Abstract
Background
Although environmental toxins, including pesticides, are suspected of contributing to the risk of amyotrophic lateral sclerosis (ALS), no data exist from large prospective investigations. This study assessed the association between exposure to chemicals and risk of ALS in a prospective cohort study.
Methods
We prospectively assessed the relation between self-report of regular exposure to 11 different chemical classes or X-rays and ALS mortality among over 1 million participants in the American Cancer Society's Cancer Prevention Study II. Follow-up from 1989 through 2004 identified 617 deaths from ALS among men and 539 among women. We calculated adjusted rate ratios (RR) using Cox proportional hazards.
Results
The RR for ALS mortality among individuals exposed to pesticides/herbicides compared to the unexposed was 1.07 (95% confidence interval (CI): 0.79-1.44), but somewhat higher after excluding those with missing duration of pesticides exposure (RR: 1.44; 95% CI: 0.89-2.31; p=0.14). A non-significant increase in ALS mortality was found among individuals who reported exposure to formaldehyde (RR: 1.34; 95% CI: 0.93-1.92). Excluding those with missing duration of formaldehyde exposure, the RR was 2.47 (95% CI: 1.58-3.86), and there was a strongly significant dose-response relation with increasing years of exposure (p-trend=0.0004).
Conclusions
We found little evidence for an association between pesticides/herbicide exposure and ALS. In contrast, we found evidence suggesting an increased risk of ALS with formaldehyde exposure. Because of the longitudinal design, this result is unlikely to be due to bias, but it should nevertheless be interpreted cautiously and needs to be independently verified.
Keywords: motor neuron disease, pesticide, formaldehyde, prospective studies, epidemiology
A contribution of environmental exposures to the etiology of amyotrophic lateral sclerosis (ALS) is suggested by geographic variation in ALS incidence, some genetic studies, and several epidemiological studies, with pesticide exposures in particular often implicated.1-7 Most of the epidemiological findings are from case-control studies, which are prone to recall bias and biased control selection.4, 8 We therefore explored the association between exposures to pesticides and other chemicals and ALS mortality in a prospective study of the Cancer Prevention Study-II (CPS-II) cohort of the American Cancer Society.9
Methods
Study population
We prospectively followed 414,493 male and 572,736 female CPS-II cohort participants who were alive as of January 1, 1989 (earlier ALS deaths were not coded separately), reported no major illness at baseline (1982), and were not missing data on age or sex.
Case ascertainment
Vital status of the study participants was determined by automated linkage with the National Death Index (NDI). Death certificates (1989-1992) or codes for cause of death (1993-2004) were obtained for over 98% of known deaths. ALS deaths were defined as an underlying or contributing cause of death on death certificates of ICD-9 (1989-1998) code 335.2 or ICD-10 (1999-2004) code G12.2 (motor neuron disease). A review of death certificates found that virtually all such deaths had a diagnosis of ALS (98.3%) or bulbar palsy (1.1%).10
Assessment of exposure
In the baseline questionnaire, participants were asked whether they were currently exposed or had been regularly exposed in the past to the series of chemical classes found in table 1. Because the risk of ALS (fully-adjusted) among participants who left blank the answer to one or more questions on chemical exposures was virtually identical to that in the corresponding unexposed group, these individuals were considered as unexposed. Exposed individuals were further categorized according to self-reported years of exposure.
Table 1.
Baseline multivariate-adjusted rate ratio (RR) of ALS by class of chemical exposure in the entire cohort and excluding individuals reporting exposure to a given chemical class but no duration (restricted cohort).
| Exposure | Cases | Person-Years (×1000) | Multivariate RRa (95% CI) Full Cohort | p-value | Multivariate RRb (95% CI) Restricted Cohort | p-value |
|---|---|---|---|---|---|---|
| Pesticides/Herbicides | ||||||
| No | 1097 | 12,917 | Ref | Ref | ||
| Yes | 59 | 661 | 1.07 (0.79, 1.43) | 0.67 | 1.44 (0.89, 2.31) | 0.14 |
| Asbestos | ||||||
| No | 1110 | 13,092 | Ref | Ref | ||
| Yes | 46 | 485 | 1.06 (0.77, 1.46) | 0.73 | 1.12 (0.60, 2.09) | 0.73 |
| Chemicals/Acids/Solvents | ||||||
| No | 1014 | 12,070 | Ref | Ref | ||
| Yes | 142 | 1,508 | 1.05 (0.86, 1.29) | 0.61 | 1.04 (0.73, 1.49) | 0.82 |
| Coal or Stone Dusts | ||||||
| No | 1111 | 13,088 | Ref | Ref | ||
| Yes | 45 | 490 | 1.03 (0.74, 1.43) | 0.86 | 0.97 (0.48, 1.95) | 0.93 |
| Coal Tar/Pitch/Asphalt | ||||||
| No | 1142 | 13,358 | Ref | Ref | ||
| Yes | 14 | 220 | 0.54 (0.29, 1.02) | 0.06 | 0.38 (0.05, 2.74) | 0.34 |
| Diesel Engine Exhaust | ||||||
| No | 1077 | 12,637 | Ref | Ref | ||
| Yes | 79 | 940 | 0.95 (0.71, 1.26) | 0.71 | 0.68 (0.33, 1.40) | 0.30 |
| Dyes | ||||||
| No | 1132 | 13,230 | Ref | Ref | ||
| Yes | 24 | 348 | 0.75 (0.47, 1.20) | 0.23 | 0.84 (0.46, 1.54) | 0.57 |
| Formaldehyde | ||||||
| No | 1120 | 13,218 | Ref | Ref | ||
| Yes | 36 | 360 | 1.34 (0.93, 1.92) | 0.11 | 2.47 (1.58, 3.86) | <0.0001 |
| Gasoline Exhaust | ||||||
| No | 1018 | 11,966 | Ref | Ref | ||
| Yes | 138 | 1,611 | 0.90 (0.73, 1.12) | 0.35 | 0.95 (0.63, 1.42) | 0.79 |
| Textile Fibers/ Dusts | ||||||
| No | 1113 | 13,005 | Ref | Ref | ||
| Yes | 43 | 573 | 0.97 (0.70, 1.34) | 0.83 | 1.23 (0.79, 1.91) | 0.36 |
| Wood Dust | ||||||
| No | 1107 | 12,980 | Ref | Ref | ||
| Yes | 49 | 598 | 0.89 (0.65, 1.20) | 0.44 | 0.96 (0.48, 1.93) | 0.91 |
| X-rays/Radioactive Material | ||||||
| No | 1118 | 13,072 | Ref | Ref | ||
| Yes | 38 | 506 | 0.90 (0.64, 1.26) | 0.53 | 0.87 (0.51, 1.50) | 0.62 |
Adjusted for age, sex, smoking (never, former, current), military service (yes/no; all women were coded as “no” because they were not asked this question), education (some high school, completed high school, vocational/trade, some college, completed college/graduate school), alcohol intake (non-drinkers and quartiles of grams per day), occupation (farmer, laboratory technician, machine assembler, programmer), vitamin E use (never, occasional, regular <10 years, regular >10 years), and all other chemical classes.
The number of cases among the exposed in these analyses were: pesticides/herbicides, 18; asbestos, 10; chemicals/acids/solvents, 36; coal or stone dusts, 8; coal tar/pitch/asphalt, 1; diesel engine exhaust, 9; dyes, 13; formaldehyde, 22; gasoline exhaust, 30; textile fibers/dusts, 22; wood dust, 8; X-rays/radioactive material, 14.
Statistical analyses
Participants contributed follow-up time from January 1, 1989 to the date of death, or December 31, 2004 (the most recent linkage with NDI), whichever came first. We used Cox proportional hazards regression stratified by both age and calendar time in single years to estimate rate ratios (RR) and 95% confidence intervals (CI) adjusted for factors possibly related to ALS as indicated (footnote, Table 1). All chemical exposures were considered in the same model simultaneously. Covariate data were obtained from responses to the CPS-II baseline questionnaire. SAS version 9.1 was used for all analyses.
Results
Between 1989 and 2004 we documented 617 ALS deaths during 5,473,411 person-years among men and 539 ALS deaths during 8,104,402 person-years among women. There was no difference in ALS mortality rate by pesticide/herbicide exposure among the entire cohort (Table 1). When excluding those participants who reported pesticide exposure but not duration of that exposure, the association was slightly stronger (Table 1). The RRs for those reporting <4 years, 4-10 years, and >10 years of exposure to pesticides/herbicides compared to the unexposed were 0.62 (95% CI: 0.09-4.45), 1.92 (95% CI: 0.71-5.19), and 1.48 (95% CI: 0.82-2.67), respectively. Because the accuracy of the ALS diagnosis may decrease at older ages,11 we repeated the analyses with follow-up only until age 75. Results of these analyses were slightly stronger, but only when excluding participants with missing duration of exposure (RR: 1.67; 95% CI: 0.93-3.02; p=0.09). No significant association was found between exposure to pesticides and ALS mortality among farmers, whether or not individuals missing duration of exposure were excluded.
Overall, we found no significant association with ALS mortality for any of the other chemical exposures, although associations with coal tar/pitch/asphalt (decreased rate) and formaldehyde (increased rate) were suggestive (Table 1). There were too few ALS deaths among people reporting coal tar exposure and the duration of that exposure (n=1) to assess any possible trend with increasing duration of exposure. There was, however, a strong dose-response relation between years of formaldehyde exposure and ALS mortality rate that was significant at p=0.0004 (Figure 1). The overall RR for ALS mortality excluding those who reported exposure but no duration was 2.47 (95% CI: 1.58-3.86; p<0.0001). While there were many more men than women who reported exposure to formaldehyde but not the duration of exposure, the overall results and dose-response relation among those who did report duration were similar for each sex. Results were similar when limiting follow-up to age 75. None of the other chemical exposures appeared to be associated with ALS (Table 2) even when excluding those reporting exposure but not duration for a given chemical class. Similarly, none of the other chemical classes exhibited any trend with increasing duration of exposure.
Figure 1.
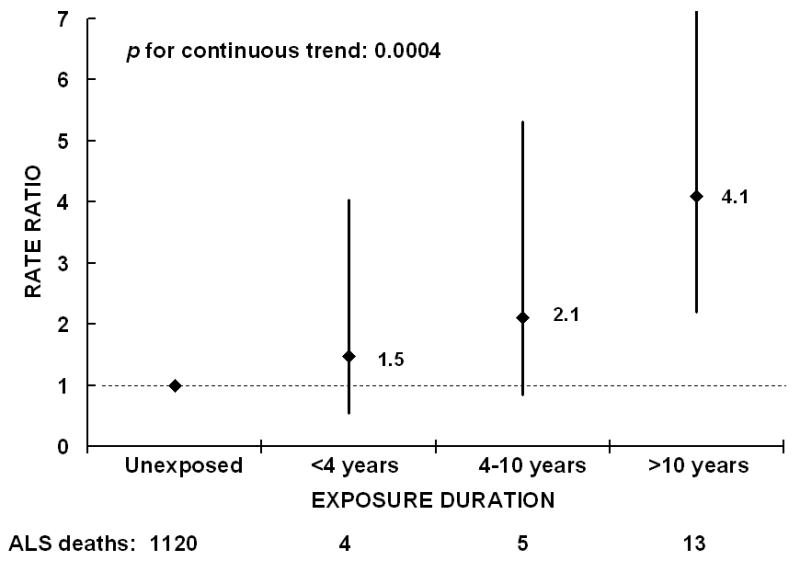
Rate ratio of Amyotrophic lateral sclerosis (ALS) mortality by years of reported exposure to formaldehyde adjusted for age, smoking, sex, military service, education, alcohol intake, occupation (farmer, laboratory technician, machine assembler, programmer), vitamin E use, and all other chemical classes, Cancer Prevention Study-II, 1989-2004. The rate ratios are indicated next to the black diamonds, and 95% confidence intervals are indicated by the vertical lines. The number of ALS deaths in each category is listed at the bottom.
Discussion
In this large prospective study we did not find an association between ALS mortality and self-reported exposure to pesticides/herbicides, although there was some indication that those reporting four or more years of such exposure may be at increased risk. The ALS death rate was also not significantly increased among individuals who reported exposure to any of the other 9 chemicals included in the survey, nor X-rays, with the possible exception of formaldehyde. Among individuals with known duration of exposure to formaldehyde, the ALS death rate was more than two times higher than among those unexposed and there was a strong and highly significant dose-response relation with years of exposure. The less pronounced increased risk of ALS with formaldehyde exposure when individuals not reporting duration of exposure were included would result if not reporting duration of exposure reflects less certain, and thus overall lower, exposure levels.
Several studies, although not all, have implicated agricultural occupations or pesticide exposures in the risk of ALS.2-5, 12 Furthermore, some reports, but not all, suggest an increased risk of ALS among people with paraoxonase-1 polymorphisms that lead to reduced organophosphate pesticide detoxification capability.7 Our prospective cohort approach is an advantage over previous case-control studies because it eliminates recall bias and avoids possible problems with control selection, but the limited exposure assessment is a disadvantage.
To our knowledge exposure to formaldehyde has not previously been suggested as a risk factor for ALS. Formaldehyde can have neurotoxic effects,13-15 including increasing oxidative stress—in part by reducing activity of superoxide dismutase—and increasing mitochondrial membrane permeability,14, 16 both of which have been implicated in ALS.17, 18 Formaldehyde exposure has generally decreased in the US since it was classified as a probable human carcinogen at high exposure levels by the US EPA in 1987, but sources of potential exposure remain widespread.19, 20 Of particular note, formaldehyde is a byproduct of cigarette smoke, which may account for up to 10-25% of indoor air formaldehyde exposure.19 Apart from age and gender, cigarette smoking is perhaps the most consistent non-genetic risk factor for ALS.1
Strengths of this study include the large sample size, prospective exposure data collection, and data on several potential confounders. The main limitations are the reliance on self-reported exposure, and lack of information about the frequency and intensity of exposures or exposure after 1982. Nor did we have information on exposure to specific pesticides. Thus we cannot exclude the possibility that a true association exists between a given chemical exposure—for example, pesticides—and ALS, but was attenuated by exposure misclassification. With respect to pesticide/herbicide exposure, however, the utility of the measure was indirectly demonstrated in a previous report of ours showing an increased risk of Parkinson's disease among those reporting exposure to pesticides/herbicides in the CPS-II Nutrition cohort,21 a subset of the full CPS-II cohort analyzed here. We also cannot rule out the possibility that the increased risk attributed to formaldehyde could be the result of exposure to some other unmeasured factor commonly associated with formaldehyde.
Additional limitations to the study include reliance on mortality rather than incidence, although any bias is likely to be small because the median survival with ALS is short (1.5-3 years)22, 23 making mortality a good surrogate for incidence. Additionally, death certificates accurately identify an estimated 70-90% of ALS or motor neuron disease cases.24, 25 Thus, a small number of ALS deaths will have been attributed to other causes in CPS-II. If people with a given chemical exposure were less likely to have ALS reported on the death certificate—perhaps related to socioeconomic status—then ALS mortality could be underestimated in that group. It is less likely that a diagnosis of ALS would be made on the death certificate in patients who did not have the disease because the diagnosis becomes manifest with disease progression. The general validity of our ALS assessment is supported by the similarity of age-specific mortality rates in our population and incidence rates in a Washington State study—with a slight lag because mortality reflects incidence at younger ages.10
In summary, in this large prospective study we found only a slight, not statistically significant suggestion of increased risk of ALS mortality by self-reported exposure to pesticides/herbicides. In contrast, we found evidence suggesting an increased risk of ALS with formaldehyde exposure, including a strong trend with increasing years of exposure. Because of the longitudinal design of the study this finding is unlikely to be due to bias, but it should nevertheless be interpreted cautiously and needs to be independently verified.
Acknowledgments
Funding: This study was supported by Research Grant W81XWH-05-1-0117 from the US Department of Defense. Dr. Weisskopf was supported by career development award K01 ES012653 from the National Institute of Environmental Health Sciences.
Footnotes
Disclosures: The authors report no conflicts of interest.
The authors have no competing interests to declare.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its licencees, to permit this article (if accepted) to be published in JNNP and any other BMJ Group products and to exploit all subsidiary rights, as set out in our licence (http://jnnp.bmjjournals.com//ifora/licence.pdf).
References
- 1.Armon C. An evidence-based medicine approach to the evaluation of the role of exogenous risk factors in sporadic amyotrophic lateral sclerosis. Neuroepidemiology. 2003;22:217–228. doi: 10.1159/000070562. [DOI] [PubMed] [Google Scholar]
- 2.Brooks BR. Risk factors in the early diagnosis of ALS: North American epidemiological studies. ALS CARE Study Group. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(Suppl 1):S19–26. doi: 10.1080/14660820052415871. [DOI] [PubMed] [Google Scholar]
- 3.McGuire V, Longstreth WT, Jr, Nelson LM, et al. Occupational exposures and amyotrophic lateral sclerosis. A population- based case-control study. Am J Epidemiol. 1997;145:1076–1088. doi: 10.1093/oxfordjournals.aje.a009070. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JD. Amyotrophic lateral sclerosis: toxins and environment. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:235–250. doi: 10.1080/14660820050515061. [DOI] [PubMed] [Google Scholar]
- 5.Morahan JM, Pamphlett R. Amyotrophic lateral sclerosis and exposure to environmental toxins: an Australian case-control study. Neuroepidemiology. 2006;27:130–135. doi: 10.1159/000095552. [DOI] [PubMed] [Google Scholar]
- 6.Nelson LM. Epidemiology of ALS. Clin Neurosci. 1996;3:327–331. [PubMed] [Google Scholar]
- 7.Schymick JC, Talbot K, Traynor BJ. Genetics of sporadic amyotrophic lateral sclerosis. Hum Mol Genet. 2007;Spec No. 2:16. R233–242. doi: 10.1093/hmg/ddm215. [DOI] [PubMed] [Google Scholar]
- 8.Armon C. Environmental risk factors for amyotrophic lateral sclerosis. Neuroepidemiology. 2001;20:2–6. doi: 10.1159/000054751. [DOI] [PubMed] [Google Scholar]
- 9.Thun MJ, Calle EE, Rodriguez C, et al. Epidemiological research at the American Cancer Society. Cancer Epidemiol Biomarkers Prev. 2000;9:861–868. [PubMed] [Google Scholar]
- 10.Weisskopf MG, McCullough ML, Calle EE, et al. Prospective study of cigarette smoking and amyotrophic lateral sclerosis. Am J Epidemiol. 2004;160:26–33. doi: 10.1093/aje/kwh179. [DOI] [PubMed] [Google Scholar]
- 11.Logroscino G, Traynor BJ, Hardiman O, et al. Descriptive epidemiology of amyotrophic lateral sclerosis: new evidence and unsolved issues. J Neurol Neurosurg Psychiatry. 2008;79:6–11. doi: 10.1136/jnnp.2006.104828. [DOI] [PubMed] [Google Scholar]
- 12.Weisskopf MG, McCullough ML, Morozova N, et al. Prospective study of occupation and amyotrophic lateral sclerosis mortality. Am J Epidemiol. 2005;162:1146–1152. doi: 10.1093/aje/kwi343. [DOI] [PubMed] [Google Scholar]
- 13.Aslan H, Songur A, Tunc AT, et al. Effects of formaldehyde exposure on granule cell number and volume of dentate gyrus: a histopathological and stereological study. Brain Res. 2006;1122:191–200. doi: 10.1016/j.brainres.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Gurel A, Coskun O, Armutcu F, et al. Vitamin E against oxidative damage caused by formaldehyde in frontal cortex and hippocampus: biochemical and histological studies. J Chem Neuroanat. 2005;29:173–178. doi: 10.1016/j.jchemneu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Nie CL, Wang XS, Liu Y, et al. Amyloid-like aggregates of neuronal tau induced by formaldehyde promote apoptosis of neuronal cells. BMC Neurosci. 2007;8:9. doi: 10.1186/1471-2202-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strubelt O, Younes M, Pentz R, et al. Mechanistic study on formaldehyde-induced hepatotoxicity. J Toxicol Environ Health. 1989;27:351–366. doi: 10.1080/15287398909531306. [DOI] [PubMed] [Google Scholar]
- 17.Andersen PM. Amyotrophic lateral sclerosis associated with mutations in the CuZn superoxide dismutase gene. Curr Neurol Neurosci Rep. 2006;6:37–46. doi: 10.1007/s11910-996-0008-9. [DOI] [PubMed] [Google Scholar]
- 18.Norenberg MD, Rao KV. The mitochondrial permeability transition in neurologic disease. Neurochem Int. 2007;50:983–997. doi: 10.1016/j.neuint.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ATSDR. Toxicological profile for Formaldehyde. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 1999. [Google Scholar]
- 20.US Consumer Product and Safety Commission. An update on formaldehyde 1997 revision. Washington DC: U.S. Government Printing Office; 1997. [Google Scholar]
- 21.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson's disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- 22.del Aguila MA, Longstreth WT, Jr, McGuire V, et al. Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology. 2003;60:813–819. doi: 10.1212/01.wnl.0000049472.47709.3b. [DOI] [PubMed] [Google Scholar]
- 23.Louwerse ES, Visser CE, Bossuyt PM, et al. Amyotrophic lateral sclerosis: mortality risk during the course of the disease and prognostic factors. The Netherlands ALS Consortium. J Neurol Sci. 1997;152 1:S10–17. doi: 10.1016/s0022-510x(97)00238-4. [DOI] [PubMed] [Google Scholar]
- 24.Buckley J, Warlow C, Smith P, et al. Motor neuron disease in England and Wales, 1959-1979. J Neurol Neurosurg Psychiatry. 1983;46:197–205. doi: 10.1136/jnnp.46.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chio A, Magnani C, Oddenino E, et al. Accuracy of death certificate diagnosis of amyotrophic lateral sclerosis. J Epidemiol Community Health. 1992;46:517–518. doi: 10.1136/jech.46.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]


