Abstract
Endogenous small interfering RNAs (endo-siRNAs) regulate diverse gene expression programs in eukaryotes by either binding and cleaving mRNA targets or mediating heterochromatin formation; however, the mechanisms of endo-siRNA biogenesis, sorting, and target regulation remain poorly understood. Here we report the identification and function of a specific class of germline-generated endo-siRNAs in Caenorhabditis elegans that are 26 nt in length and contain a guanine at the first nucleotide position (i.e., 26G RNAs). 26G RNAs regulate gene expression during spermatogenesis and zygotic development, and their biogenesis requires the ERI-1 exonuclease and the RRF-3 RNA-dependent RNA polymerase (RdRP). Remarkably, we identified two nonoverlapping subclasses of 26G RNAs that sort into specific RNA-induced silencing complexes (RISCs) and differentially regulate distinct mRNA targets. Class I 26G RNAs target genes are expressed during spermatogenesis, whereas class II 26G RNAs are maternally inherited and silence gene expression during zygotic development. These findings implicate a class of endo-siRNAs in the global regulation of transcriptional programs required for fertility and development.
Keywords: endogenous siRNA, germline, RNA interference
Small RNAs bind Argonaute/Piwi proteins in the RNA-induced silencing complex (RISC) and, through base pairing, guide RISC to silence their cognate targets. While the taxonomy of small RNAs remains fluid, they can be defined in part by nucleotide length, 5′ nucleotide composition, chemical modifications, genetic requirements for biogenesis, mode of silencing, and biological functions. For example, microRNAs are processed from double-stranded hairpin precursors by the RNase III-like enzyme Dicer to the ≈22-nt mature form containing a 5′-monophosphate nucleotide. The microRNAs associate with Argonaute (Ago) proteins in RISC and mediate translational repression and/or degradation of their target mRNAs (1). In contrast, Piwi-interacting RNAs (piRNAs) are typically longer than microRNAs, possess a uridine in the first nucleotide, and are generated by a Dicer-independent self-amplification pathway. The piRNAs bind to Piwi proteins in RISC and silence transposons (2).
Endogenous small interfering RNAs (endo-siRNAs) represent an emerging class of small RNAs described and characterized in Caenorhabditis elegans by Ambros et al. (3). These endo-siRNAs are perfectly antisense to target transcripts and require the C. elegans Dicer, DCR-1, the RNA-dependent RNA polymerase (RdRP) RRF-3, and the exonuclease ERI-1 for expression (4, 5). By large-scale pyrosequencing, Ruby et al. determined that other endo-siRNAs target transcripts associated with spermatogenesis and transposons (6). Therefore, C. elegans endo-siRNAs appear to be a diverse class of small RNAs, with distinct biological functions and genetic requirements for biogenesis. The recent discovery of endo-siRNAs derived from transposable elements, natural antisense transcripts, and hairpin RNAs in Drosophila melanogaster and Mus musculus (7–12) further supports their function in regulating endogenous gene expression.
Mutations affecting small RNA pathways frequently are associated with defective gametogenesis (13, 14). In C. elegans, mutation of dcr-1 results in severe defects in germline development, malformed unfertilized oocytes, and sterility (14–16). Similarly, mutation of prg-1 (piwi-related gene) abrogates the expression of 21U RNAs (a piwi-interacting class of small RNAs) and results in impaired germline proliferation and sterility at elevated temperatures (17–19). Small RNAs also can serve as heritable parental silencing factors to regulate filial gene expression; in D. melanogaster, misregulation of maternally inherited piRNAs results in activation of transposons and hybrid dysgenesis (20). These observations underscore the essential functions of small RNAs in germline development and cross-generational epigenetic regulation.
In this study, we identified two classes of germline-generated endo-siRNAs, the class I sperm 26G RNAs and the class II oocyte/embryo 26G RNAs, that regulate the expression of distinct sets of genes during spermatogenesis and zygotic development, respectively. Our findings indicate that the 26G endo-siRNAs not only exert a profound influence over male gametogenesis, but also are maternally inherited and act as epigenetic agents to control gene expression during zygotic development in the progeny.
Results
Deep Sequencing Revealed Germline-Enriched, eri-1-Dependent 26G endo-siRNAs.
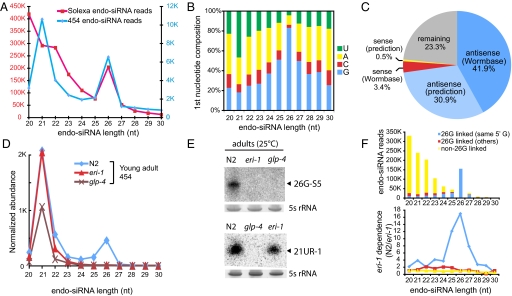
Small RNAs expressed in purified male sperm, hermaphrodite oocytes, and embryos were size selected (18–32 nt) and sequenced by high-throughput deep sequencing (Roche/454 and Illumina/Solexa). After excluding sequences corresponding to microRNAs, 21U RNAs, and putative degradation products derived from abundant noncoding RNAs (e.g., rRNAs) (Fig. S1 and SI Methods in SI Appendix), we identified 2.45 million putative endo-siRNA reads (14.8% of the total sequences) (Dataset S1). These endo-siRNAs display a bimodal length distribution with one peak clustered at ≈21 nt and the second at 26 nt (Fig. 1A). Notably, while the ≈21-nt endo-siRNAs have a first nucleotide bias for uridine observed for piRNAs in other organisms (21–23), the 26-nt endo-siRNAs preferentially start with a guanine nucleotide (Fig. 1B). Therefore, we refer to them as 26G RNAs (6).
Fig. 1.
26G RNAs are germline-enriched endogenous siRNAs. (A) Length distribution of endo-siRNAs exhibits a bimodal pattern, peaking at both 21 and 26 nt length. Small RNA libraries of mixed-stage N2 animals and purified male sperm [him-8(e1489)], oocytes [fer-1(hc1)], and N2 embryos were sequenced by Solexa/Illumina; libraries of N2 young adults, sperm, and oocytes were sequenced by Roche/454. The him-8(e1489) mutation increases the percentage of XO males to approximately 37% of the population (44) versus approximately 0.2% males in the N2 wild-type strain; the fer-1(hc1) mutation results in nonfunctional sperm at 25 °C (45), enabling purification of unfertilized oocytes. (B) First nucleotide identity of endo-siRNAs. endo-siRNAs (26-nt) have a strong preference for guanine as the first nucleotide (83%). (C) The majority of 26G RNAs are antisense to known and predicted coding transcripts. (D) Normalized length distribution of endo-siRNAs in N2, eri-1(mg366), and glp-4(bn2) young adult libraries sequenced by 454/Roche. The abundance was normalized to 100 K effective small RNA reads (excluding putative degradation products of abundant ncRNAs). (E) Northern blotting validates the lack of 26G RNA expression in eri-1(mg366) and glp-4(bn2) mutants. Total RNA from N2, eri-1(mg366), and glp-4(bn2) adult worms was probed for a 26G RNA (26G-S5) and a 21U RNA (21UR-1). The expression of the germline-derived 21U RNA (21UR-1) is not eri-1-dependent. 5S rRNA serves as the loading control. (F) endo-siRNAs were classified as 26G RNA-linked (targeting the same genes) or non-26G RNA-linked (targeting other genes or intergenic regions). Most 26G RNA-linked endo-siRNAs start with the same 5′G. A small fraction of shorter (20–24 nt) endo-siRNAs are 26G RNA-linked. The bottom panel plots the eri-1 dependence as measured by the ratio of 26G RNA sequence counts in N2 vs. eri-1.
Although 26G RNAs previously have been identified by deep sequencing of small RNAs isolated from mixed-stage N2 worms (6), little is known about their biogenesis or role in gene regulation. Mapping to the genome reveals that most 26G RNAs target protein coding genes (i.e., exons, introns, and UTRs) (77%) and exhibit a strong antisense bias (73% antisense vs. 4% sense) (Fig. 1C and SI Methods in SI Appendix). In addition, the majority of 26G RNAs are derived from exons or introns of coding transcripts target exons (97.2%) or span exon-exon junctions (0.7%), suggesting that mature mRNAs are the main targets of 26G RNAs. (Fig. S2C in SI Appendix). We also mapped independently the small RNA sequences using National Center for Biotechnology Information (NCBI) AceView (24) (www.aceview.org), a database incorporating experimentally validated transcriptome data, and show that mapping of the 26G RNAs agrees well with the analyses above (Fig. S3 and SI Methods in SI Appendix).
We next used deep sequencing to compare the endo-siRNA profiles of N2, glp-4(bn2) (25), and eri-1(mg366) (26) whole animals. The glp-4(bn2) mutant fails to proliferate its germline at nonpermissive temperature (25 °C) and therefore lacks germline-derived small RNAs. The glp-4 mutant exhibits an approximately 50% decline in 21-nt siRNA expression, but a complete loss of 26G RNAs, suggesting that 26G RNAs are exclusively derived from the germline (Fig. 1D). The eri-1(mg366) mutant also completely lacks 26G RNAs without globally affecting 21-nt endo-siRNA levels (Fig. 1D). Interestingly, we found a small fraction of ≈21-nt endo-siRNAs (4.5% total 21-nt endo-siRNAs) that appear also to be eri-1-dependent. These small RNAs largely overlap with 26G RNAs, starting with the same 5′G nucleotide (Fig. 1F). Yet, they are markedly less abundant than 26G RNAs (6.6% of the total number of 26G RNAs) (Fig. 1F). These findings suggest that, while ≈21-nt endo-siRNAs, as a whole, constitute a genetically diverse population of small RNAs, 26G RNAs represent a class of germline-enriched endo-siRNAs that exclusively depends on both germline development and eri-1 for their expression.
Two Subclasses of 26G RNAs Exhibit Different Expression Patterns.
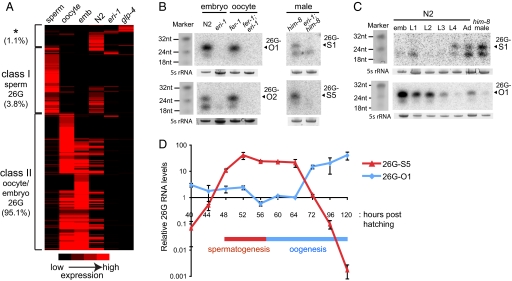
Strikingly, hierarchical clustering reveals that 98.9% of the 26G RNAs fall into two distinct classes (Fig. 2A, Fig. S1, and SI Methods in SI Appendix). Class I 26G RNAs are present in purified sperm (1,102 unique sequences; 5,960 total reads), but are not detectable in oocytes or embryos. By comparison, class II 26G RNAs are highly enriched in oocytes and embryos (2,441 unique sequences; 148,594 total reads), but are not readily detected in sperm. Both classes of 26G RNAs are present at lower levels in mixed-stage N2 and are severely depleted in glp-4(bn2) and eri-1(mg366) animals. We analyzed the expression profiles of four relatively abundant sperm 26G RNAs (26G-S1, -S4, -S5, -S6) and four oocyte/embryo 26G RNAs (26G-16, -O1, -O2, -O3) by Northern blotting and/or RT-qPCR assays (Taqman; Applied Biosystems). The stem-loop structure of the Taqman primers specifically recognizes the 3′ ends of the 26G RNAs for reverse transcription and, therefore, allows discrimination from the ≈21-nt endo-siRNAs that start with the same 5′G as the 26G RNAs. Northern blotting demonstrated that the expression of 26G RNAs is dependent on eri-1 in purified oocytes and embryos as well as in male animals (Fig. 2B). In addition, clear temporal separation in the expression of these two classes of 26G RNAs was observed (Fig. 2 C and D). The class I sperm 26G RNAs (denoted 26G-S) are only detectable in late larval (L4) and young adult stages in N2 hermaphrodites and males (Fig. 2C, top panel); furthermore, a finer time course revealed class I sperm 26G RNA expression occurs in a relatively narrow window, consistent with expression during spermatogenesis (Fig. 2D). Conversely, expression of class II oocyte/embryo 26G RNAs (denoted 26G-O) (Fig. 2C, bottom panel, and Fig. 2D) initiates during oogenesis, peaks in embryos, and progressively declines throughout the four larval stages. Consistent with the deep sequencing data, Northern blotting indicates cross-hybridization of the 26G RNA probes to a shorter ≈21-nt species (Fig. 2 B and C).
Fig. 2.
Two classes of 26G RNAs exhibit different expression patterns. (A) Hierarchical clustering reveals two major classes of 26G RNAs: Class I sperm 26G RNAs (3.8% of total reads) and class II oocyte/embryo 26G RNAs (95.1%). 26G RNA reads matching to the genome with at least two counts were included in the analysis (4,002 unique sequences; 156,204 total reads). The asterisk (*) indicates a small fraction (1.1%) of 26G RNA sequences that do not fall into either class I or II categories. (B) Both classes of 26G RNAs are dependent on eri-1 for their expression. Total RNA from N2 and eri-1(mg366) embryos and fer-1(hc1) and fer-1(hc1); eri-1(mg366) oocytes were probed for class II oocyte/embryo 26G RNAs (26G-O1, -O2). Total RNA from him-8(e1489) and eri-1(mg366); him-8(e1489) adult males were probed for class I sperm 26G RNAs (26G-S1, -S5). 5S rRNA serves as a loading control. (C) Class I and class II 26G RNAs are expressed in distinct periods during development. Total RNA from embryos, four larval stages, adult hermaphrodites, and him-8(e1489) adult males were analyzed by Northern blotting with probes for a class I sperm 26G RNA (26G-S1) and a class II oocyte/embryo 26G RNA (26G-O1). Synthetic RNA oligos stained with EtBr serve as size markers, 5S rRNA as a loading control. (D) Analysis of 26G RNA levels during germline proliferation assayed by RT-qPCR. The expression of class I sperm 26G RNA (26G-S5) and class II oocyte/embryo 26G RNA (26G-O1) correlates with the time windows for spermatogenesis and oogenesis, respectively. The x-axis represents hours post-hatching at 20 °C.
Two Subclasses of 26G RNAs Silence Distinct Sets of Targets.
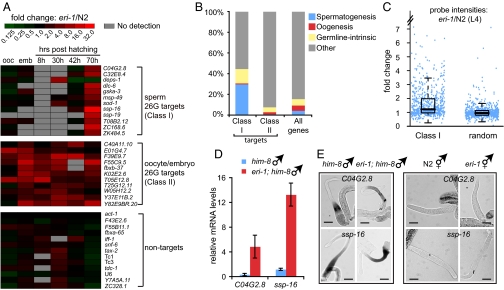
The 26G RNAs are perfectly complementary to their predicted gene targets, suggesting that they may act as canonical siRNAs to direct the cleavage of their mRNA targets. Importantly, 26G RNAs largely target a different set of genes from those targeted by shorter length (20–24 nt) endo-siRNAs (Fig. S4 in SI Appendix). Because the expression patterns of the two classes of 26G RNAs are mutually exclusive, we next asked if they differentially regulate nonoverlapping, discrete classes of target genes. Indeed, based on existing germline gene expression profiles (27), we found that predicted targets of class I sperm 26G RNAs are enriched 7-fold for genes expressed during spermatogenesis, whereas targets of class II oocyte/embryo 26G RNAs are depleted of all three classes of germline genes (spermatogenesis, oogenesis, and germline-intrinsic) (Fig. 3B). Because mutations in eri-1 abolish the expression of both classes of 26G RNAs, we used RT-qPCR to analyze the relative expression of putative 26G RNA targets in eri-1(mg366) and N2 at the following five developmental time points: embryos, and 8 h (L1), 30 h (L3), 42 h (L4), and 70 h (adult) post-hatching (Fig. 3A). While transcript levels of genes not targeted by 26G RNAs were similar in eri-1(mg366) and N2 animals (Fig. 3A, bottom panel), transcripts corresponding to 11 of the 12 genes that are targeted by class I sperm 26G RNAs and all 11 genes targeted by class II oocyte/embryo 26G RNAs are significantly elevated in eri-1(mg366) animals relative to N2 controls (Fig. 3A; see SI Methods in SI Appendix for target selection criteria). Consistent with the temporal expression pattern of class I sperm 26G RNAs, target silencing occurs in a relatively narrow window that corresponds to spermatogenesis through young adulthood (Fig. 3A and Fig. S5 in SI Appendix). By comparison, although class II oocyte/embryo 26G RNA levels steadily decline during larval development, their silencing effects persist throughout development (Fig. 3A).
Fig. 3.
Two classes of 26G RNAs silence nonoverlapping sets of mRNA transcripts. (A) Gene targets of 26G RNAs are desilenced in the eri-1(mg366) background. Differential gene expression profiles between N2 and eri-1(mg366) for 12 targets of class I sperm 26G RNAs, 11 targets of class II oocyte/embryo 26G RNAs, and 13 nontargets were measured by RT-qPCR. The level of fold upregulation is represented according to the red-green color scheme indicated in the top panel. (B) Gene class analyses of class I sperm and class II oocyte/embryo 26G RNAs. Targets of class I sperm 26G RNAs (573 genes) are significantly overrepresented in genes expressed during spermatogenesis, while targets of class II oocyte/embryo 26Gs (243 genes) are depleted of germline enriched genes. (C) Genes targeted by class I sperm 26G RNAs are upregulated in the eri-1(mg366) mutant. Each point indicates the fold change in probe intensity corresponding to predicted targets of 26G RNAs (728 probes corresponding to 589 genes). Randomly selected probes do not show significant upregulation in the eri-1(mg366) mutant. (D) Two sperm 26G RNA target mRNAs, C04G2.8 and ssp-16, are upregulated in eri-1(mg366); him-8(e1489) males relative to him-8(e1489) males. mRNA levels were quantified by RT-qPCR and normalized to act-1. (E) Loss of 26G RNA expression does not induce inappropriate ectopic expression of targets. RNA in situ hybridization of dissected gonads was performed with probes for the class I sperm 26G RNA targets C02G2.8 and ssp-16. In both wild-type and eri-1 backgrounds, mRNA expression of these two genes remained restricted to the spermatogenic gonad. No ectopic expression of the class I 26G RNA targets was observed in the hermaphrodite oogenic gonads. (Scale bar, 50 μm.)
We next asked if the eri-1-dependent regulation of 26G RNA targets could be observed at the whole-transcriptome level. Using previously reported whole-genome microarray data that compared transcript expression profiles of L4 stage eri-1 and N2 worms (28), we found that predicted targets of 26G RNAs are significantly upregulated in the eri-1(mg366) mutant (P < 0.0001, t-test) (Fig. 3C). Conversely, genes upregulated in the eri-1 mutant background also were 9-fold enriched for 26G RNA targets (SI Methods in SI Appendix). Taken together, the highly correlated expression patterns between 26G RNAs and their putative targets at the whole-transcriptome level further support the hypothesis that 26G RNAs directly regulate target gene expression in an eri-1-dependent manner.
To determine if target de-repression in eri-1(mg366) results in misexpression of target mRNAs in inappropriate tissues, we performed RNA in situ hybridization for select, relatively abundant targets (C04G2.8 and ssp-16) in dissected gonads (27). While the class I sperm 26G RNA targets C04G2.8 and spp-16 are upregulated in the eri-1 mutant (Fig. 3D), they exhibit similar expression patterns in the male spermatogenic gonads of both the him-8 and eri-1; him-8 strains (Fig. 3E). Thus, target de-silencing by class I sperm 26G RNAs in the eri-1 mutant remains restricted to the male gonad and does not result in inappropriate, ectopic expression in either the male gonads or in the oogenic gonads of eri-1 hermaphrodite animals (Fig. 3E), indicating that 26G RNAs repress target expression in their cognate cell types.
Genetic Requirements for 26G RNA Biogenesis and Function.
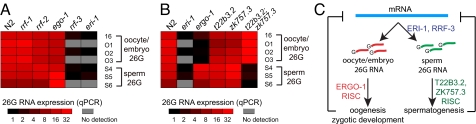
Because small RNAs that start with a guanine nucleotide are thought to be products of an RdRP (29), we asked if RdRPs could play a role in biogenesis of 26G RNAs. The C. elegans genome encodes four RdRPs (rrf-1, 2, 3, and ego-1) (30). We examined 26G RNA expression in mutants for three viable RdRPs, rrf-1(pk1419), rrf-2(ok210), and rrf-3(pk1426). Since mutations in ego-1 result in lethality (31), we used RNAi to deplete the ego-1 transcript from N2 animals. While rrf-1(pk1419), rrf-2(ok210), and ego-1(RNAi) express normal levels of 26G RNAs, both classes of 26G RNAs are abolished in rrf-3(pk1426), which results in significant upregulation of both classes of targets (Fig. 4A and Fig. S6 in SI Appendix). However, we note that RNAi-inactivation of ego-1 does not completely abolish ego-1 expression, and therefore we cannot definitively conclude that the 26G RNAs are strictly ego-1-independent. If 26G RNAs are bona fide RdRP products, then transcripts they target should serve as templates for 26G RNA production. We determined that the expression of a 26G RNA (26G-S4) is dramatically reduced when its target deps-1 is mutated and degraded by nonsense-mediated decay (see SI Results and Fig. S7 in SI Appendix). Interestingly, although 26G RNAs require the RRF-3 RdRP, they appear to possess a 5′-monophosphate, as opposed to the 5′-triphosphate of other known RdRP products (see SI Results and Fig. S8 in SI Appendix). In addition, with notable exceptions (21U RNAs), the presence of a 5′-monophosphate on a small RNA is a signature of Dicer processing. Expression analysis in the dcr-1(ok247) mutant indicates that the 26G RNAs likely require DCR-1 for biogenesis (see SI Results and Fig. S9 in SI Appendix).
Fig. 4.
Genetic requirements for 26G biogenesis and function. (A) RT-qPCR analysis of 26G RNA expression in rrf-1(pk1417), rrf-2(ok210), rrf-3(pk1426), and ego-1(RNAi). Mutation of rrf-3 abrogates the expression of both sperm and oocyte/embryo 26G RNAs, while the 26G RNAs are expressed at wild-type levels in the mutants of rrf-1 and rrf-2, as well as in RNAi-inactivation of ego-1. (B) An oogenesis-enriched Argonaute encoded by ergo-1 is required for class II oocyte/embryo 26G RNA expression but dispensable for class I sperm 26G RNA expression. The t22b3.2(tm1155); zk757.3(tm1184) double mutant is defective in sperm 26G RNAs but expresses normal levels of oocyte/embryo 26G RNAs. (C) Proposed model for 26G RNA biogenesis and function. See text for details.
The nonoverlapping identities of the two classes of 26G RNAs and the disparate targets they regulate suggested that they might be sorted into distinct RISCs. Argonaute proteins are central components of RISC and possess two conserved domains, PAZ and PIWI. Argonautes directly bind small RNAs (via both domains) and may possess target cleavage (“slicer”) activity via the PIWI domain (32). C. elegans encodes 27 potential Argonautes with diverse functions, several of which have been found to be enriched during spermatogenesis or oogenesis (27, 33). We found that an Argonaute encoded by ergo-1 (33), whose transcript is enriched during oogenesis (27), is required for the expression of class II oocyte/embryo 26G RNAs, but not for class I sperm 26G RNAs (Fig. 4B). Consistent with this finding, only targets of class II oocyte/embryo 26G RNAs were upregulated in the ergo-1(tm1860) mutant (Fig. S6 in SI Appendix). The expression of two Argonautes, T22B3.2 and its close paralog, ZK757.3 (93.1% amino acid sequence identity), are enriched during spermatogenesis (27). Although the single mutant of either t22b3.2(tm1155) or zk757.3(tm1184) maintains wild-type expression levels of both classes of 26G RNAs, mutations in both T22B3.2 and ZK757.3 abrogate the expression of class I sperm 26G RNAs, but not class II oocyte/embryo 26G RNAs (Fig. 4B). Similarly, only targets of class I sperm 26G RNAs are de-repressed in the double mutant (Fig. S6 in SI Appendix). ERGO-1, T22B3.2, and ZK757.3 all possess the Asp-Asp-His catalytic “slicer” motif (33, 34), suggesting that they are capable of directly mediating endonucleolytic cleavage of their targets. Taken together, our data suggest that distinct RISCs guide the class I and class II 26G RNAs to their cognate targets for silencing.
What are the biological functions of 26G RNA-mediated target regulation? eri-1 and rrf-3 mutants, which lack both class I and class II 26G RNAs, are temperature-sensitive (ts) sterile due to defective spermatogenesis (26, 35). While the single Argonaute mutants of T22B3.2 and ZK757.3 exhibit near wild-type levels of fertility, the double mutant, which is specifically defective in the expression of class I sperm 26G RNAs, is completely sterile at 25 °C and can be fully rescued by crossing with wild-type males (Fig. S10 A–C in SI Appendix). In contrast, the ergo-1 Argonaute mutant, which is defective in the expression of class II oocyte/embryo 26G RNAs, displays near wild-type fertility. These findings suggest that class I sperm 26G RNAs play an essential gene regulatory role during spermatogenesis. Loss of class II oocyte/embryo 26G RNAs does not result in any overt developmental phenotypes, as we did not observe any somatic defects in the eri-1, rrf-3, or ergo-1 mutant. This is consistent with the finding that endo-siRNAs recently identified in fly soma and mouse oocytes appear to be dispensable for viability and reproduction (7–11). Interestingly, mutants of eri-1, rrf-3, and ergo-1 all exhibit an enhanced response to exogenous RNAi (26, 33, 35), whereas the t22b3.2; zk757.3 double mutant does not (Fig. S10E in SI Appendix), suggesting that class II 26G RNAs may compete with the exogenous RNAi pathway for limiting common factors (4, 5).
Discussion
In this study, we characterized a class of germline-enriched endo-siRNAs that are generated by a template-dependent mechanism and require the RRF-3 RdRP and the ERI-1 exonuclease for their biogenesis. In our model, class I and class II 26G RNAs are sorted into distinct, gamete-specific RISCs during germline development and differentially target discrete classes of target genes (Fig. 4C). Class I 26G RNAs repress their target genes during spermatogenesis and mutations that abrogate their expression lead to male sterility. Class II 26G RNAs are maternally loaded and appear to be responsible for the clearance of maternal transcripts during zygotic development. In zebrafish, miR-340 clears hundreds of maternal mRNAs during the maternal-zygotic transition (36). In our model, the class II 26G RNAs not only begin to clear a subset of target maternal mRNAs that are deposited, but also act to ensure that the maternal load of mRNAs continues to be cleared during filial development. The fact that the loss of class II 26G RNAs leads to enhanced RNAi phenotypes suggests that ongoing transcript clearance competes with exogenous RNAi for limiting factors.
In exogenous RNAi, primary siRNAs derived from Dicer processing of an exogenous dsRNA trigger initiate unprimed synthesis of secondary siRNAs mediated by RdRPs (37, 38). Unlike the primary siRNAs that possess a 5′-monophosphate, these secondary siRNAs contain a 5′-triphosphate modification. Similarly, we speculate that 26G endo-siRNAs might function as 5′-monophosphorylated primary endo-siRNAs, whose biogenesis is likely dcr-1-dependent, to guide target cleavage and initiate the production of ≈21-nt secondary endo-siRNAs that further silence the 26G RNA targets. Interestingly, these ≈21-nt putative secondary siRNAs also appear to be 5′-triphosphorylated (Fig. S8 in SI Appendix) and, therefore, would be underrepresented in our deep sequencing data sets that enrich for RNAs possessing a 5′-monophosphate group.
Our study raises other interesting questions. Why are certain genes targeted by 26G RNAs? How do ERI-1 and RRF-3 participate in the biogenesis of 26G RNAs? Why do the loss of sperm 26G RNAs and consequent upregulation of targets lead to ts sterility? Further genetic and biochemical analysis may reveal additional factors and mechanisms that mediate the biogenesis, sorting, differential stability, target silencing, and developmental functions of the class I and class II 26G RNAs.
Materials and Methods
Strains and Sperm, Oocyte, and Embryo Purifications.
The Bristol N2 was used as the reference wild-type strain. Mutant alleles used in this study include: LG I: glp-4(bn2), fer-1(hc1), rrf-1(pk1417), rrf-2(ok210), deps-1(bn121), deps-1(bn124), smg-1(r861); LG II: rrf-3(pk1426); LG III: zk757.3(tm1184), dcr-1(ok247); LG IV: him-8(e1489), eri-1(mg366), t22b3.2(tm1155); LG V: ergo-1(tm1860). Sperm and oocytes from him-8(e1489) and fer-1(hc1), respectively, were purified as described (39) with some modifications. See SI Methods in SI Appendix for additional details.
RNA Analysis.
Total RNA isolation was carried out using TriReagent (Ambion) following the vendor's protocol. 5′-Monophosphate-bearing small RNA libraries were constructed as described (40). Due to limitation in sensitivity, relatively abundant 26G RNAs were selected for Northern blotting as described (41) using 5–10 μg total RNA and Starfire DNA probes (IDT). For RT-qPCR analysis of small RNAs, custom small RNA Taqman assays (Applied Biosystems) were performed following the vendor's protocol. For quantification of mRNAs, 250 ng to 1 μg total RNAs were converted into cDNAs with Multiscribe Reverse Transcriptase (Applied Biosystems) following the vendor's protocol. See SI Methods in SI Appendix for additional details.
Germline RNA in Situ Hybridization.
RNA in situ hybridization was performed with dissected gonads according to Lee and Schedl (42). Antisense cDNA fragments labeled with DIG DNA labeling mix (Roche) for C4G2.8 (547 bp) and ssp-16 (102 bp) were used as probes.
RNA Interference.
Gene inactivation by RNAi was performed as described (43) using clones from the Ahringer RNAi library.
Computational Methods.
See SI Methods in SI Appendix.
Supplementary Material
Acknowledgments.
We thank John Moran, Harrison Gabel, Chi Zhang, Tammy Wu, Kris Gunsalus, Scott Kennedy, Patrick Hu, and Allison Billi for helpful comments; Sylvia Fischer (Massachusetts General Hospital) for dcr-1(ok247) total RNA, the Caenorhabditis Genetics Center and Shohei Mitani (Tokyo Women's Medical University) for strains, Marco Marra and Martin Hirst of the British Columbia Cancer Centre for Solexa deep sequencing, and David Miller of Applied Biosystems for Taqman probes. This work was supported by a National Science Foundation CAREER Award, National Institutes of Health Grant S06 GM52588 (to D.S.C.), the Intramural Research Program of the National Institutes of Health and the National Library of Medicine (to D.T.-M. and J.T.-M.), and National Institutes of Health Grant HG004276–01 grant (to J.K.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906378106/DCSupplemental.
References
- 1.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Aravin AA, Hannon GJ, Brennecke J. The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science. 2007;318:761–764. doi: 10.1126/science.1146484. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V, Lee RC, Lavanway A, Williams PT, Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr Biol. 2003;13:807–818. doi: 10.1016/s0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 4.Duchaine TF, et al. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343–354. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Lee RC, Hammell CM, Ambros V. Interacting endogenous and exogenous RNAi pathways in Caenorhabditis elegans. RNA. 2006;12:589–597. doi: 10.1261/rna.2231506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruby JG, et al. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127:1193–1207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T, et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 8.Tam OH, et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamura K, et al. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature. 2008;453:803–806. doi: 10.1038/nature07015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawamura Y, et al. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature. 2008;453:793–797. doi: 10.1038/nature06938. [DOI] [PubMed] [Google Scholar]
- 11.Ghildiyal M, et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science. 2008;320:1077–1081. doi: 10.1126/science.1157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czech B, et al. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox DN, et al. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight SW, Bass BL. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science. 2001;293:2269–2271. doi: 10.1126/science.1062039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ketting RF, et al. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grishok A, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 17.Wang G, Reinke V. A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis. Curr Biol. 2008;18:861–867. doi: 10.1016/j.cub.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista PJ, et al. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das PP, et al. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol Cell. 2008;31:79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennecke J, et al. An epigenetic role for maternally inherited piRNAs in transposon silencing. Science. 2008;322:1387–1392. doi: 10.1126/science.1165171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aravin A, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 22.Lau NC, et al. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–367. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 23.Brennecke J, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–1103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Thierry-Mieg D, Thierry-Mieg J. AceView: A comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7(Suppl 1):S12.1–S12.14. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beanan MJ, Strome S. Characterization of a germ-line proliferation mutation in C. elegans. Development. 1992;116:755–766. doi: 10.1242/dev.116.3.755. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy S, Wang D, Ruvkun G. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature. 2004;427:645–649. doi: 10.1038/nature02302. [DOI] [PubMed] [Google Scholar]
- 27.Reinke V, Gil IS, Ward S, Kazmer K. Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development. 2004;131:311–323. doi: 10.1242/dev.00914. [DOI] [PubMed] [Google Scholar]
- 28.Asikainen S, Storvik M, Lakso M, Wong G. Whole genome microarray analysis of C. elegans rrf-3 and eri-1 mutants. FEBS Lett. 2007;581:5050–5054. doi: 10.1016/j.febslet.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 29.Makeyev EV, Bamford DH. Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol Cell. 2002;10:1417–1427. doi: 10.1016/s1097-2765(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 30.Sijen T, et al. On the role of RNA amplification in dsRNA-triggered gene silencing. Cell. 2001;107:465–476. doi: 10.1016/s0092-8674(01)00576-1. [DOI] [PubMed] [Google Scholar]
- 31.Smardon A, et al. EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C. elegans. Curr Biol. 2000;10:169–178. doi: 10.1016/s0960-9822(00)00323-7. [DOI] [PubMed] [Google Scholar]
- 32.Faehnle CR, Joshua-Tor L. Argonautes confront new small RNAs. Curr Opin Chem Biol. 2007;11:569–577. doi: 10.1016/j.cbpa.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yigit E, et al. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell. 2006;127:747–757. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 35.Simmer F, et al. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr Biol. 2002;12:1317–1319. doi: 10.1016/s0960-9822(02)01041-2. [DOI] [PubMed] [Google Scholar]
- 36.Giraldez AJ, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 37.Pak J, Fire A. Distinct populations of primary and secondary effectors during RNAi in C. elegans. Science. 2007;315:241–244. doi: 10.1126/science.1132839. [DOI] [PubMed] [Google Scholar]
- 38.Sijen T, Steiner FA, Thijssen KL, Plasterk RH. Secondary siRNAs result from unprimed RNA synthesis and form a distinct class. Science. 2007;315:244–247. doi: 10.1126/science.1136699. [DOI] [PubMed] [Google Scholar]
- 39.L'Hernault SW, Roberts TM. Cell biology of nematode sperm. Methods Cell Biol. 1995;48:273–301. doi: 10.1016/s0091-679x(08)61392-8. [DOI] [PubMed] [Google Scholar]
- 40.Lu C, Meyers BC, Green PJ. Construction of small RNA cDNA libraries for deep sequencing. Methods. 2007;43:110–117. doi: 10.1016/j.ymeth.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Pall GS, Hamilton AJ. Improved northern blot method for enhanced detection of small RNA. Nat Protoc. 2008;3:1077–1084. doi: 10.1038/nprot.2008.67. [DOI] [PubMed] [Google Scholar]
- 42.Lee MH, Schedl T. RNA in situ hybridization of dissected gonads. WormBook. 2006;14:1–7. doi: 10.1895/wormbook.1.107.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/s1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 44.Brenner S, Hodgkin J, Horvitz R. Nondisjunction mutants of the nematode C. elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward S, Argon Y, Nelson GA. Sperm morphogenesis in wild-type and fertilization-defective mutants of Caenorhabditis elegans. J Cell Biol. 1981;91:26–44. doi: 10.1083/jcb.91.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.