Abstract
Blebs are spherical membrane protrusions often observed during cell migration, cell spreading, cytokinesis, and apoptosis, both in cultured cells and in vivo. Bleb expansion is thought to be driven by the contractile actomyosin cortex, which generates hydrostatic pressure in the cytoplasm and can thus drive herniations of the plasma membrane. However, the role of cortical tension in bleb formation has not been directly tested, and despite the importance of blebbing, little is known about the mechanisms of bleb growth. In order to explore the link between cortical tension and bleb expansion, we induced bleb formation on cells with different tensions. Blebs were nucleated in a controlled manner by laser ablation of the cortex, mimicking endogenous bleb nucleation. Cortical tension was modified by treatments affecting the level of myosin activity or proteins regulating actin turnover. We show that there is a critical tension below which blebs cannot expand. Above this threshold, the maximal size of a bleb strongly depends on tension, and this dependence can be fitted with a model of the cortex as an active elastic material. Together, our observations and model allow us to relate bleb shape parameters to the underlying cellular mechanics and provide insights as to how bleb formation can be biochemically regulated during cell motility.
The cell cortex is a thin meshwork of actin filaments, myosin, and associated proteins that lies beneath the plasma membrane (1). Because of the presence of active myosin motors, which slide filaments with respect to one another in the network, the cortex is under tension. As a result, the cortex exerts pressure on the cytoplasm and can actively contract, driving cell deformations (2).
Blebs are spherical membrane protrusions that commonly occur at the cortex during cytokinesis, cell spreading, virus uptake, and apoptosis (3 –7). Moreover, increasing evidence points to an essential role for blebs as leading edge protrusions during cell migration in three-dimensional environments, particularly during embryonic development and tumor-cell dissemination (8 –11; reviewed in refs. 7, 12). Despite the importance of blebbing, very little is known about the mechanisms of bleb growth.
The life cycle of a bleb can be subdivided into three phases (7, 13). First, a bleb is nucleated, either by local detachment of the cortex from the plasma membrane or by local rupture of the cortex. In the subsequent growth phase, a membrane bulge, initially devoid of cortex, expands from the nucleation site. Finally, the cortex gradually reassembles at the bleb membrane, leading to bleb retraction.
Bleb formation is often correlated with high myosin II activity, and myosin II inhibition prevents blebbing (6, 7, 10, 14). For that reason, and because of their round shape and rapid expansion, blebs are commonly believed to be a direct mechanical consequence of the hydrostatic pressure exerted on the cytoplasm by the contractile cortex, which would drive bleb growth from places of local cortex weakening without any further regulation (7, 15, 16). However, this purely mechanical interpretation of blebbing mostly relies on indirect observations, and, to our knowledge, neither the mechanical nature of blebbing nor the link between cortex tension and bleb growth have ever been directly assessed. Moreover, a quantitative model describing the role of cortical tension in bleb expansion has not yet been established.
Here, we directly address the role of cortical tension in bleb growth by inducing bleb formation in cells with different tensions. We first show that blebs can be nucleated by local laser ablation of the actin cortex, supporting the view that bleb expansion is driven by intracellular pressure. Multiple ablations of the same cell indicate that the growth of a bleb significantly reduces pressure. We then modulate cortical tension and induce blebs in cells with different tensions. We demonstrate that there is a critical tension for bleb formation below which bleb growth cannot be induced. Above this threshold tension, bleb size increases with increasing tension. We fit our data with a theoretical model of the cortex as an elastic active gel. This model yields an estimate of cellular elasticity and gives an accurate prediction of bleb shape.
Results
Ablation of the Cell Cortex Leads to Bleb Formation.
In order to test whether bleb formation is pressure-driven, we performed local laser-ablations of the cell cortex. We hypothesized that if bleb growth is a direct consequence of cytoplasmic pressure, a bleb would grow from the site of ablation. Conversely, if bleb formation requires further regulation, ablation should not immediately trigger bleb growth.
We chose L929 fibroblasts as a model system for our study: When in suspension, these cells display an active, continuous cortical layer under the plasma membrane (17). We therefore placed the detached fibroblasts in polyethylene glycol (PEG)-coated dishes, which prevent readhesion of cells to the substrate. By using a picosecond-pulsed 405-nm laser, we then locally ablated the cell cortex (Movies S1 and S2 in the SI Appendix). Strikingly, immediately after ablation, a bleb grew from the site of cortex disruption and reached its maximal size within 10-30 s (Fig. 1 A). The bleb then remained stable for one to several minutes, followed by a retraction phase typically lasting over a minute (Movie S1 in the SI Appendix). We followed cortical dynamics after ablation in cells expressing myosin regulatory light chain (RLC)-GFP (Movie S2 in the SI Appendix); no myosin was initially present at the bleb membrane, and a myosin layer became clearly visible shortly before bleb retraction, similar to what is observed in spontaneously forming cellular blebs (13). Laser ablation thus appears to mimic the endogenous nucleation of blebs by local disruption of the cortex and supports the idea that bleb growth is driven by intracellular pressure.
Fig. 1.

Laser ablation of the cortex induces bleb growth. (A) Timelapse of a typical ablation movie. Ablation from − 5 s to 0 s. Red arrow, site of ablation; white arrows, growing bleb; white arrowheads, retracting bleb. Scale bar, 10μm. (B) Example of brightfield image analysis. The cell contour is fitted with two intersecting circles of radii r c and r b, with centers distant by a. r h, hole size; h b, bleb height. (C) Time course of bleb height (red) and hole size (blue) for a cell under control conditions (representative of N = 37 cells). 0 s, offset of ablation. Dots are individual measurements (≈ 0.2 s/frame); lines are values smoothed over 10 points.
We then analyzed bleb shape over time by using transmitted light images (see Fig. 1 B and C and Supplementary Materials and Methods in the SI Appendix). The maximal volume reached by the bleb ranged between 0.3% and 6% of the cell volume (2.73 ± 0.23%, mean ± SEM, N = 37), and the bleb height ranged from 1.4 to 4.5 μm (3.45 ± 0.11μm). The size of the bleb did not depend on the site of ablation with respect to the nucleus (Fig. S1 of the SI Appendix).
Bleb Growth Releases Cytoplasmic Pressure.
To evaluate whether the growth of a bleb actually releases pressure, we performed multiple ablations on the same cell. Indeed, if the expansion of a bleb reduces intracellular pressure, a second bleb induced shortly afterward should reach a smaller size.
Therefore, we performed experiments where two blebs were sequentially triggered at opposite sides of a cell (Fig. 2 A). We found that when the second ablation immediately followed the first one, the second bleb was significantly smaller than the first, whereas for ablations performed after the first bleb had retracted, no significant difference between the two bleb sizes was found (Fig. 2 B). These observations suggest that the growth of a bleb significantly releases intracellular pressure and that pressure returns to its initial value once the bleb has retracted.
Fig. 2.

Multiple ablations on the same cell lead to smaller blebs. (A) Time lapses from double-bleb experiments. (Top and Middle) Case 1: The second bleb is nucleated shortly after the first one (delay ΔT < 30 s), either close to it (Top) or at the opposite side of the cell (Middle). (Bottom) Case 2: The second bleb is nucleated after the first bleb is fully retracted (ΔT > 2 min). Scale bar: 10μm. (B) Plot of the ratio of maximal bleb volumes (v r2/v r1) for case 1 and case 2. Open circles, single measurements; open squares, mean ± SEM. Case 1: The ratio of volumes is 0.76 ± 0.07 (mean ± SEM) for blebs induced close to one another and 0.75 ± 0.08 for blebs formed far from one another, and significantly differs from 1 (P < 0.004 and P < 0.002 respectively). Case 2: The volume ratio is 1.13 ± 0.13 and is not significantly different from 1 (P > 0.3). Cases 1 and 2 are significantly different (P < 0.02), whereas the two distributions of case 1 are not (P > 0.9).
To assess whether the pressure release was homogenous throughout the cell or whether a pressure gradient was present, we induced the growth of a second bleb at various distances from the first one. The average decrease in size between the first and the second bleb was the same, regardless of whether the second bleb was nucleated close to or far from the first one (Fig. 2). This finding supports the view that pressure equilibrates fast when compared with the time scales of bleb expansion in this cell type.
It has been proposed that the pressure driving bleb growth is hydrostatic, resulting from tension built up in the actin cortex (7, 15). However, this pressure could also be of osmotic origin (18). We therefore sought to test the link between bleb expansion and cortical tension.
Myosin Activity and Actin-Binding Proteins Affect Cortical Tension.
In order to identify factors likely to affect cortical tension, we developed a theoretical model of the cortex by using the hydrodynamic active gel theory (19, 20) (see SI Appendix). Within this framework, cortical tension is equal to ζΔμh/2 where ζΔμ is the active stress exerted by the myosins in the cortical gel, and h is the thickness of the cortex.
We then sought to experimentally modify cortical tension. Tension was measured by using micropipette aspiration (21, 22) (Fig. 3 A). Under control conditions, L929 cells had a well-defined tension of 413.6 ± 15.2 pN/μm (mean ± SEM,N = 32, Fig. 3 B and Table S1). Provided that the cortex is tightly coupled to the membrane, the tension measured by micropipette aspiration, T, is the sum of the active cortical tension and of the membrane tension γ. In order to estimate γ, we measured the tension of cells where the cortex had been disassembled with cytochalasin D (CD). Under these conditions, tension dropped to 39.1 ± 4.2 pN/μm (N = 13, Fig. 3 C).
Fig. 3.

Cortex tension depends on myosin activity and on proteins regulating actin turnover. (A) Schematic of the micropipette aspiration setup. The parameters used in the text and the formula used to calculate tension (Laplace law) are given. The image displays a detached L929 cell aspirated into a micropipette close to the critical pressure. Scale bar, 10μm. (B) Cortex tension after treatments affecting myosin activity. (N: number of cells measured in 2–5 independent experiments). All groups were found to be significantly different from one another (two-sampled t-tests, P < 0.05), except for Y27632 2.5 μM versus Y27632 5 μM (P = 0.36), and Y27632 10 μM versus Blebbistatin (P = 0.08). (C) Cortex tension after treatments affecting actin. All groups were found to be significantly different from one another, except for control versus scrambled siRNA (P = 0.77). Protein depletion was checked by Western blotting (Fig. S4 in the SI Appendix).
We then measured cortex tension after various treatments affecting myosin II activity. Inhibition of myosin with blebbistatin decreased tension by more than 50% (Fig. 3 B). Interestingly, we could gradually decrease tension by treating the cells with increasing amounts of Y27632, which indirectly reduces myosin activity by inhibiting Rho-kinase (ROCK) (23) (Fig. 3 B and Table S1 of the SI Appendix). Finally, transfection with RhoAQ63L, a constitutively active version of the small GTPase RhoA (CA RhoA), an activator of myosin II (23), considerably increased tension to 1907.1 ± 140.7pN/μm (Fig. 3 B).
To assess the predicted dependence of tension on the thickness of the actin cortex, we then tested the influence of proteins involved in actin turnover. In addition to activating myosin II, RhoA can also enhance actin polymerization by activating formins (24); therefore, the increase in tension observed upon transfection with CA RhoA could result from the combined effect of a thicker cortex and higher myosin activity. We then investigated the effect of two proteins that regulate actin but are not involved in myosin regulation: actin depolymerizing factor (ADF)/cofilin, and gelsolin. Both depletion of gelsolin and cofilin by RNAi significantly increased the tension of L929 cells (Fig. 3 C).
Bleb Size and Growth Dynamics Depend on Cortical Tension.
We then analyzed the maximal size of blebs induced by laser ablation on cells with different tensions. Blebs induced on cells treated with CA RhoA or after cofilin depletion were bigger than blebs formed in control cells (Fig. S6 of the SI Appendix). However, as these treatments are likely to affect parameters other than cortical tension alone, we focused further analysis on cells treated with various amounts of the ROCK inhibitor Y27632.
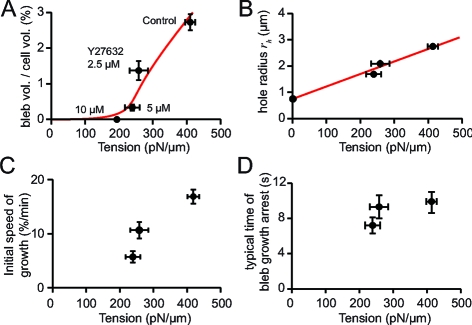
We found that for tensions lower than 200 pN/μm (cells treated with 10 μM Y27632), blebs could not be induced. At 240 pN/μm, approximately 50% of the cells formed a bleb upon ablation, but the bleb volume was eight times smaller than in control situations. For higher tensions, all cells formed a bleb, and the bleb-to-cell volume ratio, the height of the bleb, and the maximal size of the hole at the base of the bleb all increased with increasing tension (Fig. 4 A and B and Fig. S2 and Table S1 of the SI Appendix). These data indicate that there is a threshold tension for bleb formation between 200 and 240 pN/μm. Above this threshold, bleb size increases with increasing tension.
Fig. 4.
Dependence of bleb size and growth dynamics on tension. Plots of bleb parameters for cells treated with Y27632 and control conditions and reported as function of the corresponding tension. In B, C, and D there is no data point for 10 μM Y27632, because a bleb could not be induced for this condition. Values displayed: mean ± SEM unless otherwise indicated. Plots of the maximal bleb-to-cell volume ratio (A), and hole size at maximal volume ratio (B) as a function of tension. Red curves, fits of the elastic model with two free parameters the cytoplasmic elasticity and the effective cortex elastic modulus E c h. The fit yields E c h = 240 pN/μm, and Pa. The data point for T = 0 in B was used in the fitting and represents an estimate of the size of the hole made by laser ablation. (C) Initial speed of bleb growth, calculated as the rate of the time course of the bleb-to-cell volume ratio during the first 20 time points (7 to 12 s) after ablation. (D) Characteristic time for bleb growth arrest, calculated from the slope of the relation between the maximal bleb-to-cell volume ratio and the initial speed of growth. Vertical error bars, 95% confidence interval for this frame. The points were not found to be significantly different from one another.
We then asked whether the dynamics of bleb expansion also depended on cortical tension. The initial speed of bleb growth strongly increased with increasing tension (Fig. 4 C). By plotting the maximal bleb-to-cell volume ratio as a function of the initial expansion speed, we also extracted the typical time scale of bleb growth arrest (Table S1 of the SI Appendix). This time did not significantly depend on tension (Fig. 4 D), suggesting that growth arrest may result from a tension-independent mechanism.
Elastic Model for Bleb Growth.
Description of the Model.
We modeled bleb expansion as a result of the cytoplasmic pressure generated by cortical tension. Before bleb formation, intracellular pressure is given by Laplace's law P c = P e + 2T/r c, where r c is the radius of the cell and P e is the pressure in the medium. If we neglect any elastic effect, P c would still be given by Laplace's law during bleb growth, and would thus remain very close to its initial value because the radius of the cell decreases by less than 2% during bleb expansion. However, multiple ablation experiments indicate that intracellular pressure is significantly reduced during the growth of a bleb (Fig. 2).
To account for this pressure release, we incorporated the elasticity of cellular components in our description: The actin cortical gel has a Young modulus E c, and the cytoplasm has a Young modulus E i (Fig. 5). Indeed, the cytoplasm contains fluid cytosol, but it also contains membrane structures and cytoskeletal polymers, which are likely to have elastic properties (25). As a first approximation, we neglected turnover in the actin cortex on the time scale of bleb formation, and we considered that the cortex was incompressible (i.e., the Poisson ratio of the cortex νc = 0.5). The cytoplasmic elastic gel, however, must be compressible to allow for the expansion of the bleb, so that the cytoplasmic Poisson ratio νi ≠ 0.5. Finally, we assumed that the total surface of the cell can change but its volume remains constant (see Discussion) and that the membrane of the bleb is devoid of cortex throughout the expansion of the bleb, as suggested by previous work (11, 26).
Fig. 5.
Elastic model of bleb growth. Schematic of the mechanical elements involved in the Model of bleb expansion: Myosins in the cortex exert a tension that is responsible for cell contraction, expulsion of fluid cytosol into the bleb, and hole opening. The inner cytoplasmic gel and the cortex are then elastically compressed and therefore oppose cortical contraction. We assume cylindrical symmetry around the cell-bleb axis, as indicated by the curved arrow.
Bleb volume as a function of cortical tension.
In our description, the bleb reaches its maximal volume at mechanical equilibrium, when the pressure of the cytosol inside the bleb equals the pressure inside the cell. Because we assume volume conservation, as the bleb grows the volume of the cell body decreases, compressing the cytoplasm and the actin cortex. The pressure of the cytosol in the cell body is therefore given by the pressure imposed by cortical tension , minus the elastic resistance of cellular structures. The resistance is proportional to the deformation of the cell and to the total elasticity of the cytoplasm and of the cortex. The pressure in the bleb is imposed by the membrane tension, yielding P b = 2γ/r b. Mechanical equilibrium is achieved when the two pressures are equal:
where Δr c is the variation of the cell radius due to bleb formation (see SI Appendix for detailed derivation). Δr c can be related to the bleb volume, and one obtains from Eq. 1 a relationship between the equilibrium bleb-to-cell volume ratio and the cortical tension, which can be accurately fitted to the experimental data with a single adjustable parameter, the total elasticity Pa (R 2 = 0.91, Fig. 4 A), taking r c = 8.5 μm and γ = 40 pN/μm.
Strikingly, the theory predicts the existence of a threshold tension for the expansion of a bleb, as observed experimentally (Fig. 4 A). Qualitatively, the threshold results from the finite tension of the membrane. Indeed, two regimes can be distinguished, depending on whether membrane tension can resist bleb expansion. The maximal pressure reached in the bleb is on the order of 2γ/r h, where r h is the size of the hole at the base of the bleb: At intracellular pressures lower than this value, the plasma membrane limits bleb growth and the volume of the bleb remains very small; for higher intracellular pressures, the pressure in the bleb becomes negligible, bleb growth is only limited by cellular elasticity, and the bleb becomes much larger. The critical pressure for bleb expansion is therefore roughly given by T c ∼ γ r c/r h. With r h ∼ 1 μm, r c ∼ 8 μm and γ ∼ 40 pN/μm, this qualitative argument yields T c ∼ 320 pN/μm. The exact calculation, taking into account that r h depends on tension (Fig. 4 B), gives T c ∼ 200 pN/μm, in remarkable agreement with the experimental observations (Fig. 4 A).
Size of the hole in the cortex as a function of cortical tension.
To evaluate the surface elasticity of the cortex E c h, we then modeled the opening of the hole at the base of the bleb, which is driven by cortical tension and is resisted by the elasticity of the cortex. We therefore calculated the deformation of the cytoplasmic gel and actin cortex during bleb growth (see SI Appendix) and found that the elasticity of the cytoplasm is essential to account for the observations. Indeed, in the limit where cytoplasmic elasticity is neglected (), the maximal opening of the hole Δr h is on the order of . In this limit, estimating E c h from the fit for the bleb volume (Fig. 4 A), we obtain Δr h ≃ 50 nm, which is far too small to account for the experimental data (Fig. 4 B). In the opposite limit, where the elastic response is dominated by the compressibility of the cytoplasm (), the maximal opening of the hole is proportional to the tension , where r h 0 ≃ 0.75 μm is the initial hole radius (see Materials and Methods). Fitting the experimental data gives E c h ≃ 240 pN/μm (Fig. 4 B, R 2 = 0.99). From the fit of Fig. 4 A, we then obtain the cytoplasmic compressibility Pa, consistent with our assumption because h ≪ r c.
Discussion
Laser Ablation Mimics Endogenous Nucleation of Blebs.
We have shown that local ablation of the actin cortex leads to the formation of a membrane bleb (Fig. 1 A). The artificially nucleated blebs appeared identical to the blebs naturally occurring in cells (13). The induced blebs were spherical in shape and mostly devoid of intracellular structures (Movie S1 of the SI Appendix). Growth and retraction times were similar to those measured on blebs forming spontaneously (ref. 26 and Table S1 and Movie S1 of the SI Appendix). Moreover, myosin reassembled at the bleb membrane shortly before bleb retraction (Movie S2 of the SI Appendix), similar to previous observations in spontaneously blebbing cells (13). Taken together, these observations indicate that laser ablation mimics endogenous bleb nucleation by local disruption of the cortex.
Cortex Tension Depends on Motor Activity and on Actin Turnover.
Suspended L929 fibroblasts had a tension of ∼ 413 pN/μm (Fig. 3 B), similar to tensions measured in other fibroblast lines (27). Tension measurements after actin disassembly with CD give an estimation of the membrane tension: γ ∼ 40 pN/μm. This value is about 100 times higher than the typical membrane tension of a lipid vesicle (28) but comparable with the cortical tension of red blood cells (21), consistent with the observation that an erythrocytic-like cytoskeleton is observed at the plasma membrane of mammalian cells even during the expansion of blebs, when the actin cortex is absent (13).
We then showed that cortical tension of L929 fibroblasts strongly depends on myosin activity (Fig. 3 B), in agreement with previous observations in Dictyostelium discoideum cells (29 –31), where myosin II and several variants of myosin I are implicated in cortical tension. Importantly, we also showed that cortex tension depends on the level of proteins regulating actin turnover. Indeed, depletion of cofilin and gelsolin both resulted in an increase of cortical tension. ADF/cofilin enhances the rate of actin depolymerization and severs actin filaments (32); gelsolin also severs actin filaments and can moreover cap F-actin barbed ends, preventing further polymerization (33). Depletion of gelsolin or cofilin is therefore likely to result in a thicker actin cortex, which, in accordance with our model, should result in a higher tension, as observed experimentally (Fig. 3 C). A tight control of cortical tension is likely to be essential during cell motility (2), but it is also essential in tissues, where differences in cortex tensions between cell types have been shown to contribute to cell sorting (34). The mechanisms of tension regulation are still largely unknown; our results show that studies of tension regulation cannot be limited to the action of myosin motors but should also include an analysis of actin turnover.
Above a Critical Tension, Bleb Size Increases with Increasing Tension.
We show that both size and growth speed of a bleb strongly depend on cortical tension (Fig. 4), and we propose a model where the growth of a bleb results from a flow of cytoplasm generated by contractions of the actomyosin cortex. The theoretical model predicts the existence of a threshold in tension for the expansion of a bleb, as observed experimentally (Fig. 4 A). Moreover, the model accurately fits the experimentally observed dependence of bleb volume and hole radius on tension for cells treated with Y27632 (Fig. 4 A and B), yielding values of cellular parameters that are in good agreement with independent measurements of these parameters. Specifically, we find a value for the cortex effective elastic modulus E c h ≃ 240 Pa.μm. The thickness of the cortex has not been accurately measured and is likely to vary between cell lines (35, 36); however, electron microscopy observations of the cortex in Hela fibroblasts suggest a cortical thickness of about 100 nm (13). By using this value, we then get E c ≃ 2,400 Pa, in agreement with other measurements of cortex elasticity (37). The theory also predicts a cytoplasmic compressibility 6,750 Pa. The elasticity of L929 cells after depolymerization of actin has been previously measured by AFM and gives an estimated value for E i ∼ 1,200 Pa (37). We can therefore deduce the compressibility of the cytoplasm: νi ∼ 0.41, consistent with previous measurements of compressibility of cytoskeletal networks (38).
Expression of CA RhoA and depletion of cofilin are likely to result in a thicker cortex and therefore increase cellular elasticity, which resists bleb expansion. In agreement with this prediction, the blebs obtained after these treatments were smaller than would be expected with the elastic parameters inferred above from the size of blebs induced after gradual inhibition of myosin activity (Fig. S6 of the SI Appendix).
Taken together, our findings indicate that the size of a cellular bleb results from the net effect of cortical tension, which drives the expansion of the bleb, and of cellular elasticity and membrane tension, which resist expansion. All these factors can be regulated independently, which can explain how cells such as Dictyostelium, which have a relatively high tension [up to 4,000 pN/μm (29)], do not form blebs bigger than about 10% of the cellular volume (39).
Discussion of the Model of Bleb Expansion.
Our model considers the cortex as an elastic, contractile shell surrounding a poro-elastic, liquid-gel cytoplasm. Given that the suspended L929 cells are spherical, we consider that the cortex is isotropic, with homogenous thickness and tension.
We further assume that cytosolic pressure is homogenous. Previous work on blebbing cells suggests that the density of cytoplasmic structures can slow down pressure equilibration inside the cell sufficiently enough to give rise to pressure gradients on time scales of tens of seconds (25, 26). However, in L929 cells, we have observed that blebs formed near the nucleus, where we expect the cytoplasmic structures to be more crowded, and far from it had similar sizes (Fig. S1 of the SI Appendix). Moreover, experiments where two blebs were successively induced on the same cell showed that as early as 7 s after bleb initiation, the decrease in pressure due to the growth of the first bleb was independent of the distance between the blebs (Fig. 2). These observations support our assumption that in L929 cells, at time scales relevant for bleb expansion, pressure is homogenous throughout the cytoplasm.
A second assumption is that the total volume of the cell is conserved. Although the plasma membrane is permeable to water, the high osmolarity of the cell should theoretically maintain an approximately constant volume. Indeed, the total cell volume varies in order to satisfy the balance between the osmotic and hydrostatic pressures Πc − Πe = P c − P e, where Πc and Πe are the intracellular and external osmotic pressures (40). By using Van't Hoff's law, Πe = c ext Rθ, where c ext ∼ 300 mol/m3 is the osmolarity of the medium, R is the gas constant, and θ is the temperature, we get Πe ∼ 5.105 Pa and Πc = n t Rθ/V c, where n t is the total number of moles of osmolytes in the cell. The volume of the cell is then given by
Pressure variation in the cell during bleb formation is necessarily smaller than the initial intracellular excess pressure 2T/R ∼ 100 Pa, which is three orders of magnitude smaller than the osmotic pressure. By using Eq. 2, if cellular osmolarity does not change during bleb growth, the total volume change would then not exceed |ΔV c|/V c ∼ |ΔP c|/Πe ∼ 0.02 %, which is negligible compared with the typical volume of the blebs induced by laser ablation.
It is also possible that the ablation induces a local uptake or release of ions, which could drive water uptake. We have therefore verified how our results would be affected by an increase of the cell volume upon ablation and found that the experimental results cannot be accurately fit in the presence of a significant volume variation, further supporting our hypothesis of conserved volume (see SI Text and Fig. S8 of the SI Appendix).
Finally, our model assumes that the bleb membrane is devoid of an actin cortex throughout bleb expansion, in accordance with previous observations (13). However, our observation that the characteristic time of bleb growth arrest does not depend on tension (Fig. 4 D) suggests that a tension-independent mechanism may stop bleb expansion. Cortex reassembly at the bleb membrane could play such a role, as previously suggested for blebs formed by actively blebbing filamin-depleted cells (15). If bleb expansion is stalled by actin repolymerization, the maximal volume reached by the bleb will be smaller than the volume predicted by our steady-state calculation. In that case, the elastic moduli derived above would be an overestimation of the real values. A theoretical description of the dynamics of bleb expansion will be necessary to calculate the evolution of bleb size over time. In addition to cortical tension and cell elasticity, dissipation mechanisms, such as flow of cytosol through the cytoplasmic network and friction between the membrane and the cortex, will also influence bleb size.
Implications of the Critical Tension for the Regulation of Bleb Formation.
The existence of a critical tension for the expansion of a bleb has been previously hypothesized (16); however, to our knowledge, our data provide the first experimental evidence of such a threshold tension. This observation unveils a fundamental mechanism that can be used by cells for the regulation of blebbing. Indeed, regulation of bleb formation can be achieved by controlling either bleb nucleation or bleb expansion. Bleb nucleation will depend on the strength of cortex–membrane attachments and on cortex stability; we show that bleb expansion will only occur above a critical tension. It will be important to know at which level endogenous bleb formation is regulated, for example, in cells migrating by blebbing. Interestingly, reduction of cortical tension by down-regulation of myosin II can inhibit bleb-based migration (11, 10); however, as cortical tension could also be important for bleb nucleation (7), it is difficult to distinguish between the two potential regulation mechanisms. Mimicking bleb nucleation by laser ablation can be an interesting tool to figure out the level at which cellular blebbing is regulated.
Materials and Methods
Cell Preparation and Tension Measurements.
Mouse L929 fibroblasts were grown in DMEM (GIBCO, Invitrogen) supplemented with 10% FCS, 1% glutamine, and 1% penicillin-streptomycin. For all the experiments, the cells were maintained in suspension on PEG-coated glass-bottom dishes (Matttek) (ref. 17; see Supplementary Materials and Methods in the SI Appendix). We checked that detachment did not induce apoptosis in L929 cells (Fig. S3 of the SI Appendix). Details on drug treatments, plasmids, RNAi, and transfection are given in the Supplementary Materials and Methods in the SI Appendix. Tensions were measured by micropipette aspiration, as described in ref. 21 (see Supplementary Materials and Methods in the SI Appendix).
Laser Ablation and Cell Imaging.
Laser ablation experiments were performed on a scanning confocal microscope (Olympus FV1000) equipped with two scanning heads. The first one was used for standard imaging, and the second one was coupled to a laser head (LDH-P-C-405B, PicoQuant GmbH), driven by a power source (PDL 800-B, PicoQuant GmbH) delivering 405-nm picosecond pulses with a nominal power of 3 mW. For ablation, the pulsed laser was undergoing spiraling movements within a circle of 600 nm nominal diameter for 5 s. The actual ablation spot was around 1.5 μm in diameter, as estimated from fluorescence images immediately after ablation (Movie S2 of the SI Appendix). For fluorescence imaging, the pinhole size was set so as to have Z-sections of about 1μm, thickness. For transmitted light imaging, a 561-nm laser was used as a light source. Because the ablation laser saturated the transmitted light detector, transmitted images could not be acquired during ablation.
Image Processing and Data Analysis.
Images were processed by using ImageJ. They were cropped, rotated, and their contrast and brightness were manually adjusted. Bleb-shape analysis was performed on transmitted-light pictures by using MATLAB (The Math Works) (see Supplementary Materials and Methods in the SI Appendix for details).
Supplementary Material
Acknowledgments.
We thank G. Charras, S. Grill, C. P. Heisenberg, J. Howard, A. Oates, J. Prost, C. Sykes, I. M. Tolic–Norrelykke, and the members of the Paluch lab, particularly A. G. Clark, for critical reading of the manuscript and fruitful discussions. We thank the Max Planck Institute Molecular Cell Biology and Genetics (MPI-CBG) Fluorescence-Activated Cell Sorting Facility for help with the apoptosis controls; the MPI-CBG Light Microscopy Facility for development of the laser-ablation setup and for help with microscopy; and R. Chisholm (Northwestern University, Chicago, IL) for the kind gift of the RLC-GFP plasmid. This work was supported by the Polish Ministry of Science and Higher Education from science funds for the years 2006–2009, by the Max Planck Society, by the Institut Curie/ Centre National de la Recherche Scientifique, and by University Paris VI.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.J.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903353106/DCSupplemental.
References
- 1.Morone N, et al. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J Cell Biol. 2006;174:851–862. doi: 10.1083/jcb.200606007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray D, White JG. Cortical flow in animal cells. Science. 1988;239:883–888. doi: 10.1126/science.3277283. [DOI] [PubMed] [Google Scholar]
- 3.Tokumitsu T, Maramorosch K. Cytoplasmic protrusions in insect cells during mitosis in vitro. J Cell Biol. 1967;34:677–83. doi: 10.1083/jcb.34.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bereiter-Hahn J, Lück M, Miebach T, Stelzer H, Vöth M. Spreading of trypsinized cells: Cytoskeletal dynamics and energy requirements. J Cell Sci. 1990;96:171–188. doi: 10.1242/jcs.96.1.171. [DOI] [PubMed] [Google Scholar]
- 5.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 6.Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charras G, Paluch E. Blebs lead the way: How to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–736. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- 8.Trinkaus JP. Surface activity and locomotion of Fundulus deep cells during blastula and gastrula stages. Dev Biol. 1973;30:68–103. doi: 10.1016/0012-1606(73)90049-3. [DOI] [PubMed] [Google Scholar]
- 9.Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 10.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 11.Blaser H, et al. Migration of zebrafish primordial germ cells: A role for myosin contraction and cytoplasmic flow. Dev Cell. 2006;11:613–27. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charras GT, Hu CK, Coughlin M, Mitchison TJ. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175:477–90. doi: 10.1083/jcb.200602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshida K, Soldati T. Dissection of amoeboid movement into two mechanically distinct modes. J Cell Sci. 2006;119:3833–44. doi: 10.1242/jcs.03152. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham CC. Actin polymerization and intracellular solvent flow in cell surface blebbing. J Cell Biol. 1995;129:1589–1599. doi: 10.1083/jcb.129.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheetz MP, Sable JE, Döbereiner HG. Continuous membrane-cytoskeleton adhesion requires continuous accommodation to lipid and cytoskeleton dynamics. Annu Rev Biophys Biomol Struct. 2006;35:417–434. doi: 10.1146/annurev.biophys.35.040405.102017. [DOI] [PubMed] [Google Scholar]
- 17.Paluch E, Piel M, Prost J, Bornens M, Sykes C. Cortical actomyosin breakage triggers shape oscillations in cells and cell fragments. Biophys J. 2005;89:724–733. doi: 10.1529/biophysj.105.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchison TJ, Charras GT, Mahadevan L. Implications of a poroelastic cytoplasm for the dynamics of animal cell shape. Semin Cell Dev Biol. 2008;19:215–23. doi: 10.1016/j.semcdb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kruse K, Joanny JF, Jülicher F, Prost J, Sekimoto K. Asters, vortices, and rotating spirals in active gels of polar filaments. Phys Rev Lett. 2004;92:078101. doi: 10.1103/PhysRevLett.92.078101. [DOI] [PubMed] [Google Scholar]
- 20.Kruse K, Joanny JF, Jlicher F, Prost J, Sekimoto K. Generic theory of active polar gels: A paradigm for cytoskeletal dynamics. Eur Phys J E Soft Matter. 2005;16:5–16. doi: 10.1140/epje/e2005-00002-5. [DOI] [PubMed] [Google Scholar]
- 21.Evans E, Yeung A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys J. 1989;56:151–160. doi: 10.1016/S0006-3495(89)82660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochmuth RM. Micropipette aspiration of living cells. J Biomech. 2000;33:15–22. doi: 10.1016/s0021-9290(99)00175-x. [DOI] [PubMed] [Google Scholar]
- 23.Kimura K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 24.Wallar BJ, Alberts AS. The formins: Active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 2003;13:435–46. doi: 10.1016/s0962-8924(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 25.Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charras GT, Coughlin M, Mitchison TJ, Mahadevan L. Life and times of a cellular bleb. Biophys J. 2008;94:1836–53. doi: 10.1529/biophysj.107.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thoumine O, Cardoso O, Meister JJ. Changes in the mechanical properties of fibroblasts during spreading: a micromanipulation study. Eur Biophys J. 1999;28:222–234. doi: 10.1007/s002490050203. [DOI] [PubMed] [Google Scholar]
- 28.Lipowsky R, Sackmann E. Structure and Dynamics of Membranes. Amsterdam: Elsevier; 1995. [Google Scholar]
- 29.Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989;341:549–551. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- 30.Dai J, Ting-Beall HP, Hochmuth RM, Sheetz MP, Titus MA. Myosin I contributes to the generation of resting cortical tension. Biophys J. 1999;77:1168–1176. doi: 10.1016/s0006-3495(99)76968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz EC, Neuhaus EM, Kistler C, Henkel AW, Soldati T. Dictyostelium myosin IK is involved in the maintenance of cortical tension and affects motility and phagocytosis. J Cell Sci. 2000;113:621–633. doi: 10.1242/jcs.113.4.621. [DOI] [PubMed] [Google Scholar]
- 32.Carlier MF, Ressad F, Pantaloni D. Control of actin dynamics in cell motility. Role of ADF/cofilin. J Biol Chem. 1999;274:33827–33830. doi: 10.1074/jbc.274.48.33827. [DOI] [PubMed] [Google Scholar]
- 33.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 34.Krieg M, et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 35.Lang T, et al. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys J. 2000;78:2863–2877. doi: 10.1016/S0006-3495(00)76828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanakam F, Albrecht R, Eckerskorn C, Matzner M, Gerisch G. Myristoylated and non-myristoylated forms of the pH sensor protein hisactophilin II: Intracellular shuttling to plasma membrane and nucleus monitored in real time by a fusion with green fluorescent protein. EMBO J. 1996;15:2935–2943. [PMC free article] [PubMed] [Google Scholar]
- 37.Wu HW, Kuhn T, Moy VT. Mechanical properties of L929 cells measured by atomic force microscopy: effects of anticytoskeletal drugs and membrane crosslinking. Scanning. 1998;20:389–397. doi: 10.1002/sca.1998.4950200504. [DOI] [PubMed] [Google Scholar]
- 38.Mahaffy RE, Park S, Gerde E, Käs J, Shih CK. Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophys J. 2004;86:1777–1793. doi: 10.1016/S0006-3495(04)74245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langridge PD, Kay RR. Blebbing of Dictyostelium cells in response to chemoattractant. Exp Cell Res. 2006;312:2009–2017. doi: 10.1016/j.yexcr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Bereiter-Hahn J. Mechanics of crawling cells. Med Eng Phys. 2005;27:743–753. doi: 10.1016/j.medengphy.2005.04.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.