0 | 1.0 | |||
| Quartile 2 | 1.3 | 0.5, 3.1 | 1.1 | 0.4, 3.2 | 1.3 | 0.4, 4.0 |
| Quartile 3 | 2.4* | 1.1, 5.5 | 2.5* | 1.0, 6.3 | 2.1 | 0.7, 6.2 |
| Quartile 4 | 2.3* | 1.0, 5.2 | 2.4 | 0.9, 6.0 | 2.5* | 0.9, 6.9 |
Abbreviations: AOR, adjusted odds ratio; HCA, histologic chorioamnionitis.
P < 0.05.
Results are from full polytomous regression models with 4-level outcome (term = referent, medically indicated preterm delivery, premature rupture of membranes preterm delivery, and spontaneous preterm delivery), but only results from spontaneous preterm delivery are displayed in Table 5.
Adjusted for creatinine.
DISCUSSION
We found that women with higher levels of urinary catecholamines at midpregnancy were at greater risk of spontaneous preterm delivery, and the association was strengthened after exclusion of women with placental evidence of infection/inflammation and vascular complications. These findings provide compelling evidence of a potential link between a stress-related biomarker and some cases of preterm delivery.
Catecholamine release is under the control of the central nervous system (34, 35). Epinephrine is released primarily by the adrenal glands and acts as a circulating hormone (35). Norepinephrine and dopamine are neurotransmitters released into the circulation as a result of overflow from adrenergic and dopaminergic nerve endings and, to a lesser degree, following adrenal gland stimulation (35). The mechanisms that link catecholamine levels with preterm delivery may be indirect, as catecholamines affect constriction/dilatation of blood vessels, mobilization of fatty acids, and insulin secretion (36). Through more direct pathways, catecholamines can influence uterine contractions by binding to α1 (contraction) and β2 (relaxation) adrenergic receptors in the uterus (37) and, perhaps, by upregulating oxytocin receptors (38) or increasing prostaglandin production in the amnion, decidua, and myometrial tissues (39, 40). Early studies have demonstrated increased uterine contractility following administration of dopamine or norepinephrine to pregnant women at term (41, 42). β2-Adrenergic receptor agonists are used to treat premature labor, but they only delay delivery by up to 48 hours and have not proven successful in preventing preterm delivery (43). This may be due to a subsequent down regulation of β2 receptors in most women treated and, for some, underlying complications that are not adrenergic in origin. A lack of synchrony between catecholamine release and regulation of uterine adrenergic receptors might occur more often with chronic stress/arousal or in those genetically predisposed, which may account for the results presented here.
Both environmental and constitutional factors appear to influence basal catecholamine levels. Measures of allostatic load, a concept of body “wear and tear,” include catecholamine levels (44). Low socioeconomic status (45), stressful work (46–48), and chronic exposure to noise including traffic noise while sleeping (49) have all been associated with higher urinary catecholamine levels. Individuals experiencing anxiety symptoms (35) or posttraumatic stress disorder (50, 51), particularly when involving sexual abuse as a child and as an adult (52), have been found to have higher urinary catecholamine levels. Although early descriptions have often labeled epinephrine release a response to cognitive stressors and norepinephrine release a response to physical stressors, recent studies have shown that norepinephrine levels are increased with certain types of psychological arousal (53, 54) and a rise in all catecholamines levels is consistent with a generalized stress response (34).
Studies of catecholamines and psychological well-being in pregnant women are limited and typically small in scope. Among the more moderately sized studies, one found no significant increases in urinary catecholamine levels in relation to stress (55). Another reported higher urinary levels of epinephrine and norepinephrine but lower levels of dopamine in women with increased anxiety (56). There are few data on catecholamine levels and pregnancy outcome. Studies showing preterm delivery risk elevated in association with anxiety (15, 57), posttraumatic stress disorder episodes in pregnancy (58), and increased vascular reactivity (59) may be indirect evidence of an adrenergic-mediated pathway. In our analyses, adjustment for hypertensive disorders in pregnancy and history of previous preterm delivery did not alter the findings (data not presented), suggesting that maternal anxiety related to these conditions did not explain our results.
A limitation is that we measured catecholamines during only one point in the pregnancy and did not collect 24-hour samples of urine, yet outside a clinic setting, a 24-hour urine collection invites measurement error. We had the advantage of measuring stress hormones during the women's typical daily routine, and the 3 consecutive days of waking and bedtime samples allowed us to take account of intraindividual variability. Urinary levels of epinephrine and norepinephrine are considered a good indicator of plasma levels (60). Kidney synthesis of L-3,4-dihydroxyphenylalanine (L-DOPA) to dopamine accounts for a large amount of dopamine in the urine, but this is under the influence of sympathetic innervation via levels of the L-DOPA precursor in maternal blood. Therefore, it seems plausible to infer that chronic overactivation of the sympathetic system could result in higher urinary dopamine levels. Still we cannot rule out the possibility that unmeasured pathophysiologic changes act as confounders, increasing catecholamine levels and predisposing women to deliver preterm.
The specificity of our finding, catecholamine's link to spontaneous preterm delivery only, is consistent with a biologically plausible mechanism. By incorporating placental pathology findings, we were able to demonstrate that the link was not simply a result of infection/inflammation and vascular pathways to preterm delivery; this type of biologic data is rarely available in studies of stress hormones and preterm delivery risk. Our large sample size allowed us to test the robustness of our findings among women with different characteristics, and in all models our findings persisted. In future analyses we will probe, in greater detail, psychosocial and physiologic characteristics of POUCH Study women with higher catecholamine levels at midpregnancy in an effort to learn more about upstream factors.
Acknowledgments
Author affiliations: Department of Epidemiology, Michigan State University, East Lansing, Michigan (Claudia Holzman, Yan Tian, Bertha Bullen, Anjali Sapkal); Department of Pathology, Michigan State University, East Lansing, Michigan (Patricia Senagore); Department of Psychology, Saginaw Valley State University, University Center, Michigan (Eric DeVos); Department of Medicine, Michigan State University, East Lansing, Michigan (Cheryl Leece); Department of Production Animal Clinical Science, Norwegian School of Veterinary Science, Oslo, Norway (Adroaldo Zanella); Department of Pharmacology and Toxicology, Michigan State University, East Lansing, Michigan (Gregory Fink); Division of Epidemiology and Biostatistics, University of Texas School of Public Health, Houston, Texas (Mohammad H. Rahbar); and Center for Clinical and Translational Sciences, University of Texas Health Science Center, Houston, Texas (Mohammad H. Rahbar).
This work was supported by a Perinatal Epidemiological Research Initiative program grant from the March of Dimes Foundation (grants 20FY01-38 and 20-FY04-37), the National Institute of Child Health and Human Development and the National Institute of Nursing Research (grant R01 HD34543), the Thrasher Research Foundation (grant 02816-7), and the Centers for Disease Control and Prevention (grant U01 DP000143-01).
Conflict of interest: none declared.
Glossary
Abbreviations
- AOR
adjusted odds ratio
- CI
confidence interval
- MSAFP
maternal serum α-fetoprotein
- POUCH
Pregnancy Outcomes and Community Health
References
- 1.Callaghan WM, MacDorman MF, Rasmussen SA, et al. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 2.Lindström K, Winbladh B, Haglund B, et al. Preterm infants as young adults: a Swedish national cohort study. Pediatrics. 2007;120(1):70–77. doi: 10.1542/peds.2006-3260. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56(6):1–103. [PubMed] [Google Scholar]
- 4.Qin C, Dietz PM, England LJ, et al. Effects of different data-editing methods on trends in race-specific preterm delivery rates, United States, 1990–2002. Paediatr Perinat Epidemiol. 2007;21(suppl 2):41–49. doi: 10.1111/j.1365-3016.2007.00860.x. [DOI] [PubMed] [Google Scholar]
- 5.Luo ZC, Wilkins R, Kramer MS. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. CMAJ. 2006;174(10):1415–1420. doi: 10.1503/cmaj.051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fairley L, Leyland AH. Social class inequalities in perinatal outcomes: Scotland 1980–2000. J Epidemiol Community Health. 2006;60(1):31–36. doi: 10.1136/jech.2005.038380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisselmann MD, Hemström O. The contribution of maternal working conditions to socio-economic inequalities in birth outcome. Soc Sci Med. 2008;66(6):1297–1309. doi: 10.1016/j.socscimed.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87(6):590–600. doi: 10.1080/00016340802005126. [DOI] [PubMed] [Google Scholar]
- 10.Slattery MM, Geary M, Morrison JJ. Obstetric antecedents for preterm delivery. J Perinat Med. 2008;36(4):306–309. doi: 10.1515/JPM.2008.045. [DOI] [PubMed] [Google Scholar]
- 11.Moreau C, Kaminski M, Ancel PY, et al. Previous induced abortions and the risk of very preterm delivery: results of the EPIPAGE study. BJOG. 2005;112(4):430–437. doi: 10.1111/j.1471-0528.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 12.Wadhwa PD, Culhane JF, Rauh V, et al. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001;5(2):119–125. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- 13.Hogue CJ, Bremner JD. Stress model for research into preterm delivery among black women. Am J Obstet Gynecol. 2005;192(suppl 5):S47–S55. doi: 10.1016/j.ajog.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 14.Hobel CJ, Goldstein A, Barrett ES. Psychosocial stress and pregnancy outcome. Clin Obstet Gynecol. 2008;51(2):333–348. doi: 10.1097/GRF.0b013e31816f2709. [DOI] [PubMed] [Google Scholar]
- 15.Copper RL, Goldenberg RL, Das A, et al. The preterm prediction study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175(5):1286–1292. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]
- 16.Glynn LM, Schetter CD, Hobel CJ, et al. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008;27(1):43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 17.Hedegaard M, Henriksen TB, Secher NJ, et al. Do stressful life events affect duration of gestation and risk of preterm delivery? Epidemiology. 1996;7(4):339–345. doi: 10.1097/00001648-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman S, Hatch MC. Stress, social support and pregnancy outcome: a reassessment based on recent research. Paediatr Perinat Epidemiol. 1996;10(4):380–405. doi: 10.1111/j.1365-3016.1996.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu MC, Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. 2004;191(3):691–699. doi: 10.1016/j.ajog.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Lobel M, DeVincent CJ, Kaminer A, et al. The impact of prenatal maternal stress and optimistic disposition on birth outcomes in medically high-risk women. Health Psychol. 2000;19(6):544–553. doi: 10.1037//0278-6133.19.6.544. [DOI] [PubMed] [Google Scholar]
- 21.Erickson K, Thorsen P, Chrousos G, et al. Preterm birth: associated neuroendocrine, medical, and behavioral risk factors. J Clin Endocrinol Metab. 2001;86(6):2544–2552. doi: 10.1210/jcem.86.6.7607. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz RJ, Fullerton J, Brown CE, et al. Relationships of cortisol, perceived stress, genitourinary infections, and fetal fibronectin to gestational age at birth. Biol Res Nurs. 2001;3(1):39–48. doi: 10.1177/109980040100300106. [DOI] [PubMed] [Google Scholar]
- 23.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27(6):1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Mercer BM, Macpherson CA, Goldenberg RL, et al. Are women with recurrent spontaneous preterm births different from those without such history? Am J Obstet Gynecol. 2006;194(4):1176–1184. doi: 10.1016/j.ajog.2006.01.069. discussion 1184–1185. [DOI] [PubMed] [Google Scholar]
- 25.Holzman C, Bullen B, Fisher R, et al. Pregnancy outcomes and community health: the POUCH Study of preterm delivery. Paediatr Perinat Epidemiol. 2001;15(suppl 2):136–158. doi: 10.1046/j.1365-3016.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton MP, Abdalla HI, Whitfield CR. Significance of raised maternal serum alpha-fetoprotein in singleton pregnancies with normally formed fetuses. Obstet Gynecol. 1985;65(4):465–470. [PubMed] [Google Scholar]
- 27.Vogel I, Thorsen P, Curry A, et al. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand. 2005;84(6):516–525. doi: 10.1111/j.0001-6349.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 28.Jones NM, Holzman CB, Zanella AJ, et al. Assessing mid-trimester salivary cortisol levels across three consecutive days in pregnant women using an at-home collection protocol. Paediatr Perinat Epidemiol. 2006;20(5):425–437. doi: 10.1111/j.1365-3016.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 29.Hollenbach E, Schulz C, Lehnert H. Rapid and sensitive determination of catecholamines and the metabolite 3-methoxy-4-hydroxyphen-ethyleneglycol using HPLC following novel extraction procedures. Life Sci. 1998;63(9):737–750. doi: 10.1016/s0024-3205(98)00329-4. [DOI] [PubMed] [Google Scholar]
- 30.Holzman C, Lin X, Senagore P, et al. Histologic chorioamnionitis and preterm delivery. Am J Epidemiol. 2007;166(7):786–794. doi: 10.1093/aje/kwm168. [DOI] [PubMed] [Google Scholar]
- 31.Kelly R, Holzman C, Senagore P, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009;170(2):148–158. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savitz DA, Dole N, Herring AH, et al. Should spontaneous and medically indicated preterm births be separated for studying aetiology? Paediatr Perinat Epidemiol. 2005;19(2):97–105. doi: 10.1111/j.1365-3016.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- 33.SAS Institute, Inc. SAS 9.1.3 (TS1M3) Cary, NC: SAS Institute, Inc; 2007. (SAS 9.1.3 foundation with SAS Service Pack 4 Education Analytical Suite) [Google Scholar]
- 34.de Diego AM, Gandía L, García AG. A physiological view of the central and peripheral mechanisms that regulate the release of catecholamines at the adrenal medulla. Acta Physiol (Oxf) 2008;192(2):287–301. doi: 10.1111/j.1748-1716.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- 35.Kopin IJ. Avenues of investigation for the role of catecholamines in anxiety. Psychopathology. 1984;17(suppl 1):83–97. doi: 10.1159/000284081. [DOI] [PubMed] [Google Scholar]
- 36.Young JB, Landsberg L. Catecholamines and the adrenal medulla. In: Wilson JD, Foster DW, Kronenberg HM, et al., editors. Williams Textbook of Endocrinology. Philadelphia, PA: WB Saunders Company; 1998. pp. 665–728. [Google Scholar]
- 37.Nakanishi H, McLean J, Wood C, et al. The role of sympathetic nerves in control of the nonpregnant and pregnant human uterus. J Reprod Med. 1969;11:20–33. [Google Scholar]
- 38.Engstrom T, Bratholm P, Christensen NJ, et al. Up-regulation of oxytocin receptors in non-pregnant rat myometrium by isoproterenol: effects of steroids. J Endocrinol. 1999;161(3):403–411. doi: 10.1677/joe.0.1610403. [DOI] [PubMed] [Google Scholar]
- 39.Tada K, Kudo T, Kishimoto Y. Effects of L-dopa or dopamine on human decidual prostaglandin synthesis. Acta Med Okayama. 1991;45(5):333–338. doi: 10.18926/AMO/32193. [DOI] [PubMed] [Google Scholar]
- 40.Quaas L, Zahradnik HP. The effects of alpha- and beta-adrenergic stimulation on contractility and prostaglandin (prostaglandins E2 and F2 alpha and 6-keto-prostaglandin F1 alpha) production of pregnant human myometrial strips. Am J Obstet Gynecol. 1985;152(7 pt 1):852–856. doi: 10.1016/s0002-9378(85)80076-4. [DOI] [PubMed] [Google Scholar]
- 41.Urban J, Radwan J, Laudański T, et al. Dopamine influence on human uterine activity at term pregnancy. Br J Obstet Gynaecol. 1982;89(6):451–455. doi: 10.1111/j.1471-0528.1982.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 42.Zuspan FP, Cibils LA, Pose SV. Myometrial and cardiovascular responses to alterations in plasma epinephrine and norepinephrine. Am J Obstet Gynecol. 1962;84:841–851. doi: 10.1016/0002-9378(62)90057-1. [DOI] [PubMed] [Google Scholar]
- 43.Anotayanonth S, Subhedar NV, Garner P, et al. Betamimetics for inhibiting preterm labour. Cochrane Database Syst Rev. 2004;(4):CD004352. doi: 10.1002/14651858.CD004352.pub2. [DOI] [PubMed] [Google Scholar]
- 44.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 45.Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006;68(3):414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 46.Katz VL, Jenkins T, Haley L, et al. Catecholamine levels in pregnant physicians and nurses: a pilot study of stress and pregnancy. Obstet Gynecol. 1991;77(3):338–342. [PubMed] [Google Scholar]
- 47.van der Beek AJ, Meijman TF, Frings-Dresen MH, et al. Lorry drivers’ work stress evaluated by catecholamines excreted in urine. Occup Environ Med. 1995;52(7):464–469. doi: 10.1136/oem.52.7.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujiwara K, Tsukishima E, Kasai S, et al. Urinary catecholamines and salivary cortisol on workdays and days off in relation to job strain among female health care providers. Scand J Work Environ Health. 2004;30(2):129–138. doi: 10.5271/sjweh.770. [DOI] [PubMed] [Google Scholar]
- 49.Babisch W, Fromme H, Beyer A, et al. Increased catecholamine levels in urine in subjects exposed to road traffic noise: the role of stress hormones in noise research. Environ Int. 2001;26(7–8):475–481. doi: 10.1016/s0160-4120(01)00030-7. [DOI] [PubMed] [Google Scholar]
- 50.Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57(2):105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Breslau N. Neurobiological research on sleep and stress hormones in epidemiological samples. Ann N Y Acad Sci. 2006 doi: 10.1196/annals.1364.017. 1071 (Psychobiology of Posttraumatic Stress Disorder: A Decade of Progress):221–230. [DOI] [PubMed] [Google Scholar]
- 52.Friedman MJ, Jalowiec J, McHugo G, et al. Adult sexual abuse is associated with elevated neurohormone levels among women with PTSD due to childhood sexual abuse. J Trauma Stress. 2007;20(4):611–617. doi: 10.1002/jts.20221. [DOI] [PubMed] [Google Scholar]
- 53.Weber CS, Thayer JF, Rudat M, et al. Emotional irritation before mental stress is associated with enhanced peripheral norepinephrine. Scand J Psychol. 2007;48(6):459–466. doi: 10.1111/j.1467-9450.2007.00612.x. [DOI] [PubMed] [Google Scholar]
- 54.Hughes JW, Watkins L, Blumenthal JA, et al. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res. 2004;57(4):353–358. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 55.Petraglia F, Hatch MC, Lapinski R, et al. Lack of effect of psychosocial stress on maternal corticotropin-releasing factor and catecholamine levels at 28 weeks’ gestation. J Soc Gynecol Investig. 2001;8(2):83–88. [PubMed] [Google Scholar]
- 56.Field T, Diego M, Hernandez-Reif M, et al. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depress Anxiety. 2003;17(3):140–151. doi: 10.1002/da.10071. [DOI] [PubMed] [Google Scholar]
- 57.Dayan J, Creveuil C, Herlicoviez M, et al. Role of anxiety and depression in the onset of spontaneous preterm labor. Am J Epidemiol. 2002;155(4):293–301. doi: 10.1093/aje/155.4.293. [DOI] [PubMed] [Google Scholar]
- 58.Rogal SS, Poschman K, Belanger K, et al. Effects of posttraumatic stress disorder on pregnancy outcomes. J Affect Disord. 2007;102(1–3):137–143. doi: 10.1016/j.jad.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCubbin JA, Lawson EJ, Cox S, et al. Prenatal maternal blood pressure response to stress predicts birth weight and gestational age: a preliminary study. Am J Obstet Gynecol. 1996;175(3 pt 1):706–712. doi: 10.1053/ob.1996.v175.a74286. [DOI] [PubMed] [Google Scholar]
- 60.Akerstedt T, Gillberg M, Hjemdahl P, et al. Comparison of urinary and plasma catecholamine responses to mental stress. Acta Physiol Scand. 1983;117(1):19–26. doi: 10.1111/j.1748-1716.1983.tb07174.x. [DOI] [PubMed] [Google Scholar]