Abstract
Background
The worldwide prevalence of metabolic syndrome is increasing and has been associated with chronic kidney disease. Kidney pathological findings in patients with metabolic syndrome have not been well described, as was explored in this study.
Study Design
Cross-sectional study.
Setting & Participants
We retrospectively screened clinical information for 146 patients who underwent elective nephrectomy for renal cell carcinoma between January 2005 and March 2007 at Brigham and Women’s Hospital, Boston, MA. Twelve patients with metabolic syndrome were identified. Twelve age- and sex-matched patients who did not have any of the criteria for metabolic syndrome were used as controls.
Predictor
Presence of metabolic syndrome defined by using Adult Treatment Panel III criteria.
Outcomes
Histological characteristics in each group, decrease in kidney function at 1-year follow-up.
Measurements
Two pathologists blinded to the clinical diagnosis independently evaluated nephrectomy specimens using Banff criteria to objectively assess histological characteristics.
Results
Baseline characteristics were similar between the 2 groups. On histopathologic examination, patients with metabolic syndrome compared with controls had a greater prevalence of tubular atrophy (P = 0.006), interstitial fibrosis (P = 0.001), and arterial sclerosis (P = 0.001), suggesting microvascular disease. Patients with metabolic syndrome had greater global (P = 0.04) and segmental glomerulosclerosis (P = 0.05). Glomerular volume and cross-sectional surface area were not different. The combined end point of tubular atrophy greater than 5%, interstitial fibrosis greater than 5%, and presence of arterial sclerosis was more prevalent in patients with metabolic syndrome (P = 0.003; odds ratio, 33; confidence interval, 2.9 to 374.3) than controls. After 1 year, estimated glomerular filtration rate was significantly lower in patients with metabolic syndrome compared with controls (P = 0.03).
Limitations
Small sample size, retrospective design.
Conclusions
We report a high prevalence of microvascular disease in patients with metabolic syndrome. There was a steeper decrease in kidney function over time in patients with metabolic syndrome, suggesting limited renal reserve. Aggressive screening and management may be warranted in patients with metabolic syndrome to protect kidney function.
INDEX WORDS: Vascular, metabolic syndrome, kidney pathology
Metabolic syndrome, or syndrome X, was first described in 1988 by Reaven1 as a clustering of cardiovascular disease risk factors. Since then, there has been great interest in research related to metabolic syndrome.2 The prevalence of metabolic syndrome in the United States is increasing and now affects almost one-third of the US adult population.3 Furthermore, given the acculturation of western diet in the developing world, the burden of metabolic syndrome appears to be increasing worldwide.3,4 Metabolic syndrome represents a clustering of metabolic abnormalities and is associated with the risk of coronary heart disease, stroke, and cardiovascular- related mortality.5,6 The Adult Treatment Panel (ATP) III defines metabolic syndrome as the presence of any 3 of 5 traits: abdominal obesity, high blood pressure (BP), abnormalities in triglyceride and high-density lipoprotein cholesterol (HDL-C) levels, and fasting hyperglycemia.7,8 In addition, metabolic syndrome is associated with increased risk of type 2 diabetes, 9 nonalcoholic fatty liver,10 hyperuricemia, and gout.11
The association of obesity and chronic kidney disease (CKD) has been reported extensively.12,13 Patients with morbid obesity characteristically develop glomerulomegaly on kidney biopsy.14,15 The association of metabolic syndrome with CKD has recently been reported.16–19 To date, kidney pathological characteristics in individuals with metabolic syndrome have not been described in detail and were the objective of the present study. In addition, we evaluated the impact of nephrectomy on kidney function in patients with metabolic syndrome and controls.
METHODS
Participants
Clinical records of patients who underwent nephrectomy at Brigham and Women’s Hospital, Boston, MA, between January 2005 and March 2007 were screened. The Partners Healthcare Institutional Review Board approved the study. Informed consent was waived because of the retrospective design of the study. In each case, tissue had been submitted from non-neoplastic kidney parenchyma distant to the tumor for evaluation by the kidney pathology service per department routine protocol. Transplant nephrectomy specimens were excluded from the initial selection. A total of 146 patients were identified, and their medical records were reviewed to determine the presence or absence of metabolic syndrome.We excluded patients with serum creatinine level greater than 1.4 mg/dL in men and greater than 1.2 mg/dL in women. This was to exclude those with advanced CKD because kidney histological characteristics in those with advanced CKD would undermine findings related to metabolic syndrome. Estimated glomerular filtration rate (eGFR) was calculated by using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation: 186 × (serum creatinine)−1.154 × (age)−0.203 × 0.742 (if female) × 1.212 (if African American).20 Most patients did not have a urine protein-creatinine ratio measured at the time of nephrectomy. Thus, we have not included this parameter in the analysis.
Metabolic syndrome was defined in our study according to National Cholesterol Education Program ATP III guidelines. According to these guidelines, the diagnosis of metabolic syndrome is based on the presence of any 3 of the following criteria: (1) abdominal obesity, defined as waist circumference greater than 102 cm in men and greater than 88 cm in women; (2) serum triglyceride level of 1.7 mmol/L or greater or drug treatment for increased triglyceride level; (3) serum HDL-C level less than 1 mmol/L in men and less than 1.3 mmol/L in women or drug treatment for low HDL-C level; (4) BP of 130/85 mm Hg or greater or drug treatment for increased BP; and (5) fasting plasma glucose level of 6.1 mmol/L or greater or drug treatment for increased blood glucose level. Patients’ records were reviewed for age, sex, race, and laboratory features within 30 days before nephrectomy. To attenuate the confounding influence of overt diabetes on kidney histological characteristics, we included only patients documented as not having a diagnosis of diabetes mellitus or use of diabetic medication. Laboratory measurements included fasting glucose, lipid profile, serum creatinine, eGFR, and urine analysis. Serial recordings of a minimum of 2 separate BP readings were recorded. Age- and sex-matched controls with no evidence of abnormal laboratory parameters were identified from the remaining patients. Twelve patients met diagnostic criteria for metabolic syndrome. We evaluated 12 age- and sex-matched healthy controls (referred to as controls) who did not meet the aforementioned criteria for metabolic syndrome.
Pathology
Two experienced kidney pathologists evaluated the pathology material independently (M.P.A. and A.F.). Both were blinded to clinical information about patients. Data were tabulated according to a scoring sheet, and when there was discordance, the independent opinion of a third senior pathologist was sought (H.G.R.). Histological sections of randomly sampled non-neoplastic kidney parenchyma submitted were processed for light microscopy according to standard techniques. Three-micron thick sections were stained with hematoxylin-eosin and periodic acid–Schiff, trichrome, and Jones’ methenamine silver stains. Detailed examination of glomerular, tubular, interstitial, and vascular pathological specimens was performed for all patients. Glomerular architecture, hypercellularity, mesangial expansion, inflammatory changes, nodular glomerulosclerosis, and other glomerular abnormalities were scored. Total number of viable and sclerosed glomeruli inclusive of subcortical scarred glomeruli present in the 1 section of non-neoplastic renal parenchyma was counted, and percentage of globally or segmentally sclerosed glomeruli was determined. Amounts of tubular atrophy and interstitial fibrosis were estimated and expressed as percentage of cortical involvement. The presence of vascular fibrous intimal thickening and arteriolar hyalinosis was recorded and graded as mild (1+), moderately severe (2+), or severe (3+). Afferent and efferent arterioles were examined for hyalinosis. Criteria for grading tubular atrophy, interstitial fibrosis, vascular sclerosis, and arteriolar hyalinosis were scored according to the Banff pathological classification, namely on a 0, 1, 2, and 3 scale reflecting none, mild, moderate, and severe changes, respectively. 21
Morphometric evaluation of glomerular diameter and total glomerular area was performed by using the National Institutes of Health Image J analyzer program,22 which involved digital image capture and computer-assisted image analysis. A single calibrated digital image capture system was used. In an unbiased systematic sampling fashion, an average of 40 viable glomeruli examined per case was electronically captured as JPEG files. At a later occasion, using the Image J software, glomeruli were outlined and the resulting planar surface area was converted from square pixels into square microns (mm2) by using a calibrated standard. Glomerular volume was calculated using the formula described by Weibel and Gomez, in which glomerular volume = glomerular area1.5 × 1.38/1.01, where 1.38 is β, the shape coefficient for a sphere, and 1.01 is the size distribution coefficient assuming a 10% coefficient of variation.23
Statistical Analysis
Baseline data were analyzed by using the following statistical methods: continuous data were analyzed by using independent-sample nonparametric tests and categorical data were analyzed by using Fisher exact test. A nonparametric method (Mann-Whitney U test) was used to compare patients with metabolic syndrome and controls. Spearman correlation coefficients were calculated for pairs of continuous variables. A specified composite end point of interstitial fibrosis, tubular atrophy, and medial sclerosis with respect to metabolic syndrome status was analyzed. Significance was reported for P < 0.05. SPSS, version 15 (SPSS Inc, Chicago, IL), was used to analyze the data.
RESULTS
Baseline characteristics are listed in Table 1. Twelve individuals with metabolic syndrome and 12 controls without metabolic syndrome were analyzed. There was no significant difference between the 2 groups by age, sex, race, smoking status, alcohol use, hemoglobin level, serum creatinine level (1.1 ± 0.28 versus 1.0 ± 0.18 mg/dL for metabolic syndrome versus controls, P = 0.2), and eGFR (62 ± 13.8 versus 71 ± 16.3 mL/min/1.73 m2 for metabolic syndrome versus controls, P = 0.1). Two control individuals had a body mass index (BMI) greater than 30 kg/m2. Similarly, 2 individuals with metabolic syndrome had a BMI less than 30 kg/m2.
Table 1.
Clinical and Kidney Pathological Characteristics in Participants With Metabolic Syndrome and Controls
| Variable | Metabolic Syndrome (n = 12) | Controls (n = 12) | P |
|---|---|---|---|
| Age (y) | 68.6 ± 8.4 | 62.2 ± 11.9 | 0.1 |
| Men | 8 (67) | 7 (58) | 0.5 |
| White race | 8 (67) | 9 (75) | 0.5 |
| History of smoking tobacco | 5 (42) | 7 (58) | 0.4 |
| History of alcohol use | 4 (33) | 6 (50) | 0.6 |
| Creatinine (mg/dL) | 1.1 ± 0.28 | 1.0 ± 0.18 | 0.1 |
| Glomerular filtration rate (mL/min/1.73 m2) | 61 ± 13.8 | 71 ± 16.3 | 0.1 |
| Hemoglobin (g/dL) | 12.7 ± 1.9 | 13.3 ± 2.0 | 0.4 |
Note: Values expressed as mean ± SD or number (percent). Conversion factors for units: serum creatinine in mg/dL to µmol/L, ×88.4, glomerular filtration rate in mL/min/1.73 m2 to mL/s/1.73 m2, ×0.01667, hemoglobin in g/dL to g/L, ×10.
Comparison between the 2 groups according to metabolic syndrome parameters is listed in Table 2. Individuals with metabolic syndrome had significantly greater weight (93.9 ± 18.4 versus 80.3 ± 12.9 kg; P = 0.04), greater BMI (31.4 ± 6.1 versus 27.0 ± 3.0 kg/m2; P = 0.04), lower serum HDL-C level (35.1 ± 8.6 versus 50.5 ± 8.2 mg/dL; P < 0.001), greater serum triglyceride level (264.4 ± 90 versus 150 ± 72 mg/dL; P = 0.01), greater fasting glucose level (136.2 ± 23 versus 88.1 ± 11 mg/dL; P < 0.001), and greater systolic BP (143 ± 14 versus 123 ± 12 mm Hg; P < 0.001) than controls. Median BMI, triglyceride, HDL-C, fasting glucose, and systolic BP values were similar to mean values.
Table 2.
Clinical Characteristics by Metabolic Syndrome Status
| Variable | Metabolic Syndrome (n = 12) | Controls (n = 12) | P |
|---|---|---|---|
| Weight (kg) | 93.9 ± 18.4 | 80.3 ± 12.9 | 0.04 |
| Height (m) | 1.72 ± 0.1 | 1.71 ± 0.8 | 0.6 |
| Body mass index (kg/m2) | 31.4 ± 6.1 | 27.0 ± 3.0 | 0.04 |
| Serum high-density lipoprotein cholesterol (mg/dL) | 35.1 ± 8.6 | 50.5 ± 8.2 | <0.001 |
| Fasting serum triglycerides (mg/dL) | 264.4 ± 90 | 150 ± 72 | 0.01 |
| Fasting serum glucose (mg/dL) | 136.2 ± 23 | 88.1 ± 11 | <0.001 |
| Systolic blood pressure (mm Hg) | 143 ± 14 | 123 ± 12 | <0.001 |
| Diastolic blood pressure (mm Hg) | 77 ± 6 | 73 ± 7 | 0.1 |
| Use of lipid-lowering agents | 3 | 0 | |
| Use of antihypertensive | 6 | 0 | |
| β-Blockers | 4 | 0 | |
| Angiotensin-converting enzyme inhibitors | 2 | 0 |
Note: Conversion factors for units: serum high-density lipoprotein cholesterol in mg/dL to mmol/L, ×0.02586, triglycerides in mg/dL to mmol/L, ×0.01129, glucose in mg/dL to mmol/L, ×0.05551.
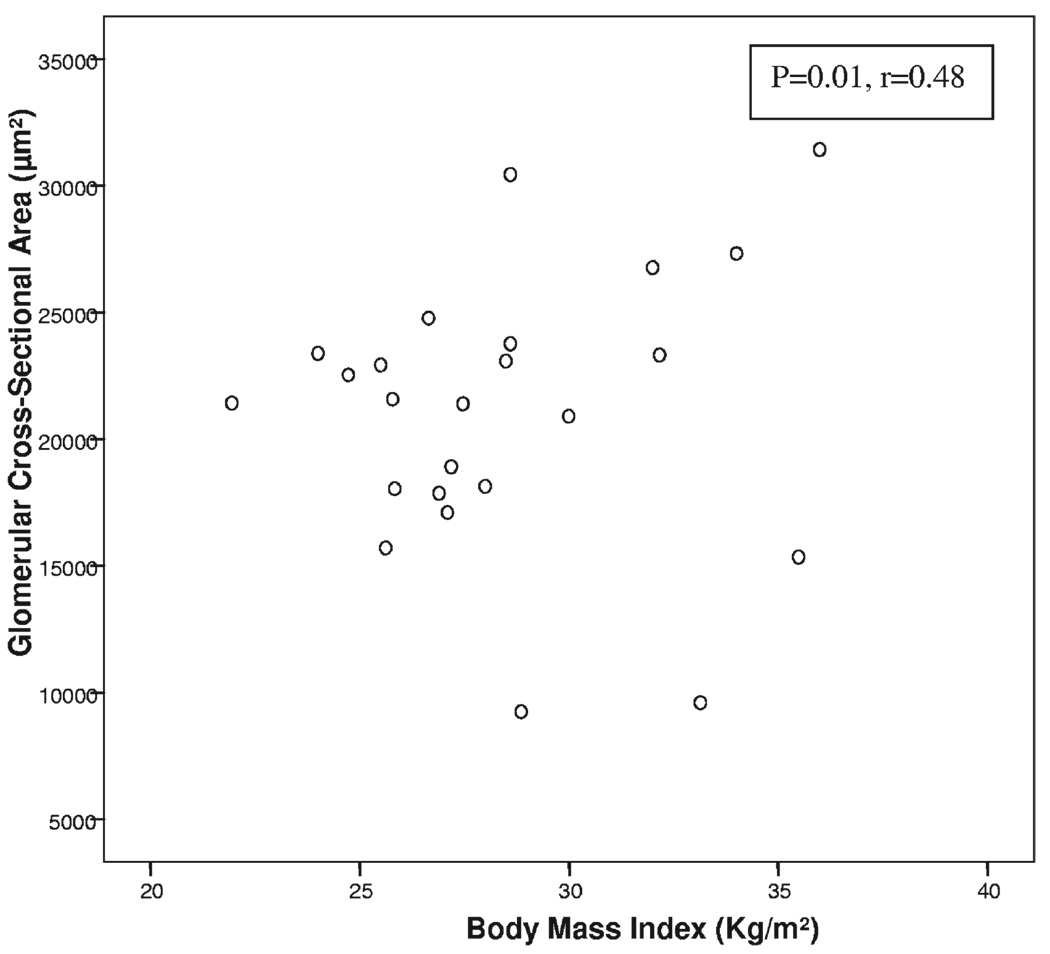
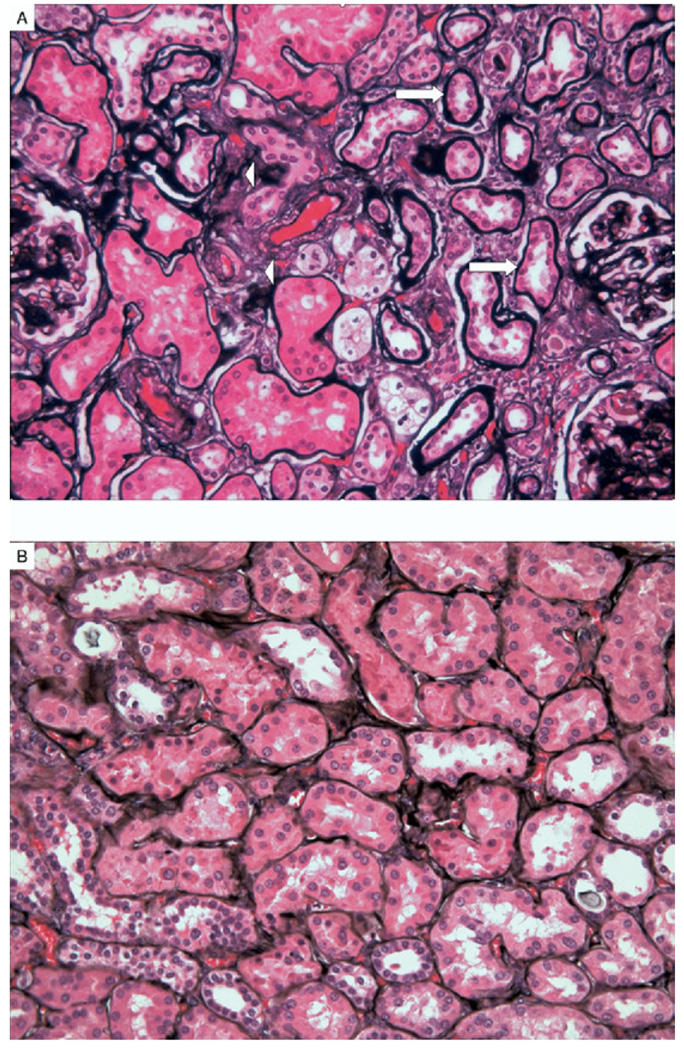
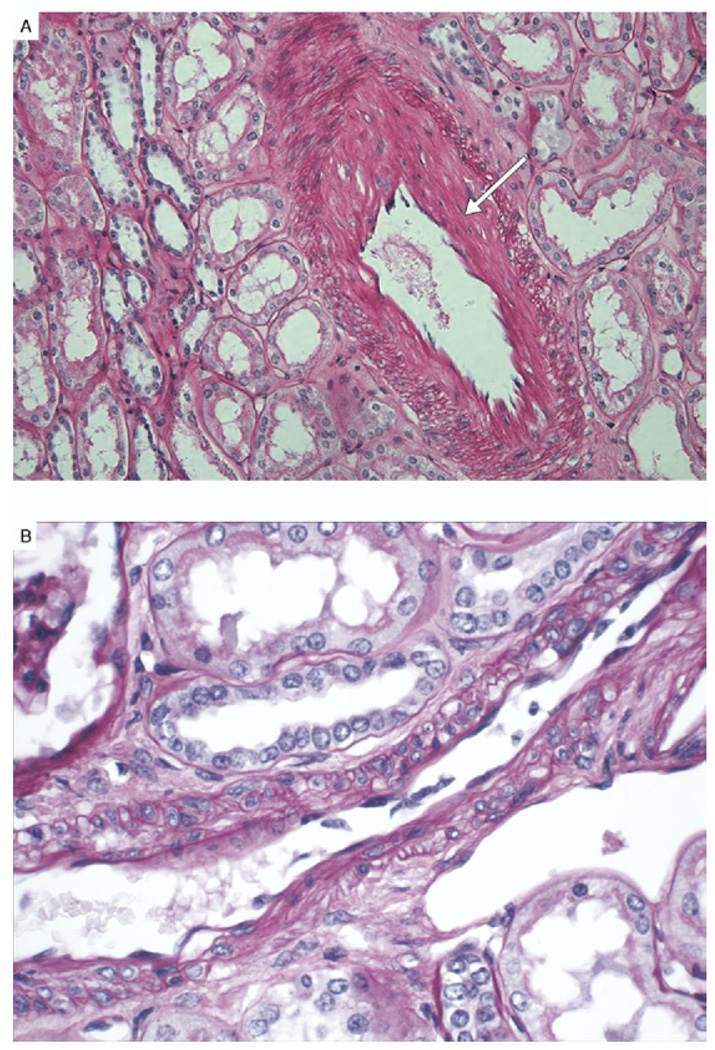
Kidney pathological findings in patients with metabolic syndrome and controls are listed in Table 3. The number of glomeruli evaluated was not different between groups. We evaluated glomerular cross-sectional area in the 2 groups and found good correlation with BMI (P = 0.01; r = 0.48; Fig 1). The difference in mean glomerular cross-sectional area (21,356 ± 7,387 µm2 in patients with metabolic syndrome versus 20,721 ± 2,802 µm2 in controls; P = 0.8) and mean glomerular volume (6.8 × 108 µm3 in metabolic syndrome versus 5.9 × 108 µm3 in controls; P = 0.5) did not reach statistical significance between the 2 groups. Glomerular sclerosis, as global glomerulosclerosis (22% ± 24% versus 8% ± 9%; P = 0.04) and segmental glomerulosclerosis (12% ± 9% versus 6.2% ± 8%; P = 0.05), was greater in patients with metabolic syndrome. Although mesangial expansion (P = 0.3) did not reach significance between groups, nodular glomerulosclerosis (4 versus 0; P = 0.03) was significantly more frequent in patients with metabolic syndrome. Tubular atrophy (16.6% ± 13% versus 3.7% ± 5.6%; P = 0.006), interstitial fibrosis (20% ± 12.5% versus 5.4% ± 4.9%; P = 0.001), and moderate to severe arterial sclerosis (9 patients with metabolic syndrome versus 1 control; P = 0.001) were more prevalent in patients with metabolic syndrome. Of note, arteriolar hyalinosis was not appreciated in those with metabolic syndrome (P = 0.2). Representative pathological characteristics for patients with metabolic syndrome and controls are shown in Fig 2 and Fig 3.
Table 3.
Kidney Pathological Findings by Metabolic Syndrome Status
| Variable | Metabolic Syndrome (n = 12) | Healthy Controls (n = 12) | P |
|---|---|---|---|
| Glomerular area (µm2) | 21,356.7 ± 7,387 | 20,721.45 ± 2,802 | 0.8 |
| Glomerular diameter (µm2) | 190.5 ± 35.5 | 194.14 ± 17.11 | 0.8 |
| Glomerular volume (µm3) | 6.8 × 108 ± 4.0 × 108 | 5.9 × 108 ± 1.5 × 108 | 0.5 |
| Global sclerosis (%) | 22.5 ± 24.6 (60) | 8.2 ± 9.2 (25) | 0.04 |
| Segmental sclerosis (%) | 12.2 ± 9.8 (25) | 6.2 ± 8.8 (18) | 0.05 |
| Tubular atrophy (%) | 16.67 ± 13.2 (40) | 3.75 ± 5.6 (15) | 0.006 |
| Interstitial fibrosis (%) | 20.0 ± 12.6 (35) | 5.4 ± 4.9 (15) | 0.001 |
| Moderate to severe arterial sclerosis | 9 (75) | 1 (8) | 0.001 |
| Nodules | 4 (33) | 0 (0) | 0.03 |
| Hyalinosis | 3 (25) | 2 (17) | 0.2 |
| No. of glomeruli visualized | 192 ± 102 | 214 ± 120 | 0.8 |
Note: Values expressed as mean ± SD or number (percent).
Figure 1.
Glomerular cross-sectional area by body mass index irrespective of metabolic syndrome status in 24 patients.
Figure 2.
(A) Representative figure of tubular atrophy (arrows) and interstitial fibrosis (arrowheads) in patients with metabolic syndrome. (B) Controls without significant abnormality. (Jones’ silver methenamine stain; original magnification ×40 high-power field.)
Figure 3.
(A) Representative figure of arterial sclerosis (arrow) in patients with metabolic syndrome. (B) Controls without significant abnormality. (Periodic acid–Schiff stain; original magnification ×40 high-power field.)
Analysis of Composite of Tubular Atrophy, Interstitial Fibrosis, and Medial Sclerosis With Metabolic Syndrome
For further analysis, we selected each of the highly significant outcome variables and categorized them as follows: tubular atrophy greater than 5% or less than 5%, interstitial fibrosis greater than 5% or less than 5%, and presence or absence of moderate to severe vascular sclerosis. Individuals in the metabolic syndrome group had a greater prevalence of tubular atrophy (P = 0.01), interstitial fibrosis (P = 0.03), and arterial sclerosis (P = 0.003). The composite of tubular atrophy, interstitial fibrosis, and medial sclerosis was more prevalent in patients with metabolic syndrome compared with controls (P = 0.003; odds ratio, 33; confidence interval, 2.9 to 374.3).
Correlation of Serum Uric Acid With Metabolic Syndrome
In a subset of patients (n = 5 with metabolic syndrome and n = 3 controls), serum uric acid was measured within 90 days before surgery. Serum uric acid level was significantly increased in patients with metabolic syndrome compared with controls (7.6 ± 1.4 versus 4.8 ± 0.7 mg/dL; P = 0.05).
Effect of Nephrectomy on Kidney Function
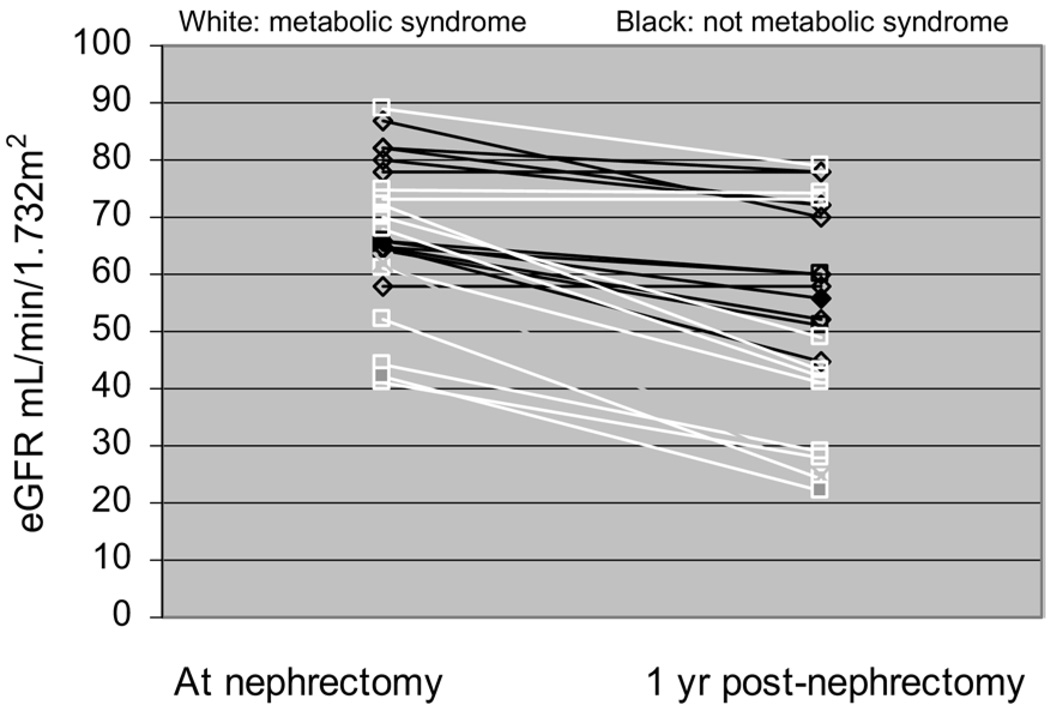
All individuals underwent planned nephrectomy without perioperative complications. We evaluated kidney function at a median follow-up of 1 year after nephrectomy. Patients were not exposed to nephrotoxic chemotherapy during this period. At 1 year of follow-up, eGFR by means of the MDRD Study equation was significantly lower in patients with metabolic syndrome compared with controls (44.0 ± 20 versus 62.0 ± 11 mL/min/1.73 m2, metabolic syndrome versus controls; P = 0.03; Fig 4). Serum creatinine level did not reach statistical significance between patients with metabolic syndrome and controls at 1 year (1.7 ± 0.67 versus 1.3 ± 0.3 mg/dL; P = 0.1). There was no statistically significant difference in prenephrectomy and 1-year follow-up eGFR in controls (71.5 ± 9.5 versus 62 ± 11 mL/min/1.73 m2 prenephrectomy versus at 1 year; P = 0.06). In contrast, individuals with metabolic syndrome had a significant decrease in eGFR (62.5 ± 15 versus 44.0 ± 20 mL/min/1.73 m2 prenephrectomy versus at 1 year; P = 0.02; Fig 4).
Figure 4.
Mean estimated glomerular filtration rate (eGFR) at the time of nephrectomy and after 1 year by metabolic syndrome status. eGFR at 1 year was significantly different between groups (P = 0.027). eGFR decreased in patients with metabolic syndrome (P = 0.02), but not in controls (P = 0.06).
DISCUSSION
The principal findings in this study were that patients with metabolic syndrome had greater tubular atrophy, interstitial fibrosis, and arterial sclerosis than matched controls, suggesting evidence of vascular damage. Patients with metabolic syndrome also had global and focal segmental sclerosis. Glomerular volume was not different between groups. One year after nephrectomy, the kidney function decrease was greater in patients with metabolic syndrome compared with controls. In conclusion, the presence of metabolic syndrome appears to adversely affect kidney parenchyma, resulting in limited kidney reserve in affected individuals.
Epidemiological evidence is emerging linking metabolic syndrome with CKD.19,24,25 The Third National Health and Nutritional Examination Survey (NHANES III) reported an increased risk of CKD (defined as eGFR < 60 mL/min/1.73 m2 and/or microalbuminuria) in patients with metabolic syndrome according to ATP III guidelines (odds ratios, 2.6 and 1.9, respectively). Furthermore, there was a graded increase in risk of worsening kidney function with an increase in number of components of metabolic syndrome.26 In our study, kidney function at baseline (serum creatinine and eGFR) was remarkably similar in both groups with metabolic syndrome and controls. However, despite controlling for age and sex, we were able to identify evidence of pronounced microvascular disease in patients with metabolic syndrome. This emphasizes the lack of sensitivity of serum creatinine assay in detecting early kidney disease and further emphasizes the importance of using a prediction equation, such as the MDRD Study equation.
In our study, tubulointerstitial changes of fibrosis and tubular atrophy were prominent kidney lesions in patients with metabolic syndrome. There may be a number of mechanisms that could explain these findings. In animal studies, hyperlipidemia has been shown to induce interstitial fibrosis and tubular atrophy.27,28 The mechanism underlying kidney injury in these animal models appears to be a combination of oxidative stress through increased reactive oxygen species, effects of profibrotic factor transforming growth factor β (TGF-β), chemokine monocyte chemoattractant protein 1 (HCP-1), and an increase in desmin-positive myofibroblasts.29–31 The role of hyperglycemia in contributing to tubulointerstitial injury has also been described.32–34 Impaired glucose tolerance and diabetes mellitus are increasingly recognized as proinflammatory states.35 More recently, midkine, a retinoic acid–responsive factor expressed in proximal tubules, has been identified as a novel key molecule that has a critical role in the tubulointerstitial inflammation associated with diabetic nephropathy.36,37 Tubulointerstitial changes also have been observed in patients with hypertension. Angiotensin II has emerged as a regulator of tubulointerstitial cell kinetics in hypertensive rats.38 Angiotensin II exerts its effects by increasing reactive oxygen species and downregulating nitric oxide synthase.39,40 It is plausible that vascular damage preceded and resulted in subsequent tubulointerstitial injury. Thus, our data are consistent with prior animal studies.
The kidney lesion of metabolic syndrome was also characterized by microvascular changes: arterial and arteriolar sclerosis. Interestingly, arteriolar hyalinosis, one of the earliest vascular changes noted in diabetes, was not appreciated in our patients with metabolic syndrome. Thus, the abnormalities we observed may not be explainable on the basis of hyperglycemia alone. Greater BPs were also observed in those with metabolic syndrome and could also have potentiated the observed vascular insult. Experimental evidence suggests that uric acid has an important role in vascular injury in fructose-induced metabolic syndrome and endothelial dysfunction by inhibiting nitric oxide production.41–43 Although we did not have serum uric acid data for all patients, we observed a significantly greater serum uric acid level in patients with metabolic syndrome compared with controls (P = 0.05).
Obesity-related glomerular changes have also been described recently in humans. A study of kidney biopsy changes in 95 extremely obese patients with a mean BMI of 52 kg/m2 undergoing bariatric surgery reported a greater prevalence of glomerulomegaly, podocyte hypertrophy, mesangial matrix, and mesangial cell proliferation.15 Another study compared kidney histological changes in obese (mean BMI, 37.6 kg/m2) and nonobese (mean BMI, 24.8 kg/m2) kidney transplant donors. Obese patients had a greater glomerular cross-sectional area compared with nonobese donors (23,604 versus 20,878 µm2).14 In contrast, our study did not include individuals with extreme obesity; mean BMI was 31.4 kg/m2 in patients with metabolic syndrome and 27.0 kg/m2 in controls. Glomerular cross-sectional area correlated with BMI (P = 0.01; r = 0.48) and thus was consistent with previous studies. We observed glomerular abnormalities with greater degrees of glomerulosclerosis and segmental sclerosis in patients with metabolic syndrome. Although studies have documented the presence of focal segmental sclerosis as a feature in patients with obesity-related kidney disease, it is not a consistent finding.44 Finally, smoking status has been associated with nodular sclerosis.45 We found that 2 of 3 patients with nodules in the metabolic-syndrome group were smokers. Because of a relatively small sample size, it was not statistically significant.
Progression of kidney damage in the contralateral kidney after nephrectomy has been observed. 46 The proposed mechanism probably is lower nephron mass available after nephrectomy. 47 In our study, the significant decrease in eGFR in those with metabolic syndrome has important clinical consequences. This is specifically true in patients undergoing nephrectomy for urogenital neoplasm or those being considered as kidney transplant donors. Because studies have shown improved kidney outcome by aggressively treating hyperglycemia,48,49 dyslipidemia, 50,51 and hypertension,52 our study supports the importance of the screening and management of metabolic syndrome.
Our study has strengths and limitations. Detailed anthropometrical measurements, such as waist and hip girth, were not available for all patients. However, we classified patients who met clinical criteria for metabolic syndrome as defined by the ATP III guidelines. Our study is a comparative analysis with its inherent confounding bias. The study had a relatively small sample size. However, given the findings, we do not believe that increasing the sample size would have changed results. Glomerular cross-sectional area was not different between groups, possibly because the groups did not have markedly different BMIs. In line with previous studies, 14 glomerular cross-sectional area correlated with BMI in our study. The inability to obtain baseline data for microalbuminuria is also a limitation because it could have served as a more sensitive index of kidney injury. Although remote, it is possible that the extent and duration of malignancy might have influenced renal pathological changes and baseline kidney function. We had complete follow up for only 1 year. However, we found striking differences in kidney function decline between groups within this short time.
In conclusion, we report kidney pathological findings in patients with metabolic syndrome. We observed a high prevalence of vascular sclerosis, tubular atrophy, and interstitial fibrosis in patients with metabolic syndrome. These changes collectively suggest a primary vascular insult. Glomerular volume was not different between groups, likely reflecting less marked differences in BMI between groups. Furthermore, patients with metabolic syndrome had a more rapid decrease in kidney function. Screening and management of patients with metabolic syndrome is warranted, especially if these patients are undergoing elective nephrectomy.
ACKNOWLEDGEMENTS
We thank Dr Joel Henderson for assistance and instruction in the use of Image J.
Support: Dr Patel was supported by National Institutes of Health grant no. T32-DK007527-23. Dr. Florez and Dr Alexander were recipients of the International Society of Nephrology scholarship.
Footnotes
Financial Disclosure: None.
REFERENCES
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—A new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 4.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–375. doi: 10.1016/j.ecl.2004.03.005. table of contents. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 6.Obunai K, Jani S, Dangas GD. Cardiovascular morbidity and mortality of the metabolic syndrome. Med Clin North Am. 2007;91:1169–1184. doi: 10.1016/j.mcna.2007.06.003. x. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 9.Resnick HE, Jones K, Ruotolo G, et al. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: The Strong Heart Study. Diabetes Care. 2003;26:861–867. doi: 10.2337/diacare.26.3.861. [DOI] [PubMed] [Google Scholar]
- 10.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Lin WY, Liu CS, Li TC, et al. In addition to insulin resistance and obesity, hyperuricemia is strongly associated with metabolic syndrome using different definitions in Chinese populations:Apopulation-based study (Taichung Community Health Study) Ann Rheum Dis. 2008;67:432–433. doi: 10.1136/ard.2007.073601. [DOI] [PubMed] [Google Scholar]
- 12.Hollenberg NK. Obesity and the kidney: Why is the kidney at risk? Kidney Int. 2007;71:187–188. doi: 10.1038/sj.ki.5002029. [DOI] [PubMed] [Google Scholar]
- 13.Ritz E. Metabolic syndrome: An emerging threat to renal function. Clin J Am Soc Nephrol. 2007;2:869–871. doi: 10.2215/CJN.02350607. [DOI] [PubMed] [Google Scholar]
- 14.Rea DJ, Heimbach JK, Grande JP, et al. Glomerular volume and renal histology in obese and non-obese living kidney donors. Kidney Int. 2006;70:1636–1641. doi: 10.1038/sj.ki.5001799. [DOI] [PubMed] [Google Scholar]
- 15.Serra A, Romero R, Lopez D, et al. Renal injury in the extremely obese patients with normal renal function. Kidney Int. 2008;73:947–955. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 16.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 17.Kanauchi M, Kanauchi K, Kimura K, Inoue T, Saito Y. Associations of chronic kidney disease with the metabolic syndrome in non-diabetic elderly. Nephrol Dial Transplant. 2006;21:3608–3609. doi: 10.1093/ndt/gfl435. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya T, Kiyohara Y. Albuminuria and chronic kidney disease in association with the metabolic syndrome. J Cardio metab Syndr. 2007;2:104–107. doi: 10.1111/j.1559-4564.2007.05734.x. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka H, Shiohira Y, Uezu Y, Higa A, Iseki K. Metabolic syndrome and chronic kidney disease in Okinawa, Japan. Kidney Int. 2006;69:369–374. doi: 10.1038/sj.ki.5000050. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Greene T, Kusek J, Beck G. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:55A. (abstr) [Google Scholar]
- 21.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 22.Rasband WS. [Accessed June 18, 2008];Bethesda, MD: US National Institutes of Health; Image J. 1997–2007 Available at: http://rsb.info.nih.gov/ij/
- 23.Weibel ER. Elementary introduction to stereological principles. In: Wiebel ER, editor. Stereological methods. Practical methods for Biological morphometry. London, UK: Academic Press; 1979. pp. 44–45. [Google Scholar]
- 24.Toprak O, Cirit M, Yesil M, et al. Metabolic syndrome as a risk factor for contrast-induced nephropathy in non-diabetic elderly patients with renal impairment. Kidney Blood Press Res. 2006;29:2–9. doi: 10.1159/000092481. [DOI] [PubMed] [Google Scholar]
- 25.Tozawa M, Iseki C, Tokashiki K, et al. Metabolic syndrome and risk of developing chronic kidney disease in Japanese adults. Hypertens Res. 2007;30:937–943. doi: 10.1291/hypres.30.937. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 27.Eddy AA. Interstitial fibrosis in hypercholesterolemic rats: Role of oxidation, matrix synthesis, and proteolytic cascades. Kidney Int. 1998;53:1182–1189. doi: 10.1046/j.1523-1755.1998.00889.x. [DOI] [PubMed] [Google Scholar]
- 28.Grone HJ, Hohbach J, Grone EF. Modulation of glomerular sclerosis and interstitial fibrosis by native and modified lipoproteins. Kidney Int Suppl. 1996;54:S18–S22. [PubMed] [Google Scholar]
- 29.Scheuer H, Gwinner W, Hohbach J, et al. Oxidant stress in hyperlipidemia-induced renal damage. Am J Physiol Renal Physiol. 2000;278:F63–F74. doi: 10.1152/ajprenal.2000.278.1.F63. [DOI] [PubMed] [Google Scholar]
- 30.Joles JA, Kunter U, Janssen U, et al. Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J Am Soc Nephrol. 2000;11:669–683. doi: 10.1681/ASN.V114669. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez J, Wu P, Packer CS, Temm C, Kelly KJ. Lipotoxic and inflammatory phenotypes in rats with uncontrolled metabolic syndrome and nephropathy. Am J Physiol Renal Physiol. 2007;293:F670–F679. doi: 10.1152/ajprenal.00021.2007. [DOI] [PubMed] [Google Scholar]
- 32.Ziyadeh FN. Mediators of diabetic renal disease: The case for TGF-beta as the major mediator. J Am Soc Nephrol. 2004;15 suppl 1:S55–S57. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]
- 33.Singh DK, Winocour P, Farrington K. Mechanisms of disease: The hypoxic tubular hypothesis of diabetic nephropathy. Nat Clin Pract Nephrol. 2008;4:216–226. doi: 10.1038/ncpneph0757. [DOI] [PubMed] [Google Scholar]
- 34.Ueno M, Kawashima S, Nishi S, et al. Tubulointerstitial lesions in non-insulin dependent diabetes mellitus. Kidney Int Suppl. 1997;63:S191–S194. [PubMed] [Google Scholar]
- 35.Al-Aly Z. Medial vascular calcification in diabetes mellitus and chronic kidney disease: The role of inflammation. Cardiovasc Hematol Disord Drug Targets. 2007;7:1–6. doi: 10.2174/187152907780059047. [DOI] [PubMed] [Google Scholar]
- 36.Kosugi T, Yuzawa Y, Sato W, et al. Midkine is involved in tubulointerstitial inflammation associated with diabetic nephropathy. Lab Invest. 2007;87:903–913. doi: 10.1038/labinvest.3700599. [DOI] [PubMed] [Google Scholar]
- 37.Sato W, Kadomatsu K, Yuzawa Y, et al. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol. 2001;167:3463–3469. doi: 10.4049/jimmunol.167.6.3463. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi S, Moriya H, Nakabayashi I, Nishiyama J, Fukuda T. Angiotensin II and IGF-I may interact to regulate tubulointerstitial cell kinetics and phenotypic changes in hypertensive rats. Hypertens Res. 2002;25:257–269. doi: 10.1291/hypres.25.257. [DOI] [PubMed] [Google Scholar]
- 39.Cao Z, Cooper ME. Role of angiotensin II in tubulointerstitial injury. Semin Nephrol. 2001;21:554–562. doi: 10.1053/snep.2001.26794. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa T, Kang DH, Ohashi R, et al. Tubulointerstitial disease: Role of ischemia and microvascular disease. Curr Opin Nephrol Hypertens. 2003;12:233–241. doi: 10.1097/00041552-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Lozada LG, Tapia E, Jimenez A, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:F423–F429. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 43.Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 44.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 45.Nasr SH, D’Agati VD. Nodular glomerulosclerosis in the nondiabetic smoker. J Am Soc Nephrol. 2007;18:2032–2036. doi: 10.1681/ASN.2006121328. [DOI] [PubMed] [Google Scholar]
- 46.Bijol V, Mendez GP, Hurwitz S, Rennke HG, Nose V. Evaluation of the nonneoplastic pathology in tumor nephrectomy specimens: Predicting the risk of progressive renal failure. Am J Surg Pathol. 2006;30:575–584. doi: 10.1097/01.pas.0000194296.74097.87. [DOI] [PubMed] [Google Scholar]
- 47.Brenner BM, Mackenzie HS. Nephron mass as a risk factor for progression of renal disease. Kidney Int Suppl. 1997;63:S124–S127. [PubMed] [Google Scholar]
- 48.Patel A, MacMahon S, Chalmers S, et al. for ADVANCE Collaborative Group: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 49.Gaede P, Lund-Andersen H, Parving HH. Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 50.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: A randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 51.Tonelli M, Moye L, Sacks FM, Cole T, Curhan GC. Effect of pravastatin on loss of renal function in people with moderate chronic renal insufficiency and cardiovascular disease. J Am Soc Nephrol. 2003;14:1605–1613. doi: 10.1097/01.asn.0000068461.45784.2f. [DOI] [PubMed] [Google Scholar]
- 52.Thomas MC, Atkins RC. Blood pressure lowering for the prevention and treatment of diabetic kidney disease. Drugs. 2006;66:2213–2234. doi: 10.2165/00003495-200666170-00005. [DOI] [PubMed] [Google Scholar]