Abstract
The phenotype of HIV-associated Neurocognitive Disorders (HAND) in the developed world has changed with the broad institution of HAART and with aging of the HIV+ population. Extrapyramidal motor signs were a prominent feature of HAND as defined in the early stages of the epidemic but has not be re-evaluated in the era of HAART. Moreover, the contribution of aging to extrapyramidal motor signs in the context of HIV remains undefined. We examined these questions among the 229 HIV+ participants in the Hawaii Aging with HIV Cohort compared to age-, gender-, and ethnicity-matched HIV-negative controls. Extrapyramidal motor signs were quantified using the motor exam of the Unified Parkinson’s Disease Rating Scale (UPDRSmotor) and compared to concurrent neuropsychological and clinical cognitive diagnostic categorization. The mean UPDRSmotor score increased with older age (1.68 vs. 3.35, p<0.001) and with HIV status (1.18 vs. 3.56, p<0.001). Age group (p=0.024), HIV status (p<0.001), and the interaction between age and HIV (p=0.026) were significantly associated with UPDRSmotor score. Among HIV+ patients, the mean UPDRSmotor score increased with worsening cognitive diagnostic category (p<0.001) where it was 2.06 (2.31) in normal cognition (n=110), 3.21 (3.48) in MCMD (n=84), and 5.72 (5.01) in HAD (n=37). We conclude that extrapyramidal motor signs are increased in HIV in the era of HAART and that the impact of HIV on extrapyramidal motor signs is exacerbated by aging. These results highlight the importance of a careful neurological examination in the evaluation of HIV patients.
Keywords: HIV, age, UPDRS
Introduction
The basal ganglia and frontal white matter are preferential sites of neuropathology in HIV (Glass et al, 1995; Neuen-Jacob et al, 1993). Imaging studies confirm this pattern with subcortical white matter hyper-intensities in MRI fluid-attenuated inversion recovery (FLAIR) sequences (Harrison et al, 1998; Pomara et al, 2001), altered anisotropy in the frontal white matter in diffusion tensor imaging (DTI) (Filippi et al, 2001) and altered inflammatory metabolites in MRI spectroscopy (MRS) (Suwanwelaa et al, 2000; Yiannoutsos et al, 2004). Positron emission tomography (PET) studies of HAD patients suggest that higher plasma HIV RNA levels are associated with reduced dopamine transporters (DAT) within the caudate and putamen (Wang et al, 2004). There is also evidence of atrophy and reduced blood flow in the caudate of cognitively impaired HIV+ patients (Ances et al, 2006).
Consistent with this neuropathology, motor impairment and particularly extrapyramidal signs were prominent in the Minor Cognitive Motor Disorder (MCMD) and HAD diagnostic criteria originally defined in 1991 (Working Group 1991). However, the phenotype of HAD has changed within the era of HAART (Brew, 2004; Sacktor, 2002), prompting a re-evaluation of characteristics and risk factors (McArthur et al, 2003). In particular, a large group of older HIV patients has emerged, among whom the effects of normal aging may interact with the effects HIV on motor function. This interaction could alter the sensitivity of extrapyramidal motor signs with respect to HAD (Luther and Wilkin, 2007).
Studies that include clinical evaluations or clinical diagnoses identify aging as a risk for HAD in the pre-HAART and HAART eras (Chiesi et al, 1996; Janssen et al, 1992; Valcour et al, 2004a). However, several recent neuropsychological testing studies provide mixed results regarding increased risk for neuropsychological impairment in aged HIV patients in the absence of AIDS (Becker et al, 2004; Cherner et al, 2004; Kissel et al, 2005). Since extrapyramidal motor signs increase risk for HAD (Stern et al, 2001), these discrepant results provide additional motivation for clarifying the prevalence of extrapyramidal motor signs in HIV in a way that adequately addresses the impact of normal aging on motor function.
The Hawaii Aging with HIV Cohort (HAHC) was designed specifically to address the neurological epidemiology of aging with HIV infection. Previous work identified an increased risk for dementia (Valcour et al, 2004a) and for neuropathy (Watters et al, 2004) associated with aging. In this study, we examined the association of older age, HIV infection, and the interaction of HIV and older age with extrapyramidal motor signs. We also display the frequency of extrapyramidal motor signs among groups with various levels of cognitive impairment. We hypothesized that the mean number of extrapyramidal motor signs would be higher in the HIV+ than in the HIV-negative group, higher in the older than in the younger group, and that the magnitude of the effect observed in the HIV+ group would depend on their age group.
Results
Five hundred-sixteen individuals were enrolled into the targeted age cohorts. Individuals with external factors thought to confound our ability to interpret neuropsychological data (e.g. active substance abuse, anxiety, focal motor findings, stroke, etc) were not included, resulting in 433 evaluable cases, 229 (52.8%) of whom were HIV+. By study design, we enrolled individuals who were over 50 years of age (n = 121) or under 40 years of age (n = 108). Cohort members in the older and younger HIV groups were closely matched to HIV-negative control groups on age, education, ethnicity and gender. The older HIV+ group reported higher mean years of educational attainment when compared to the younger HIV+ group (table 1).
Table 1.
Demographic characteristics of the Hawaii Aging with HIV Cohort. Within each age strata, the HIV (+) and HIV (−) groups were equivalent on age, education and gender distribution.
| Younger Groups | Older Groups | |||
|---|---|---|---|---|
| HIV (−) | HIV (+) | HIV (−) | HIV (+) | |
| sample size | 98 | 108 | 106 | 121 |
| age, mean (SD) * | 34.9 (5.1) | 35.0 (4.8) | 55.4 (5.2) | 55.5 (5.4) |
| % female* | 25 | 28 | 10 | 8 |
| Education* | 13.3 (1.9) | 13.7 (2.3) | 14.8 (2.5) | 15.2 (2.7) |
| CD4 count | 433 (220) | ----- | 483 (263) | ----- |
| % detectable VL | 55% | ----- | 47% | ----- |
HIV groups differed by age (p<0.001), gender (p=0.002), and education (p=0.011).
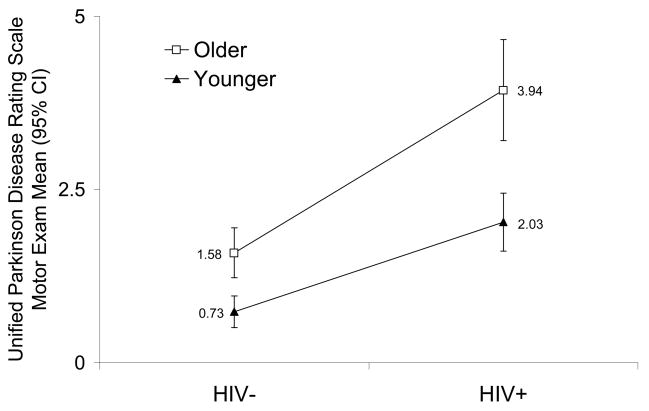
The sizes of the effects (β coefficients) for the main study design factors were all clinically substantive and statistically significant in a regression model: HIV+ status (β = 1.30, SE = 0.37, p < 0.05), older age group (β = 0.85, SE = 0.38, p < 0.001) and the interaction between HIV+ status and older age group (β = 1.15, SE =0.52, p < 0.05). Among seronegative controls, the mean (SD) UPDRSmotor scores were 1.58 (1.89) compared to 0.73 (1.16) (p < 0.001), whereas in the HIV+ groups, the scores were 4.04 (4.13) compared to 2.04 (2.21) (p < 0.001) for older and younger groups, respectively (figure 1).
Figure 1.
Unified Parkinson Disease Rating Scale Motor Exam (UPDRSmotor) by HAHC Arm
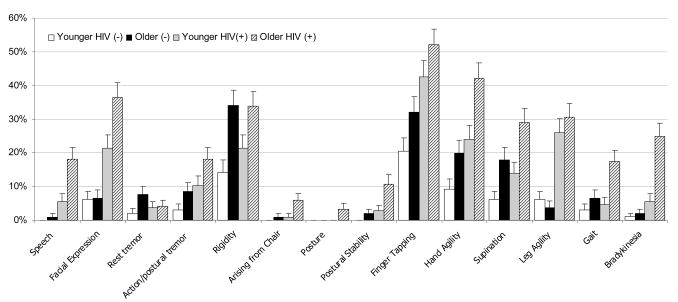
Among HIV+ subjects, the best predictors of the UPDRSmotor score were slowness of hand movements (hand agility), body bradykinesia, action/postural tremor, and facial expression (hypomimia) (figure 2). These four signs accounted for 83% of the variance in the total scores. The least useful extrapyramidal sign was resting tremor.
Figure 2.
Frequency of extrapyramidal motor signs by study arm.
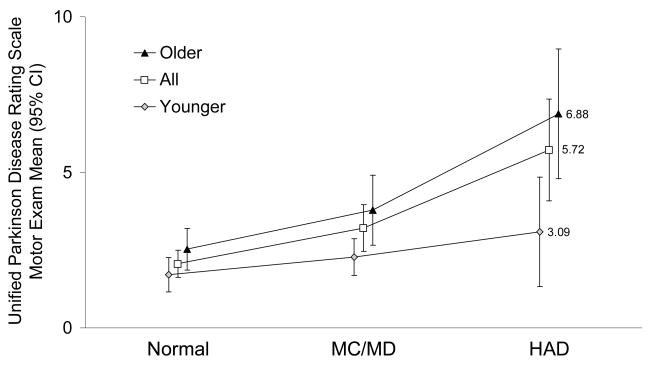
The UPDRSmotor scores among all HIV+ cohort members varied by cognitive diagnosis (p < 0.001). The size of the effect is illustrated by the differences in the mean score among members with normal cognition which was 2.06, compared to 3.21 for those with MCMD, and 5.72 among those with HAD. The rise in UPDRSmotor scores with diagnosis severity was more marked among older members, noting mean scores of 2.51 among those with normal cognition, 3.57 for those with MCMD, and 6.88 for those with HAD (p < 0.001, figure 3).
Figure 3.
Unified Parkinson Disease Rating Scale Motor Exam by Age Group and Cognitive Diagnosis
Extrapyramidal signs were commonly identified with 184 (79.7%) of HIV+ participants, and in 108 (52.9%) of controls having at least one sign. Having multiple extrapyramidal signs was more discriminating. An increase in the number of signs correlated with both HIV seropositivity and with cognitive status among HIV+ individuals. Three or more signs were found in 40.7% of HIV+ participants, compared to 15.7% of controls (OR = 3.69, CI: 2.28 –6.04, p < 0.001). . Among HIV+ members with normal cognition 28% had three or more signs, while 45% of members with MCMD, and 68% of members with HAD had three of more signs (p < 0.0001). Having at least three extrapyramidal signs was associated with cognitive impairment and a diagnosis of either MCMD or HAD among HIV+ members of all ages (OR = 2.99, CI: 1.61 – 5.41, p < 0.001). This association was stronger among older members (OR = 4.38, CI: 1.94 – 10.39, p < 0.001).
Discussion
Extrapyramidal motor impairment is a well-documented aspect of HIV-related cognitive disorders in patients with untreated disease. These findings are consistent with HIV-specific neuropathology within subcortical and deep grey matter structures that subserve motor functions. The presence of multiple extrapyramidal signs among older patients correlates to dementia in seronegative elders compared to those with only one or no extrapyramidal signs (Richards et al, 1993). Our results suggest that extrapyramidal signs continue to be associated with HIV cognitive disorders; although, readers are cautioned that the consensus diagnostic categorization in this study was not blinded to neurological examination findings. A prospective blinded study would be required to determine the extent to which motor findings impact the diagnoses in HAND. In this cohort, three or more extrapyramidal signs on the UPDRS motor exam were associated with HAD (OR = 2.99 overall, 4.38 after age 50). We also found that the magnitude of the association between HIV and extrapyramidal signs increases with age, a finding that may have particular relevance as this aged population grows in size.
In this study, bradykinesia and postural/action tremor appeared to most strongly associate with aging in HIV; however, bradykinesia for tasks requiring rapid repetitive motor synchrony was the most common extrapyramidal sign among HAD patients. This is consistent with the involvement of frontal white matter and basal ganglia in HIV neuropathology. It may also be suggestive of increased incidence of cerebrovascular risk factors and associated white matter abnormalities among older HIV patients, a finding identified in this cohort (McMurtray et al, 2007; Valcour et al, 2006). Specifically, insulin resistance and systolic blood pressure correlate to the severity of white matter abnormalities in this cohort, while HIV-related markers (CD4 lymphocyte count, CD4 nadir count) do not. One must also consider other age-related brain changes that may impact extrapyramidal motor findings in HIV, including immunologic, vascular, and intrinsic brain protection and signaling processes (Valcour et al, 2004b).
Four extrapyramidal signs, in particular, captured over two-thirds of the variability in the UPDRS neurological examination motor scores: hypomimia, postural/action tremor, rapid movement of hands, and body bradykinesia. Since neurological examinations are resource-intensive, our results provide some direction regarding tests most sensitive in the era of HAART that could be validated in future work as a brief neurological assessment alternative to comprehensive examinations.
In summary, we conclude that extrapyramidal motor signs remain an important consequence of HIV infection in the era of HAART and are a sensitive correlate to HIV-associated neurocognitive impairment. We found that the size of the association between HIV and extrapyramidal motor signs depends, in part, on age. This finding may have important implications for an aging HIV population. We caution that our results are neutral with respect to etiology and must remain so until research that meets the formidable methodological challenges required to tease apart the effects of concurrent cerebrovascular disease, normal aging, and HIV, is conducted.
Materials and Methods
Population tested
The Hawaii Aging with HIV Cohort (HAHC) is a prospective longitudinal assessment of older (age greater than 50) and younger (age 20–40) HIV-1 seropositive patients, with age-, gender-, education- and ethnicity-matched seronegative controls. Individuals were broadly recruited from all islands of Hawaii, capturing approximately 10% of patients living with HIV in the state and resulting in demographic data that roughly matches that of the Hawaii State Department of Health for those age groups (Health;). Exclusion criteria have been previously described and briefly include CNS opportunistic conditions, traumatic brain injury with loss of consciousness, learning disability, and comorbid major neuropsychiatric disorders which could impact cognition. Additional exclusionary criteria for this motor assessment included urine drug screen positive for psychostimulants, current substance dependency, use of neuroleptic medications, myopathy, myelopathy, or cerebrovascular events (TIA or stroke). Patients diagnosed with HAD received MRI of the brain, serum B12 level, thyroid function testing, serologies for syphilis, and lumbar puncture, when indicated, to exclude conditions other than HIV which might affect cognitive performance. If such conditions were found, the patients were excluded from this analysis. The cognitive outcomes of this cohort have been previously published (Valcour et al, 2004a). In the full cohort utilized for this analysis and among the 121 older individuals, 25 (20.7%) met research criteria for HAD and an additional 52 (43.0%) met criteria for MCMD. Among the 108 younger individuals, 12 (11.1%) met criteria for HAD and 32 (29.6%) met criteria for MCMD.
Assessment of Motor Signs
All participants were evaluated with the macroneurological examination employed in HIV studies of the Adult AIDS Clinical Trials Group (AACTG, NIAID/NIH) and which includes the motor portion (part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS) (Gancher, 1997). This assessment includes 14 parameters (Table 2), each of which are scored on a 5-point scale (0 – normal, 1 – minimal, 2 – slight, 3 – moderate, 4 – marked abnormality). All neurologic examinations were performed by one of two HIV investigators (MRW and VGV). The neuropsychological test battery and cognitive diagnostic criteria have been previously described (Valcour et al, 2004a). Briefly, American Academy of Neurology HIV diagnostic criteria were employed in a weekly consensus conference with HIV colleagues from Johns Hopkins University (Working Group 1991). Consensus conference diagnosis was reached using all available cognitive and neurological data, including the neurological examination. Extrapyramidal signs were not a requirement for diagnosis, but were considered to be evidence for disease when appropriate.
Table 2.
Fourteen motor aspects assessed in the macroneurological examination
| Speech |
| Facial expression |
| Tremor at rest |
| Action or postural tremor of hands |
| Rigidity |
| Arise from chair |
| Posture |
| Postural stability Bradykinesia |
| Finger tapping |
| Hand opening/closing |
| Hand pronation/supination |
| Heel tapping |
| Gait |
| Body bradykinesia |
Data Management and Statistical Analyses
All participants signed IRB-approved informed consent forms. Data were entered in duplicate by separate research associates. We tested hypotheses regarding the main HIV-aging interaction effects in a multiple regression model using maximum likelihood estimation. We tested hypotheses regarding the association between having three or more extrapyramidal signs and the frequency of MCMD and HAD at baseline using Pearson Chi-Square tests. The 95% confidence intervals (CI) around the odds ratios (OR) in these tests were estimated using exact methods. All p-values were adjusted for multiple comparisons using bootstrap resampling.
Footnotes
Disclosures: none
References
- 1.Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 41:778–85. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- 2.Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, Kim J, Wolf R, Lawler K, Kolson DL, Detre JA. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66:862–6. doi: 10.1212/01.wnl.0000203524.57993.e2. [DOI] [PubMed] [Google Scholar]
- 3.Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. Aids. 2004;18(Suppl 1):S11–8. [PubMed] [Google Scholar]
- 4.Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. Aids. 2004;18(Suppl 1):S75–8. [PubMed] [Google Scholar]
- 5.Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, Grant I, Heaton RK. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. Aids. 2004;18(Suppl 1):S27–34. [PubMed] [Google Scholar]
- 6.Chiesi A, Vella S, Dally LG, Pedersen C, Danner S, Johnson AM, Schwander S, Goebel FD, Glauser M, Antunes F, et al. Epidemiology of AIDS dementia complex in Europe. AIDS in Europe Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;11:39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR Am J Neuroradiol. 2001;22:277–83. [PMC free article] [PubMed] [Google Scholar]
- 8.Gancher S. Scales for the assessment of movement disorders. In: Herndon R, editor. Handbook of neurologic rating scales. Demos Vermande; New York: 1997. pp. 81–123. [Google Scholar]
- 9.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–62. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 10.Harrison MJ, Newman SP, Hall-Craggs MA, Fowler CJ, Miller R, Kendall BE, Paley M, Wilkinson I, Sweeney B, Lunn S, Carter S, Williams I. Evidence of CNS impairment in HIV infection: clinical, neuropsychological, EEG, and MRI/MRS study. J Neurol Neurosurg Psychiatry. 1998;65:301–7. doi: 10.1136/jnnp.65.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Health; HDo. HIV/AIDS surveillance semi-annual report. Honolulu: 2003. [Google Scholar]
- 12.Janssen RS, Nwanyanwu OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency virus encephalopathy in the United States. Neurology. 1992;42:1472–6. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- 13.Kissel EC, Pukay-Martin ND, Bornstein RA. The relationship between age and cognitive function in HIV-infected men. J Neuropsychiatry Clin Neurosci. 2005;17:180–4. doi: 10.1176/jnp.17.2.180. [DOI] [PubMed] [Google Scholar]
- 14.Luther VP, Wilkin AM. HIV infection in older adults. Clin Geriatr Med. 2007;23:567–83. vii. doi: 10.1016/j.cger.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 15.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–21. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 16.McMurtray A, Nakamoto B, Shikuma C, Valcour V. Small-Vessel Vascular Disease in Human Immunodeficiency Virus Infection: The Hawaii Aging with HIV Cohort Study. Cerebrovasc Dis. 2007;24:236–241. doi: 10.1159/000104484. [DOI] [PubMed] [Google Scholar]
- 17.Neuen-Jacob E, Arendt G, Wendtland B, Jacob B, Schneeweis M, Wechsler W. Frequency and topographical distribution of CD68-positive macrophages and HIV-1 core proteins in HIV-associated brain lesions. Clin Neuropathol. 1993;12:315–24. [PubMed] [Google Scholar]
- 18.Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 19.Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology. 1993;43:2184–8. doi: 10.1212/wnl.43.11.2184. [DOI] [PubMed] [Google Scholar]
- 20.Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–21. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- 21.Stern Y, McDermott MP, Albert S, Palumbo D, Selnes OA, McArthur J, Sacktor N, Schifitto G, Kieburtz K, Epstein L, Marder KS. Factors associated with incident human immunodeficiency virus-dementia. Arch Neurol. 2001;58:473–9. doi: 10.1001/archneur.58.3.473. [DOI] [PubMed] [Google Scholar]
- 22.Suwanwelaa N, Phanuphak P, Phanthumchinda K, Suwanwela NC, Tantivatana J, Ruxrungtham K, Suttipan J, Wangsuphachart S, Hanvanich M. Magnetic resonance spectroscopy of the brain in neurologically asymptomatic HIV-infected patients. Magn Reson Imaging. 2000;18:859–65. doi: 10.1016/s0730-725x(00)00173-9. [DOI] [PubMed] [Google Scholar]
- 23.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004a;63:822–7. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, Williams AE, Shikuma CM. Insulin Resistance Is Associated With Cognition Among HIV-1-Infected Patients: The Hawaii Aging With HIV Cohort. J Acquir Immune Defic Syndr. 2006;43:405–410. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- 25.Valcour VG, Shikuma CM, Watters MR, Sacktor NC. Cognitive impairment in older HIV-1-seropositive individuals: prevalence and potential mechanisms. Aids. 2004b;18(Suppl 1):S79–86. doi: 10.1097/00002030-200401001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–8. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- 27.Watters MR, Poff PW, Shiramizu BT, Holck PS, Fast KM, Shikuma CM, Valcour VG. Symptomatic distal sensory polyneuropathy in HIV after age 50. Neurology. 2004;62:1378–83. doi: 10.1212/01.wnl.0000120622.91018.ea. [DOI] [PubMed] [Google Scholar]
- 28.Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, Meyerhoff DJ, Jarvik JG, Kolson D, Schifitto G, Ellis RJ, Swindells S, Simpson DM, Miller EN, Gonzalez RG, Navia BA. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23:928–35. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]