Abstract
The relationship between prenatal cocaine use and preschooler’s physical and cognitive development and behavioral characteristics was examined, controlling for other influences on child development. On average, children were 38.5 months old, women were 29.4 years old, had 12.3 years of education, and 47% were African American. During the first trimester, 18% of the women were frequent cocaine users (≥ 1 line/day). First trimester cocaine exposure predicted decreased head circumference at 3 years and lower scores on the short-term memory subscale of the Stanford-Binet Intelligence Scale (SBIS) [74]. There was no significant relationship between prenatal cocaine use and the other SBIS scales. First trimester cocaine use also predicted more total, internalizing, and externalizing behavior problems on the Child Behavior Checklist [3] and higher scores on the fussy/difficult scale of the Infant Characteristics Questionnaire [6]. Children who were exposed to cocaine throughout pregnancy had more behavior problems and were more fussy compared to children of women who never used cocaine prenatally. A repeated measures analysis showed that children of first trimester cocaine users became more fussy over time. These detrimental effects on growth and behavior are consistent with other reports in the literature and with the hypothesis that prenatal cocaine exposure affects development through changes in neurotransmitter systems.
Keywords: prenatal cocaine exposure, preschool age, growth, cognitive development, temperament, behavior problems
1. Introduction
The effects of prenatal cocaine exposure (PCE) on child development are an important public health issue, with rates of prenatal cocaine use ranging from 1% in the National Pregnancy and Health Survey [52] to 2.6% in a partially rural sample from Florida [31] to 8% in our longitudinal study of prenatal cocaine exposure (PCE) [57] to 18% in an inner-city sample from Boston [80]. It is important to determine whether the associations between PCE and neonatal and infant development that have been reported persist into the preschool period (3 to 5 years), or whether new relations will emerge. The outcomes that are typically investigated are growth, cognitive development, and behavior.
Griffith, Azuma, and Chasnoff [35] found that 3-year head circumference was smaller in two drug-exposed groups compared with a non-drug-exposed group, although the two drug groups did not differ from each other. In a report from an adoption counseling service, PCE was associated with reduced head circumference and height at approximately 4 to 5 years [54]. By contrast, other researchers have reported that PCE was not associated with preschool growth [13,40,44,46].
There have been reports of relationships between PCE and specific areas of cognitive functioning in 3- to 4-year-olds, including short-term memory [11], verbal reasoning [11,35], abstract-visual reasoning [12], performance IQ [53], and visual-spatial and arithmetic scores [72]. In general, PCE has not been found to be associated with deficits in global preschool cognitive development, as measured by scales such as the Bayley Scales of Infant Development (BSID-II [7]), the Stanford-Binet Intelligence Scale (SBIS) [74], the Wechsler Preschool and Primary Scale of Intelligence-Revised [77], and the McCarthy Scales of Children’s Abilities [48] [1,5,8,32,37,40,49,51,53,72], although Lewis et al. [44] reported a relationship between PCE and lower mental and psychomotor scores on the BSID-II and Bennett et al. [12] reported that PCE predicted lower composite SBIS scores for exposed boys only.
The literature is also inconsistent in the behavior domain. PCE has been reported to be associated with increased caregiver-reported behavior problems in preschoolers, particularly externalizing behavior [4,35], with aggression [9], with shorter latency to frustration and increased disruptive behaviors in exposed boys [29], and with poorer attention on laboratory vigilance measures [1,5,10]. By contrast, no significant relations were found between PCE and caregiver’s ratings of preschooler’s behavior problems [2,11,76], nor with trained observer’s ratings on the BSID-II Behavior Rating Scale [49].
The inconsistencies in this literature could be due to a number of methodological factors, including failure to control for differences between exposure groups, different sample characteristics, especially in terms of patterns of drug use, differential rates of attrition between exposure groups, and high rates of subject loss across time [57,63]. The research presented here is a longitudinal study of prenatal cocaine use that was designed to address these factors. We can also address the timing of PCE because multiple interviews were conducted to obtain trimester-specific substance use data. This report evaluates the relations between PCE and preschool physical, cognitive, and behavioral development. Based on our findings at the 1-year follow-up [60] and on those in the literature, we hypothesized that the strongest association would be with PCE and behavior problems, and that this relation would remain significant after adjusting for covariates of cocaine use. We further hypothesized that cocaine use during the first trimester would have an impact on outcome, as would continued use throughout pregnancy.
2. Methods
2.1. Study Design
The sample consisted of women and children participating in a longitudinal investigation of the effects of PCE. Written consent was obtained according to guidelines established by the University of Pittsburgh’s Institutional Review Board and by the Research Review and Human Experimentation Committee of Magee-Womens Hospital. A Confidentiality Certificate, obtained from the Department of Health and Human Services, assured participants that their responses could not be subpoenaed.
Women who were at least 18 years of age were initially interviewed when they came for prenatal care during their fourth or fifth prenatal month. Women in this prenatal care sample were not enrolled if they did not initiate prenatal care by the fifth month of pregnancy. During the initial interview, information was obtained about cocaine, crack, alcohol, tobacco, marijuana, and other illicit drug use in the year prior to pregnancy and during the first trimester. All women who reported any cocaine or crack use during the first trimester were enrolled. The next woman interviewed who reported no cocaine or crack use during both the year prior to pregnancy and the first trimester was also enrolled. The selected sample was interviewed at 7 months and at 24 hours post-delivery about substance use during their second and third trimesters, respectively. Techniques used to enhance the accuracy of reporting can be found in previous publications [26,60]. All infants were examined at delivery by study nurses who were unaware of prenatal exposure status. At 1 year postpartum, growth, mental and motor development, and temperament were assessed [60].
At the 3-year phase, the child’s growth was measured and his/her medical history was obtained from the mother. Cognitive development was assessed with the Stanford-Binet Intelligence Scale - 4th Edition (SBIS) [74], which consists of the following scales: verbal reasoning (VR), reflecting verbal comprehension and reasoning; abstract/visual reasoning (AVR), reflecting visual-perceptual analysis; quantitative reasoning (QR), reflecting early math concepts; short-term memory (STM), including visual and auditory short-term recall; and the composite score [28]. These scores are normed to have an average of 100 and standard deviation of 16. After the assessment, examiners completed the SBIS global ratings, which reflect the examiner’s perception of the child’s responsiveness and activity during the test. The examiners were bachelor’s or master’s level research staff who had extensive experience administering standardized child assessments. They were trained to reliability and supervised by a developmental psychologist (GAR). Examiners were blind to prenatal and current substance use status. Periodic reliability checks were conducted to maintain consistent administration and scoring.
At 3 years, mothers were interviewed about substance use during the last year and their demographic and psychological characteristics, household composition, and social support (how often have contact with friends and relatives; someone to turn to in times of need; support received in role as a mother; satisfaction with help received). Maternal depression was assessed using the Center for Epidemiological Studies - Depression Scale (CES-D) [56] and anxiety and hostility were measured by the Spielberger State-Trait Anxiety Inventory (STAI) [73]. The Home Screening Questionnaire (HSQ) [20] was used to measure aspects of the home environment that correlate with cognitive development.
The mother’s view of the child’s temperament was measured using the Infant Characteristics Questionnaire (ICQ) [6]. This scale, also used at the 1-year phase, describes four dimensions of temperament: fussy/difficult (ease of becoming upset), unadaptable (how child responds to new situations), persistent (how child reacts to discipline), and unsociable (interest in people). Cronbach’s alphas for the current sample were 0.70, 0.71, 0.74, and 0.28 for the four dimensions, respectively. The unsociable scale was eliminated from the analyses because of the low alpha. The Child Behavior Checklist/2-3 (CBCL) [3] was also completed by the caregivers at 3 years. The mean T total behavior problems score for the non-referred sample described by Achenbach [3] is 50 and for the clinically-referred sample is 63.8 [3].
2.2. Sample Characteristics
Women were recruited for the study between March 1988 and December 1992. Ninety percent of those approached agreed to participate. Medical chart reviews of a random sample of women who refused to participate indicated that only 5% had a history of drug use during the current pregnancy. A total of 320 women met the inclusion criteria and were enrolled into the study. Between enrollment at the 4th or 5th month of pregnancy and delivery, 20 subjects were eliminated for the following reasons: home delivery, miscarriage/abortion/fetal death, moved, lost to follow-up, and refused. Women could miss the 7-month interview, but not the delivery interview, and still remain in the study. Thus, delivery assessments were completed on 300 women. Four pairs of twins and one child with Trisomy 21 were excluded from additional follow-up, resulting in a birth cohort of 295 mothers and infants.
By 3 years, 32 subjects were lost to follow-up: 4 children died, 2 mothers lost custody and the children could not be traced, 13 families moved out of state, 8 mothers refused to participate, and 5 were missed. The 263 subjects interviewed at the 3-year follow-up represented 89% of the birth cohort. Seven percent of the children were not in maternal custody at 3 years, in which case the current caretaker was interviewed. The majority of these caregivers were relatives of the child (father, grandparent, aunt).
Three children with severe disabilities (autism, Alagille Syndrome, Tourette’s Syndrome) and 2 children whose SBIS testing conditions were rated as seriously detrimental due to maternal interference in the testing were excluded from the analyses, resulting in an analysis cohort of 258 mothers and children. The mothers who were not included in the 3-year analysis (N=37) were shorter (63.6 vs. 64.5 inches, p < .05) and had higher CES-D scores at delivery (46.4 vs. 41.4, p < .05) than those who were included in the analysis (N=258). There were no other significant differences in maternal sociodemographic, prenatal substance use, or infant birth characteristics between those who were and were not included in this analysis.
The median child age at assessment was 37 months (mean = 38.5 months; SD = 4.3; range = 33 - 59). Ninety percent were seen by 44 months of age. Fifty-two percent of the children were male. The average weight was 33.7 pounds (range = 23 - 71), height was 38.3 inches (range = 34 - 44), and head circumference was 500 mm (range = 457 - 559). The mean SBIS scores were: VR - 94; AVR - 90; QR - 94; STM - 101; and composite - 93 (range = 67 - 118). At 3 years, 25% of the children had attended preschool or child care.
At the 3-year follow-up, the women were, on average, 29.4 years old (range = 21 - 67), 53% were Caucasian, and 47% were African American. Women had a median family income of $700 per month (range = $0 - $5,366/month) and an average educational level of 12.3 years (range = 8 - 18). Thirty-two percent were married, 44% had a man living in the household (includes husband, boyfriend, father or stepfather of the child), and 44% worked or went to school.
2.3. Data Analysis
Cocaine and crack use were reported in lines, rocks, or grams and then converted into gram equivalents. One line was estimated as 1/30th (0.03) of a gram; one rock of crack was estimated to be equivalent to 0.2 g. These estimates were based on information obtained from toxicology laboratories and law enforcement officials in Pittsburgh, PA. Cocaine/crack use was then expressed as average grams/day. For analytic purposes, first trimester cocaine use was defined as use/no use and as frequent use (≥ 1 line/day) versus non-frequent use (< 1 line/day; includes non-users and occasional users). Cocaine use was dichotomized into use/no use for the second and third trimesters and for the 3-year postpartum phase because of the small number of women who used during those time periods.
Alcohol and marijuana use were expressed as average number of drinks or joints per day, respectively, and were log transformed to reduce skewness. Tobacco was expressed as number of cigarettes per day. The alcohol, marijuana, and tobacco variables were ascertained separately for each trimester of pregnancy, as well as at 3 years postpartum, and were used as continuous variables in the analyses. Further details about the calculation of the first trimester substance use variables can be found in Day and Robles [26].
The outcomes for the growth analyses were weight, height, and head circumference. The SBIS outcomes were the VR, AVR, STM, and composite scores. The QR score was eliminated from the analyses because 51% of the children did not complete the subtest due to failure to establish a basal level. The ICQ variables were fussy/difficult, unadaptable, and persistent. Although Bates et al. [6] showed a different factor structure for the 24-month version of the ICQ compared to the 12-month version, we chose to use the same item grouping as we did at 1 year [60] so that we were consistent across follow-up phases. It was also necessary to use the same structure for the repeated measures analysis.
To reduce Type I error rate due to multiple tests on the same outcome, the first set of regression analyses on the CBCL was with the total, internalizing, and externalizing scales. If PCE was a significant predictor of these three scales, then further analyses were conducted with the withdrawn, anxious/depressed, aggressive, sleep, somatic, and destructive behavior scales. T scores were used for the total, internalizing, and externalizing scales and raw scores were used for the subscales, as recommended by Achenbach [3].
Stepwise multiple regression analyses were used to select significant covariates for the final model. To explore the stability of the variables entering the model, the initial significance level for entry and removal was 0.10. For the final regression models, variables that were significant at an alpha of 0.05 were retained. Child characteristics, maternal sociodemographic, psychosocial and environmental characteristics, and prenatal and 3-year substance use were considered as potential covariates (Table 1). These domains were selected because of associations with the outcomes and/or PCE based on initial bivariate analyses and based on the literature and prior analyses of these data. The covariates that were retained for the final models are indicated in Table 1. Because this is a study of a potential teratogen, all regressions were run separately by trimester to assess the effects of exposure during each time period. Type I error rate was not adjusted for the number of multiple measures within each domain. Therefore, the overall p value may be slightly larger than reported.
Table 1.
Variables considered for inclusion as covariates
| 3-Year Child Characteristics |
| Age at assessmenta (for growth analyses only) |
| Gendera |
| Number of illnesses |
| Number of injuries |
| Number of hospitalizations |
| Preschool attendancea |
| 3-Year Maternal Demographic and Environmental Characteristics |
| Adult male in household |
| Agea |
| Anxiety/Depression |
| Custody (maternal vs. non-maternal) |
| Educationa |
| Family income |
| Heighta (for growth analyses only) |
| Home Screening Questionnairea |
| Hostilitya |
| Marital statusa |
| Number of siblingsa |
| Racea |
| Social supporta |
| Work statusa |
| 3-Year Maternal Substance Use |
| Alcohola |
| Cocainea |
| Marijuanaa |
| Other illicit drugs |
| Tobaccoa |
| Prenatal Substance Use (for each trimester) |
| Alcohola |
| Cocainea |
| Marijuanaa |
| Other illicit drugs |
| Tobaccoa |
Variable was retained in final model
Residuals and the modified Cook’s statistic [19] were used to identify possible outliers and influential points. This report includes only those relations with PCE that remained stable after removal of the influential cases. There was one influential point for the SBIS, one for the ICQ, and 3 for the CBCL. The tolerance of each predictor was examined to assure that the estimated regression slopes were not unstable due to multicollinearity. In summary, regression analyses were conducted with 3 growth outcomes, 4 SBIS outcomes, 3 CBCL scores (for the first runs and then with 6 subscales), and 3 ICQ scores. Each domain was analyzed separately by the three trimesters.
To investigate the impact of duration of cocaine use, a descriptive group analysis was conducted. Three groups were defined: women who abstained from cocaine throughout pregnancy (“Never”) (N=141); those who used first trimester only (“Stopped”) (N=62); and those who used both first and third trimesters (“Continued”) (N=28). Analysis of covariance was used in order to adjust for covariates. Two comparisons were made: The never used group to the stopped group and the never used group to the continued group, with an alpha level of 0.025 for each comparison in order to limit the overall Type I error rate to 0.05.
We also conducted a repeated measures analysis on the ICQ fussy/difficult scale to test two questions: 1) Was fussiness in the stopped and continued groups significantly higher at 1 and 3 years than in the never used group; and 2) Was the magnitude of change in fussiness from 1 to 3 years similar for the three groups? Within this repeated measures model, we controlled for significant covariates of the fussy/difficult scale that were identified at each phase. We also measured growth at both phases but we did not do repeated measures analyses on the growth parameters because of our previously published paper using longitudinal growth analyses [59].
3. Results
3.1. Descriptive Analyses
The prevalence of cocaine/crack use is shown in Table 2. During the first trimester, 18.2% of the women were frequent users (≥ 1 line of powder cocaine per day, or the equivalent in crack). By the third trimester, 6.3% of the women were frequent users. Only 13% of the women who used first trimester also used second and third trimester. All of the women who used cocaine second and third trimester had used first trimester. The prevalence of frequent cocaine use did not change significantly during the first and third postpartum years from that of the third trimester. The mean level of cocaine use for the women who used during the first trimester was 0.23 g/day, or ~8 lines/day (range = < 1 line/month - 4 g/day). During the second and third trimesters, the mean level of use for the users was 0.17 g/day (~6 lines/day) (range = < 1 line/month - 1 g/day) and 0.14 g/day (~5 lines/day) (range = < 1 line/month - 2 g/day), respectively. The mean level of cocaine use for the women who used at the 3-year follow-up phase was 0.22 g/day, or ~7 lines/day (range = < 1 line/month - 3 g/day).
Table 2.
Prevalence of Cocaine Use (%)
| Level of Use | |||
|---|---|---|---|
| Time Period | None | Occasionala | Frequentb |
| Year prior to pregnancy (N = 258) | 55.4 | 23.6 | 20.9 |
| First trimester (N = 258) | 58.5 | 23.3 | 18.2 |
| Second trimester (N = 232) | 92.7 | 2.6 | 4.7 |
| Third trimester (N = 255) | 89.0 | 4.7 | 6.3 |
| One-year follow-up (N = 244) | 84.8 | 8.2 | 7.0 |
| Three-year follow-up (N = 256) | 87.1 | 6.6 | 6.2 |
Occasional: > 0 and < 1 line/day of cocaine or gram equivalent of crack
Frequent: ≥ 1 line/day of cocaine or gram equivalent of crack
The characteristics associated with first trimester cocaine use are shown in Table 3. Women who used ≥ 1 line of cocaine/day during the first trimester were significantly more likely to be older, African American, single, and to have lower family incomes than the women who did not use first trimester. Frequent first trimester cocaine users also used more alcohol, cigarettes, marijuana, and other illicit drugs than did first trimester non-cocaine users. Children of mothers who were frequent users of cocaine during the first trimester had lower SBIS AVR, STM, and composite scores, and were rated as more fussy/difficult by their mothers on the ICQ than were those who were not exposed first trimester. These descriptive analyses did not control for covariates of PCE.
Table 3.
Maternal and Child Characteristics Associated with Levels of First Trimester Cocaine Use
| No cocaine use 1st trimester | Occasional use 1st trimestera | Frequent cocaine use 1st trimesterb | |
|---|---|---|---|
| First Trimester | n = 151 | n = 60 | n = 47 |
| Maternal Characteristics | |||
| Mean (SD) Age (yrs) | 24.1 (4.8) | 25.3 (5.6) | 27.2 (4.7)*** |
| Mean (SD) Education (yrs) | 12.1 (1.3) | 11.8 (1.2) | 11.7 (1.2) |
| % Caucasian | 58.3 | 58.3 | 29.8** |
| % Married | 25.8 | 11.7 | 12.8* |
| % Work/Attend School | 45.7 | 36.7 | 31.9 |
| % Family Income < $500/mo | 40.0 | 56.4 | 62.2* |
| % Drink Alcohol | 56.3 | 93.3 | 85.1*** |
| Mean (SD) # Drinks/Day | .30 (0.7) | 1.7 (2.3) | 2.3 (3.2)*** |
| % Smoke Cigarettes | 44.4 | 70.0 | 87.2*** |
| Mean (SD) # Cigarettes/Day | 6.0 (8.6) | 10.3 (10.3) | 11.3 (9.2)*** |
| % Use Marijuana | 16.6 | 56.7 | 53.2*** |
| Mean (SD) # Joints/Day | .07 (0.3) | .40 (0.7) | .70 (1.6)*** |
| % Use Other Drugs (excluding cocaine) | 3.3 | 11.7 | 10.6* |
| 3-Year Child Characteristics: | |||
| Mean (SD) | |||
| Age (mos) | 38.7 (3.8) | 38.1 (4.7) | 38.0 (4.9) |
| Weight (lbs) | 34.2 (6.4) | 32.6 (4.5) | 33.1 (4.5) |
| Height (ins) | 38.4 (1.8) | 38.0 (1.9) | 38.2 (1.8) |
| Head circumference (mm) | 502.2 (16.6) | 502.0 (15.4) | 498.1 (15.3) |
| Stanford-Binetc: VR | 94.5 (11.1) | 95.1 (11.1) | 90.7 (8.1) |
| AVR | 89.9 (11.0) | 93.4 (12.6) | 87.9 (10.1)* |
| STM | 100.3 (11.3) | 104.8 (10.9) | 97.7 (12.9)** |
| CSCORE | 93.4 (9.9) | 95.4 (10.1) | 90.3 (8.2)* |
| ICQ Scale: Fussy/Difficult | 31.6 (6.4) | 34.7 (6.7) | 34.6 (5.6)*** |
| ICQ Scale: Unadaptable | 16.7 (4.5) | 17.1 (4.6) | 18.1 (4.4) |
| ICQ Scale: Persistent | 12.4 (3.4) | 12.9 (3.3) | 12.4 (3.0) |
| CBCL: Total | 52.0 (9.0) | 54.3 (10.1) | 54.1 (8.9) |
| Internalizing | 51.8 (9.5) | 53.7 (10.7) | 55.4 (9.5) |
| Externalizing | 51.0 (8.9) | 52.7 (9.8) | 52.3 (8.8) |
Occasional: > 0 and < 1 line/day of cocaine or gram equivalent of crack
Frequent: ≥ 1 line/day of cocaine or gram equivalent of crack
VR=verbal reasoning; AVR=abstract/visual reasoning; STM=short-term memory; CSCORE=composite score
p < .05: Overall significance using F test for continuous variables and Chi-square test for dichotomous variables.
p < .01: Overall significance using F test for continuous variables and Chi-square test for dichotomous variables.
p < .001: Overall significance using F test for continuous variables and Chi-square test for dichotomous variables.
Women who continued to use cocaine throughout pregnancy were more likely to be African American, single, to have lower incomes, and to use more alcohol, cigarettes, and marijuana than women who stopped or never used during pregnancy (Table 4). The children who were exposed throughout pregnancy had lower SBIS VR scores, were more fussy/difficult, and had more internalizing behavior problems on the CBCL than children who were exposed first trimester only or who were never exposed. These analyses were not adjusted for any covariates of PCE.
Table 4.
Maternal and Child Characteristics Associated with Duration of Prenatal Cocaine Use
| Never Useda | Stoppedb | Continuedc | |
|---|---|---|---|
| Third Trimester | n = 141 | n = 62 | n = 28 |
| Maternal Characteristics | |||
| Mean (SD) Education (yrs) | 12.1 (1.4) | 12.0 (1.3) | 11.9 (1.0) |
| % Caucasian | 60.3 | 64.5 | 10.7*** |
| % Married | 29.8 | 14.5 | 10.7* |
| % Work/Attend School | 16.3 | 14.5 | 7.1 |
| % Family Income < $500/mo | 43.5 | 59.7 | 78.6** |
| % Drink Alcohol | 30.5 | 53.2 | 60.7*** |
| Mean (SD) # Drinks/Day | .04 (0.2) | .13 (0.3) | 1.2 (1.9)*** |
| % Smoke Cigarettes | 36.7 | 71.0 | 77.8*** |
| Mean (SD) # Cigarettes/Day | 5.4 (8.7) | 9.8 (10.0) | 8.4 (7.3)** |
| % Use Marijuana | 6.4 | 16.1 | 32.1*** |
| Mean (SD) # Joints/Day | .02 (0.1) | .10 (0.4) | .07 (0.2) |
| 3-Year Child Characteristics: | |||
| Mean (SD) | |||
| Age (mos) | 38.7 (3.9) | 38.0 (4.3) | 38.7 (5.4) |
| Weight (lbs) | 34.3 (6.5) | 32.8 (4.7) | 33.3 (4.1) |
| Height (ins) | 38.4 (1.8) | 38.2 (1.8) | 38.1 (2.1) |
| Head circumference (mm) | 502.0 (16.4) | 500.0 (15.0) | 498.8 (16.7) |
| Stanford-Binetd: VR | 94.9 (11.2) | 94.7 (10.5) | 88.9 (7.8)* |
| AVR | 90.0 (11.1) | 93.2 (12.3) | 87.8 (11.6) |
| STM | 100.6 (11.4) | 102.9 (13.4) | 99.2 (11.0) |
| CSCORE | 93.6 (10.0) | 94.9 (10.2) | 89.9 (8.3) |
| ICQ Scale: Fussy/Difficult | 31.3 (6.4) | 34.4 (6.1) | 35.4 (6.9)*** |
| ICQ Scale: Unadaptable | 16.7 (4.4) | 17.0 (4.4) | 17.5 (5.0) |
| ICQ Scale: Persistent | 12.2 (3.4) | 12.7 (3.1) | 13.0 (3.0) |
| CBCL: Total | 52.0 (9.1) | 54.0 (9.4) | 55.6 (8.9) |
| Internalizing | 51.9 (9.5) | 53.5 (10.0) | 57.4 (10.0)* |
| Externalizing | 51.0 (8.9) | 52.7 (9.2) | 53.0 (8.8) |
Did not use cocaine during any trimester
Used cocaine first trimester only
Used cocaine both first and third trimesters
VR=verbal reasoning; AVR=abstract/visual reasoning; STM=short-term memory; CSCORE=composite score
p < .5: Overall significance using F test for continuous variables and Chi-square test for dichotomous variables.
p < .01: Overall significance using F test for continuous variables and Chi-square test for dichotomous variables.
p < .001: Overall significance using F test for continuous variables and Chi-square test for dichotomous variables.
Women who used cocaine/crack at the 3-year phase reported more symptoms of hostility, provided less stimulating home environments, and used more alcohol, tobacco, marijuana, and other illicit drugs than women who did not use cocaine at 3 years (Table 5).
Table 5.
Current Caregiver Characteristics Associated with Cocaine Use at 3-Year Follow-Up
| No Cocaine Use at 3 Years | Cocaine Use at 3 Years | |
|---|---|---|
| N= 223 | N= 33 | |
| Mean (SD) Age (yrs) | 29.3 (6.7) | 29.8 (5.2) |
| Mean (SD) Education (yrs) | 12.3 (1.5) | 11.9 (1.2) |
| % Caucasian | 53.4 | 51.5 |
| % Married | 33.6 | 24.2 |
| % Work/Attend School | 45.7 | 30.3 |
| Mean (SD) Family Income/Month ($) | 1001 (773) | 889 (621) |
| % in Maternal Custody | 6 | 7 |
| Mean (SD) Depression (total CES-D score) [56] | 20.2 (8.8) | 23.0 (10.1) |
| Mean (SD) Hostility (total STAI score) [73] | 16.7 (4.4) | 20.0 (5.3)*** |
| Mean (SD) Home Screening Questionnaire [20] | 39.4 (5.7) | 34.7 (7.6)** |
| % Drink Alcohol | 78 | 97* |
| Mean (SD) # Drinks/Day | 0.88 (2.1) | 2.6 (3.7)* |
| % Smoke Cigarettes | 53 | 85*** |
| Mean (SD) # Cigarettes/Day | 7.9 (9.6) | 12.6 (12.1)** |
| % Use Marijuana | 17 | 54*** |
| Mean (SD) # Joints/Day | 0.12 (0.5) | 0.38 (0.7) |
| % Use Other Drugs (excluding cocaine) | 4.5 | 21.2*** |
p < .05: Overall significance using t-test for continuous variables and Chi-square test for dichotomous variables.
p < .01: Overall significance using t-test for continuous variables and Chi-square test for dichotomous variables.
p < .001: Overall significance using t-test for continuous variables and Chi-square test for dichotomous variables.
3.2. Regression Analyses
Exposure to ≥ 1 line of cocaine/day during the first trimester predicted decreased head circumference at 3 years (Table 6), as did second trimester use (defined as use/no use). The average head circumference for children of frequent first trimester users was 4.6 mm smaller than that of children of women who were not frequent users. The effect size for second trimester use was 8.9 mm. There were no effects of PCE on 3-year weight or height. Significant positive predictors of growth included older child age at assessment, gender (male), and maternal height. Significant negative predictors were prenatal tobacco use and current marijuana and tobacco use. The amount of variance explained by the models was 17% for weight, 36% for height, and 24% for head circumference. When birth head circumference was added to the model, the relation between PCE and 3-year head circumference became non-significant, indicating that the association between PCE and 3-year head circumference was mediated by the effects of PCE on birth head circumference.
Table 6.
| Raw Beta | Standardized Regression Coefficient | p value | |
|---|---|---|---|
| Weight (Total R2 = .17) | |||
| Child age at assessment | .43 | .31 | .00 |
| Maternal height | .42 | .21 | .00 |
| Genderc | 1.78 | .15 | .01 |
| First trimester tobacco use | -.07 | -.12 | .03 |
| Height (Total R2 = .36) | |||
| Child age at assessment | .24 | .54 | .00 |
| Maternal height | .14 | .22 | .00 |
| Current tobacco use | -.02 | -.10 | .03 |
| Head Circumferenced (Total R2 = .24) | |||
| Gender | 12.91 | .41 | .00 |
| Child age at assessment | .71 | .18 | .00 |
| Current marijuana use | -9.16 | -.15 | .01 |
| Maternal height | .76 | .14 | .01 |
| Prenatal cocaine use | |||
| First trimester (frequente vs. not frequent) | -4.59 | -.11 | .03 |
| Second trimester (use vs. no use) | -8.93 | -.14 | .01 |
Listed in order of standardized regression coefficient, an indication of the magnitude of the effect.
Each trimester was analyzed separately but all are presented together for convenience. In cases where more than one trimester was significant, the betas for the other variables were essentially the same across models. If more than one trimester was significant, the values presented are those for the first trimester model.
0=female; 1=male
Measured in millimeters
Frequent=≥ 1 line/day; Not frequent=< 1 line/day
First trimester frequent cocaine use significantly predicted the SBIS STM score, with an effect size of 3.6 points (Table 7). PCE did not predict the other SBIS scores. Higher HSQ scores, Caucasian race, and older maternal age predicted higher SBIS scores. Lower SBIS scores were predicted by more siblings, male gender, higher maternal hostility, and prenatal alcohol, marijuana, and tobacco. The amount of variance explained by the models ranged from 8 to 29% for the SBIS scores.
Table 7.
| Raw Beta | Standardized Regression Coefficient | p value | |
|---|---|---|---|
| Verbal Reasoning (Total R2 = .29) | |||
| Raced | 6.17 | .28 | .00 |
| Home Screening Questionnaire | .48 | .26 | .00 |
| Second trimester alcohol use | -4.16 | -.12 | .02 |
| Number of siblings | -1.15 | -.12 | .02 |
| Maternal age | .18 | .11 | .03 |
| Abstract/Visual Reasoning (Total R2 =.18) | |||
| Race | 4.57 | .20 | .00 |
| Home Screening Questionnaire | 0.35 | .19 | .00 |
| Gendere | -3.51 | -.16 | .01 |
| First trimester illicit drugs | 7.04 | .15 | .01 |
| First trimester marijuana use | -4.09 | -.13 | .02 |
| Short-Term Memory (Total R2 = .08) | |||
| Home Screening Questionnaire | 0.30 | .15 | .01 |
| Gender | -3.03 | -.13 | .02 |
| First trimester cocaine use (frequentf vs. not frequent) | -3.58 | -.12 | .03 |
| Prenatal tobacco use | |||
| 2nd trimester | -0.15 | -.12 | .04 |
| 3rd trimester | -0.18 | -.15 | .01 |
| Hostility | -0.28 | -.11 | .05 |
| Composite Score (Total R2 = .20) | |||
| Home Screening Questionnaire | 0.42 | .27 | .00 |
| Race | 3.95 | .20 | .00 |
| First trimester marijuana use | -4.01 | -.14 | .01 |
| Gender | -2.77 | -.14 | .01 |
Thorndike et al. [74]
Listed in order of standardized regression coefficient, an indication of the magnitude of the effect.
Each trimester was analyzed separately but all are presented together for convenience. In cases where more than one trimester was significant, the betas for the other variables were essentially the same across models. If more than one trimester was significant, the values presented are those for the first trimester model.
0=African American; 1=Caucasian
0=female; 1=male
Frequent=≥ 1 line/day; not frequent=< 1 line/day
First trimester cocaine exposure was a significant predictor of the total, internalizing, externalizing, withdrawn, anxious/depressed, and aggressive CBCL scales (Table 8). Third trimester cocaine use was a significant predictor of the total, internalizing, and withdrawn scales. The effect sizes for the total, internalizing, and externalizing scales ranged from ~2.5 to 5 points. There were no significant relations between PCE and the sleep, somatic, or destructive behavior scales. Increased maternal hostility was a significant predictor of more behavior problems. Older maternal age, not working, better home environment, more social support, preschool attendance, current alcohol use, and prenatal tobacco use predicted fewer behavior problems. First trimester cocaine use was a significant predictor of the ICQ fussy/difficult factor, with an effect size of 3 points (Table 8). There were no significant relations between PCE and the ICQ persistent or unadaptable scales. Less difficult temperament was predicted by higher HSQ scores. The amount of variance explained by the models ranged from 17 to 24% for the CBCL scores and from 8 to 10% for the ICQ scales.
Table 8.
| Raw Beta | Standardized Regression Coefficient | p value | |
|---|---|---|---|
| CHILD BEHAVIOR CHECKLISTc | |||
| Total (Total R2 = 0.24) | |||
| Hostility | 0.57 | .28 | .00 |
| Maternal age | -0.27 | -.18 | .00 |
| Prenatal cocaine use | |||
| 1st trimester (use vs. no use) | 3.22 | .17 | .01 |
| 1st trimester (frequentd vs. not frequent) | 2.49 | .10 | .05 |
| 3rd trimester (use vs. no use) | 4.23 | .14 | .01 |
| Work statuse | -3.18 | -.17 | .01 |
| Home Screening Questionnaire | -0.25 | -.16 | .01 |
| Current alcohol use | -1.90 | -.12 | .03 |
| First trimester tobacco use | -0.12 | -.12 | .02 |
| Internalizing (Total R2 = 0.23).02 | |||
| Hostility | 0.50 | .23 | .00 |
| First trimester tobacco use | -0.18 | -.17 | .00 |
| Work status | -3.29 | -.16 | .01 |
| Home Screening Questionnaire | -0.26 | -.15 | .01 |
| Prenatal cocaine use | |||
| 1st trimester (use vs. no use) | 3.04 | .15 | .01 |
| 1st trimester (frequent vs. not frequent) | 4.07 | .16 | .01 |
| 3rd trimester (use vs. no use) | 5.15 | .16 | .01 |
| Social support | -1.95 | -.12 | .03 |
| Maternal age | -0.18 | -.12 | .03 |
| Externalizing (Total R2 = 0.17) | |||
| Hostility | 0.42 | .21 | .00 |
| Maternal age | -0.27 | -.19 | .00 |
| Home Screening Questionnaire | -0.24 | -.15 | .01 |
| Work status | -2.74 | -.15 | .01 |
| Current alcohol use | -2.25 | -.15 | .02 |
| First trimester cocaine use (use vs. no use) | 2.28 | .12 | .04 |
| First trimester marijuana use | 2.78 | .10 | .05 |
| Withdrawn (Total R2 = 0.19) | |||
| Home Screening Questionnaire | -0.12 | -.21 | .00 |
| Work status | -1.20 | -.18 | .01 |
| Prenatal cocaine use | |||
| 1st trimester (use vs. no use) | 1.21 | .18 | .01 |
| 1st trimester (frequent vs. not frequent) | 1.29 | .15 | .01 |
| 3rd trimester (use vs. no use) | 2.82 | .26 | .00 |
| Third trimester alcohol use | -1.69 | -.17 | .01 |
| Hostility | 0.12 | .17 | .01 |
| First trimester tobacco use | -0.06 | -.15 | .01 |
| Maternal age | -0.07 | -.13 | .03 |
| Current alcohol use | -0.77 | -.14 | .02 |
| Anxious/Depressed (Total R2 = 0.23) | |||
| Hostility | 0.20 | .33 | .00 |
| Home Screening Questionnaire | -0.07 | -.15 | .01 |
| Preschool attendanceg | -1.02 | -.15 | .01 |
| First trimester cocaine use (frequent vs. not frequent) | 0.96 | .13 | .02 |
| Social Support | -0.55 | -.11 | .03 |
| First trimester tobacco use | -0.03 | -.11 | .03 |
| Aggressive (Total R2 = 0.18) | |||
| Hostility | 0.28 | .24 | .00 |
| Work status | -2.24 | -.20 | .00 |
| First trimester cocaine use (use vs. no use) | 2.03 | .18 | .01 |
| Maternal age | -0.14 | -.17 | .01 |
| Current alcohol use | -1.21 | -.13 | .02 |
| First trimester tobacco use | -0.07 | -.13 | .03 |
| First trimester marijuana use | 1.98 | .12 | .02 |
| Genderf | 1.10 | .10 | .05 |
| INFANT CHARACTERISTICS QUESTIONNAIREh | |||
| Fussy/difficult (Total R2 = 0.10) | |||
| First trimester cocaine use (use vs. no use) | 2.83 | .22 | .00 |
| Home Screening Questionnaire | -0.22 | -.21 | .00 |
| Unadaptable (Total R2 = 0.09) | |||
| Home Screening Questionnaire | -0.13 | -.18 | .01 |
| Maternal education | -0.49 | -.16 | .01 |
| Maternal age | 0.09 | .12 | .03 |
| Persistent (Total R2 = 0.08) | |||
| Home Screening Questionnaire | -0.15 | -.28 | .00 |
| Gender | 0.81 | .12 | .03 |
| Race | 0.75 | .11 | .05 |
Listed in order of standardized regression coefficient, an indication of the magnitude of the effect.
Each trimester was analyzed separately but all are presented together for convenience. In cases where more than one trimester was significant, the betas for the other variables were essentially the same across models. If more than one trimester was significant, the values presented are those for the first trimester model.
Achenbach [3]
Frequent=≥ 1 line/day; not frequent=< 1 line/day
0=No work/school; 1=Works and/or attends school
0=female; 1=male
0=No; 1=Yes
Bates et al. [6]
3.3. Group Analysis
The group analysis was limited to those outcomes for which PCE was a significant predictor in the regression analysis. Analysis of covariance was used, adjusting for the significant covariates from the regression analyses. Children who were exposed throughout pregnancy had significantly more total, internalizing, and withdrawn behavior problems and were more fussy/difficult compared to those with no PCE. In addition, children who were exposed first trimester only were more fussy/difficult than those with no PCE. There were no group differences in head circumference, STM, externalizing, aggressive, or anxious/depressed behavior problems.
3.4. Repeated Measures Analysis
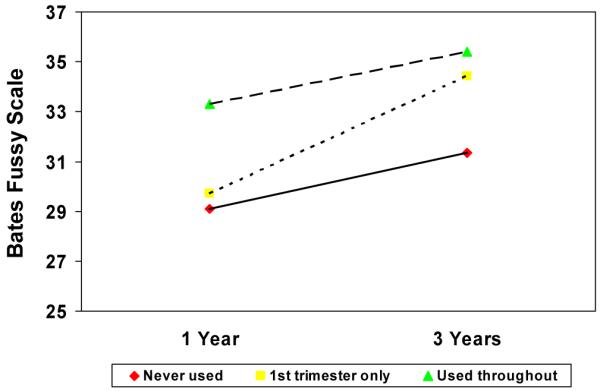
A repeated measures analysis was used to explore the relations over time between temperament and PCE, adjusting for significant predictors from the regressions. Previously, we found a significant association between PCE and the fussy/difficult scale at 1 year [44], and at 3 years, as shown in Table 8. Figure 1 shows that children who were exposed throughout pregnancy were fussier at 1 year and continued to be more fussy at 3 years of age than those whose mothers stopped using or never used. There was also a significant time by PCE interaction: Children who were exposed first trimester only were not different from the non-exposed group at 1 year, but by 3 years of age, a significant difference between groups was found.
Figure 1. Effect of prenatal cocaine use and age on child temperament.
4. Discussion
This report investigated the relationship between PCE and growth, cognitive development, behavior, and temperament at 3 years of age. PCE predicted reduced 3-year head circumference, consistent with our findings at 1 year [60] and with reports from others [35,54]. However, birth head circumference was found to mediate this relationship. There were no significant relationships between PCE and 3-year weight or height, also consistent with our 1-year findings [60] and with previous research [13,40,44]. First trimester cocaine use predicted lower short-term memory scores, which has been reported previously by some researchers [11], but not by others [35,40]. The lack of significant findings on the SBIS composite score is consistent with other reports [11,35,40], and is an indication that the effects of PCE on cognitive function are subtle and should be measured by focused neuropsychological assessments.
As we predicted, the strongest associations were between PCE and behavior. The relations between PCE and behavior and temperament were consistent across phases and type of analysis. Children with PCE were rated as more fussy/difficult and had higher behavior problem scores than non-exposed children, controlling for prenatal and current maternal substance use, sociodemographic and environmental factors, and child characteristics. There was also an effect of duration: Children who were exposed throughout pregnancy had the highest behavior problem scores and the highest fussy/difficult scores. Although there have been no publications, to our knowledge, investigating the associations between PCE and preschool temperament, our findings of increased behavior problems are consistent with reports of increased externalizing behavior problems [4,35] and aggressive behaviors [9].
We found that first trimester cocaine use was a significant predictor of neurobehavioral and neurophysiological changes at birth [61,66], temperament at 1 year [60], and memory, temperament, and behavior at 3 years. Laboratory studies demonstrate that PCE alters neural development, affecting brain function and behavior. PCE has a direct effect on the development of monoaminergic systems in rats [50,64,67,78], which have a regulatory role in neuronal proliferation and migration [15,43,45] and disrupt the development of cortical architecture [68,79]. These direct PCE effects on the developing nervous system implicate changes in brain structure and function as an underlying mechanism for our reported effects of PCE.
We expect the effects of PCE to be specific rather than global because PCE acts on the developing brain primarily through its effects on dopamine neurons. Animal models have found effects of PCE on cognitive abilities, motor performance, reward, mood, and stress reactivity, behaviors that are regulated by the dopamine and norepinephrine neurotransmitter systems [36]. PCE effects would be expected to occur in brain regions such as the prefrontal cortex that express dopamine receptors and receive dopaminergic projections from the midbrain [34]. Thus, behavioral changes would be expected in domains that rely on the function of the dopamine systems in the brain such as attention, arousal, mood, state regulation, and executive functioning [17,18,22,29].
We also found that continued cocaine use throughout pregnancy was associated with changes in neonatal neurobehavioral status [61], infant temperament [60], and preschool temperament and behavior problems. Among the continuously exposed children, the effects on temperament were evident early in development, while among those who were exposed first trimester only, the effects appeared later in development. It is not possible to determine the independent effects of cocaine use during the third trimester in human samples because all of the third trimester users also used first trimester.
Our findings regarding the associations between other predictors, including other prenatal drug use, and the reported outcomes are consistent with other reports in the literature. We found that gender and maternal height were associated with growth, as did Karlberg et al. [39] and Kuczmarski et al. [41]. Correlates of cognitive development such as home environment and race have also been found by other investigators [14,55], and in the behavior domain, we found that home environment was a correlate of temperament, as found by other researchers [16,47]. In terms of other prenatal drug exposure, we found that prenatal tobacco exposure was a significant predictor of decreased weight at 3 years: The findings in this area are inconsistent and await further study [21]. There were no relations between prenatal marijuana exposure and 3-year growth, a finding that is consistent with the literature [23,33]. We found that prenatal marijuana exposure was a predictor of 3-year cognitive development, consistent with the findings of Day et al. [25]. It is important to note that the associations between PCE and the outcomes are independent of the associations with the other drug exposures, as these other relations were statistically controlled.
The strengths of this study include its prospective design, large number of subjects, good follow-up rates, and statistical control for confounding factors, including other drugs. We can assess the effects of light to moderate levels of PCE and of the timing and duration of exposure on development. Most other studies aggregate cocaine use across pregnancy and do not look at the timing of exposure. Thus, our study makes a unique contribution by investigating the effects of PCE during the first trimester of pregnancy. Women enrolled in this study received prenatal care by their fourth or fifth month of pregnancy, were interviewed at the same time points, at frequent intervals to minimize recall bias, and with instruments that were reliable and valid [24,27,58]. This prenatal care sample represents the typical pattern of substance use in a general population of pregnant women and allows us to study the effects of exposure to cocaine early in pregnancy. One drawback of the lower prevalence of second and third trimester cocaine use is a potential lack of power to detect effects during these trimesters. We did find some effects of use later in pregnancy, but we may have detected more effects with a higher prevalence of use.
A potential limitation of the study is that biological measures were not used to document drug use. It is possible that some women who used drugs denied use and were misclassified. This would reduce the differences between groups and would not affect the significant findings. However, biological screening fails to detect many cocaine users because of the short time period for detection [38,75]. Our interviews identified a higher percentage of users than did urine screening [62], a finding also reported by others [42,80]. Detailed, confidential interviewing is an effective way to identify users and to characterize the quantity, timing, and pattern of use [57,63].
Another issue is whether maternal ratings of behavior and temperament reflect the child’s behavior or the mother’s perception of the child’s behavior. For example, women who use cocaine could be more depressed than women who do not and they might perceive their child’s behavior differently. However, PCE and maternal depression were not related in our study. Other studies also have not found differences in psychological characteristics between women who used cocaine prenatally and those who did not [44,65,71]. Maternal hostility was associated with child temperament, but when we controlled for maternal hostility, PCE was still a significant predictor of temperament.
The findings presented here controlled for maternal socioeconomic and home environment characteristics. Another potential limitation is that there may be other socioeconomic differences associated with PCE that we did not measure, such as exposure to violence and community characteristics. In later follow-up phases, we added these broad environmental measures and will be able to address this issue.
The effect sizes that we detected were small and would not be seen as clinically significant in an individual child. Furthermore, the mean scores for the SBIS and CBCL were within the average range. However, significant differences were found between groups of children and indicate that, on average, children exposed to cocaine prenatally will have reduced growth, lower memory scores, and higher behavior problem scores compared to those who were not exposed. These differences, which were found when other covariates of cocaine use and child development were controlled, are an indication that prenatal cocaine exposure has an effect on development.
In conclusion, this is a unique study of a representative sample of women from a prenatal clinic who used lower levels of substances early in pregnancy, which is an understudied population. This report documents that light to moderate levels of prenatal cocaine use are negatively associated with child growth, memory, behavior, and temperament. The associations with temperament are particularly important as previous literature has shown that aspects of child temperament, such as difficultness and unadaptability, are associated with later behavior problems [30,69,70]. Future reports from this cohort will address the development of the children at 7 and 10 years of age, when we continued to evaluate physical, cognitive, and behavioral development.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants DA05460 and DA06839 (G. Richardson, Principal Investigator).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Accornero V, Amado A, Morrow C, Xue L, Anthony J, Bandstra E. Impact of prenatal cocaine exposure on attention and response inhibition as assessed by continuous performance tests. J Dev Behav Pediatr. 2007;28:195–205. doi: 10.1097/01.DBP.0000268560.72580.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Accornero V, Morrow C, Bandstra E, Johnson A, Anthony J. Behavioral outcome of preschoolers exposed prenatally to cocaine: role of maternal behavioral health. J Pediatr Psychol. 2002;27:259–269. doi: 10.1093/jpepsy/27.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Achenbach T. Manual for the Child Behavior Checklist/2-3 and 1992 Profile. University of Vermont Department of Psychiatry; Burlington, VT: 1992. [Google Scholar]
- [4].Bada H, Das A, Bauer C, Shankaran S, Lester B, LaGasse L, Hammond J, Wright L, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:348–359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- [5].Bandstra E, Morrow C, Anthony J, Accornero V, Fried P. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001;23:545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- [6].Bates J, Freeland C, Lounsbury M. Measurement of infant difficultness. Child Dev. 1979;50:794–803. [PubMed] [Google Scholar]
- [7].Bayley N. Manual for the Bayley Scales of Infant Development. Second Edition Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- [8].Behnke M, Eyler F, Warner T, Garvan C, Hou W, Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: preschool development at 3 years of age. J Pediatr Psychol. 2006;31:41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bendersky M, Gambini G, Lastella A, Bennett D, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. J Dev Behav Pediatr. 2003;24:345–351. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bennett D, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002;38:648–658. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bennett D, Bendersky M, Lewis M. Children’s cognitive ability from 4 to 9 years old as a function of prenatal cocaine exposure, environmental risk, and maternal verbal intelligence. Dev Psychol. 2008;44:919–928. doi: 10.1037/0012-1649.44.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Betancourt L, Fischer R, Giannetta J, Malmud E, Brodsky N, Hurt H. Problem-solving ability of inner-city children with and without in utero cocaine exposure. J Dev Behav Pediatr. 1999;20:418–424. doi: 10.1097/00004703-199912000-00003. [DOI] [PubMed] [Google Scholar]
- [14].Brooks-Gunn J, Klebanov P, Duncan G. Ethnic differences in children’s intelligence test scores: Role of economic deprivation, home environment, and maternal characteristics. Child Dev. 1996;67:396–408. [PubMed] [Google Scholar]
- [15].Buznikov G, Lambert H, Lauder J. Serotonin and serotonin-like substances as regulators of early embryogenesis and morphogenesis. Cell Tissue Res. 2001;305:177–186. doi: 10.1007/s004410100408. [DOI] [PubMed] [Google Scholar]
- [16].Carey W, McDevitt S. Prevention and Early Intervention: Individual Differences as Risk Factors for the Mental Health of Children. Brunner/Mazel Publishers; New York: 1994. [Google Scholar]
- [17].Casey B, Castellano F, Giedd J, Marsh W, Hamburger S, Schubert A, Vauss Y, Vaituzis A, Dickstein D, Sarfatti S, Rapoport J. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- [18].Chiriboga C, Starr D, Kuhn L, Wasserman G. Prenatal cocaine exposure and prolonged focus attention. Poor infant information processing ability or precocious maturation of attentional systems? Dev Neurosci. 2009;31:149–58. doi: 10.1159/000207502. [DOI] [PubMed] [Google Scholar]
- [19].Cook R, Weisberg S. Residuals and Influence in Regression. Chapman and Hall; New York: 1982. [Google Scholar]
- [20].Coons C, Gay E, Fandal A, Ker C, Frankenburg W. The Home Screening Questionnaire Reference Manual. Denver Developmental Materials, Inc.; Denver, CO: 1981. [Google Scholar]
- [21].Cornelius M, Goldschmidt L, Day N, Larkby C. Alcohol, tobacco and marijuana use among pregnant teenagers: 6-year follow-up of offspring growth effects. Neurotoxicol Teratol. 2002;24:703–710. doi: 10.1016/s0892-0362(02)00271-4. [DOI] [PubMed] [Google Scholar]
- [22].Dahl R. The regulation of sleep and arousal: Development and psychopathology. Dev Psychopathol. 1996;8:3–27. [Google Scholar]
- [23].Day N, Cornelius M, Goldschmidt L, Richardson G, Robles N, Taylor P. The effects of prenatal tobacco and marijuana use on offspring growth form birth through 3 years of age. Neurotoxicol Teratol. 1992;14:407–414. doi: 10.1016/0892-0362(92)90051-b. [DOI] [PubMed] [Google Scholar]
- [24].Day N, Jasperse D, Richardson GA, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M. Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 1989;84:536–541. [PubMed] [Google Scholar]
- [25].Day N, Richardson G, Goldschmidt L, Robles N, Taylor P, Stoffer D, Cornelius M, Geva D. Effect of prenatal marijuana exposure on the cognitive development of offspring at age three. Neurotoxicol Teratol. 1994;16:169–175. doi: 10.1016/0892-0362(94)90114-7. [DOI] [PubMed] [Google Scholar]
- [26].Day N, Robles N. Methodological issues in the measurement of substance use. Ann N Y Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- [27].Day N, Sambamoorthi U, Taylor P, Richardson GA, Robles N, Jhon Y, Scher M, Stoffer D, Cornelius M, Jasperse D. Prenatal marijuana use and neonatal outcome. Neurotoxicol Teratol. 1991;13:329–334. doi: 10.1016/0892-0362(91)90079-c. [DOI] [PubMed] [Google Scholar]
- [28].Delaney E, Hopkins T. The Stanford-Binet Intelligence Scale: Fourth Edition Examiner’s Handbook. Riverside Publishing Company; Chicago, IL: 1987. [Google Scholar]
- [29].Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Earls F, Jung K. Temperament and home environment characteristics as causal factors in the early development of childhood psychopathology. J Am Acad Child Adolesc Psychiatry. 1987;26:491–498. doi: 10.1097/00004583-198707000-00005. [DOI] [PubMed] [Google Scholar]
- [31].Eyler F, Behnke M, Conlon M, Woods N, Frentzen B. Prenatal cocaine use: a comparison of neonates matched on maternal risk factors. Neurotoxicol Teratol. 1994;16:81–87. doi: 10.1016/0892-0362(94)90012-4. [DOI] [PubMed] [Google Scholar]
- [32].Frank D, Rose-Jacobs R, Beeghly M, Wilbur M, Bellinger D, Cabral H. Level of prenatal cocaine exposure and 48-month IQ: importance of preschool enrichment. Neurotoxicol Teratol. 2005;27:15–28. doi: 10.1016/j.ntt.2004.09.003. [DOI] [PubMed] [Google Scholar]
- [33].Fried P, Watkinson B, Gray R. Growth from birth to early adolescence in offspring prenatally exposed to cigarettes and marijuana. Neurotoxicol Teratol. 1999;21:513–525. doi: 10.1016/s0892-0362(99)00009-4. [DOI] [PubMed] [Google Scholar]
- [34].Goldman-Rakic P, Lidow M, Gallager D. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Griffith D, Azuma S, Chasnoff I. Three-year outcome of children exposed prenatally to drugs. J Am Acad Child Adolesc Psychiatry. 1994;33:20–27. doi: 10.1097/00004583-199401000-00004. [DOI] [PubMed] [Google Scholar]
- [36].Harvey J. Cocaine effects on the developing brain: Current status. Neurosci Biobehav Rev. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- [37].Hurt H, Malmud E, Betancourt L, Brodsky N, Giannetta J. A prospective comparison of developmental outcome of children with in utero cocaine exposure and controls using the Battelle Developmental Inventory. J Dev Behav Pediatr. 2001;22:27–34. doi: 10.1097/00004703-200102000-00005. [DOI] [PubMed] [Google Scholar]
- [38].Julien R. A Primer of Drug Action. Seventh Edition W.H. Freeman Company; New York: 1995. [Google Scholar]
- [39].Karlberg J, Lawrence C, Albertsson-Wikland K. Prediction of final height in short, normal and tall children. Acta Paediatr Supppl. 1994;406:3–10. doi: 10.1111/j.1651-2227.1994.tb13411.x. [DOI] [PubMed] [Google Scholar]
- [40].Kilbride H, Castor C, Hoffman E, Fuger K. Thirty-six-month outcome of prenatal cocaine exposure for term or near-term infants: impact of early case management. J Dev Behav Pediatr. 2000;21:19–26. doi: 10.1097/00004703-200002000-00004. [DOI] [PubMed] [Google Scholar]
- [41].Kuczmarski R, Ogden C, Grummer-Strawn L, Flegal K, Guo S, Wei R, Mei Z, Curtin L, Roche A, Johnson C. Adv Data. Vol. 31. 2000. CDC growth charts: United Sates; pp. 1–27. [PubMed] [Google Scholar]
- [42].Lester B, ElSohly M, Wright L, Smeriglio V, Verter J, Bauer C, Shankaran S, Bada H, Walls H, Huestis M, Finnegan L, Maza P. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107:309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- [43].Levitt P. Prenatal effects of drugs of abuse on brain development. Drug Alcohol Depend. 1998;51:109–125. doi: 10.1016/s0376-8716(98)00070-2. [DOI] [PubMed] [Google Scholar]
- [44].Lewis M, Misra S, Johnson H, Rosen T. Neurological and developmental outcomes of prenatally cocaine-exposed offspring from 12 to 36 months. Am J Drug Alcohol Abuse. 2004;30:299–320. doi: 10.1081/ada-120037380. [DOI] [PubMed] [Google Scholar]
- [45].Lidow M, Wang F. Neurotransmitter receptors in the developing cerebral cortex. Crit Rev Neurobiol. 1995;9:395–418. [PubMed] [Google Scholar]
- [46].Lumeng J, Cabral H, Gannon K, Heeren T, Frank D. Pre-natal exposures to cocaine and alcohol and physical growth patterns to age 8 years. Neurotoxicol Teratol. 2007;29:446–457. doi: 10.1016/j.ntt.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mangelsdorf S, Schoppe S, Buur H. The meaning of parental reports: A contextual approach to the study of temperament and behavior problems in childhood. In: Molfese V, Molfese D, editors. Temperament and Personality Development Across the Life Span. L. Erlbaum Associates Publishers; Mahwah, NJ: 2000. pp. 121–140. [Google Scholar]
- [48].McCarthy D. Manual for the McCarthy Scales of Children’s Abilities. Psychological Corporation; New York: 1972. [Google Scholar]
- [49].Messinger D, Bauer C, Das A, Seifer R, Lester B, LaGasse L, Wright L, Shankaran S, Bada H, Smeriglio V, Langer J, Beeghly M, Poole W. The Maternal Lifestyle Study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113:1677–1685. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- [50].Meyer J, Shearman L, Collins L, Maguire R. Cocaine binding sites in fetal rat brain: implications for prenatal cocaine action. Psychopharmacology. 1993;112:445–451. doi: 10.1007/BF02244892. [DOI] [PubMed] [Google Scholar]
- [51].Morrow C, Vogel A, Anthony J, Ofir A, Dausa A, Bandstra E. Expressive and receptive language functioning in preschool children with prenatal cocaine exposure. J Pediatr Psychol. 2004;29:543–554. doi: 10.1093/jpepsy/jsh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].National Institute on Drug Abuse . National Pregnancy and Health Survey: Drug Use Among Women Delivering Livebirths, 1992. Rockville, MD: 1996. National Institutes of Health Publication No. 96-3819. [Google Scholar]
- [53].Nelson S, Lerner E, Needlman R, Salvator A, Singer L. Cocaine, anemia, and neurodevelopmental outcomes in children: a longitudinal study. J Dev Behav Pediatr. 2004;25:1–9. doi: 10.1097/00004703-200402000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nulman I, Rovet J, Greenbaum R, Loebstein M, Wolpin J, Pace-Asciak P, Koren G. Neurodevelopment of adopted children exposed in utero to cocaine: the Toronto Adoption Study. Clin Invest Med. 2001;24:129–137. [PubMed] [Google Scholar]
- [55].Plomin R, Spinath F. Intelligence: Genetics, genes, and genomics. J Personal Soc Psychol. 2004;86:112–129. doi: 10.1037/0022-3514.86.1.112. [DOI] [PubMed] [Google Scholar]
- [56].Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- [57].Richardson GA, Day N, McGauhey P. The impact of prenatal marijuana and cocaine use on the infant and child. Clin Obstet Gynecol. 1993;36:302–318. doi: 10.1097/00003081-199306000-00010. [DOI] [PubMed] [Google Scholar]
- [58].Richardson GA, Day N, Taylor P. The effect of prenatal alcohol, marijuana, and tobacco exposure on neonatal behavior. Infant Behav Dev. 1989;12:199–209. [Google Scholar]
- [59].Richardson GA, Goldschmidt L, Larkby C. Effects of prenatal cocaine exposure on growth: a longitudinal analysis. Pediatrics. 2007;120:1017–1027. doi: 10.1542/peds.2006-3482. [DOI] [PubMed] [Google Scholar]
- [60].Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 2008;30:96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Richardson GA, Hamel S, Goldschmidt L, Day N. Effects of prenatal cocaine use on neonatal neurobehavioral status. Neurotoxicol Teratol. 1996;18:519–528. doi: 10.1016/0892-0362(96)00062-1. [DOI] [PubMed] [Google Scholar]
- [62].Richardson GA, Hamel S, Goldschmidt L, Day N. Growth of infants prenatally exposed to cocaine/crack: a comparison of a prenatal care and a no prenatal care sample. Pediatrics. 1999;104:e18. doi: 10.1542/peds.104.2.e18. [DOI] [PubMed] [Google Scholar]
- [63].Richardson GA, Huestis M, Day N. Assessing in utero exposure to cannabis and cocaine. In: Bellinger DC, editor. Human Developmental Neurotoxicology. Taylor & Francis Group; New York: 2006. pp. 287–302. [Google Scholar]
- [64].Ronnekleiv O, Fang Y, Choi W, Chai L. Changes in the midbrain-rostral forebrain dopamine circuitry in the cocaine-exposed primate fetal brain. Ann N Y Acad Sci. 1998;846:165–181. [PubMed] [Google Scholar]
- [65].Salisbury A, Lester B, Seifer R, LaGasse L, Bauer C, Shankaran S, Bada H, Wright L, Liu J, Poole K. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007;29:331–340. doi: 10.1016/j.ntt.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Scher M, Richardson GA, Day N. Effects of prenatal cocaine/crack exposure on EEG-sleep studies at birth and one year. Pediatrics. 2000;105:39–48. doi: 10.1542/peds.105.1.39. [DOI] [PubMed] [Google Scholar]
- [67].Seidler F, Slotkin T. Fetal cocaine exposure causes persistent noradrenergic hyperactivity in rat brain regions: effects on neurotransmitter turnover and receptors. J Pharmacol Exp Ther. 1992;263:413–421. [PubMed] [Google Scholar]
- [68].Seidler F, Temple S, McCook E, Slotkin T. Cocaine inhibits central noradrenergic and dopaminergic activity during the critical developmental period in which catecholamines influence cell development. Brain Res. 1995;85:48–53. doi: 10.1016/0165-3806(94)00186-4. [DOI] [PubMed] [Google Scholar]
- [69].Shaw D, Owens E, Giovannelli J, Winslow E. Infant and toddler pathways leading to early externalizing disorders. J Am Acad Child Adolesc Psychiatry. 2001;40:36–43. doi: 10.1097/00004583-200101000-00014. [DOI] [PubMed] [Google Scholar]
- [70].Shaw D, Vondra J, Hommerding K, Keenan K, Dunn M. Chronic family adversity and early child behavior problems: a longitudinal study of low income families. J Child Psychol Psychiatry. 1994;35:1109–1122. doi: 10.1111/j.1469-7610.1994.tb01812.x. [DOI] [PubMed] [Google Scholar]
- [71].Singer L, Arendt R, Farkas K, Minnes S, Huang J, Yamashita T. Relationship of prenatal cocaine exposure and maternal postpartum psychological distress to child developmental outcome. Dev Psychopathol. 1997;9:473–489. doi: 10.1017/s0954579497001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Singer L, Minnes S, Short E, Arendt R, Farkas K, Lewis B, Klein N, Russ S, Min M, Kirchner H. Cognitive outcomes of preschool children with prenatal cocaine exposure. JAMA. 2004;291:2448–2456. doi: 10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc.; Palo Alto, CA: 1970. [Google Scholar]
- [74].Thorndike R, Hagen E, Sattler J. The Stanford-Binet Intelligence Scale. Fourth Edition Riverside Publishing Company; Chicago, IL: 1986. [Google Scholar]
- [75].Verebey K. In: Cocaine abuse detection by laboratory methods, Cocaine - A Clinician’s Handbook. Washton AM, Gold MS, editors. Guilford Press; New York: 1987. pp. 214–228. [Google Scholar]
- [76].Warner T, Behnke M, Hou W, Garvan C, Wobie K, Eyler F. Predicting caregiver-reported behavior problems in cocaine-exposed children at 3 years. J Dev Behav Pediatr. 2006;27:83–92. doi: 10.1097/00004703-200604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised. The Psychological Corporation; San Antonio, TX: 1989. [Google Scholar]
- [78].Whitaker-Azmitia P. Role of the neurotrophic properties of serotonin in the delay of brain maturation induced by cocaine. Ann N Y Acad Sci. 1998;846:158–164. doi: 10.1111/j.1749-6632.1998.tb09734.x. [DOI] [PubMed] [Google Scholar]
- [79].Zachor D, Cherkes J, Fay C, Ocrant I. Cocaine differentially inhibits neuronal differentiation and proliferation in vitro. J Clin Invest. 1994;93:1179–1185. doi: 10.1172/JCI117071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zuckerman B, Frank D, Hingson R, Amaro H, Levenson S, Kayne H, Parker S, Vinci R, Aboagye K, Fried L, Cabral H, Timperi R, Bauchner H. Effects of maternal marijuana and cocaine use on fetal growth. N Engl J Med. 1989;320:762–768. doi: 10.1056/NEJM198903233201203. [DOI] [PubMed] [Google Scholar]


