Abstract
The present work describes a new-not yet described-interaction between the Human immunodeficiency virus type 1 (HIV-1) Rev protein and the cellular lens epithelium-derived growth factor p75 (LEDGF/p75) protein in-vitro and in virus-infected cells. Here we show, for the first time, that formation of a Rev-LEDGF/p75 complex is a crucial step in regulating the viral cDNA integration. Co-immunoprecipitation experiments at various times after virus infection revealed that first an integrase(IN)-LEDGF/p75 complex is formed, which is then replaced by a Rev-LEDGF/p75 and Rev-IN complexes. This was supported by in-vitro experiments showing that Rev promotes dissociation of the IN-LEDGF/p75 complex. Combination of the viral IN and the cellular LEDGF/p75 is required for proper integration of the viral cDNA into the host chromosomal DNA. Our findings suggest a new mechanism demonstrating that integration of HIV-1 cDNA is regulated by an interplay between viral Rev and the host-cell LEDGF/p75 proteins.
Introduction
Integration of the HIV genome into the host-cell chromosome is a central event in the viral replication cycle (1). The viral integrase enzyme (IN), which integrates the viral cDNA into the host chromosome, is one of the key components of the pre-integration complex (PIC) (2). In addition to the IN, the cellular protein lens epithelium-derived growth factor (LEDGF/p75), was also shown to be required for promoting integration of the viral DNA (3, 4). The contribution of LEDGF/p75 to the tethering of IN to the host chromatin, and thus to the whole integration process, has been demonstrated by the dramatic decrease in HIV-1 replication in siRNA LEDGF/p75-knockdown cells (5–8).
Only a small percentage of the cDNA molecules become part of the chromosomal DNA, leaving the large majority as unintegrated copies (9, 10). In some cases, the level of non-integrated HIV DNA can reach up to 99% of total viral DNA (10). A quantitative estimation of integration revealed that out of a total of 15 to 21 copies of cDNA, only one or two molecules are integrated per cell (9). Why the number of integration events is that low remains an enigma. It may be that, in addition to the stimulatory LEDGF/p75 protein, the integration process is subjected to inhibition by another regulatory factor. Our recent data suggest that this factor may be the viral Rev protein (11, 12).
The Rev protein is known to promote nuclear export of singly spliced and unspliced viral RNA in the late phase of viral infection (13). However, it was well established that Rev is also transcribed during the early phase of infection before integration of the viral DNA, namely from unintegrated DNA which has the capacity to synthesize all classes of viral transcripts (14–18). Recently it has also been clearly demonstrated that the amount of Rev transcribed from the unintegrated DNA can reach up to 70% of that transcribed by the integrated DNA (18). We have recently proposed that an early transcribed Rev interacts with IN, thereby decreasing the degree of integration (16). The ability of Rev to inhibit the integration of cDNA in-vivo was further confirmed by experiments showing that infection of cultured cells by Rev-deficient HIV results in a relatively high number of integration events (about 10 integrations/cell) (16). On the other hand, practically no integration was observed when Rev-expressing cells were infected with WT HIV (16). Thus, in addition to its function in the late phase of replication, Rev may play a central role in inhibiting/regulating the integration process (16).
In the present work, we studied the functional relations between the stimulatory LEDGF/p75 and the inhibitory Rev proteins. Based on in-vitro quantitative experiments as well as on co-immunoprecipitation (co-IP) of virus-infected cells, we suggest that Rev can remove the LEDGF/p75 protein from its association with IN, resulting in the formation of two complexes: the non-active Rev-IN (11) and the—as yet unobserved—complex between viral Rev and the cellular LEDGF/p75. We conclude that integration of viral cDNA is regulated by the interplay between the viral IN and Rev proteins and the cellular LEDGF/p75 protein.
Materials and methods
Cells
Monolayer adherent HEK293T cells, HEK293T cells overexpressing Rev (Rev10+ cells) and HeLa MAGI cells (TZM-bl) (19, 20) were grown in Dulbecco’s modified Eagle’s Medium (DMEM). The T-lymphocyte cell lines Sup-T1 and H9 were grown in RPMI 1640 medium. Cells other than the Rev10+ cells were provided by the NIH Reagent Program, Division of AIDS, NIAID, NIH, Bethesda, MD, USA. Cells were incubated at 37°C in a 5% CO2 atmosphere. All media were supplemented with 10% (v/v) fetal calf serum, 0.3 g/l L-glutamine, 100 U/ml penicillin and 100 U/ml streptomycin (Biological Industries, Beit Haemek, Israel). HeLaP4/shp75Cl15 cells, a generous gift of Prof. Z. Debyser (Molecular Medicine, K.U. Leuven, Flanders, Belgium), were grown as described in (4). Rev10+ and LEDGF/p75-knockdown Rev-expressing cells were generated by transfection into HEK293T and HeLaP4/shp75Cl15 cells, respectively (21) with pcDNA3.1 plasmid bearing the full Rev.
Viruses
WT HIV-1 (HXB2 (22)) and ΔEnv (23), as well as the IN mutant D64N D116N (24), were generated by transfection into HEK293T cells (21) of the virus-containing plasmid or co-transfected with a plasmid containing VSV-G (11). ΔRev pLAIY47H2 (25) and Rev M10 (26) HIVs were generated by transfection into Rev10+ cells. Viruses were harvested and stored as described in (11). The pLAIY47H2 (25) viruses were a generous gift from Prof. B. Berkhout (Department of Human Retrovirology, Academic Medical Center, University of Amsterdam, The Netherlands), and the IN mutant D64N D116N virus was a generous gift from Prof. A. Engelman (Department of Cancer Immunology and AIDS Dana-Farber Cancer Institute and Division of AIDS, Harvard Medical School, Boston, MA, USA).
Infection of cultured cells
Cultured lymphocytes were infected exactly as described in (11). Cultured HEK293T cells, Rev10+ cells and HeLa MAGI cells (TZM-bl) were grown for 24 h before infection, then the medium was discarded and cells were incubated at different multiplicity of infections (MOI) with the indicated virus for 2 h at 37°C. Cells were washed three times with PBS and incubated in DMEM.
Peptide synthesis and purification
Peptides were synthesized on an Applied Biosystems (ABI) 433A peptide synthesizer and purification was performed on a Gilson HPLC using a reverse-phase C8 semi-preparative column (ACE, Advanced Chromatography Technologies, USA) as described in (11).
Protein expression and purification
Expression and purification of histidine-tagged Rev-GFP was performed as previously described (27). The histidine-tagged IN and LEDGF/p75 expression vectors were a generous gift from Prof. A. Engelman and their expression and purification were performed essentially as described previously (28, 29). GST-Tat was expressed and purified as described previously (30).
ELISA-based binding assays
Protein-peptide, protein-protein and protein-DNA binding was estimated using an ELISA-based binding assay exactly as described previously (31). Briefly, Maxisorp plates (Nunc) were incubated at room temperature for 2 h with 200 ml of 10 μg/ml synthetic peptide/recombinant protein in carbonate buffer. After incubation, the solution was removed, the plates were washed three times with PBS, and 200 μl of 10% BSA (Sigma) in PBS (w/v) was added for 2h at room temperature. After rewashing with PBS, tested BSA-biotinilited (Bb), peptide or protein (alone or biotinilated) or biotinilated DNA were added for further incubation for 1h at room temperature. Following three washes with PBS, the concentration of bound molecules was estimated after the addition of streptavidin-horseradish peroxidase (HRP) conjugate (Sigma), as described previously (32), or of anti-GFP mouse antibody (Santa Cruz) which was then interacted with rabbit anti-mouse IgG antibody conjugated to HRP. The enzymatic activity of HRP was estimated by monitoring the product’s optical density (OD) at 490 nm using an ELISA plate reader (Tecan Sunrise Swizerland). Each measurement was performed in duplicate. For dissociation from and binding to a complex after binding of the first protein to the Maxisorp plate, the binding partner was incubated for 1 h at room temperature and after three washes with PBS, the dissociated component was added and its binding to the complex, as well the amount of remaining bound complex, were estimated separately as described above.
In-vitro IN activity assay
Quantitative determination of IN activity was performed exactly as described previously (12) using a previously described assay system (33, 34).
Plasmids construction
All of the plasmids used in this study were constructed exactly as described in (12). The coding sequences for full-length HIV-1 IN, Rev, LEDGF/p75 and Tat were amplified by PCR and inserted in-frame into the corresponding sites of the GN- and GC-linkers (12).
Bimolecular fluorescence complementation (BiFC)
The above-described plasmids were transformed into the yeast strain EGY48 (Clontech) and the subsequent steps, including visualization by confocal microscope (MRC 1024 confocal imaging system, Bio-Rad), were as previously described (35) (12).
Study of in-vivo protein-protein interactions by co-IP
The co-IP experiments were conducted essentially as described previously (36) with several modifications. Briefly, cells were infected with a MOI of 15 for the indicated viruses. Cells were harvested at different times PI, washed three times in PBS and lysed by the addition of PBS containing 1% (v/v) Triton X-100 for whole-cell lysate. Cytoplasm, nuclei and PIC were isolated as described below. Half of the lysate or the isolated fraction was subjected to SDS-PAGE and immunoblotted with either a monoclonal anti-Rev antibody (α-Rev) (37) or antiserum raised against IN amino acids 276–288 (α-IN) (NIH AIDS Research & Reference Reagent Program catalog number 758), or anti-LEDGF/p75 (α-LEDGF/p75) (R&D Systems) or anti-actin (α-actin) antibody (Santa Cruz), and the complementary HRP-conjugated secondary antibodies (Jackson).
The remaining lysate or isolated fractions were incubated for 1 h at 4°C with either the α-Rev, α-IN, α-LEDGF/p75 or α-actin antibodies. Following a 3-h incubation with protein G-agarose beads (Santa Cruz) at 4°C, the samples were washed three times with PBS containing 1% (v/v) Nonidet P-40. SDS buffer was added to the samples and after boiling and subjecting to SDS-PAGE, the membranes were immunoblotted with either α-Rev, α-IN, α-LEDGF/p75 or α-actin antibodies, and the complementary HRP-conjugated secondary antibodies.
When peptides were used, cells were incubated with 150 μM of the indicated peptide for 2 h prior to infection.
Quantitative estimation of the bands was performed by Image Gauge V3.46 software (Fujifilm).
Isolation of cytoplasm, nuclei and PIC from infected cells
The various fractions were obtained from virus-infected cells essentially as described previously (38) with several modifications. Briefly, cells were harvested and washed twice in buffer A (20 mM Hepes pH 7.3, 150 mM KCl, 5 mM MgCl2, 1 mM DTT and 0.1 mM PMSF). Cells were then suspended in 200 μl of buffer A with 0.025% (w/v) digitonin and incubated at room temperature for 10 min. Cells were centrifuged for 3 min at 1000g at room temperature. The supernatant was then centrifuged at 8000g and separated into supernatant (cytoplasm) and pellet (nuclei) and stored at − 70°C. For PIC isolation, an equal volume of buffer B (20 mM Hepes pH 7.4, 5 mM MgCl2, 1 mM DTT and 0.1 mM PMSF) was added to the cytoplasm fraction. Samples were incubated for 10 min at room temperature and then centrifuged for 10 min at 2000g. The supernatant was discarded and the pellet, containing the PIC aggregates, was stored at − 70°C.
Cytoplasm, nuclei and PICfraction Analysis
Cytoplasm and nuclei fractions were analyzed by western blot as described above. For detection of fraction specific protein ant actin antibody (Santa Cruz)and anti histone H3 antibody (abcam) were used.
For the anlysis of the PIC a total viral DNA was estimated by real time PCR as described below as well as integration of the PIC fraction in-vitro as described at (39).
Quantitative analysis of copy numbers of HIV-1 DNA integrated into the cellular genome
The integration reaction, as well as the integration events, were performed exactly as described previously (11).
Quantitation of total viral DNA
Total viral DNA was estimated using SYBR green real-time quantitative PCR 12 h PI, exactly as described in (40).
Quantitative estimation of HIV-1 infection by determination of extracellular p24
The amount of p24 protein was estimated in the cell medium exactly as described previously (12).
Immunostaining
HeLaP4/shp75Cl15 cells were grown on chamber slides (Nunc), then infected with ΔRev HIV-1 at a MOI of 25. Cells were fixed 16 h PI exactly as described previously (41) and immunostained essentially as described previously (41) with some modifications. Briefly, after fixation, cells were blocked with 5% IgG-free BSA (Jackson) in PBS for 60 min. For detection of HIV-1 IN and Rev and the host LEDGF/p75, the cells were incubated with 1:50 rabbit α-IN (NIH AIDS Research & Reference Reagent Program catalog number 758), 1:50 rat α-Rev (37) and 1:100 goat α-LEDGF/p75 (R&D Systems) at room temperature for 60 min each. Cells were washed five times with PBS + 0.05% (v/v) Tween 20 between antibodies. Then the cells were incubated with the following secondary antibodies: Cy2-conjugated anti-rat, Cy3-conjugated anti-rabbit and Cy5-conjugated anti-goat (Jackson) (all diluted 1:100) at room temperature for 60 min each, with five washes with PBS + 0.05% Tween 20 between antibodies. For detection of DNA, cells were stained with DAPI according to the manufacturer's protocol. Slides were prepared with Mounting Media (Bio-Rad) and immunofluorescent cells were detected with an Olympus confocal microscope.
Statistic analysis
p < 0.05, calculated from at least 3 repetitions for Real time analysis p < 0.01, ± stand for standard deviation.
For further experimental details see supplementary Materials and Methods. All supplementary materials are available online at www.molmed.org).
Results
Viral Rev and cellular LEDGF/p75 proteins interact under in-vitro conditions and in yeast cells
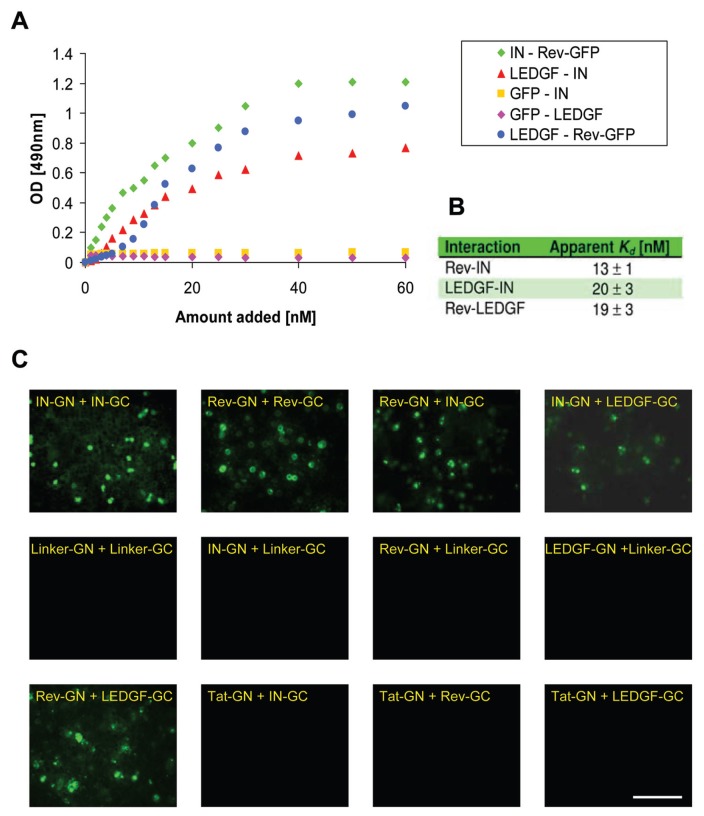
Both the host cell’s LEDGF/p75 and the viral Rev have been found to interact with the viral IN protein, affecting its biological function (3, 4, 11, 16). Using an ELISA-based system, a Rev-LEDGF/p75 interaction was also observed with an apparent Kd in the low nanomolar range, similar to that observed for the IN-LEDGF/p75 and Rev-IN interactions (Figure 1A and B). Due to the relatively low solubility (42) of recombinant Rev, we have used—in our in-vitro experiments—a Rev-GFP conjugate (27) (Figure 1A). Specificity of the Rev-LEDGF/p75 interaction could be inferred from the observation that GFP alone fails to interact with LEDGF/p75 (Figure 1A). The Rev and LEDGF/p75 proteins were also able to interact within the intracellular environment of yeast cells, as shown by the bimolecular fluorescence complementation (BiFC) experiments depicted in Figure 1C. As can be seen restoration of fluorescence was observed only in samples bearing two known interacting proteins such as IN-IN (1), Rev–Rev (13), Rev–IN (12) and IN-LEDGF/p75 (8) (Figure 1C), indicating the specificity of the Rev-LEDGF/p75 interaction. Furthermore the fact that restoration of fluorescence demonstrates specific protein-protein interaction is strengthen by the control experiments showing that no fluorescence was restored when cells were transformed with the linker plasmids containing only the half GFP (GN or GC) or when such linkers were co-expressed with Rev, IN or LEDGF/p75 conjugated to the second half of the GFP (Figure 1C). Also no interaction was observed when a different early expressed protein namely Tat (15, 17) was co-expressed with either one of those proteins (Figure 1C).
Figure 1.
Interaction between the HIV Rev and the cellular LEDGF/p75 proteins under in-vitro and in-vivo conditions. (A) An ELISA assay system was used to determine the Rev(−GFP)-LEDGF/p75, Rev(−GFP)-IN and IN-LEDGF/p75 interactions. LEDGF/p75 or IN were bound to ELISA plate and the binding of Rev(−GFP) to LEDGF/p75 ( ) or to IN (
) or to IN ( ) as well as the binding of LEDGF/p75 to IN (
) as well as the binding of LEDGF/p75 to IN ( ) was estimated as described in Materials and Methods. For control the binding of the GFP to LEDGF/p75 (
) was estimated as described in Materials and Methods. For control the binding of the GFP to LEDGF/p75 ( ) and to IN (
) and to IN ( ) were studied. (B) Apparent Kd as calculated from the results presented in (A). (C) The BiFC method was used in yeast cells to demonstrate Rev-LEDGF/p75 interactions. Cells were transfected with the indicated expression vectors and following incubation appearance of fluorescence, due to protein complementation, was visualized by fluorescent confocal microscopy. The Linker plasmid contain only the half GFP (GN or GC) and a linker as described in Materials and methods. The scale bar represents 1 μm. All other details as described in Materials and methods.
) were studied. (B) Apparent Kd as calculated from the results presented in (A). (C) The BiFC method was used in yeast cells to demonstrate Rev-LEDGF/p75 interactions. Cells were transfected with the indicated expression vectors and following incubation appearance of fluorescence, due to protein complementation, was visualized by fluorescent confocal microscopy. The Linker plasmid contain only the half GFP (GN or GC) and a linker as described in Materials and methods. The scale bar represents 1 μm. All other details as described in Materials and methods.
Rev promotes in-vitro dissociation of IN-LEDGF/p75 complex
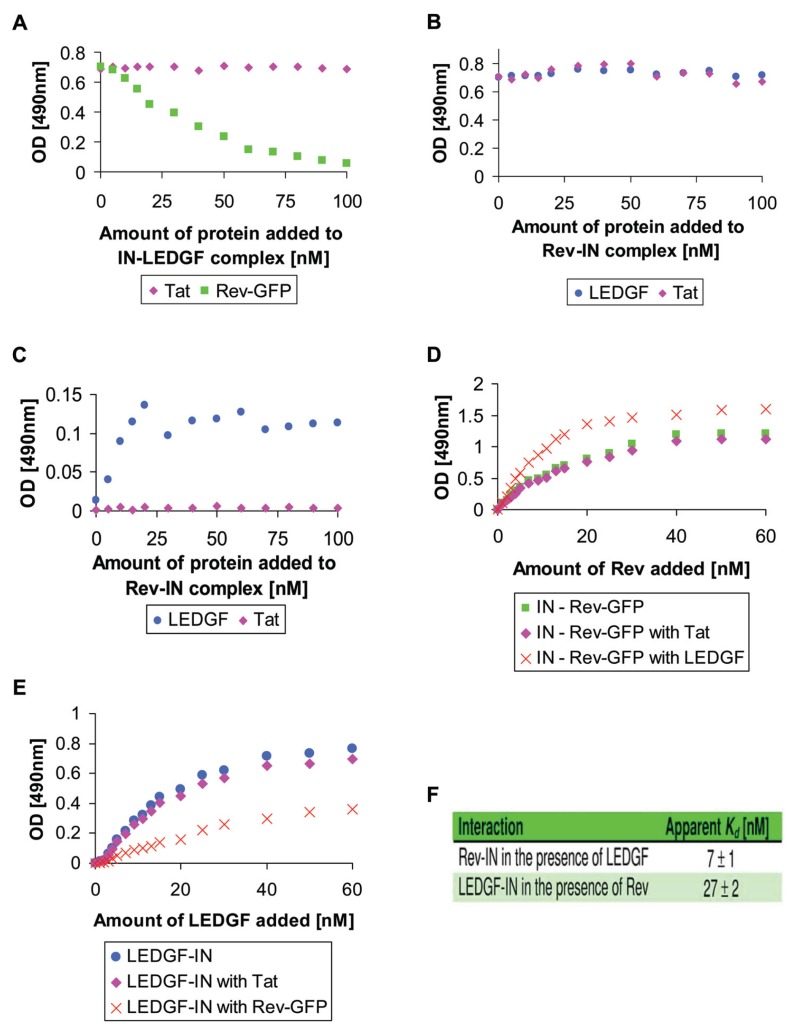
The results in Figure 2A show that addition of the Rev-GFP conjugate to pre-formed IN-LEDGF/p75 complex promotes removal of the bound LEDGF/p75. This disruption of the complex was probably due to the ability of Rev to interact with both LEDGF/p75 (Figure 1) and IN (12). GFP does not interact with either LEDGF/p75 (Figure 1) or IN (11). Specificity of Rev's binding ability could also be inferred from the observation that the HIV-1 Tat protein failed to promote the IN-LEDGF/p75 dissociation (Figure 2A). On the other hand, LEDGF/p75 did not promote dissociation of the Rev-IN complex (Figure 2B), probably due to its lower ability to bind that complex (Figure 2C).
Figure 2.
Rev promotes dissociation of the IN-LEDGF/p75 complex while the LEDGF/p75 fails to promote dissociation of the Rev-IN complex. (A) Rev-GFP conjugate ( ) and Tat (
) and Tat ( ) were added to plate-bound IN-LEDGF/p75 complex and the amount of bound LEDGF/p75 was estimated. (B) LEDGF/p75 (
) were added to plate-bound IN-LEDGF/p75 complex and the amount of bound LEDGF/p75 was estimated. (B) LEDGF/p75 ( ) and Tat (
) and Tat ( ) were added to plate-bound Rev(−GFP)-IN complex and the amount of bound Rev-GFP was estimated. (C) Same as in B but the amount of bound LEDGF/p75 and Tat was estimated. (D) Rev-GFP (
) were added to plate-bound Rev(−GFP)-IN complex and the amount of bound Rev-GFP was estimated. (C) Same as in B but the amount of bound LEDGF/p75 and Tat was estimated. (D) Rev-GFP ( ), Rev-GFP and LEDGF/p75 (
), Rev-GFP and LEDGF/p75 ( ) or Rev-GFP and Tat (
) or Rev-GFP and Tat ( ) were added to plate-bound IN and the amount of bound Rev-GFP was estimated. (E) LEDGF/p75 (
) were added to plate-bound IN and the amount of bound Rev-GFP was estimated. (E) LEDGF/p75 ( ), Rev-GFP and LEDGF/p75 (
), Rev-GFP and LEDGF/p75 ( ) or LEDGF/p75 and Tat (
) or LEDGF/p75 and Tat ( ) were added to plate-bound IN and the amount of bound LEDGF/p75 was estimated. (F) Apparent Kd as calculated from the changes in affinity presented in (D) and (E). All other details are described in Materials and Methods.
) were added to plate-bound IN and the amount of bound LEDGF/p75 was estimated. (F) Apparent Kd as calculated from the changes in affinity presented in (D) and (E). All other details are described in Materials and Methods.
Our binding experiments, revealed slightly higher affinity of the Rev protein to the IN-LEDGF/p75 complex than to IN alone (Figure 2D and F), while LEDGF/p75 showed lower affinity to the Rev-IN complex (Figure 2E and F). For characterization of the domains mediating the Rev-LEDGF/p75 interaction see supplementary data and supplementary Figure S1.
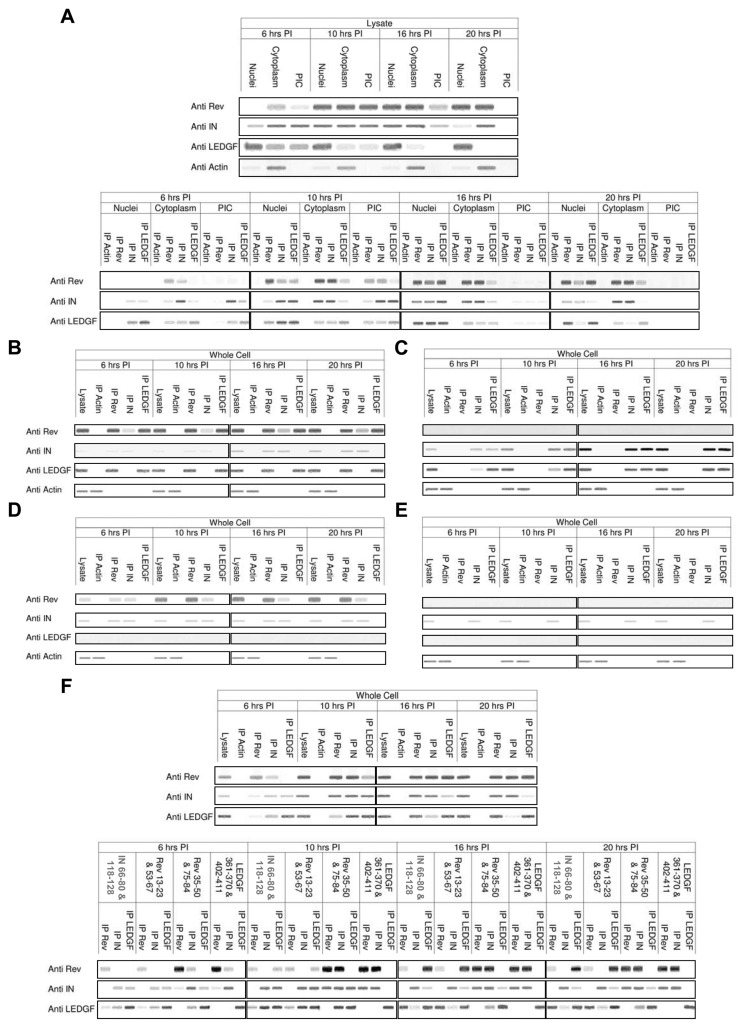
Rev promotes dissociation of the IN-LEDGF/p75 complex in virus-infected cells
Western blot analysis revealed the presence of IN, LEDGF/p75 and Rev within the cytoplasm and the viral PIC in infected cells at 6h and 16h post infection (PI) (Figure 3A). These results demonstrate that Rev expressed from unintegrated DNA can be translocated into the PIC (see also (16)). Analysis of the cytoplasm, nuclei and PIC fractions) revealed IN enzymatic activity of the PIC fraction and the presence of actin only in the cytoplasm and chromatin only in the nuclei fractions (see supplementary data and supplementary Figure S2).
Figure 3.
Rev induced disruption of the IN-LEDGF/p75 complex. (A) The presence of Rev, IN, LEDGF/p75 and Actin (control) was detected by western blots in cell lysates obtained from HIV-1-infected H9 lymphocytes at different times after infection using the appropriate antibodies. Rev, IN, LEDGF/p75 and Actin were also precipitated from the cytoplasm, nuclei and PIC fractions obtained from HIV-1-infected H9 lymphocytes at different times PI by the indicated specific antibodies. (B) Co-IP of Rev-IN and Rev-LEDGF/p75, but not IN-LEDGF/p75, can be observed in Rev10+ cells. (C) Only IN-LEDGF/p75 co-IP can be observed in cells infected with ΔRev HIV-1. (D) Only Rev-IN co-IP can be observed in LEDGF/p75-knockdown cells infected with WT HIV-1. (E) In LEDGF/p75-knockdown cells infected with ΔRev HIV-1, only IN can be detected. (F) Co-IP of Rev-IN, IN-LEDGF/p75 and Rev-LEDGF/p75, which is observed in infected cell lysates, is specifically interrupted by pretreating the cells with 150 μM of specific peptides that contain the interaction-mediating sequences. Quantitative estimation of the bands, performed by Image Gauge V3.46 software, is presented in supplementary Table 1A–F, respectively. All other details are described in Materials and Methods.
It also should be noted that the western blot analysis revealed (Figure 3A) that at early stages of viral infection (at 6h and 10h PI) the LEDGF/p75 protein is present in all these three fractions but predominantly is seen in the nuclei. At later periods it is only localized within the nuclei due to its karyophilic properties.(43). The cytoplasm and PIC presence of LEDGF/p75 at early stages of infection may be due to its interaction with the IN protein which is localized at these stages in cytoplasm and within the PIC. This is indicated by the co-IP experiments demonstrating the presence of IN-LEDGF/p75 complexes in these fractions (Figure 3). At the later time of infection the increasing amounts of Rev, expressed from unitegrated viral cDNA, promote dissociation of the IN-LEDGF/p75 complex as is evident from the co-IP experiment (Figure 3). It is our assumption that disruption of this complex may allow translocation of LEDGF/p75 into the nuclei of the infected cells.
Our co-IP experiments (Figure 3) revealed that a Rev-LEDGF/p75 complex is present also in virus-infected cells. As can be seen Rev-LEDGF/p75 and IN-LEDGF/p75 complexes were present, in almost the same amounts at 10–16 h PI, but not at 6 hrs PI (Figure 3A). At 20 h PI, the amount of IN-LEDGF/p75 complex was reduced, in parallel with an increase in the Rev-LEDGF/p75 complex (Figure 3A and supplementary Table 1).
In virus-infected Rev-expressing cells (Rev10+), the IN-LEDGF/p75 complex could not be detected (Figure 3B). The presence of overexpressed Rev may either induce the dissociation or disrupt the formation of the IN-LEDGF/p75 complex, confirming the results obtained in-vitro (Figure 2). On the other hand, the formation of Rev-IN and Rev-LEDGF/p75 complexes was not affected in Rev10+ cells, as demonstrated by the co-IP experiments (Figure 3B).
Only IN-LEDGF/p75 complex was detected in cells infected with the ΔRev virus (Figure 3C). Correspondingly, only Rev-IN complex was observed in LEDGF/p75-knockdown cells (Figure 3D). As expected, no complex between IN and either LEDGF/p75 or Rev was detected in LEDGF/p75-knockdown cells infected with the ΔRev virus (Figure 3E), supporting the validity of our experimental system. Our experiments also demonstrated that the formation of either Rev-LEDGF/p75 or Rev-IN complexes does not require the presence of a functional Rev as was demonstrated following infection with a Rev-M10 mutated HIV ((26) and not shown).
Specific disruption of the Rev, IN and LEDGF/p75 interaction by peptides derived from the interaction domains
The use of peptides in the co-IP experiments verified the specificity of the domains, which mediate the Rev-LEDGF/p75, Rev-IN and IN-LEDGF/p75 interactions, as were characterized by the in-vitro binding studies (see also supplementary data and supplementary Figure S1).
The IN-binding Rev-derived peptides, Rev 13–23 and Rev 53–67 ((12) and supplementary Figure S1), failed to disrupt the Rev-LEDGF/p75 and the IN-LEDGF/p75 complexes but were able to disrupt the Rev-IN complex (Figure 3F). Recently, we reported the selection of two IN-derived peptides—IN 66–80 and IN 118–128, designated INr-1 and 2, respectively—which specifically interact with the Rev protein and are able to promote dissociation of the Rev-IN complex in-vitro (11). As can be seen in Figure 3F, indeed these two peptides prevented co-IP of Rev and IN in the HIV-infected cells. Similar to the IN binding Rev derived, these peptides failed to disrupt the Rev-LEDGF/p75 or IN-LEDGF/p75 complexes (Figure 3F).
On the other hand, the LEDGF/p75-binding Rev-derived peptides, Rev 35–50 and Rev 75–84 selected in the present work (supplementary Figure S1), were able to specifically prevent co-IP of the Rev-LEDGF/p75 complex (Figure 3F). The LEDGF/p75 derived peptides, LEDGF 361–370 and LEDGF 402–411, which were shown to mediate the binding of LEDGF/p75 to both IN (44) and Rev (supplementary Figure S1), disrupted formation of the IN-LEDGF/p75 and Rev-LEDGF/p75 complexes but not of the Rev-IN complex (Figure 3F).
Integration of viral cDNA is regulated by the interplay between viral Rev and host-cell LEDGF/p75 proteins
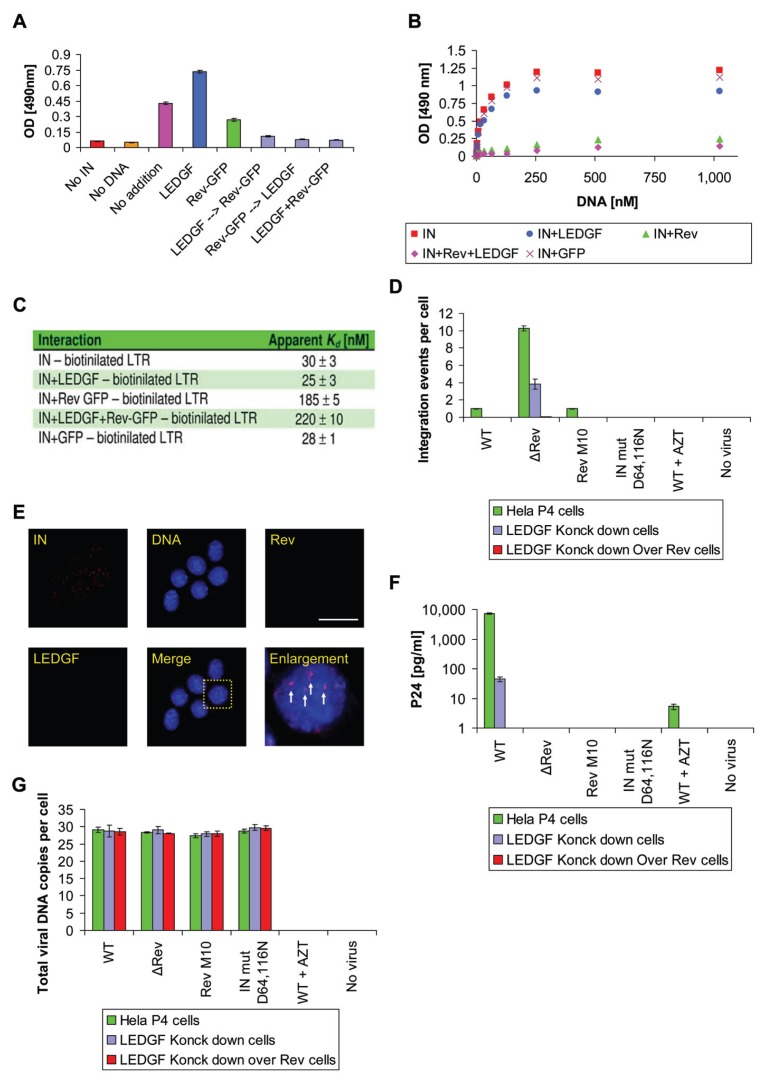
A) In-Vitro
As previously shown (11), the interaction of the recombinant Rev(−GFP) protein with IN causes partial inhibition of IN’s enzymatic activity in-vitro (Figure 4A), The presence of Rev significantly blocked the interaction between the viral IN and its DNA substrate (Figure 4B and C). The apparent Kd of the IN-DNA interaction increased from about 30 nM to 185 nM in the presence of Rev (Figure 4C). On the other hand, and as has been observed previously (45), the LEDGF/p75 protein stimulated IN activity (Figure 4A). The addition of both Rev and LEDGF/p75 to IN, regardless of the order of addition, caused further inhibition of integration, above that observed with Rev(−GFP) alone (Figure 4A). This is probably due to the increased affinity of the inhibitory Rev protein to IN in the presence of LEDGF/p75 (Figure 2D and F). The addition of LEDGF/p75 slightly reduced the affinity of the Rev-IN complex to DNA (Figure 4B and C). Thus it is clear that even in the presence of excess LEDGF/p75, the inhibitory effect of Rev prevails may be due to direct effect on the IN activity regardless of its affinity to DNA. From these results, as well as from previous ones (11, 16), it appears that both the inhibitory Rev and the stimulatory LEDGF/p75 affect the enzymatic activity of IN in-vitro.
Figure 4.
The Effect of the combination of LEDGF/p75 and Rev on IN enzymatic activity in-vitro and in LEDGF/p75-knockdown cells. (A) IN (390 nM) was added to the substrate DNA and then either LEDGF/p75, Rev-GFP or both—in the order indicated by arrows—were added or as control non of them were added (no addition) and IN enzymatic activity was determined as described in Materials and methods. (B) An ELISA system was used to determine binding of biotinilated DNA to plate-bound IN ( ), IN+LEDGF/p75 (
), IN+LEDGF/p75 ( ), IN+Rev−GFP (
), IN+Rev−GFP ( ), IN+Rev−GFP+LEDGF/p75 (
), IN+Rev−GFP+LEDGF/p75 ( ), and IN+GFP (
), and IN+GFP ( ) used as a control. Binding was estimated as described in Materials and methods. (C) Apparent Kd as calculated for the results presented in (B). (D) Hela P4 (
) used as a control. Binding was estimated as described in Materials and methods. (C) Apparent Kd as calculated for the results presented in (B). (D) Hela P4 ( ), LEDGF/p75-knockdown (
), LEDGF/p75-knockdown ( ) and LEDGF/p75-knockdown-Rev-expressing (
) and LEDGF/p75-knockdown-Rev-expressing ( ) cells were infected by the indicated viruses (at a MOI of 1) and integration events per cell were determined at 24 h PI. (E) LEDGF/p75-knockdown cells were infected with ΔRev HIV-1, and IN (red), DNA (blue), Rev (green) and LEDGF/p75 (gray) were immunostained as described in Materials and methods. Scale bar represents 10 μm. (F) Same as (D) except that p24 production was determined at 72 h PI. (G) Same as (D) except that viral cDNA was determined at 12 h PI. AZT was added at 2 μM.
) cells were infected by the indicated viruses (at a MOI of 1) and integration events per cell were determined at 24 h PI. (E) LEDGF/p75-knockdown cells were infected with ΔRev HIV-1, and IN (red), DNA (blue), Rev (green) and LEDGF/p75 (gray) were immunostained as described in Materials and methods. Scale bar represents 10 μm. (F) Same as (D) except that p24 production was determined at 72 h PI. (G) Same as (D) except that viral cDNA was determined at 12 h PI. AZT was added at 2 μM.
B) In-Vivo
To further clarify the relations between the inhibitory Rev and the stimulatory LEDGF/p75, we infected cells lacking the LEDGF/p75 protein with a ΔRev HIV. This resulted in four integration events/cell (Figure 4D). In contrast, practically no integration was observed when LEDGF/p75-knockdown cells with overexpressed Rev were infected by the wt HIV (Figure 4D). Similarly, almost no integration was observed when both types of LEDGF/p75-knockdown cells (+/− Rev expression) were infected by WT HIV (Figure 4D). Fluorescence immunostaining clearly demonstrated the presence of IN within the intranuclear space of the LEDGF/p75-knockdown cells infected by the ΔRev virus, indicating nuclear import of IN in the absence of LEDGF/p75 and Rev proteins (Figure 4E).
To confirm that the viruses used in the present work are indeed infective and to study the correlation between infection and cDNA intracellular integration by the viruses used, HeLa P4 cells were infected with the following viruses: WT, ΔRev, Rev M10, and the IN-defective virus IN D64N D116N HIV (see Materials and methods and Figure 4D, F and G). As expected, viral p24 was detected only in systems in which both the integration process occurred and a functional Rev was present (Figure 4F). The different degrees of integration were not due to variations in the amount of viral cDNA present within the infected cells (Figure 4G). Also, as evidenced by the results depicted in Figure 4, the addition of AZT completely prevented integration, as well as the appearance of viral DNA, clearly demonstrating the requirement for reverse-transcribed DNA for the observed infection.
Discussion
Disruption of the IN-LEDGF/p75 interaction by Rev inhibits the integration process
Our present results clearly demonstrate for the first time that, in addition the previously described interaction with viral IN (6, 8), the LEDGF/p75 protein also interacts with the viral Rev. Formation of a Rev-LEDGF/p75 complex was demonstrated in-vitro using recombinant proteins, as well as by BiFC assay in yeast cells and by co-IP experiments in virus-infected cells. Using LEDGF/p75-derived peptides, we clearly show that the same protein domains can mediate binding of LEDGF/p75 to either IN and Rev (For a summary of the domains which mediate the formation of the various complexes and their ability of peptides bearing these domains to disrupt these complexes see Fig 5A and B). Therefore, it is not surprising that the Rev protein is able to induce dissociation of the IN-LEDGF/p75 complex in-vitro and in virus infected cells. Interference with the formation of the IN-LEDGF/p75 complex in-vivo was especially evident in viral Rev10+ cells: due to the presence of overexpressed Rev no IN-LEDGF/p75 complex could be detected by the co-IP experiments. It is our assumption that in these cells, Rev interacts with LEDGF/p75 as well as with IN, thereby preventing the IN-LEDGF/p75 interaction and consequently the integration process (see the proposed model in Figure 5C). The central role of Rev in regulating the integration process was also evident when comparing the results obtained using LEDGF/p75-deficient cells infected with WT and ΔRev viruses (Figure 4D).
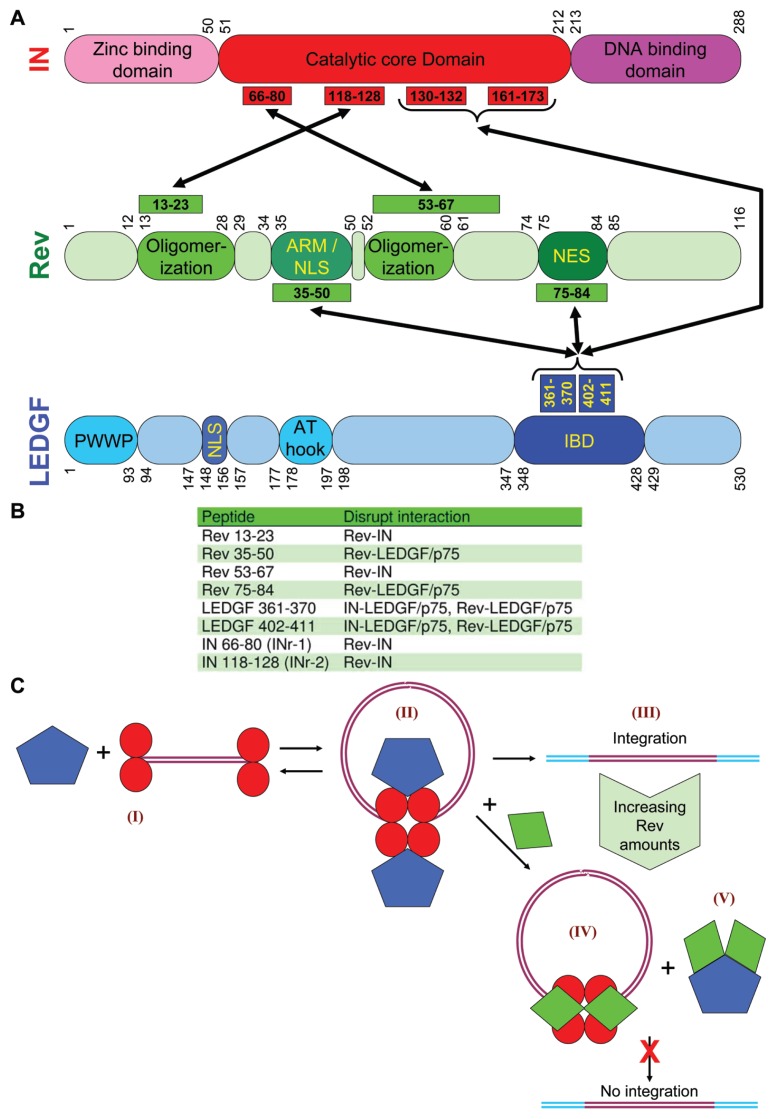
Figure 5.
Summary of the results obtained on the Rev-LEDGF/p75 interaction and a proposed integration model. (A) A scheme illustrating the interacting sequences of IN (red), Rev (green) and LEDGF/p75 (blue), as was revealed mainly by the ELISA based assay system, which mediate the binding between these proteins. (B) A table summarizing the abilities of the various IN-, Rev- and LEDGF/p75-derived peptides to specifically disrupt IN-LEDGF/p75, Rev-IN and Rev-LEDGF/p75 interactions. (C) A proposed model of the HIV-1 integration mechanism. IN (red), LEDGF/p75 (blue), Rev (green), viral DNA (purple) and host cell DNA (light blue). Following virus infection, LEDGF/p75 interacts with an IN (dimer)-DNA (I) complex resulting in the formation of a IN-LEDGF/p75 (tetramer)-DNA complex in the viral PIC (II) (47). Despite the presence of numerous IN-LEDGF/p75 (tetramer)-DNA complexes, only 1–2 copies of the viral DNA are integrated into the host chromosomal DNA (III). Most of the IN-LEDGF/p75 complexes are dissociated by increasing amounts of transcribed Rev, resulting in the formation of inhibitory Rev-IN-DNA complex (IV) and a Rev-LEDGF/p75 complex (V).
A novel view of the HIV integration process
Our view of the integration process of HIV-1 cDNA, which is based on the present as well as on previous results (3, 4, 11, 16) is summarized in Figure 5C. From all these results, it appears that the integration process is regulated by interplay between the viral IN, Rev and the host-cell LEDGF/p75.
IN-LEDGF/p75 complex was observed in the present work in the cytoplasm, nuclei as well as in the PIC of HIV-infected cells at early stages after infection, confirming previous observations (46, 47). In this early period, very little, if any, Rev-IN complex could be detected. Based on these observations, it appears that after infection, a IN-LEDGF/p75(-DNA) complex is formed either within the viral PIC or within the host cell nucleus. Potentially most, if not all, the viral cDNA of the nuclei localized IN-LEDGF/p75(-DNA) complexes should be integrated into host chromosomal DNA. Indeed, as is demonstrated in the present work and was observed before (16), about 10 integration events/cell were obtained in the absence of Rev as for example following infection with a ΔRev virus. On the other hand, only about 1–2 integration events/cell were obtained following infection with a wt virus (9, 16). These results as well as of a previous work (16) clearly indicate that this limited number of integration is due to the ability of Rev to interact with both, the IN and LEDGF/p75 proteins. It appears that the combination of these two interactions is required to completely block any further integration beyond the 1–2 integration events/cells. It is our assumption that this limited integration occurs—by IN-LEDGF/p75(-DNA) complexes—prior to the accumulation of sufficient amount of inhibitory Rev transcribed from unintegrated viral DNA (16, 18). At a later period of infection, increasing amounts of Rev-IN and Rev-LEDGF/p75 complexes could be observed with a concomitant decrease in the IN-LEDGF/p75 complex reaching a phase where none of these complexes could be detected in the PIC. This may be due to the fact most of the PIC contents had already been translocated into the nuclei of the infected cells. The view that viral DNA integration is controlled by interaction of Rev with both IN and LEDGF is further supported by the experiments showing that almost no integration is observed in LEDGF knockdown cells infected with a wt HIV (3, 4) while as many as 4 integrations/cell were observed following infection of the same cells with a ΔRev virus.
Our previous (11, 16) and present results strongly suggest that the viral Rev protein is an IN inhibitor that promotes termination of the integration process and/or prevents it. In addition, our in-vitro and co-IP experiments suggest that the viral Rev protein may either interfere with the IN-LEDGF/p75 interaction or displace the LEDGF/p75 protein from the already formed IN-LEDGF/p75 complex by interacting with both IN and LEDGF/p75. From the in-vitro experiments, it appears that the affinities of LEDGF/p75 to IN and to Rev are almost the same, exhibiting a Kd of about 20 nM (Figure 1B). This may indicate that in the presence of high excess Rev, a Rev-LEDGF/p75 and Rev-IN complexes—rather than an IN-LEDGF/p75 complex—are formed. The same domain mediates binding of LEDGF/p75 to Rev and to IN (Figure 5A and B), making Rev a potent competitive inhibitor of IN-LEDGF/p75 binding. Moreover, the Kd of the Rev-IN interaction is 13 nM, almost the same as the affinity of Rev to LEDGF/p75 (19 nM). However, the binding affinity of Rev to the IN-LEDGF/p75 complex is relatively high, with a Kd of about 7 nM, while the affinity of LEDGF/p75 to the Rev-IN complex is somewhat lower, showing a Kd of 27 nM (Figure 2F), favoring the formation of an inactive Rev-IN complex once LEDGF/p75 is removed (Figure 1B and Figure 5C). These results indicate that relative amounts of these three partners as well as time of their appearance are crucial for their activity in regulating integration of the viral DNA. Evidently, detailed information regarding their intracellular concentrations will allow a better understanding of the above-mentioned interactions and regulation of integration. A model summarizing our view of the sequence of events leading to limitation of HIV-1 cDNA integration and of the involvement of LEDGF/p75 and Rev in regulating the integration events is presented in Figure 5C. Also attempts are currently being made in our laboratory to obtain mutants of HIV-1 bearing which either fail to interact with IN or with LEDGF/p75 leaving all it other biological activities intact. Such virus will be of tremendous help for getting a better understanding of the suggested interplay between the Rev, IN and LEDGF/p75 proteins.
Supplementary Material
Acknowledgments
This work was supported by the Israeli Science Foundation (AL) and by a starting grant from the European Research Council (ERC) (to AF).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
References
- 1.Esposito D, Craigie R. HIV integrase structure and function. Adv Virus Res. 1999;52:319–333. doi: 10.1016/s0065-3527(08)60304-8. [DOI] [PubMed] [Google Scholar]
- 2.Sherman MP, Greene WC. Slipping through the door: HIV entry into the nucleus. Microbes Infect. 2002;4:67–73. doi: 10.1016/s1286-4579(01)01511-8. [DOI] [PubMed] [Google Scholar]
- 3.Llano M, et al. An essential role for LEDGF/p75 in HIV integration. Science. 2006;314:461–464. doi: 10.1126/science.1132319. [DOI] [PubMed] [Google Scholar]
- 4.Vandekerckhove L, et al. Transient and stable knockdown of the integrase cofactor LEDGF/p75 reveals its role in the replication cycle of human immunodeficiency virus. J Virol. 2006;80:1886–1896. doi: 10.1128/JVI.80.4.1886-1896.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emiliani S, et al. Integrase mutants defective for interaction with LEDGF/p75 are impaired in chromosome tethering and HIV-1 replication. J Biol Chem. 2005;280:25517–25523. doi: 10.1074/jbc.M501378200. [DOI] [PubMed] [Google Scholar]
- 6.Llano M, et al. LEDGF/p75 determines cellular trafficking of diverse lentiviral but not murine oncoretroviral integrase proteins and is a component of functional lentiviral preintegration complexes. J Virol. 2004;78:9524–9537. doi: 10.1128/JVI.78.17.9524-9537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maertens G, Cherepanov P, Debyser Z, Engelborghs Y, Engelman A. Identification and characterization of a functional nuclear localization signal in the HIV-1 integrase interactor LEDGF/p75. J Biol Chem. 2004;279:33421–33429. doi: 10.1074/jbc.M404700200. [DOI] [PubMed] [Google Scholar]
- 8.Maertens G, et al. LEDGF/p75 is essential for nuclear and chromosomal targeting of HIV-1 integrase in human cells. J Biol Chem. 2003;278:33528–33539. doi: 10.1074/jbc.M303594200. [DOI] [PubMed] [Google Scholar]
- 9.Butler SL, Hansen MS, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]
- 10.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 11.Levin A, et al. Peptides derived from HIV-1 integrase that bind Rev stimulate viral genome integration. PLoS ONE. 2009;4:e 4155. doi: 10.1371/journal.pone.0004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbluh J, et al. Interaction between HIV-1 Rev and integrase proteins: a basis for the development of anti-HIV peptides. J Biol Chem. 2007;282:15743–15753. doi: 10.1074/jbc.M609864200. [DOI] [PubMed] [Google Scholar]
- 13.Pollard VW, Malim MH. The HIV-1 Rev protein. Annu Rev Microbiol. 1998;52:491–532. doi: 10.1146/annurev.micro.52.1.491. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y. HIV-1 gene expression: lessons from provirus and non-integrated DNA. Retrovirology. 2004;1:13. doi: 10.1186/1742-4690-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Marsh JW. Early transcription from nonintegrated DNA in human immunodeficiency virus infection. J Virol. 2003;77:10376–10382. doi: 10.1128/JVI.77.19.10376-10382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin A, et al. Novel Regulation of HIV-1 Replication and Pathogenicity: Rev Inhibition of Integration. In press Protein Engineering Design and Selection. 2009 doi: 10.1093/protein/gzp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly J, et al. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology. 2008;372:300–312. doi: 10.1016/j.virol.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer SR, Yu D, Biancotto A, Margolis LB, Wu Y. Measurement of human immunodeficiency virus type 1 preintegration transcription by using Rev-dependent Rev-CEM cells reveals a sizable transcribing DNA population comparable to that from proviral templates. J Virol. 2009;83:8662–8673. doi: 10.1128/JVI.00874-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derdeyn CA, et al. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cullen BR. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 22.Ratner L, et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 23.Gummuluru S, Kinsey CM, Emerman M. An in vitro rapid-turnover assay for human immunodeficiency virus type 1 replication selects for cell-to-cell spread of virus. J Virol. 2000;74:10882–10891. doi: 10.1128/jvi.74.23.10882-10891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima N, Lu R, Engelman A. Human immunodeficiency virus type 1 replication in the absence of integrase-mediated dna recombination: definition of permissive and nonpermissive T-cell lines. J Virol. 2001;75:7944–7955. doi: 10.1128/JVI.75.17.7944-7955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verhoef K, Koper M, Berkhout B. Determination of the minimal amount of Tat activity required for human immunodeficiency virus type 1 replication. Virology. 1997;237:228–236. doi: 10.1006/viro.1997.8786. [DOI] [PubMed] [Google Scholar]
- 26.Bahner I, et al. Comparison of trans-dominant inhibitory mutant human immunodeficiency virus type 1 genes expressed by retroviral vectors in human T lymphocytes. J Virol. 1993;67:3199–3207. doi: 10.1128/jvi.67.6.3199-3207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fineberg K, et al. Inhibition of nuclear import mediated by the Rev-arginine rich motif by RNA molecules. Biochemistry. 2003;42:2625–2633. doi: 10.1021/bi0206199. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins TM, Engelman A, Ghirlando R, Craigie R. A soluble active mutant of HIV-1 integrase: involvement of both the core and carboxyl-terminal domains in multimerization. J Biol Chem. 1996;271:7712–7718. doi: 10.1074/jbc.271.13.7712. [DOI] [PubMed] [Google Scholar]
- 29.Turlure F, Maertens G, Rahman S, Cherepanov P, Engelman A. A tripartite DNA-binding element, comprised of the nuclear localization signal and two AT-hook motifs, mediates the association of LEDGF/p75 with chromatin in vivo. Nucleic Acids Res. 2006;34:1653–1675. doi: 10.1093/nar/gkl052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhim H, Echetebu CO, Herrmann CH, Rice AP. Wild-type and mutant HIV-1 and HIV-2 Tat proteins expressed in Escherichia coli as fusions with glutathione S-transferase. J Acquir Immune Defic Syndr. 1994;7:1116–1121. [PubMed] [Google Scholar]
- 31.Rosenbluh J, et al. Positively charged peptides can interact with each other, as revealed by solid phase binding assays. Anal Biochem. 2006;352:157–168. doi: 10.1016/j.ab.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Melchior F, Paschal B, Evans J, Gerace L. Inhibition of nuclear protein import by nonhydrolyzable analogues of GTP and identification of the small GTPase Ran/TC4 as an essential transport factor. J Cell Biol. 1993;123:1649–1659. doi: 10.1083/jcb.123.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craigie R, Mizuuchi K, Bushman FD, Engelman A. A rapid in vitro assay for HIV DNA integration. Nucleic Acids Res. 1991;19:2729–2734. doi: 10.1093/nar/19.10.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang Y, Rhodes D, Bushman F. Rapid microtiter assays for poxvirus topoisomerase, mammalian type IB topoisomerase and HIV-1 integrase: application to inhibitor isolation. Nucleic Acids Res. 2000;28:4884–4892. doi: 10.1093/nar/28.24.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kass G, et al. Permeabilized mammalian cells as an experimental system for nuclear import of geminiviral karyophilic proteins and of synthetic peptides derived from their nuclear localization signal regions. J Gen Virol. 2006;87:2709–2720. doi: 10.1099/vir.0.82021-0. [DOI] [PubMed] [Google Scholar]
- 36.Iordanskiy S, et al. Heat shock protein 70 protects cells from cell cycle arrest and apoptosis induced by human immunodeficiency virus type 1 viral protein R. J Virol. 2004;78:9697–9704. doi: 10.1128/JVI.78.18.9697-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer-Hammerle S, et al. Identification of a novel Rev-interacting cellular protein. BMC Cell Biol. 2005;6:20. doi: 10.1186/1471-2121-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Scadden DT, Crumpacker CS. Primitive hematopoietic cells resist HIV-1 infection via p21. J Clin Invest. 2007;117:473–481. doi: 10.1172/JCI28971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iordanskiy S, Berro R, Altieri M, Kashanchi F, Bukrinsky M. Intracytoplasmic maturation of the human immunodeficiency virus type 1 reverse transcription complexes determines their capacity to integrate into chromatin. Retrovirology. 2006;3:4. doi: 10.1186/1742-4690-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casabianca A, et al. Fast and sensitive quantitative detection of HIV DNA in whole blood leucocytes by SYBR green I real-time PCR assay. Mol Cell Probes. 2007;21:368–378. doi: 10.1016/j.mcp.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Levin A, Kutznetova L, Kahana R, Rubinstein-Guini M, Stram Y. Highly effective inhibition of Akabane virus replication by siRNA genes. Virus Res. 2006;120:121–127. doi: 10.1016/j.virusres.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Watts NR, et al. Three-dimensional structure of HIV-1 Rev protein filaments. J Struct Biol. 1998;121:41–52. doi: 10.1006/jsbi.1998.3964. [DOI] [PubMed] [Google Scholar]
- 43.Singh DP, Kimura A, Chylack LT, Jr, Shinohara T. Lens epithelium-derived growth factor (LEDGF/p75) and p52 are derived from a single gene by alternative splicing. Gene. 2000;242:265–273. doi: 10.1016/s0378-1119(99)00506-5. [DOI] [PubMed] [Google Scholar]
- 44.Hayouka Z, et al. Inhibiting HIV-1 integrase by shifting its oligomerization equilibrium. Proc Natl Acad Sci U S A. 2007;104:8316–8321. doi: 10.1073/pnas.0700781104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandey KK, Sinha S, Grandgenett DP. Transcriptional coactivator LEDGF/p75 modulates human immunodeficiency virus type 1 integrase-mediated concerted integration. J Virol. 2007;81:3969–3979. doi: 10.1128/JVI.02322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engelman A, Cherepanov P. The lentiviral integrase binding protein LEDGF/p75 and HIV-1 replication. PLoS Pathog. 2008;4:e1000046. doi: 10.1371/journal.ppat.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poeschla EM. Integrase, LEDGF/p75 and HIV replication. Cell Mol Life Sci. 2008;65:1403–1424. doi: 10.1007/s00018-008-7540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.