Abstract
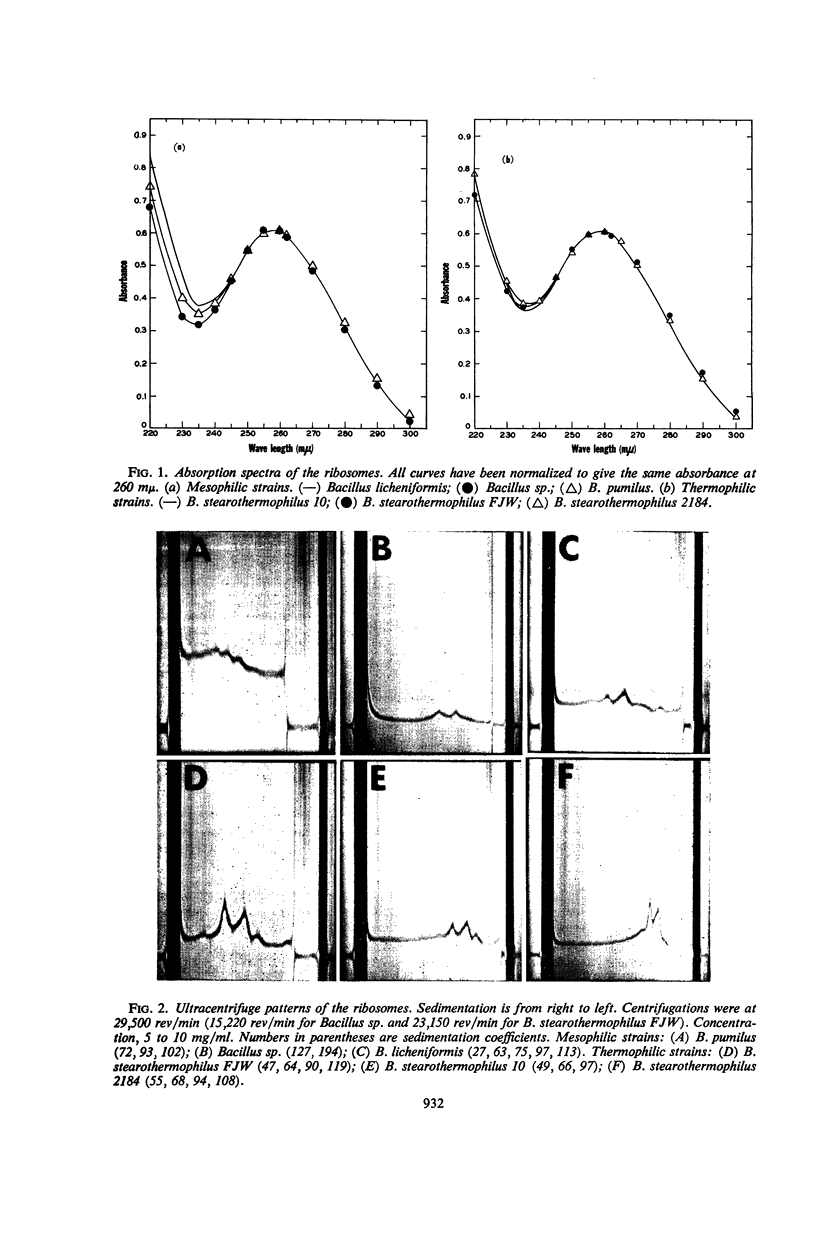
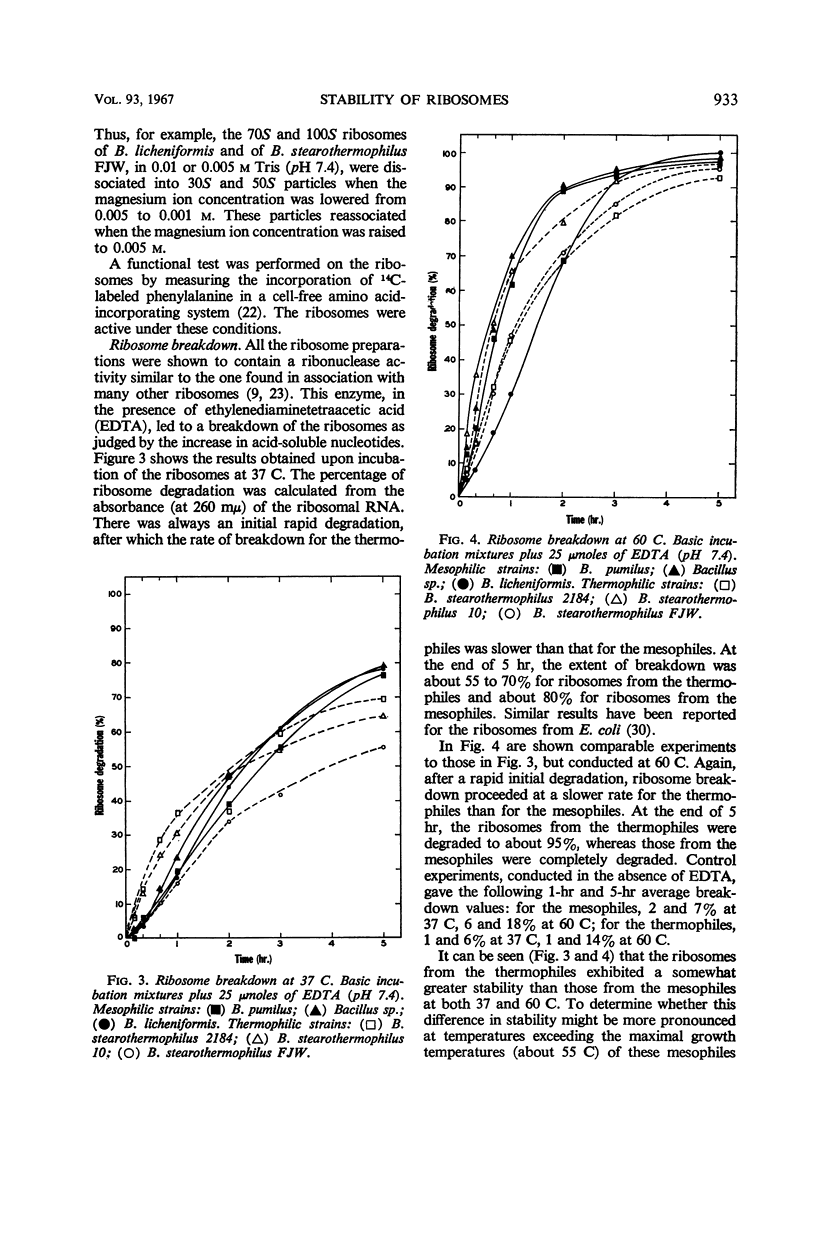
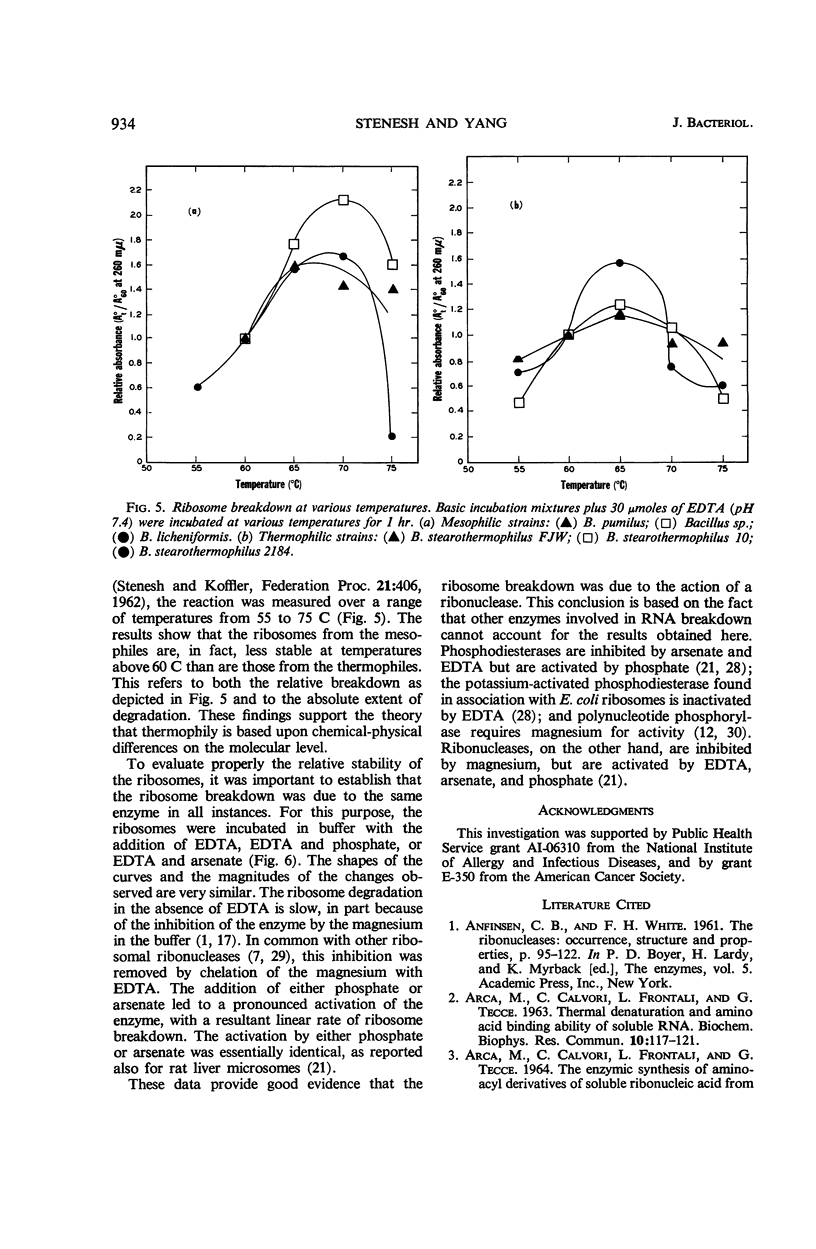
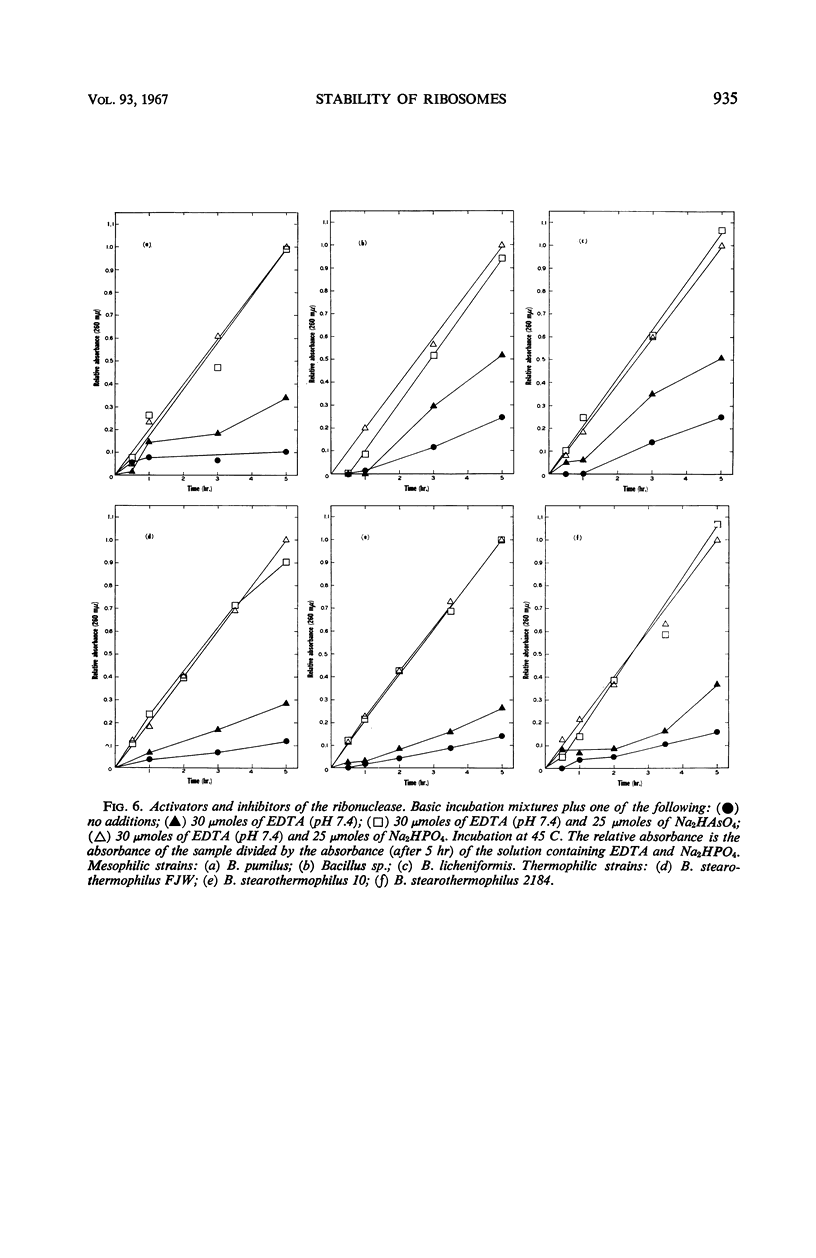
Ribosomes were isolated from three mesophilic and three thermophilic strains of Bacillus. The ribosomes consisted of about 55% protein and 45% ribonucleic acid. Average ratios for the absorbance at 260/235 and 260/280 mμ were 1.77 and 1.92 for the mesophiles and 1.63 and 1.84 for the thermophiles. Ultracentrifugation revealed mainly components with sedimentation coefficients of about 30, 50, 70, 100, and 120S. All the preparations were shown to contain a ribonuclease which, in the presence of ethylenediaminetetraacetic acid, led to ribosome breakdown as measured by the increase in acid-soluble nucleotides. The stability of the ribosomes from the thermophiles was consistently greater than that of the ribosomes from the mesophiles. After 5 hr at 37 C, the breakdown was about 80% for the ribosomes from the mesophiles and 55 to 70% for those from the thermophiles. At 60 C, the ribosomes from the mesophiles were broken down slightly more and at a faster rate than those from the thermophiles. At temperatures above 60 C, the breakdown was again more pronounced for the ribosomes from the mesophiles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCA M., CALVORI C., FRONTALI L., TECCE G. Thermal denaturation and amino acid binding ability of soluble RNA. Biochem Biophys Res Commun. 1963 Jan 31;10:117–121. doi: 10.1016/0006-291x(63)90035-4. [DOI] [PubMed] [Google Scholar]
- Bausum H. T., Matney T. S. Boundary Between Bacterial Mesophilism and Thermophilism. J Bacteriol. 1965 Jul;90(1):50–53. doi: 10.1128/jb.90.1.50-53.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr Purification and properties of an alpha-amylase from facultative thermophilic bacteria. Arch Biochem Biophys. 1955 Jan;54(1):154–161. doi: 10.1016/0003-9861(55)90018-7. [DOI] [PubMed] [Google Scholar]
- DICKMAN S. R., TRUPIN K. M. Bound and latent mouse-pancreas ribonucleases. Biochim Biophys Acta. 1958 Oct;30(1):200–201. doi: 10.1016/0006-3002(58)90267-1. [DOI] [PubMed] [Google Scholar]
- ELSON D. Latent ribonuclease activity in a ribonucleoprotein. Biochim Biophys Acta. 1958 Jan;27(1):216–217. doi: 10.1016/0006-3002(58)90320-2. [DOI] [PubMed] [Google Scholar]
- ELSON D. Preparation and properties of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:362–371. doi: 10.1016/0006-3002(59)90178-7. [DOI] [PubMed] [Google Scholar]
- Imsande J., Caston J. D. Synthesis of protein with a cell-free system from Bacillus cereus 569. J Mol Biol. 1966 Mar;16(1):28–41. doi: 10.1016/s0022-2836(66)80260-7. [DOI] [PubMed] [Google Scholar]
- Koffler H., Mallett G. E., Adye J. MOLECULAR BASIS OF BIOLOGICAL STABILITY TO HIGH TEMPERATURES. Proc Natl Acad Sci U S A. 1957 Jun 15;43(6):464–477. doi: 10.1073/pnas.43.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANNING G. B., CAMPBELL L. L. Thermostable alpha-amylase of Bacillus stearothermophilus. I. Crystallization and some general properties. J Biol Chem. 1961 Nov;236:2952–2957. [PubMed] [Google Scholar]
- MORAIS R., DE LAMIRANDE G. AUTODEGRADATION OF RIBONUCLEIC ACID OF RAT-LIVER MICROSOMES. Biochim Biophys Acta. 1965 Jan 11;95:40–47. doi: 10.1016/0005-2787(65)90208-x. [DOI] [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L. Characterization of a thermophilic bacteriophage for Bacillus stearothermophilus. J Bacteriol. 1966 Jan;91(1):340–348. doi: 10.1128/jb.91.1.340-348.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L. Ribonucleic acid and ribosomes of Bacillus stearothermophilus. J Bacteriol. 1966 Jan;91(1):332–339. doi: 10.1128/jb.91.1.332-339.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F., Tolbert G. Purification and properties of a potassium-activated phosphodiesterase (RNAase II) from Escherichia coli. Biochemistry. 1965 Jul;4(7):1319–1330. doi: 10.1021/bi00883a016. [DOI] [PubMed] [Google Scholar]
- WADE H. E. The autodegradation of ribonucleoprotein in Escherichia coli. Biochem J. 1961 Mar;78:457–472. doi: 10.1042/bj0780457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade H. E., Lovett S., Robinson H. K. The autodegradation of 32-P-labelled ribosomes from Escherichia coli. Biochem J. 1964 Oct;93(1):121–128. doi: 10.1042/bj0930121. [DOI] [PMC free article] [PubMed] [Google Scholar]




