Abstract
The efficacy of a recombinant subunit West Nile (WN) vaccine candidate was determined in a hamster model of encephalitis. Animals included young, aged, and immunocompromised animals in an effort to simulate key groups at risk of WN virus–induced disease. Groups of aged (12 month old), weanling, and adult hamsters rendered leukopenic after immunization were immunized subcutaneously with a WN virus recombinant envelope protein (WN-80E) with or without WN virus non-structural protein 1 (NS1) mixed with adjuvant or adjuvant alone. A challenge dose of wild-type WN virus was administered to produce 40–100% mortality in the control hamsters. The recombinant antigen preparations containing WN-80E with or without WN NS1 gave similar results. Hamsters in both groups had a strong antibody response after immunization, and none of the aged or weanling animals became ill or developed detectable viremia after challenge with WN virus at 2 weeks after booster vaccination. However, mortality among the control animals (administered adjuvant without antigen) at 2 weeks after booster challenge was 40–60%. In hamsters rendered leukopenic after immunization, survival rates up to 80% were observed, and a low-level viremia was detected in the vaccinated and challenged hamsters. The survival rate was significantly (P < 0.05) higher in animals vaccinated with a higher dose of WN-80E than a lower dose. The addition of NS1 did not significantly affect survival after challenge. In contrast, all of the control animals that received adjuvant only developed a very high level of viremia, and the mortality rate was 100%. These findings indicate that the recombinant WN vaccines induced antibody in and afforded protection to young and aged hamsters and immunosuppressed hamsters.
INTRODUCTION
West Nile virus (WNV), a member of the Japanese encephalitis serogroup within the family Flaviridae, genus Flavivirus, is an important mosquito-borne human pathogen that causes life-threatening neurologic disease. The public health importance of this disease has increased markedly during the last decade in WNV-endemic areas in Europe and the Middle East. In addition, since 1999, WNV has spread rapidly throughout North America, causing epidemics of WNV encephalitis, meningitis, and acute flaccid paralysis in naive populations.1,2
Although ~80% of WNV infections are asymptomatic, severe neurologic symptoms developed in ~0.7% of human infections. Overall, 9–14% of the neurologic cases of WN reported to the CDC in 1999–2006 resulted in fatal outcomes. Advanced age and immunosuppression are the main risk factors of severe or fatal WNV infection. The incidence of severe WNV neurologic disease in persons > 65 years old is estimated to be ~3–4 times higher and in immunosuppressed organ transplant recipients 60 times higher than in the general population.3,4 Children have a lower risk of developing neuroinvasive disease, but encephalitis, meningitis, and flaccid paralysis have been reported in immunocompetent children as well.5,6
Since the isolation of WNV in 1937 from a febrile woman in Uganda,7 no human vaccine or specific treatments against WNV have been developed. Recently several candidate WN vaccines in various stages of research and development were reported.8–11
Our recent studies showed that a new recombinant subunit WN vaccine candidate, developed by Hawaii Biotech (Aiea, HI), induced antibody in hamsters and afforded protection against a lethal challenge dose of WNV.12,13 The hamster model was used in the efficacy studies because the level of viremia achieved after infection was much higher than in mice models of WNV infection14 (Siirin and others, unpublished data). The elicited immune response was protective in hamsters for at least 1 year.13 Because key targets for WN vaccination are the elderly, the very young, and immunocompromised individuals, this study was conducted to assess vaccine immunogenicity and efficacy in animal populations designed to mimic these human target populations. As reported herein, the candidate WN vaccine induced antibody and afforded protection against a lethal challenge dose of WNV in old and young as well as in immunosuppressed hamsters.
MATERIALS AND METHODS
Animals
Female Syrian golden hamsters (Mesocricetus auratus) were obtained from Harlan Sprague-Dawley, Indianapolis, IN, and were used in experiments at 4 and 9 weeks and 12 months of age. Animals were cared for in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council) under an animal care and use protocol approved by the University of Texas Medical Branch. All work with infected animals was carried out in an AAALAC accredited biosafety level 3 facility.
Virus
WNV strain NY385-99, 13, originally isolated from a dead bird at the Bronx Zoo in 1999 and passaged three times in Vero cells, was used to infect the animals. The virus challenge doses were 105 PFUs for weanling and aged hamsters and 102 PFUs for immunosuppressed animals and were administered intraperitoneally (IP) at 100 µL/animal.
Vaccines and adjuvant
The production and purification of the WNV vaccine is described in detail elsewhere.12 Briefly, WNV carboxy-truncated envelope protein (WN-80E) and nonstructural protein 1 (NS1) were produced in an insect cell (Drosophila) expression system. The proteins were purified by immunoaffinity chromatography (IAC), using monoclonal antibodies that target the envelope protein and are flavivirus group specific (for the WN-80E) or target the NS1 protein and are flavivirus group specific (for NS1). For vaccination of hamsters, 1 or 5 µg of WN-80E ± 1 or 5 µg of NS1 was used as the immunizing dose per animal (Table 1). The antigens were mixed with GPI-0100 adjuvant (250 or 75 µg; Hawaii Biotech). The adjuvant control vaccine was formulated to include “mock” antigen. This material was prepared by subjecting culture supernatants from induced Drosophila cells transformed with plasmids lacking the genes encoding the specific antigens to the same purification scheme used for the WN-80E protein. The purpose of including this material with adjuvant was to control for any possible non-specific immunostimulatory effects of potential contaminants from the cell cultures co-purified with the antigens. Each hamster was inoculated twice by the subcutaneous route with 0.5 mL of vaccine formulation at 4-week intervals.
TABLE 1.
Experimental design for the evaluation of WN candidate vaccine WN-80E with or without the WN NS1 protein in groups of aged, weanling, and leukopenic hamsters
| Experiment | Group number | Number of animals | Adjuvant GPI-0100 (µg) | WN immunogens |
Challenge | Observation period (days) | ||
|---|---|---|---|---|---|---|---|---|
| WN-80E (µg) | NS1 (µg) | Mock (µg) | ||||||
| Aged hamster model | 1 | 15 | 250 | 1 | 0 | 0 | + | 30 |
| 2 | 15 | 250 | 1 | 1 | 0 | + | ||
| 3 | 15 | 250 | 0 | 0 | 1 | + | ||
| Weanling hamster model | 1 | 15 | 250 | 1 | 0 | 0 | + | 30 |
| 2 | 15 | 250 | 1 | 1 | 0 | + | ||
| 3 | 15 | 250 | 0 | 0 | 1 | + | ||
| 4 | 15 | 75 | 1 | 0 | 0 | + | ||
| 5 | 15 | 75 | 1 | 1 | 0 | + | ||
| 6 | 15 | 75 | 0 | 0 | 1 | + | ||
| Leukopenic hamster model | 1 | 15 | 250 | 1 | 0 | 0 | + | 50 |
| 2 | 15 | 250 | 1 | 1 | 0 | + | ||
| 3 | 15 | 250 | 1 | 5 | 0 | + | ||
| 4 | 15 | 250 | 5 | 0 | 0 | + | ||
| 5 | 15 | 250 | 5 | 1 | 0 | + | ||
| 6 | 15 | 250 | 5 | 5 | 0 | + | ||
| 7 | 10 | 250 | 0 | 0 | 10 | + | ||
| 8 | 10 | 250 | 0 | 0 | 10 | − | ||
| 9* | 3 | 250 | 0 | 0 | 10 | − | ||
Group 9: non-leukopenic controls (did not receive cyclophosphamide).
Experimental design
The animals were randomly assigned into the vaccination groups (Table 1). Two weeks after the second inoculation with WN vaccine, blood samples were obtained from all hamsters for assay of hemagglutination inhibition (HI), complement fixation (CF), and viral neutralizing (plaque reduction neutralization test [PRNT]) antibodies. Immediately after the blood samples were obtained, all animals were challenged by the IP route with 105 or 102 PFU of WNV as appropriate. After inoculation with WNV, six randomly selected hamsters in each group of weanling and aged hamsters were bled daily (200 µL from retroorbital sinus complex) for 6 consecutive days to determine the level and duration of viremia. Leukopenic hamsters (Groups 1–7) were bled on Days 1, 2, 3, 5, 7, 9, 12, and 15 after challenge. All animals were examined daily for signs of illness, and on Day 30 after challenge, blood samples were obtained from all surviving animals, diluted, and stored as described below until tested for HI, CF, and PRNT antibodies.
Immunosuppression of animals
Cyclophosphamide (Cytoxan; Baxter Healthcare, Princeton, NJ), a known immunosuppressive agent, was given IP at 4-day intervals in doses of 100 mg/kg. The first two doses of cyclophosphamide were given 5 and 1 days before challenge. Overall, 10 doses of cyclophosphamide were given over a 37-day interval to maintain immunosuppression. To monitor the level of immunosuppression, white blood cell (WBC), neutrophil, and lymphocyte counts were done on the serial blood samples from control uninfected animals, using a Hemavet HV950FS multispecies hematology analyzer (Drew Scientific, Oxford, CT), following the manufacturer’s instructions.
Virus titration and antibody determinations
Blood samples from the hamsters were processed differently, depending on their use. Whole blood for virus titration and PRNT antibody was collected from the retro-orbital sinus (100 µL) and was diluted 1:10 in phosphate-buffered saline, pH 7.4 (PBS), containing 10% heat-inactivated fetal bovine serum (FBS); these samples were frozen at −80°C immediately after collection until tested. Blood for HI and CF antibody determinations (100 µL) was obtained by the same method and was diluted 1:10 in saline without buffer. This suspension was centrifuged to sediment the blood cells, and the supernatant was aspirated and stored at −20°C until tested for HI and CF antibodies.
Serial dilutions (10−1–10−7) of hamster blood from six randomly selected animals per group were tested for virus in 24-well microplate cultures of Vero cells. Four microplate wells were inoculated with 100 µL of each dilution. A double overlay system was used, consisting of 2% Noble agar and minimal essential medium with Earle’s salts (MEM) containing 2% FBS, 0.21% NaHCO3, 2 mmol/L l-glutamine, 100 units/mL of penicillin, 100 µg/mL of streptomycin, and 0.015% DEAE-dextran. The second overlay contained 1.5% of neutral red solution (cat no. N2889; 0.005% final concentration of neutral red; Sigma, St. Louis, MO) and was added on Day 2 after inoculation. Plaques were read on the fourth day after inoculation; virus titers were calculated as the number of PFUs per milliliter of blood.
Antibodies to WNV were measured by HI, CF, and PRNT tests, as described previously.15,16 Antigens for the HI and CF tests were prepared from brains of newborn mice inoculated intracerebrally with WNV; infected brains were treated by the sucrose-acetone extraction method16 and inactivated with β-propiolactone.17 Hamster specimens were tested by HI at serial 2-fold dilutions from 1:20 to 1:5,120 at pH 6.6 with 4 units of antigen and a 1:200 dilution of goose erythrocytes. CF tests were performed by a microtechnique16 with 2 full units of guinea pig complement and antigen titers of 1:32 in its optimal amount of fixation. Titers were recorded as the highest dilutions giving +3 or +4 fixation of complement on a scale of 0 to +4.
PRNT tests were performed by a previously described technique13 in 24-well, Vero microplate cultures, using a fixed inoculum of WNV (~100 PFUs) against varying dilutions (1:20–1:20,480) of blood samples. Hamster blood was diluted in PBS containing 1% normal guinea pig serum (Sigma). The virus inoculum was mixed with an equal volume of each dilution, and the mixture was incubated overnight at 4°C. The following day, 50 µL of the diluted blood-virus mixture was injected into Vero microplate cultures, using two wells per dilution. Virus plaques were read 4 days later; ≥ 90% plaque reduction was used as the endpoint.
Histologic evaluation of tissues
After fixation, tissues samples were processed for routine paraffin embedding and sectioning. Tissues sections of 4- to 5-µm thickness were made, stained with hematoxylin and eosin (H&E), and evaluated microscopically; additional sections were prepared for immunoperoxidase staining by direct primary antibody labeling, as described previously.14 The primary antibody was a mouse hyperimmune ascitic fluid (MIAF), prepared against WNV, with a working dilution of 1:200.
Statistical analysis
Log-rank tests or Fisher exact probability tests were used to analyze survival data. Differences between viral titers and antibody responses were analyzed using the Wilcoxon rank-sum test or unpaired t tests. Differences were considered to be significant at P < 0.05.
RESULTS
Immunogenicity and protective efficacy in aged and weanling hamsters with WN-80E or WN-80E + NS1 antigens
Antibody response
Table 2 compares the HI, CF, and PRNT antibody responses in aged and weanling hamsters (immunized and control groups) 2 weeks after booster vaccination and before challenge with WNV. Both aged and weanling animals vaccinated with WN-80E or WN-80E + NS1 and 250 µg GPI-0100 adjuvant developed comparable levels of antibody responses. However, titers of specific WNV antibodies in weanling hamsters vaccinated with WN-80E or WN-80E + NS1 and 75 µg of adjuvant were lower than with 250 µg of adjuvant.
TABLE 2.
Immunogenicity and protective efficacy of a WN candidate vaccine in aged and weanling golden hamsters based on challenge with wild-type WN virus
| Experiment | Group | Immunogens | Adjuvant GPI-0100 | WNV reciprocal geometric mean titers (titer range) before challenge |
No. sick but recovered | Survival |
|||
|---|---|---|---|---|---|---|---|---|---|
| CF | HI | PRNT | No. survivals/total | Percent | |||||
| Aged animals | 1 | 1 µg WN-80E | 250 µg | 88 (10–640) | 111 (20–320) | 640 (160–> 1,280) | 0 | 15/15 | 100 |
| 2 | 1µg WN-80E + 1 µg NS1 | 39 (< 10–320) | 52 (< 10–320) | 854 (160–> 1,280) | 0 | 15/15 | 100 | ||
| 3 | Mock | < 10 | < 10 | < 20 | 4 | 9/15 | 60 | ||
| Weanling animals | 1 | 1 µg WN-80E | 250 µg | 68(<10–160) | 118 (< 10–320) | 1,114 (< 20–10,240) | 0 | 15/15 | 100 |
| 2 | 1µg WN-80E + 1 µg NS1 | 192 (40–320) | 211 (40–320) | 1,404 (640–2,560) | 0 | 15/15 | 100 | ||
| 3 | Mock | < 10 | < 10 | < 20 | 5 | 9/15 | 60 | ||
| 4 | 1µg WN-80E | 75 µg | 17(<10–160) | 22 (< 10–160) | 211 (40–2560) | 0 | 15/15 | 100 | |
| 5 | 1µg WN-80E + 1 µg NS1 | 12 (< 10–320) | 14 (< 10–320) | 101 (20–5120) | 0 | 15/15 | 100 | ||
| 6 | Mock | < 10 | < 10 | < 20 | 3 | 6/15 | 40 | ||
Clinical manifestations and mortality
None of the aged or weanling vaccinated animals died or showed any signs of illness during the 30-day observation period after challenge (Table 2). In contrast, 40–60% of unvaccinated animals in the aged and weanling experiments died; 27% of aged and 20–33% of weanling animals in control groups were sick but recovered.
Viremia
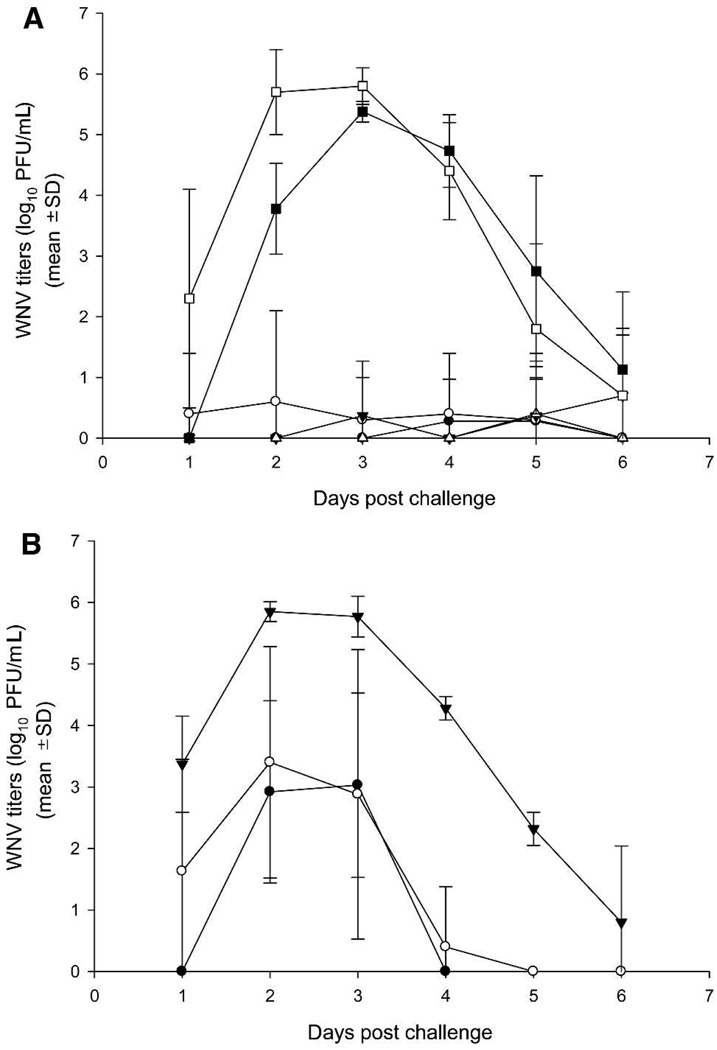
A low level of viremia was detected only in single animals of each vaccinated group in aged and weanling hamsters that received 250 µg of GPI-0100 adjuvant with both formulations of vaccine (Figure 1A), whereas all control animals in both experiments showed high levels of viremia (~20 log10 PFU/mL, cumulative values for Days 1–6 after challenge, for all control animals). There was no correlation between disease status and viremia level in control animals. In aged animals, the pattern of viremia in the control group was typical for naïve animals,14 but viremia was delayed for 1 day. The majority of weanling hamsters vaccinated with WN-80E or WN-80E + NS1 and the low dose of adjuvant (75 µg; Figure 1B) showed a higher level of viremia (2–3 logs) compared with animals that received the high dose of adjuvant (250 µg). However, even with the lower dose of adjuvant, the viremia levels were suppressed in vaccinees compared with the control animals (5–6 logs of virus).
FIGURE 1.
West Nile viremia in aged and weanling hamsters. Arithmetic means ± SD are plotted. A, 250 µg of GPI = 0100 adjuvant: Aged animals: ■ = control; ● = WN-80E; ▼ = WN-80E + NS1. Weanling animals: □ = control; ○ = WN-80E; △= WN-80E + NS1. B, 75 µg of GPI-0100 adjuvant. Weanling animals: ● = WN-80E; ○ = WN-80E + NS1; ▼ = mock control.
Protection of leukopenic hamsters with WN-80E or WN-80E + NS1 antigens
Effect of cyclophosphamide treatment
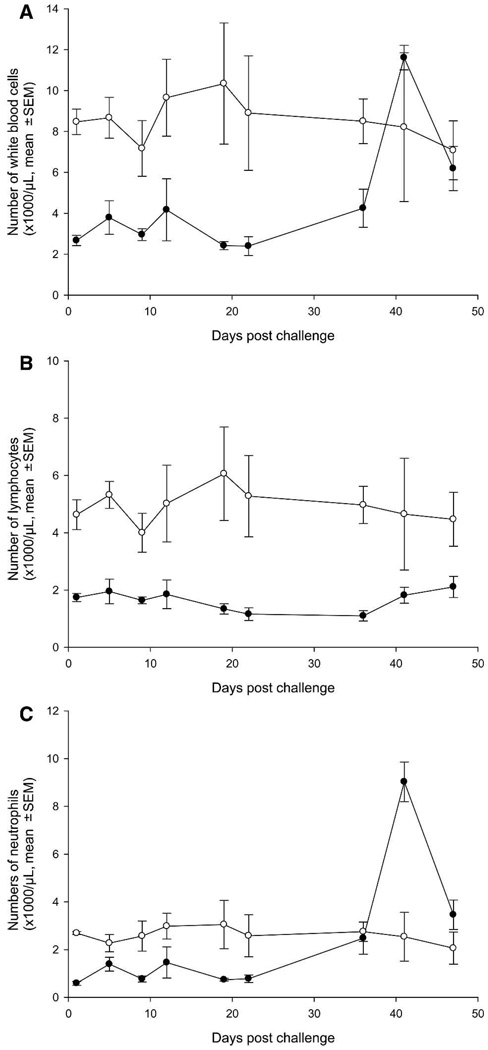
Ten mock vaccinated hamsters in the leukopenia control group (Group 8; not challenged) were treated with cyclophosphamide every 4 days and received 10 doses of cyclophosphamide along with vaccinated animals to monitor the level of leukopenia and document any adverse effects caused by the cyclophosphamide treatment (in the absence of viral challenge). The results showed that the hamsters had a 2- to 3-fold decreased level of total white blood cells (WBCs; Figure 2A) compared with normal hamsters (not cyclophosphamide treated; Group 9) during treatment with cyclophosphamide and a 2- to 5-fold decreased level of lymphocytes (Figure 2B). Although the WBCs increased rapidly after the last dose of cyclophosphamide (Day 31), the increase in WBCs was mainly caused by neutrophilia (Figure 2C), because the level of lymphocytes remained low even 19 days after the last dosing with cyclophosphamide, indicating that the animals were immunosuppressed throughout the observation period.
FIGURE 2.
A–C, Leukocyte counts in leukopenia control and normal hamsters. ○ = mock vaccinated control animals (n = 3); ● = mock vaccinated animals treated with cyclophosphamide (n = 6).
Common overt effects of cyclophosphamide treatment such as hair shedding and minor skin lesions appeared after seven doses (Day 19 after challenge) and became more pronounced in some animals after administration of the ninth dose of cyclophosphamide. Erythema of abdomen and foot pads (more pronounced on hind limbs) and weight loss of 1.4–3% developed in hamsters after 9–10 administrations of cyclophosphamide on Days 27–31 after challenge, and thereafter cyclophosphamide treatment was stopped. Other than these effects, the animals remained healthy throughout the observation period.
Antibody response
Table 3 compares the HI, CF, and PRNT antibody response of hamsters 2 weeks after booster vaccination. The antibody titers were somewhat higher (~2-fold) in the cohort vaccinated with 5 µg of WN-80E (Groups 4–6) than in the cohort vaccinated with 1 µg of WN-80E (Groups 1–3). The inclusion of NS1 had no significant effect on antibody titers. None of the control animals (Groups 7–9) had detectable antibodies against the WNV antigen.
TABLE 3.
Immunogenicity and protective efficacy of a WN candidate vaccine in leukopenic golden hamsters based on challenge with wild-type WN virus
| Group | Immunogens | Cyclophosphamide treatment | WNV reciprocal geometric mean titers (±95% CI) prior to challenge |
Survival |
|||
|---|---|---|---|---|---|---|---|
| CF | HI | PRNT | No. survivals/total | Percentage | |||
| 1 | 1µg WN–80E | + | 84 (43–125) | 70 (29–111) | 192 (24–360) | 6/15 | 40 |
| 2 | 1µg WN–80E + 1 µg NS1 | + | 53 (12–94) | 50 (11–89) | 279 (175–383) | 5/15 | 33 |
| 3 | 1µg WN–80E + 5 µg NS1 | + | 61 (34–86) | 61 (40–82) | 243 (92–394) | 8/15 | 53 |
| 4 | 5µg WN–80E | + | 153 (142–164) | 88 (74–102) | 508 (290–726) | 10/15 | 67 |
| 5 | 5µg WN–80E + 1 µg NS1 | + | 101 (74–128) | 73 (48–98) | 442 (264–620) | 12/15 | 80 |
| 6 | 5µg WN–80E + 5 µg NS1 | + | 96 (72–120) | 80 (56–104) | 368 (215–521) | 11/15 | 73 |
| 7 | Mock (adjuvant control) | + | < 10 | < 10 | < 20 | 0/10 | 0 |
| 8 | Mock (control of leukopenia) | + | < 10 | < 10 | < 20 | 10/10 | 100 |
| 9 | Mock (adjuvant control) | − | < 10 | < 10 | < 20 | 3/3 | 100 |
Clinical manifestations and mortality
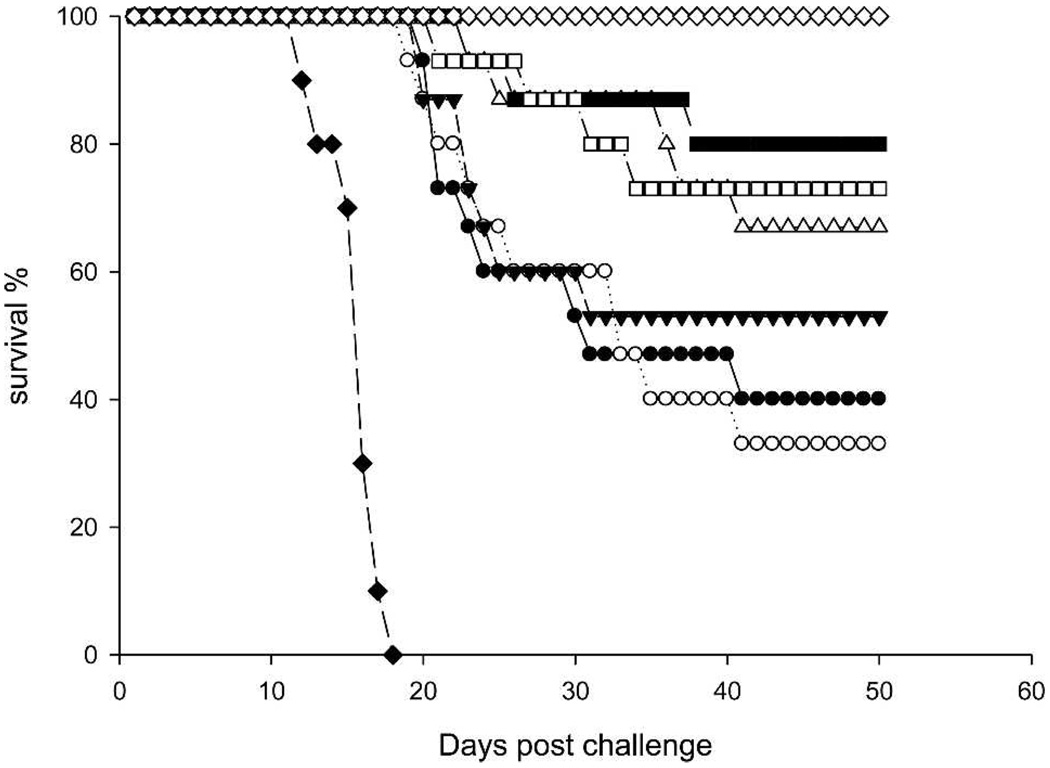
The mock-vaccinated animals in the virus control group (Group 7) appeared lethargic by the seventh day after challenge. Between Days 7 and 14, all animals in this group developed neurologic signs, including hind limb paralysis, tremors, difficulty walking, loss of balance, somnolence, and coma. Figure 3 compares the survival in WNV-infected groups. By Day 18, all of the hamsters in Group 7 were dead. In contrast, all vaccinated animals remained normal throughout Day 15 after challenge. Several animals in Groups 2 and 3 became lethargic on Day 16. The deaths in vaccinated animals occurred between Days 18 and 40 after challenge. By Day 20 and thereafter, a significant difference in survival rate (P < 0.05) was established between the two cohorts of animals that received either 1 or 5 µg of WN-80E antigen (regardless of the dose of NS1 antigen added to the vaccine). Thirty-three percent to 53% of the animals in the first cohort (1-µg dose of WN-80E) and 67–80% in the second cohort (5-µg dose of WN-80E) survived infection and remained well through Day 50 after challenge. Only two animals from Group 2 showed neurologic signs of disease after Day 40. One of those animals become lethargic on Days 40–42 but recovered, and another became lethargic on Day 48 and remained sick on the last day of observation (Day 50).
FIGURE 3.
Survival rates of leukopenic hamsters. ◆ = control Group 7 mock vaccinated, challenged; ◇ = control Group 8 mock vaccinated, not challenged; ● = Group 1 vaccinated with 1 µg WN-80E; ○ = Group 2 vaccinated with 1 µg WN-80E + 1 µg NS-1; ▼ = Group 3 vaccinated with 1 µg WN-80E + 5 µg NS-1; △= Group 4 vaccinated with 5 µg WN-80E; ■ = Group 5 vaccinated with 5 µg WN-80E + 1 µg NS-1; □ = Group 6 vaccinated with 5 µg WN-80E + 5 µg NS-1.
Level and duration of viremia
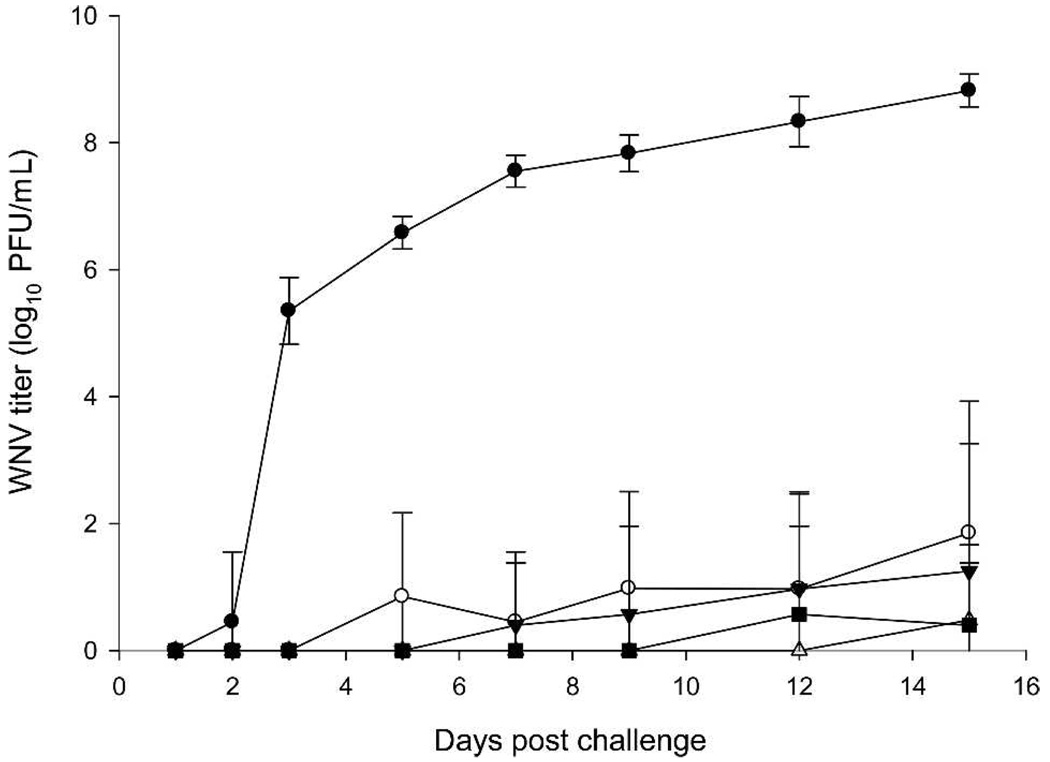
Figure 4 shows the pattern of viremia. Viremia was detected in all control Group 7 hamsters within 2 days after challenge and was continuously increasing up to 8.8 log10 PFU/mL on Day 15. After Day 15, there were no blood samples collected. In contrast, only a low level of viremia was detected in both cohorts of vaccinated animals: in two and three hamsters of Groups 1 and 2, respectively, and only single animals in Groups 4 and 6. In the animals of the first cohort vaccinated with 1 µg of WN-80E, viremia started on Days 3–5, whereas in the second cohort vaccinated with 5 µg of WN-80E, viremia was delayed until Days 12–15. No viremia was detected in animals of Groups 3 and 5.
FIGURE 4.
WN viremia in leukopenic hamsters. Arithmetic means ± SD are plotted. ● = Control Group 7 (mock vaccinated, challenged); ○ = Group 1 vaccinated with 1 µg WN-80E; ▼ = Group 2 vaccinated with 1 µg WN-80E + 1 µg NS-1; △ = Group 4 vaccinated with 5 µg WN-80E; ■ = Group 6 vaccinated with 5 µg WN-80E + 5 µg NS-1.
Histopathology and immunohistochemistry
Histopathologic examination of tissues from cyclophosphamide-treated, uninfected hamsters (control Group 8) on Day 50 after infection did not show any pathologic abnormalities (data not shown). Depletion of white pulp in the spleen with lymphocyte re-population areas around arterioles was typical in all cyclophosphamide-treated animals.
Figure 5 shows representative photomicrographs taken of immunoperoxidase-stained sections. In immunized animals that survived WNV infection and were killed 50 days after challenge, there was no obvious antigen staining in brain tissue (Figure 5C), although the virus was occasionally observed in renal tubules (Figure 5D). In contrast, WNV antigen was readily shown in the brain tissue of immunized hamsters that died (Figure 5A and B). Occasional antigen distribution was seen in liver, heart, and kidney (data not shown). Less intensive antigen staining was detected in the brain of animals in the adjuvant control group (Group 7) with more intensive viral antigen distribution in the visceral organs (heart, lung, liver, kidney). This result may be caused by more rapid death in the adjuvant control group compared with immunized animals that died.
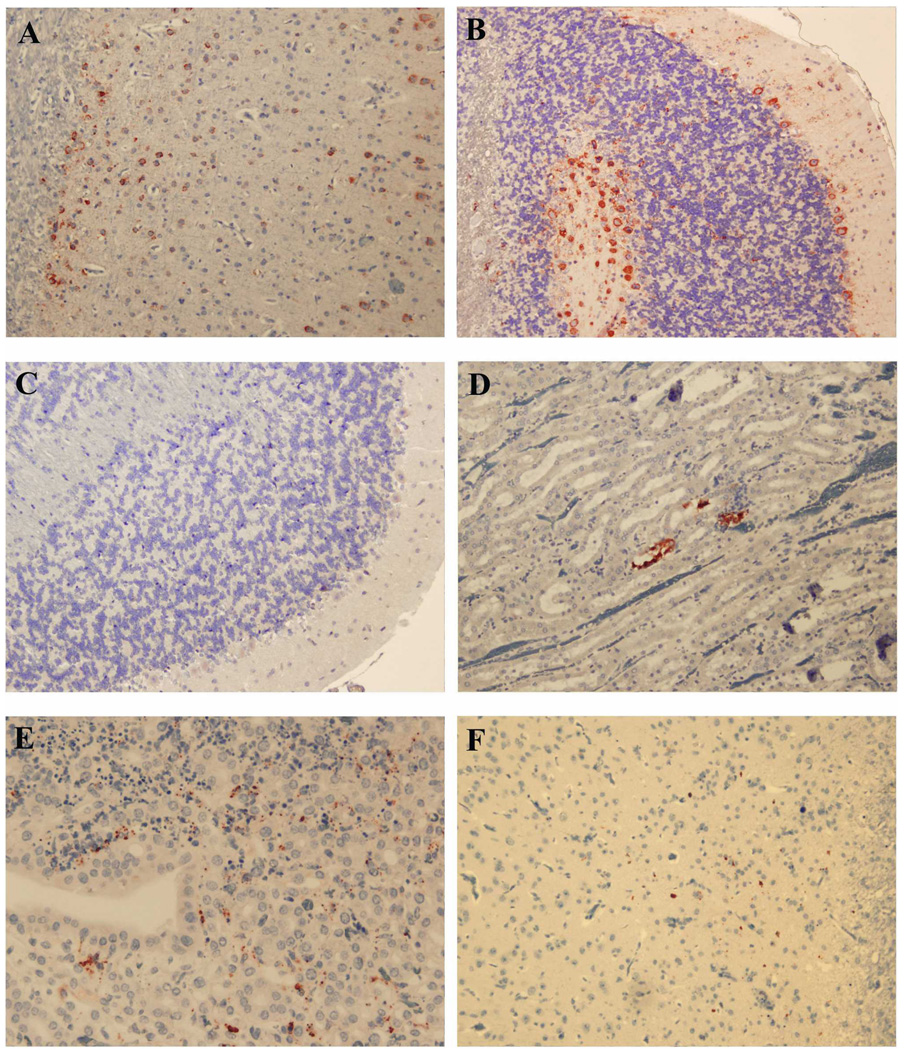
FIGURE 5.
WN viral antigen distribution in vaccinated hamsters that died after virus challenge (A and B) or survived 50 days after challenge (C–F). Antigen appears as red stain. A, Antigen staining in neurons of cerebral cortex (Group 4; magnification, ×200). B, Cerebellum, antigen staining in Purkinje cells and neurons of granular layers (Group 6; magnification, ×100). C, Cerebellum (Group 6; magnification, ×100). D, Kidney of clinically healthy hamster from Group 1, renal tubules (magnification, ×100). E, Kidney of sick hamster 843 from Group 2 (magnification, ×200). F, Single focus of infected neurons in cerebral cortex of sick hamster 843 (magnification, ×200).
Clinical disease was also associated with viral antigen staining. Tubular epithelium of medulla and cortex and also renal glomerular cells from a Group 2 animal (Hamster 843), the only immunized animal that had clinical signs of illness on Day 50 after challenge, were positive for the WNV antigen. Broad areas of renal necrosis in this animal correlated with WNV antigen distribution (Figure 5E). Weak antigen staining was also detected in the brain cortex (Figure 5F), cerebellum, and spleen of this hamster.
DISCUSSION
Although it is known that advanced age is the main risk factor for severe WNV neurologic diseases in humans,3 most of the animal models for the study of WNV infection use young adult animals. This study showed that a recombinant WNV subunit vaccine provided 100% protection in aged animals (~14 months old at the time of infection and 15 months old at the termination of the experiment). Because this species has a normal life span of ~24 months, these animals would be considered well past the midpoint of their lifespan. The vaccinated hamsters not only survived challenge, they showed no signs of clinical disease and appeared normal. Laboratory data (WNV-specific antibody titers and the absence of viremia) supported the clinical findings.
The increased susceptibility of older individuals to WNV infection is suspected to be the result of a waning immune response capability (immunosenescence). There are other populations with potentially weaker immune systems who also may be at greater risk of serious WNV infection, such as the very young. The studies reported herein document the protective efficacy of the adjuvanted WN-80E antigen vaccine in a weanling hamster model and the aged animal model. Again, 100% protection was achieved by vaccination, with no clinical signs of disease present in vaccinated weanling animals. Using the standard dose of adjuvant (250 µg) in this model, antibody titers and viremia assessments were similar to those in the aged animals (as well as young adult animals13). However, using a lower dose of adjuvant (75 µg) in weanling animals resulted in significantly decreased antibody titers and increased levels of viremia in vaccinated animals (but still 2–3 logs less virus PFU/mL than the control non-vaccinated animals). Nevertheless, protection against lethal WNV encephalitis was still 100% with no clinical disease evident in any animal vaccinated using the lower dose of adjuvant. This protection may suggest that even the lower antibody levels observed are adequate to inhibit viremia sufficiently for prevention of disease and consequent mortality.
In an animal model designed to simulate iatrogenic immunosuppression, animals were vaccinated and rendered immunocompromised by administration of the cytotoxic and immunosuppressive drug cyclophosphamide that is routinely applied to treat cancer and organ transplant patients. Animals were infected with WNV, and immunosuppression was maintained by subsequent repeated doses of the drug. Immunosuppression was monitored by leukocyte enumeration in treated animals. In this model, 100% mortality was observed in non-vaccinated infected animals, whereas vaccinated animals showed up to 80% survival, depending on the vaccine dosages used. Antibody titers and viremia assessments supported the clinical findings as well. Further support for the efficacy of the vaccine was provided by the histologic and immunohistochemical analysis of brain and visceral organs from vaccinated hamsters that survived infection compared with non-vaccinated animals (and vaccinated animals that died). In vaccinated animals surviving infection, antigen staining was not apparent in brain tissue (with the exception of only one animal) and only infrequently in renal tubules. In contrast, intensive WNV antigen staining was seen in brain tissue of all animals that died and also in liver, heart, and kidneys.
Previous studies13 have shown that a vaccine formulation with WN-NS1 as the only immunogen (i.e., without WN-80E) was capable of providing partial protection against lethal WN encephalitis and significantly decreased viremia after live viral challenge. Thus, WN-NS1 was included in some of the vaccine formulations tested in the models described herein in an attempt to determine whether the inclusion of this protein in a vaccine would enhance its protective capability. The results did not provide any evidence that the combination of NS1 with WN-80E yielded a vaccine with enhanced efficacy compared with a vaccine with WN-80E as the only immunogen.
The efficacy of the subunit WN vaccine is particularly important in these animal models because they represent the best available simulations of the particular patient populations at high risk of severe or lethal WNV infections. Many of the alternative vaccine candidates currently in various stages of research and development are based on live virus vaccine platforms.8,9,18,19 Live virus vaccines may be contraindicated in these patient populations because of increased risk of systemic viral dissemination, resulting in disease manifestation. Thus, the availability of a safe and effective non-infectious vaccine would have great value for the prevention of WNV disease in the most susceptible target populations.
Acknowledgments
The authors thank Tania Garron and Liang Xiaodong for technical contributions to this study.
Financial support: This work was supported in part by Contracts NO1-AI25489 and NO1-AI30027 and Grant 9 R44 NS52139-02A1 from the National Institutes of Health.
Contributor Information
Marina T. Siirin, University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555.
Amelia P. A. Travassos da Rosa, University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555.
Patrick Newman, University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555..
Carolyn Weeks-Levy, Hawaii Biotech Inc., 99-193 Aiea Heights Drive, Suite 200, Aiea, HI 96701..
Beth-Ann Coller, Hawaii Biotech Inc., 99-193 Aiea Heights Drive, Suite 200, Aiea, HI 96701..
Shu-Yuan Xiao, University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555..
Michael M. Lieberman, Hawaii Biotech Inc., 99-193 Aiea Heights Drive, Suite 200, Aiea, HI 96701.
Douglas M. Watts, University of Texas Medical Branch, 301 University Boulevard, Galveston, TX 77555.
REFERENCES
- 1.Zeller HG, Schuffenecker I. West Nile virus: an overview or its spread in Europe and the Mediterranean basin in contast to its speread in the Americas. Eur J Clin Microbiol Infect Dis. 2004;23:147–156. doi: 10.1007/s10096-003-1085-1. [DOI] [PubMed] [Google Scholar]
- 2.Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warner RD, Kimbrough RC, Alexander JL, Rush Pierce J, Jr, Ward T, Martinelli LP. Human West Nile virus neuro-invasive disease in Texas, 2003 epidemic. Regional Differences Ann Epidemiol. 2006;16:749–755. doi: 10.1016/j.annepidem.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Kumar D, Drebot MA, Wong SJ, Lim G, Artsob H, Buck P, Humar A. A seroprevalence study of West Nile virus infection in solid organ transplant recipients. Am J Transplant. 2004;4:1883–1888. doi: 10.1111/j.1600-6143.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 5.Civen R, Villacorte F, Robles DT, Dassey DE, Croker C, Boren-stein L, Harvey SM, Mascola L. West Nile virus infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75–78. doi: 10.1097/01.inf.0000195629.77618.30. [DOI] [PubMed] [Google Scholar]
- 6.Braun LE, Tsuchida T, Spiegel H. Meningoencephalitis in a child complicated by myocarditis, quadriparesis and respiratory failure. Pediatr Infect Dis J. 2006;25:853–856. doi: 10.1097/01.inf.0000234058.31683.70. [DOI] [PubMed] [Google Scholar]
- 7.Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from blood of a native of Uganda. Am J Trop Med. 1940;20:471–492. [Google Scholar]
- 8.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, McCarthy K, Johnson C, Ermak T, Shin S, Arroyo J, Guirakhoo F, Kennedy JS, Ennis FA, Green S, Bedford P. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci USA. 2006;103:6694–6699. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pletnev AG, Swayne DE, Speicher J, Rumyantsev AA, Murphy BR. Chimeric West Nile/dengue virus vaccine candidate: preclinical evaluation in mice, geese and monkeys for safety and immunogenicity. Vaccine. 2006;24:6392–6404. doi: 10.1016/j.vaccine.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Martina BE, Koraka P, van den Doel P, van Amerongen G, Rimmelzwaan GF, Osterhaus AD. Immunization with West Nile virus envelope domain III protects mice against lethal infection with homologous and heterologous virus. Vaccine. 2008;26:153–157. doi: 10.1016/j.vaccine.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, Andrews CA, Xu Q, Davis BS, Nason M, Fay M, Koup RA, Roederer M, Bailer RT, Gomez PL, Mascola JR, Chang GJ, Nabel GJ, Graham BS. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196:1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman MM, Clements DE, Ogata S, Wang G, Corpuz G, Wong T, Martyak T, Gilson L, Coller BA, Leung J, Watts DM, Tesh RB, Siirin M, Travassos da Rosa AP, Humphreys T, Weeks-Levy C. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine. 2007;25:414–423. doi: 10.1016/j.vaccine.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts DM, Tesh RB, Siirin M, Travassos da Rosa AP, Newman PC, Clements DE, Ogata S, Coller BA, Weeks-Levy C, Lieberman MM. Efficacy and durability of a recombinant subunit West Nile vaccine candidate in protecting hamsters from West Nile encephalitis. Vaccine. 2007;25:2913–2918. doi: 10.1016/j.vaccine.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao SY, Guzman H, Zhang H, Travassos da Rosa AP, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis. 2001;7:714–721. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tesh RB, Arroyo J, Travassos Da Rosa AP, Guzman H, Xiao SY, Monath TP. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg Infect Dis. 2002;8:1392–1397. doi: 10.3201/eid0812.020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Schmidt NJ, Emmons RW, editors. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. Sixth edition. Washington, DC: American Public Health Association; 1989. pp. 797–856. [Google Scholar]
- 17.Sever JL, Castellano GA, Pelon W, Huebner RJ, Wolman F. Inactivation of the infectivity of viral hemagglutinating antigens with the use of betaprone (propiolactone) J Lab Clin Med. 1964;64:983–988. [PubMed] [Google Scholar]
- 18.Lustig S, Olshevsky U, Ben-Nathan D, Lachmi BE, Malkinson M, Kobiler D, Halevy M. A live attenuated WNV strain as a potential veterinary vaccine. Viral Immunol. 2000;13:401–410. doi: 10.1089/vim.2000.13.401. [DOI] [PubMed] [Google Scholar]
- 19.Minke JM, Siger L, Karaca K, Austgen L, Gordy P, Bowen R, Renshaw RW, Loosmore S, Audonnet JC, Nordgren B. Recombinant canarypox vaccine carrying the prM/E genes of West Nile virus protects horses against a West Nile virus-mosquito challenge. Arch Virol Suppl. 2004;18:221–230. doi: 10.1007/978-3-7091-0572-6_20. [DOI] [PubMed] [Google Scholar]