Abstract
Inflammatory activation of microglia in response to neurodegenerative changes in diseases such as Alzheimer's disease (AD) and Parkinson's disease has been extensively described. These observations have suggested that inflammation could be contributing to disease progression. In this paper, the potential role of CD200 and CD200 receptor (CD200R), whose known functions are to activate anti-inflammatory pathways and induce immune tolerance through binding of CD200 to CD200 receptor (CD200R), was studied in AD. Quantitative studies showed a significant decrease in CD200 protein and mRNA in AD hippocampus and inferior temporal gyrus, but not cerebellum. Immunohistochemistry of brain tissue sections of hippocampus, superior frontal gyrus, inferior temporal gyrus and cerebellum from AD and non-demented cases demonstrated a predominant, though heterogeneous, neuronal localization for CD200. Decreased neuronal expression was apparent in brain regions affected by AD pathology. There was also a significant decrease in CD200R mRNA expression in AD hippocampus and inferior temporal gyrus, but not cerebellum. Low expression of CD200R by microglia was confirmed at the mRNA and protein level using cultured human microglia compared to blood-derived macrophages. Treatment of microglia and macrophages with interleukin-4 and interleukin-13 significantly increased expression of CD200R. Expression of these cytokines was not generally detectable in brain. These data indicate that the anti-inflammatory CD200/CD200R system may be deficient in AD brains. Mechanisms aimed at increasing levels of CD200 and CD200R could have therapeutic potential for controlling inflammation in human neurodegenerative diseases.
Keywords: Human, In vitro, Gene expression, Immunohistochemistry, Anti-inflammatory
Introduction
The role of inflammation in contributing to neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) has been intensively studied since the identification of prominent responses by microglia (brain resident macrophages) to degenerative structures in brain tissues affected by these diseases (Neuroinflammation Working Group, 2000;Hirsch et al., 2003). Pathological studies of AD indicated that the presence of activated microglia, reactive astrocytes, and complement activation, particularly in association with amyloid beta (Aβ)-containing plaques, demonstrated that a type of chronic inflammation was ongoing (McGeer and McGeer, 2003;Xiang et al., 2006;Sastre, Klockgether, and Heneka, 2006). A range of in vitro studies using cultured microglia from humans or rodents demonstrated that aggregated Aβ peptide could activate microglia to a proinflammatory state (Walker et al., 2006;Chen et al., 2005;Combs et al., 2001;Giulian et al., 1998;Gan et al., 2004;Yan et al., 1996). As activated microglia have the potential to produce a wide range of neurotoxic molecules, it was hoped that anti-inflammatory therapies might provide new targets for treating this disease (Launer, 2003;MacKenzie and Munoz, 1998;McGeer, Schulzer, and McGeer, 1996). Similarly, pronounced microglial responses were observed in the substantia nigra of PD patients, again establishing a neuroinflammatory component to this disease (Hirsch et al., 2003;McGeer, Itagaki, and McGeer, 1988;McGeer et al., 1988;Teismann et al., 2003). Since clinical trials with anti-inflammatory agents did not generally show effectiveness at slowing the progression of AD, other inflammatory therapeutic targets need to be considered. These targets can include enhancing the function of endogenous immune regulatory molecules. One such immunoregulatory system involves CD200 and CD200 receptor (CD200R). CD200 is a type-1 membrane glycoprotein of the immunoglobulin superfamily (IgSF) of cell surface proteins. It contains 2 IgSF domains and is expressed in a variety of lymphoid and non-lymphoid cells, including kidney glomeruli, vascular endothelium, and subsets of neurons. It was shown that CD200 was expressed by various populations of neurons in rodent brains (Barclay et al., 2002;Wright et al., 2001), but its neuroanatomy and biochemistry in human brain, and involvement in human neurodegenerative diseases such as AD, has not been studied extensively. CD200 (previously known as OX2) was studied for a number of years before being identified as the ligand for a myeloid cell receptor that became designated CD200 receptor (CD200R) (Wright et al., 2003;Wright et al., 2000). CD200R is a closely related molecule to CD200, also having two IgSF domains (Vieites et al., 2003), and is primarily expressed by myeloid cells (e.g. macrophages, neutrophils, monocytes and microglia) (Gorczynski et al., 2004;Voehringer et al., 2004;Vieites et al., 2003;Hatherley and Barclay, 2004). CD200R is a highly glycosylated protein with a molecular weight ranging from 60-110 kD depending on the degree of glycosylation and expressing cell type; four separate CD200R-related genes have been identified in humans (Wright et al., 2003).
There is a growing body of data on the significance of CD200/CD200R in modulating tissue inflammation in various inflammatory diseases (Barclay et al., 2002;Elward and Gasque, 2003;Gorczynski et al., 2002b;Gorczynski et al., 2002a). Their interaction is also involved in inducing immune tolerance, and the prevention of tissue rejection (Clark et al., 2003;Rosenblum et al., 2004). In addition, increased expression of CD200 has been demonstrated in a number of cancers (Kretz-Rommel et al., 2007;Moreaux et al., 2008;Siva et al., 2007). For example, increased expression of CD200 in melanoma cells correlated with their metastatic potential, similarly CD200 expression by multiple myelomas correlated with the survival outcome of patients (Petermann et al., 2007;Siva et al., 2007;Moreaux et al., 2006). Increased CD200 expression appears to enhance the ability of cancer cells to escape immunological removal. CD200 has many features of related cell adhesion molecules; however, there is no evidence that it can activate intracellular signaling pathways. It has been shown that an interaction between the extracellular domains of CD200 and CD200R is necessary for the activation of anti-inflammatory signals by CD200R (Chen and Gorczynski, 2005;Hatherley and Barclay, 2004). Activation of the ERK, JNK, and p38 mitogen activated protein kinase (MAPK) pathways was inhibited by CD200R engagement with CD200 (Zhang et al., 2004). It has been demonstrated that responsiveness to CD200 was dependent on the level of expression of CD200R (Jenmalm et al., 2006). Monocytic cell lines that expressed high, medium, low or very low amounts of CD200R were treated with CD200 and then challenged with interferon-γ (IFN-γ). The low and very low CD200R-expressing cells showed minimal inhibitory response to CD200, measured as reduction in secretion of interleukin (IL)-8, unless CD200R was further crosslinked by antibody, while cells expressing medium to high levels of CD200R showed significant inhibitory response to applied CD200 (Jenmalm et al., 2006).
The significance of CD200/CD200R interactions has been demonstrated from a number of animal studies. One study showed a decrease in CD200 mRNA expression in the hippocampus of rats with increasing age (Frank et al., 2005). Another study showed that mice lacking the CD200 gene had significantly greater numbers of activated monocytes/macrophages under constitutive conditions, and macrophage/microglial activation and inflammatory damage were exacerbated in these animals following injurious treatments (e.g. collagen-induced arthritis, facial nerve transaction, induction of experimental allergic encephalomyelitis (EAE)) (Hoek et al., 2000). Similarly, mice lacking CD200R1 expression showed enhanced tumor necrosis factor-α production in response to lipopolysaccharide, and a lack of ability by CD200 to suppress this inflammatory response (Boudakov et al., 2007). It was recently shown that the reduced susceptibility to EAE by the wld mutant mouse strain was due to enhanced neuronal expression of CD200, which aided in suppression of CNS inflammation (Chitnis et al., 2007). Blocking CD200 in wld mice with antibodies restored EAE pathology to control levels. In mice treated to develop experimental autoimmune uveoretinitis, both CD200 knockout mice and wild type mice treated with antibody to block CD200-CD200R interactions had enhanced inflammation and tissue damage compared to controls. By comparison, in the same model, wild type mice treated with CD200R agonist antibodies showed less tissue damage and inflammation (Banerjee and Dick, 2004;Copland et al., 2007). Interestingly, CD200 knockout mice that had been infected with toxoplasma in a murine model of Toxoplasma encephalitis were able to clear the parasite more effectively due to the stronger inflammatory response that occurred (Deckert et al., 2006). A recent study employing mRNA expression analysis of laser-dissected active and inactive multiple sclerosis lesions demonstrated downregulated expression of CD200 in both types of lesions, while CD200R expression was unaffected (Koning et al., 2007).
In this report, we compared the expression of CD200 and CD200R in brain tissues affected by AD and demonstrated a deficit of both. The expression patterns of CD200 were examined in human AD and ND brains in relation to AD pathology. We also demonstrated that CD200R expression by human microglia is significantly lower than by human macrophages, but that its expression in both cell types can be increased by the anti-inflammatory cytokines IL-4 and IL-13. Expression of both anti-inflammatory cytokines is generally lacking in human elderly brains.
Materials and Methods
Human Brain Tissues
Human brain tissue samples from non-demented (ND) and AD cases that had been diagnosed by a neuropathologist were obtained from the Sun Health Research Institute Brain Bank. Brain tissues were donated to the Sun Health Research Institute Brain and Body donation program with informed consent and the approval of the Sun Health Corporation Institutional Review Board (IRB). A total of 59 cases were used in this study; 28 of these cases were diagnosed as ND (10 female: 18 male with mean age 86.0 y). These cases were diagnosed as not meeting the pathological criteria of AD, but most had certain numbers of senile plaques and/or neurofibrillary tangles. There were 31 cases with a pathological diagnosis of AD (16 female: 15 male with mean age 84.4 y). For each case, a total plaque score and tangle score was obtained. These scores, on a scale of 0 to 15, represent estimations of the numbers of Thioflavin-S positive plaques or tangles in 5 brain regions (hippocampus, frontal cortex, temporal cortex, parietal cortex and entorhinal cortex) with each brain region being rated as 0, 1, 2, or 3. Included in these numbers were frontal and occipital cortex samples from 9 cases, which were solely used for microglia isolation. Each brain was processed in a standardized manner within 3.4 h of recorded death. Following removal from the skull, the brain was cut into 1-cm thick coronal slices. The coronal slices from the right hemisphere were frozen on dry ice and stored at -80°C, while those from the left hemisphere were fixed for 48 h in 4% paraformaldehyde. The fixed tissue was then cryoprotected and stored at -20°C in 30% glycol/30% glycerol solution (in 0.1 M Phosphate buffer, pH 7.3) until sectioned.
Tissue Processing for Biochemical and Histological Studies
Tissue from the hippocampus, inferior temporal gyrus and cerebellum from the right hemisphere of each case was used for preparation of protein extracts for western blot and for extraction of RNA. Fixed blocks containing hippocampus, superior frontal gyrus, inferior temporal gyrus and cerebellum were dissected from the appropriate coronal slices. From these, 20 μm tissue sections were cut using a freezing sliding microtome and stored at -20°C in the glycol/glycerol solution.
Antibody Reagents
A custom antibody to CD200 (designated A2522) was prepared by Anaspec Inc (San Jose, CA). Rabbits were immunized with the peptide LYWKRHRNQDREP (corresponding to amino acids 257 to 269 of CD200) conjugated to keyhole limpet hemocyanin. The antibody was used for immunohistochemistry at 1:20,000 dilution, and for western blot analyses at 1:10,000 dilution. In addition, some of the western blot studies were carried out using a goat polyclonal antibody to recombinant glycosylated full-length CD200 (R & D Systems, Minneapolis, MN: 1:1000). Additional antibodies to CD200 used in this studies were mouse monoclonals SP100 (Biospark, Mississauga, ON - 1:100), and MAB 2724 (4 μg/ml - R & D Systems), which were used for confirmatory purposes in immunohistochemistry studies. A custom antibody to CD200R was also prepared by Anaspec Inc. Rabbits were immunized with the peptide sequence SYTEKNNPLYDTTNKVK (amino acids 296-310 in the C-terminus region of CD200R1) conjugated to KLH. This sequence is not found in CD200R2, CD200R3 or CD200R4. The CD200R antibody (designated A2524) was used for western blot studies at 1:10,000 dilution. An antibody to NeuN (mouse monoclonal; Chemicon, Temecula, CA: 1:500) was used to identify neurons, an antibody to GFAP (rabbit polyclonal: DAKO: 1:5000) to identify activated astrocytes, antibody LN3 (mouse monoclonal; MP Biomedicals, Solon, OH: 1:750) that recognizes HLA-DR on activated microglia/macrophages, antibody 3D6 (mouse monoclonal; Elan Pharmaceuticals, South San Francisco, CA: 1:3000) that recognizes Aβ peptide (not amyloid precursor protein), and antibody AT8 (mouse monoclonal; Innogenetics, Alpharetta, GA: 1:5000) that recognizes phosphorylated serine on tau and identifies neurofibrillary tangles.
Immunohistochemistry Procedures for Antigen Localization
Single or double immunohistochemistry were carried out to localize antigens in tissue sections essentially as described in our previous publications (Lue et al., 2001). Immunolocalization of antigens was carried out primarily using nickel-enhanced diaminobenizidine (DAB), a substrate for horseradish peroxidase, to produce a black/purple reaction product. Dual-color immunohistochemistry used the same procedures with sections being reacted for the second time with DAB substrate without nickel to produce a brown reaction product. For confocal microscopy, tissue sections were processed in the same manner for antigen localization using the listed primary antibodies, but for fluorescent detection, sections were subsequently incubated with Alexa 488-conjugated or Alexa 568-conjugated anti-immunoglobulin species antibodies (Invitrogen, Carlsbad, CA). Following mounting, these brain sections were counterstained with 1% Sudan black in 70% ethanol solution (Schnell, Staines, and Wessendorf, 1999) to block autofluorescence, and coverslipped with fluorescent mounting media. Confocal microscopy was carried out using an Olympus IX70 microscope with argon and krypton lasers running Fluoview 2 software. Images were collected as three-dimensional scans using Z-series scanning.
Preparation of CD200 and CD200R Stably Transfected Cell Lines
For use in characterizing custom antibodies, HEK-293 cell lines that had been stably transfected with expression plasmids for CD200 (catalog number EX-Z2639-M01), CD200R1 (EX-T3432-M01) (both obtained from Genecopoeia Inc, Germantown, MD) or control vector were isolated. SH-SY5Y neuronal cells stably transfected with CD200 plasmid were also isolated.
Western Immunoblot Analyses
Protein extracts were prepared from brain or cultured cell samples by sonication in protein extraction buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 1% (w/v) sodium dodecyl sulfate (SDS) and protease inhibitors (Complete Mini - Roche Biochemicals, Indianapolis, IN). Equal amounts of protein extract were diluted in NuPAGE LDS sample buffer (Invitrogen, Carlsbad, CA) containing 0.1 M dithiothreitol (DTT), heated at 70°C for 10 min and separated on 4-12% NuPAGE Bis-Tris gels using MOPS running buffer. Proteins were transferred to nitrocellulose membranes according to the manufacturer's suggested procedures (Invitrogen). Following drying, membranes were blocked in 5% skim milk solution dissolved in Tris-buffered saline containing 0.05% Tween 20 (TBST-50 mM Tris-HCl (pH 8.0), 250 mM NaCl, 0.05% (w/v) Tween 20), and then reacted for 18 hours in appropriate dilutions of antibodies in TBST containing 2% milk. Following washes with TBST, bound antibody was detected by reaction for 2 h with the appropriate horseradish peroxidase (HRP) labeled anti-immunoglobulin (Pierce Chemical Company, Rockland, IL. at 1:10,000 dilution). Bound immune complexes were detected by reaction of membranes with HRP chemiluminescent substrate (Supersignal Dura, Pierce) and by imaging using a Fluorochem 8900 imaging system (Alpha Innotech, San Leandro, CA). Protein loading on brain samples was determined by staining of reacted membranes with 1% Ponceau S solution to measure total protein, or with an antibody to β-actin (mouse monoclonal: Sigma: 1:5000 dilution) for cell culture samples. Intensities of bands on chemiluminescence detected and stained membranes were quantified using Alpha-Ease FC software (Alpha Innotech).
RNA Isolation and cDNA Preparation
Frozen tissue samples (100-200 mg) or cell pellets were sonicated briefly in Trizol reagent (Invitrogen), mixed with 0.2 volumes of chloroform and centrifuged for 15 min at 10,000g. The clear layer was removed, precipitated with isopropanol, and recovered by centrifugation. The recovered RNA pellet was washed with 70% ethanol and dissolved in diethyl pyrocarbonate-treated water. The concentration and purity of each sample was determined using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE). The integrity of selected samples was confirmed using agarose gel electrophoresis. For cDNA preparation, up to 2 μg of each RNA sample was pretreated with TurboDNAse (1 unit; Ambion, Austin, TX) to remove contaminating DNA, and then reverse transcribed using the Superscript III reverse transcription kit (Invitrogen) employing random hexamer primers, according to the manufacturer's instructions. For some studies, RNA was purified using RNeasy plus purification kits (Qiagen, Valencia, CA) and reverse transcribed, without TurboDNase pretreatment, using QuantiTect reverse transcription kits (Qiagen) according to the manufacturer's instructions.
Reverse Transcription Polymerase Chain Reaction: Gel-Based and Real Time Detection
Samples were analyzed for gene expression by standard and real time polymerase chain reaction (PCR) techniques. For standard analysis, 1-2 μl of cDNA was mixed with 0.25 μM primers, 0.25 units of enzyme (Tempase, GeneChoice, MD) and amplified in 25 μl reactions using an Eppendorf thermal cycler. Primer sequences used for CD200R - sense sequence: CATCGTGGGATTCATTTGGT, and anti-sense sequence - CTCGTTTGTTGTTGTTTCTTGG. These primers amplified a cDNA fragment of 249 bp of the membrane forms of CD200R1. Primer sequences used for β-actin; sense sequence: TCCTATGTGGGCGACGAG, and anti-sense sequence; ATGGCTGGGGTGTTGAAG. These primers amplified a cDNA fragment of 242 bp. Primer sequences used for interleukin (IL)-4 - sense sequence: CTTCCCCCTCTGTTCTTCCT, and anti-sense sequence: GGCAGCGAGTGTCCTTCT. These primers amplified two transcript variants of IL-4 producing cDNA fragments of 199 bp and 247 bp.
The relative level of CD200 mRNA in each brain sample was measured by real-time PCR procedures using a Mx3000P (Stratagene, La Jolla, CA) real time PCR machine employing the gene specific predesigned Taqman Gene Expression assay (Applied Biosystems, Foster City, CA) for CD200 (Assay number Hs000245978_m1). Amplifications were run for 45 cycles and data collected and analyzed using Mx3000P software. The relative amounts of transcripts were calculated using the standard curve method. Standards were prepared from pooled cDNAs that were serially diluted fold over a 2-3 log concentration range. Expression levels for CD200 were normalized using 18S ribosomal RNA (18S RNA: Assay number Hs 99999901_s1) levels in each sample measured in the same manner. As the premade Taqman Gene Expression assays for CD200R did not show sufficient sensitivity, the relative levels of CD200R mRNA were measured by real-time PCR procedures using SYBR green detection, with expression levels being normalized using 18S ribosomal RNA levels for brain samples or β-actin mRNA levels for cell culture experiments. For SYBR green detection, 2 μl of cDNA were mixed with 2x SYBR green master mix (Superarray Bioscience, Frederick, MD) and each primer at 1 μM concentration. The relative amounts of transcript were calculated as described above. Samples were amplified for 45 cycles (95°C for 15 sec; 60°C for 30 sec). At the end of each run, a thermal denaturation profile was generated to verify the single identity of amplified DNA.
Tissue Fractionation Procedures
To prepare different fractions from brain tissue, samples of gray matter from inferior temporal gyrus of 2 AD cases and 2 ND cases were processed as follows. Tissue samples (around 100 mg) were sonicated in 4 volumes of TE buffer (10 mM Tris, pH 7.5, 1 mM EDTA containing protease inhibitors). Tissue extracts were centrifuged (18,000g/30 min at 4°C), and the soluble fractions separated from the insoluble pellets. The pellets were extracted by brief sonication in 4 volumes of TE containing 1% Triton X100 and protease inhibitors, and then centrifuged as described above. The soluble portions, which represent the membrane-soluble fractions, were separated from the pellets. The pellets were then extracted with TE containing 1% SDS. All samples were adjusted to contain equal amounts of proteins and then analyzed by western immunoblot procedures.
Isolation and Culture of Human Brain Microglia and Human Blood Macrophages
Microglia were isolated from samples of frontal and occipital cortex from postmortem elderly human brains, using our published procedures. (Walker et al., 2006) Microglia were cultured for 12-14 d prior to subculture and use in experiments. For comparison, human macrophages were cultured from donated human blood samples that had been collected with IRB approval. Leukocytes were purified from whole blood by centrifugation through Histopaque gradients (Sigma). Banded white blood cells were recovered, washed twice by centrifugation and plated in T-75 flasks at 80 × 106 white blood cells/flask using RPMI 1640 media containing 1% fetal bovine serum (FBS) and 1% human AB serum. Macrophages develop from monocytes and attach to the flask over a 1-week culture period; during this time the non-adherent lymphocytes are not removed. To prepare macrophages for experiments, cultures were rinsed twice with Hanks Balanced Salt Solution (HBSS) to remove non-adherent cells, treated with 0.25% trypsin and subcultured (105 cells/well) in 12-well culture plates. As a model for human neurons, we used differentiated neuronal cells derived from the SH-SY5Y neuroblastoma cell line. To produce them, undifferentiated neuroblastoma cells were plated (2× 105 cells/well) in collagen-I coated culture plate wells in DMEM/F12 media containing 10% FBS, then cells were treated with 10-5 M retinoic acid (Sigma) for 1 week then with 50 ng/ml brain-derived neurotrophic factor (BDNF) (Peprotech, Rocky Hill, NJ) for 1 week. For gene expression experiments, macrophages or microglia were stimulated with IL-4, IL-13, IL-12, IFN-γ (all from Peprotech), IL-1β or IL-18 (R and D Systems) at the indicated concentrations in serum-free media for 24 hours.
Statistical Analysis
Results for quantitative PCR and western blots for brain samples are expressed as relative units (mean ± standard error of mean for each disease group). Results for cell culture experiments are expressed as relative units (mean ± standard error of mean of 3-4 replicate samples per treatment); each experiment was carried out at least 3 times. Statistical analysis between two groups (for example AD versus ND) was performed by the student's t test using GraphPad Prism 4 software (San Diego, CA). Statistical analysis between more than two groups (cell culture experiments) was carried out by one way Analysis of Variance using GraphPad Prism 4 and the appropriate post-hoc test of significance. Pearson correlation analysis was performed using GraphPad Prism 4 to determine if there was significant correlation between CD200 and CD200R mRNA levels in hippocampus or inferior temporal gyrus and brain plaque or tangle scores. Significance between groups or between treatments was assumed if P < 0.05 was obtained.
Results
Immunolocalization of CD200 in Human Elderly Brain
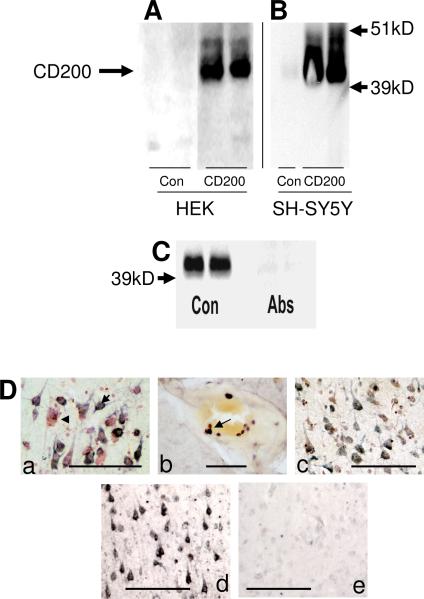
The aim of this study was to characterize CD200 and CD200 receptor expression in elderly human brains, and to demonstrate whether there was a deficit in AD of one or both. Studies on CD200 expression in human brain have not previously been reported except to demonstrate neuronal expression in cerebellum, a brain region not affected by AD (Wright et al., 2001). Most of our studies at the protein level on human brain tissues (immunohistochemistry and immunoblot) were carried out using a custom antibody (A2522) prepared against a C-terminal peptide sequence of CD200. To confirm specificity of this antibody, HEK cells stably transfected with a CD200 expression vector (or control vector) were prepared. Fig. 1A shows that this antibody detected a protein band of approximately 43 kD, the identified molecular weight for CD200, that was only detectable in CD200-transfected cells. For comparison, we also characterized the specificity of a commercial anti-CD200 antibody prepared against extracellular glycosylated recombinant CD200. This antibody, used to probe blots of control and CD200-transfected SH-SY5Y neuronal cells (Fig. 1B), also recognized an abundant protein of 43 kD in the CD200-transfected SH-SY5Y cells. A faint band of the same molecular weight was also detectable in untransfected SH-SY5Y cells, consistent with the protein being expressed by neuronal cells. Western blots of brain extracts from inferior temporal gyrus were reacted with CD200 antibody A2522 preabsorbed with bovine serum albumin (Con) or antibody preabsorbed with an excess of immunizing peptide (Abs) (Fig. 1C). Similar to Fig. 1A and 1B, a band of approximately 43 kD was identified in the brain samples, and this band was not present on matching blots probed with peptide-absorbed antibody (Fig. 1C). Staining fixed tissue sections of human brains using antibody A2522 showed that CD200 was primarily localized to neurons (Fig. 1D-a), but this panel shows that not all neurons stained with equal intensity. This antibody also stained round cells (presumptive lymphocytes) in blood vessels (Fig. 1D-b). As antibody A2522 produced the most robust staining on fixed tissue sections, most immunohistochemistry studies in this report were carried out using this antibody, though the same pattern of neuronal immunostaining was confirmed with monoclonal antibodies MAB2724 (Fig. 1D-c) and SP14 (not shown). The specificity of the immunostaining with antibody A2522 was demonstrated by comparing sections reacted with non-absorbed antibody (Fig. 1D-d), which showed neuronal staining, with sections reacted with antibody that had been preabsorbed with CD200 immunizing peptide, which showed that the neuronal staining pattern was almost completely abolished (Fig. 1D-e).
Figure 1.
Characterization of antibodies to CD200 in cell cultures and brain. A) Western immunoblot of control transfected (HEK-Con) and CD200 transfected (HEK-CD200) protein extracts reacted with rabbit custom antibody to CD200. B) Western immunoblot of control or CD200 transfected SH-SY5Y cells, and control and CD200 transfected HEK cells probed with goat polyclonal antibody to recombinant CD200. C) Immunoblot of control human brain protein extract showing absorption of specific reactivity (Abs) when CD200 antibody (1:10,000 dilution) was preabsorbed with 250 μg of immunizing peptide. CD200 specific band (43 kD) present in sample (Con) reacted with unabsorbed custom rabbit CD200 antibody. D). a) Immunohistochemical staining of section of hippocampus with antibody A2522 to CD200. Section was counterstained with neutral red and show heterogeneous neuronal staining. Arrow indicates CD200 reactive neurons, while arrowhead indicates a neuron with no CD200 immunoreactivity. b). Immunohistochemical staining of subset of round cells with antibody A2522 to CD200 (presumptive lymphocytes) in vessel of AD brain section. c) Immunohistochemical staining of hippocampus with monoclonal antibody MAB2724 showing similar neuronal staining pattern as antibody A2522 to CD200. d) Staining of section of inferior temporal gyrus with antibody A2522 preabsorbed with control peptide. e) Staining of parallel section of inferior temporal gyrus with antibody A2522 preabsorbed with CD200 immunizing peptide. Absorption of CD200 antibody A2522 with immunizing peptide removes neuronal staining. Scale bars represent 10 micron.
Cellular Localization of CD200 in Human Brain Tissues
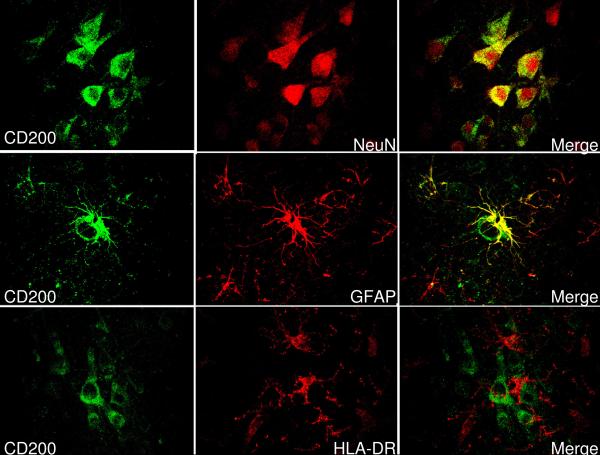
Confocal microscopy was performed with double-labeled tissue sections to identify cell types that were immunoreactive for CD200. Sections from inferior temporal gyrus from ND cases were stained with A2522 to localize CD200 (green color- Fig. 2). Parallel sections were also stained with an antibody to NeuN to identify neurons, with an antibody to GFAP to identify astrocytes, and with antibody LN3 to identify microglia (red color - Fig. 2). Merging of the respective confocal images showed that most CD200 staining colocalized with NeuN staining, confirming neuronal expression (Fig. 2 top row: CD200:NeuN:merge). Some highly reactive astrocytes closely opposed to a CD200 positive neuron also strongly stained for CD200 (Fig. 2 middle row: CD200:GFAP:merge). CD200 staining did not colocalize with staining for microglia, identified with LN3 antibody to HLA-DR (Fig. 2 bottom row: CD200:HLA-DR:merge). Contrary to other reports, we could not observe CD200 immunoreactivity colocalizing with CD31-positive endothelial cells in blood vessels (data not shown).
Figure 2.
Confocal micrographs showing colocalization of CD200 immunoreactivity with cell type markers. Left hand panels (green) represent sections of inferior temporal gyrus from an ND case stained with antibody to identify CD200. Top panels show that most CD200 immunoreactivity colocalizes with cells demonstrating reactivity to neuronal marker NeuN (red)(see merge). Middle panels show that CD200 immunoreactivity (green) colocalizes with some GFAP positive astrocytes (red) (see merge). Lower panels show that CD200 immunoreactivity (green) does not colocalize with HLA-DR immunoreactive (red) microglia, though there is a close association between some of these cells.
Relative Expression of CD200 Protein and mRNA in Alzheimer's Disease Brains
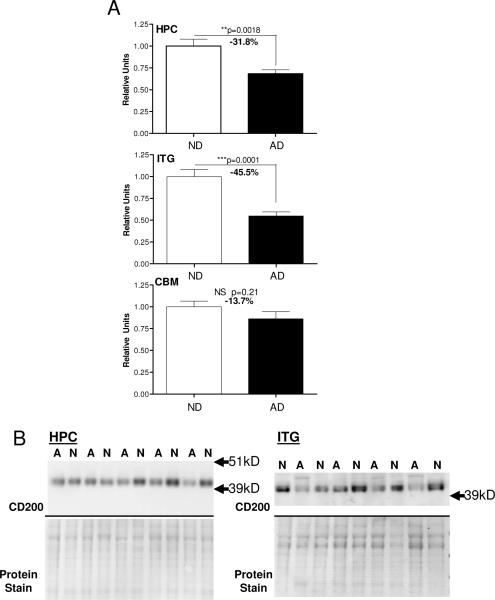
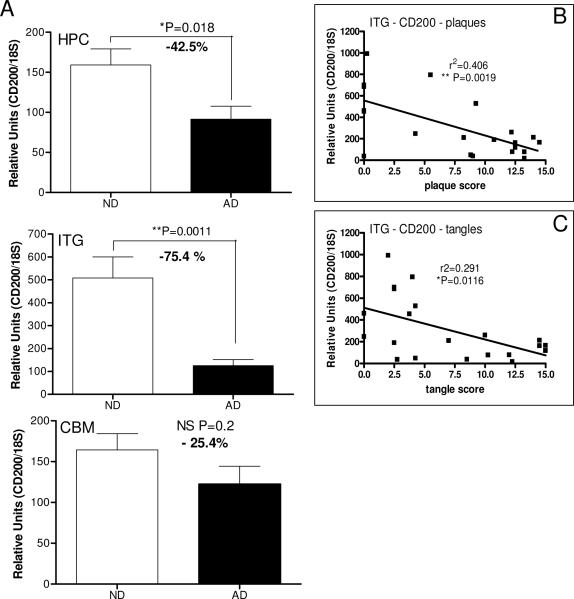
Using samples prepared from hippocampus, inferior temporal gyrus (gray matter) and cerebellum from AD and ND cases, measurements were made of CD200 protein by western immunoblot analyses (Fig. 3). Results demonstrated that there was significantly less CD200 protein in the hippocampus and inferior temporal gyrus from the neuropathologically confirmed AD cases, brain regions that show significant AD pathology, but there was no difference in levels in cerebellum, a brain region generally spared AD pathology. These findings were confirmed at the RNA level (Fig. 4); real time PCR analyses demonstrated a significant decrease in CD200 mRNA levels in samples from hippocampus and inferior temporal gyrus from AD cases compared to ND cases, but not in cerebellum samples (Fig. 4). Correlation analyses were carried out to determine whether CD200 mRNA expression levels correlated with total plaque or tangle scores. There was a significant negative correlation between CD200 mRNA levels and plaque score (r2 = 0.406; P=0.0019) (Fig. 4 - ITG-plaque) or tangle score (r2 = 0.291; P= 0.0116) (Fig. 4 - ITG-tangle) for inferior temporal gyrus samples, but not for hippocampus samples. These correlations are not strong and only reach significance using the Pearson correlation analysis, but not using the Spearman correlation analysis, which assumes non-parametric distribution. Although there appears to be correlation between CD200 expression and these AD hallmark features, other factors could be involved in levels of CD200 mRNA expression.
Figure 3.
Decreased CD200 protein in AD brains in regions with AD pathology. A: Relative levels of CD200 protein in hippocampus (HPC) (n=23 cases; ND - 11 cases; AD - 12 cases), inferior temporal gyrus (ITG) (n-21 cases; ND - 10 cases; AD - 11 cases) and cerebellum (CBM) (n-21 cases; ND - 10 cases; AD - 11 cases) in tissue samples from non-demented (ND) and Alzheimer's disease (AD) cases. Results were normalized for total amount of protein present in each lane as measured by densitometry of Ponceau-S stained membranes. B: Western blots of CD200 and corresponding stained membranes show representative results for some of the samples from hippocampus and inferior temporal gyrus. (lane designation: A; AD case; N; ND case).
Figure 4.
Relative expression of CD200 mRNA in hippocampus (HPC) (n=20 cases; ND - 9 cases; AD - 11 cases), inferior temporal gyrus (ITG) (n-21 cases; ND - 10 cases; AD - 11 cases) and cerebellum (CBM) (n-22 cases; ND - 10 cases; AD - 12 cases) in tissue samples from non-demented (ND) and Alzheimer's disease (AD) cases. Levels of CD200 mRNA were measured by real time PCR with expression levels normalized for 18S RNA. Linear regression analysis indicated that there was a significant negative correlation between ITG mRNA levels for CD200 and total plaque score (ITG-plaque)(P=0.0019)(n=21 cases), and between ITG mRNA CD200 mRNA levels and total tangle scores (ITG-tangle)(P=0.0116)(n = 21 cases).
CD200 Immunolocalization in ND and AD Brains
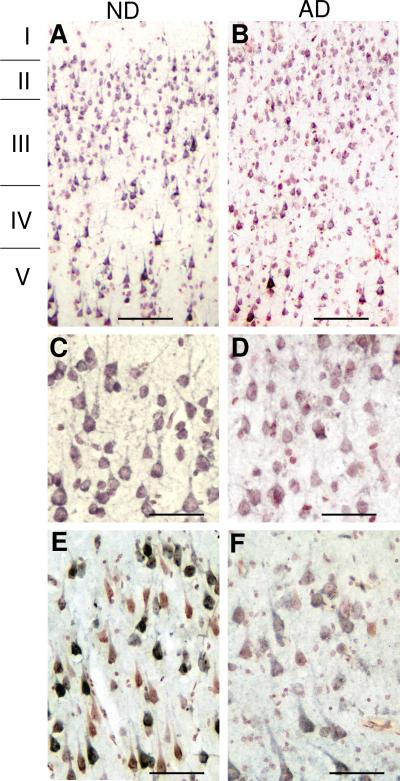
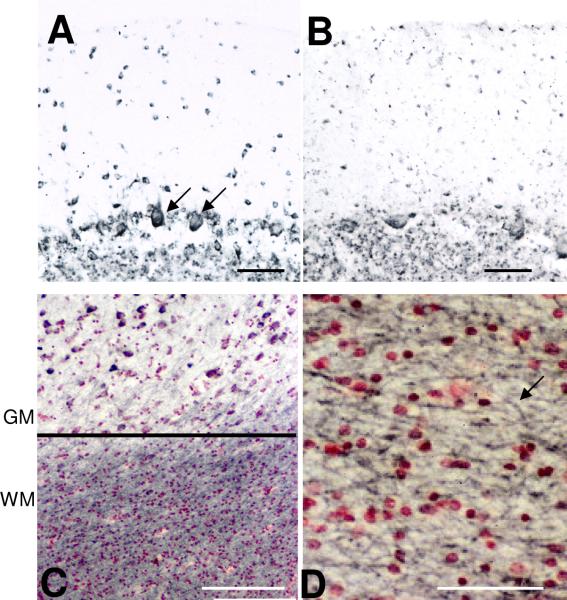
We examined the distribution of CD200 immunoreactivity using antibody A2522 in the hippocampus and inferior temporal gyrus of a series of ND and AD cases to determine whether there was evidence for lower levels of neuronal staining in the AD cases. The results presented are representative of most cases examined. Low and higher power micrographs of inferior temporal gyrus (Fig. 5 - A and C (ND) and B and D (AD)) demonstrate a weaker intensity of neuronal staining for CD200 in AD cases, though a distinct pattern of staining was apparent in different cortical layers. Both AD and ND sections showed strong CD200 staining in larger pyramidal neurons in layer IV and V. A similar observation can be seen in the cornu Ammonus (CA) 1-2 subdivision of hippocampus of an AD and ND case (Fig. 5 E and F). In agreement with an earlier study (Wright et al., 2001), CD200 expression was detectable in Purkinje neurons, both in an ND case (Fig. 6A) and in an AD case (Fig. 6B). CD200 immunoreactivity was also present in the molecular layer and granular layer of this brain region. Strong CD200 staining was present in white matter of both AD and ND sections (Fig. 6C). Higher magnification of the white matter demonstrated strong CD200 staining on myelinated fibers (Fig. 6D). This indicates that CD200 could also have a role in protecting oligodendrocytes and myelinated fibers from inflammation. (Koning et al., 2007).
Figure 5.
Immunohistochemistry of ND and AD brain sections demonstrating distribution of neuronal immunoreactivity of CD200. A and B: Lower power magnifications of inferior temporal gyrus showing distribution of staining of CD200 in ND (A) and AD (B) section through cortical layers I to V. Scale bars represent 20 micron. C and D: Higher power magnifications of corresponding inferior temporal gyrus sections. Scale bars represent 10 micron. E and F: CD200 immunoreactivity in hippocampus sections (CA1-2) of ND and AD case showing heterogeneous distribution of immunoreactivity. Scale bars represent 10 micron.
Figure 6.
A and B Figures demonstrate similar immunoreactivity of CD200 in cerebellum of ND (A) and AD (B). Positive staining is observed in cells in molecular and granular layers, as well as Purkinje neurons. C: Strong immunoreactivity for CD200 in white matter (WM) region of inferior temporal cortex compared to gray matter (GM). D: Higher magnification shows CD200 staining of fibers of white matter. Scale bars represent 10 micron (panels A, B, and D) and 20 micron (panel C).
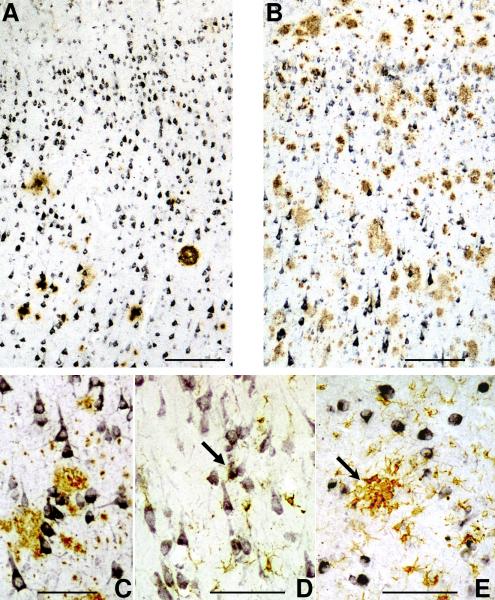
Double immunohistochemistry was employed to determine the localization between CD200 immunoreactive structures and Aβ plaques or activated microglia. Although we had shown (Fig. 5) that there was an overall decrease of CD200 immunoreactivity in neurons in AD inferior temporal gyrus, in sections that were double stained to show CD200 (purple) and Aβ plaques (brown), expression of CD200 in larger pyramidal neurons in layers IV and V (Fig. 7B-ND and 7C-AD) did not appear to be directly affected by the presence of plaques. The corresponding low plaque ND section (Fig. 7A) also had strongly expressing pyramidal neurons in layers IV and V. In hippocampal sections from AD cases, double staining for CD200 and HLA-DR showed that highly activated microglia clusters did not appear to be associated with CD200 positive neurons (Fig. 7E). In ND sections, some HLA-DR positive microglia with a more ramified, less-activated, morphology, were closely associated with neurons (Fig. 7D).
Figure 7.
A-C: Relationship of CD200 stained neurons with Aβ plaques. Inferior temporal gyrus sections from A) ND and B) AD double stained for CD200 (purple) and Aβ (brown). Both sections show layers IV and V. In panel B, neuronal staining for CD200 was not reduced in pyramidal neurons closely opposed to Aβ plaques, shown at higher magnification in panel C. Scale bars represent 20 micron in panels A and B and 10 micron in panel C.
D and E: Sections of hippocampus from ND (D) and AD (E) showing double staining for CD200 (purple) and HLA-DR as a marker for activated microglia (brown). (D) Weakly stained, less reactive microglia closely associated with CD200 immunoreactive neurons in ND case (arrow), but in AD case (E), strongly reactive microglial clusters (arrow) is not associated with CD200 immunoreactive neurons. Scale bars represent 10 micron.
Biochemical Localization of CD200 in Brain Samples
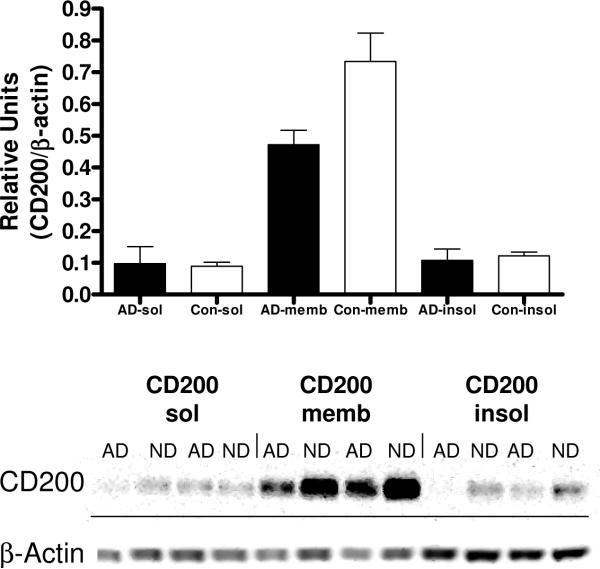
To address whether there was a soluble form of CD200 in brain, and whether the majority of CD200 was localized in cellular membranes, fractionation of brain samples (2 samples from AD brains; 2 samples from ND brains) was carried out. Western blot analyses of the buffer soluble fractions (sol), membrane fractions (memb) (protein extractable using non-ionic detergent Triton X-100) and SDS-extractable (insol) fractions showed that the majority of CD200 was extracted in the Triton fraction (Fig. 8), indicating a membrane location. Some of the CD200 protein could be detected in the soluble fraction; however, as this protein had the same molecular size as the membrane form, this can not be taken as evidence for a soluble form of CD200. To date, there is no evidence for the existence of alternative transcripts of CD200 lacking the transmembrane domain; however, it has been observed that CD200 can be readily shed from the surface of blood cells upon storage (Clark and Chaouat, 2005).
Figure 8.
Fractionation of brain tissue samples (inferior temporal gyrus) into soluble (sol), Triton X100 extractable (memb), and SDS extractable (insol) fractions demonstrate majority of CD200 localized to Triton X100 extractable membrane fraction. Equal amounts of protein in each fraction was analyzed by western blot for CD200 and normalized for β-actin.
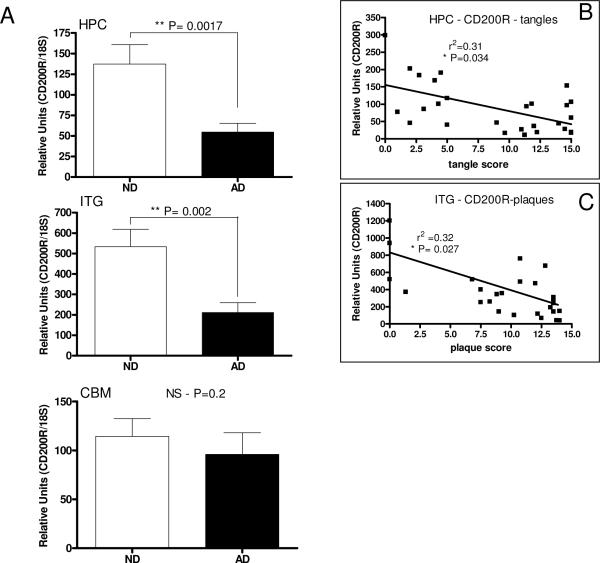
Relative Expression of CD200R mRNA in Alzheimer's Disease Brains
Using cDNA samples prepared from hippocampus, inferior temporal gyrus (gray matter) and cerebellum from AD and ND cases, measurements were made of CD200R mRNA by real time PCR (Fig. 9). Results demonstrated that there was significantly less CD200R mRNA in hippocampus and inferior temporal gyrus samples from AD cases, but not in cerebellum samples. There was a weak but significant negative correlation between values for CD200R mRNA in hippocampus and the total tangle score of the brain (r2 = 0.31; P= 0.0342), and a negative correlation between inferior temporal gyrus values for CD200R mRNA and total plaque score (r2 = 0.324; P=0.027) using Pearson correlation analysis, but not if these parameters were reversed.
Figure 9.
Decreased expression of CD200R mRNA in human brains affected by AD pathology. A: Real time PCR analyses of CD200R mRNA expression in brain samples hippocampus (HPC) (n=27 cases; ND - 11 cases; AD - 16 cases), inferior temporal gyrus (ITG) (n-27 cases; ND - 13 cases; AD - 14 cases) and cerebellum (CBM) (n-22 cases; ND - 10 cases; AD - 10 cases) in tissue samples from non-demented (ND) and Alzheimer's disease (AD) cases showed significant decrease in expression in HPC and ITG, brain regions affected by AD pathology, but not in CBM, a brain region usually spared significant pathology. Regression analysis demonstrated a significant negative correlation between HPC levels of CD200R and total brain tangle score (B) (P=0.034), and between ITG levels of CD200R and total brain plaque score (C) (P=0.027) (n=27 cases).
Expression of CD200R in Human Neural-Derived Cells and Blood Macrophages
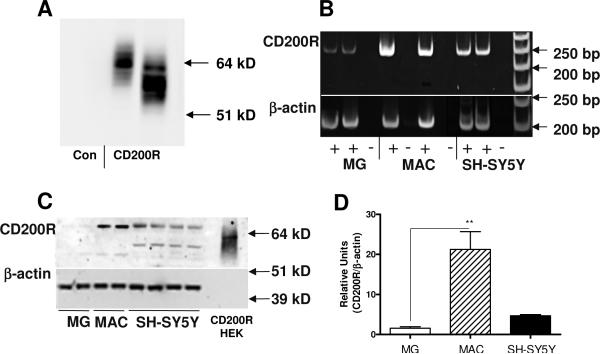
To characterize CD200R protein expression in cells, we utilized our custom prepared CD200R antibody, which strongly detected CD200R protein in HEK cells transfected with a CD200R expression plasmid, but not in control transfected cells (Fig. 10A). The molecular weight of CD200R varied in different CD200R-expressing isolates (Fig. 10A), likely due to differing amounts of protein glycosylation. Analyses of human postmortem brain-derived microglia and human blood-derived macrophages for CD200R expression demonstrated that macrophages expressed significantly higher amounts of CD200R mRNA (Fig. 10B) and CD200R protein (Fig. 10 C and D) than microglia. From these studies, we surprisingly demonstrated that neuronal cells derived from the SH-SY5Y neuroblastoma cell line expressed CD200R mRNA (Fig. 10B) and protein (Fig. 10 C and D) at higher levels than microglia. The molecular weight of CD200R protein expressed by microglia, macrophages and neuronal cells was approximately 68 kD. Expression of CD200R mRNA by neuronal cells derived from the LAN5 neuroblastoma cell line was also observed (data not shown). This is the first demonstration of potential neuronal expression of CD200R; its expression is generally considered to be restricted to myeloid cells, though a recent study demonstrated CD200R immunostaining of astrocytes and oligodendrocytes in mice (Chitnis et al., 2007).
Figure 10.
Human macrophages express significantly greater amounts of CD200R mRNA and protein than human microglia. A: Characterization of custom made antibody to CD200R (A2524) using HEK cell line transfected with control vector (Con) or CD200R expression plasmids. Antibody recognized band only in CD200R plasmid transfected cells. Molecular weight of CD200R varied between different HEK isolates. B: CD200R mRNA is expressed by microglia (MG), macrophages (MAC) and differentiated SH-SY5Y neuronal cells. Expression of CD200R mRNA by microglia is noticeably less than by macrophages. C: Detection of CD200R protein with molecular weight of 66-68 kD by western blot in extracts of human microglia (MG), macrophages (MAC), and SH-SY5Y neuronal cells, and in CD200R transfected HEK cells (CD200R-HEK - at 1% of protein level compared to other cells). Protein bands were detected with custom antibody to CD200R (A2524). D: Quantitative measurements of western immunoblots showed that macrophages express significantly greater amounts of CD200R protein than human microglia.
Regulation of CD200R Expression
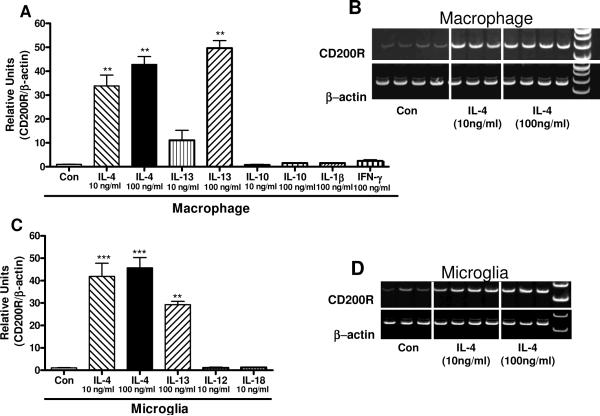
Augmenting expression of CD200R by microglia or macrophages without promoting proinflammatory activation could increase the anti-inflammatory signaling mediated by CD200R. Treatment of macrophages (Fig. 11A) or microglia (Fig. 11C) with the anti-inflammatory cytokines IL-4 and IL-13 resulted in significantly increased expression of CD200R mRNA. Measurements were made by real time PCR (Fig. 11A and 11C), but the corresponding PCR gel images for IL-4 treatments also demonstrate strong induction of CD200R mRNA expression (Fig. 11 B and D). By comparison, treatment of macrophages with the anti-inflammatory cytokine IL-10, or the proinflammatory cytokines IL-1β, TNF-α or IFN-γ, and treatment of microglia with the proinflammatory cytokines IL-12 or IL-18 (Fig. 11D) did not increase CD200R mRNA expression.
Figure 11.
Induction of CD200R mRNA expression by interleukin-4 and interleukin-13 in human macrophages and microglia. A: Bar chart showing results of real time PCR analyses for CD200R mRNA expression in human macrophages treated with 10 ng/ml and 100 ng/ml of IL-4, IL-13 and IL-10, and 100 ng/ml of IL-1β and IFN-γ for 24 hours. CD200R expression values were normalized for expression of β-actin mRNA. Results show significant induction of CD200R mRNA in a dose response manner for IL-4 and IL-13, but not IL-10, IL-1β or IFN-γ. B: Representative gel image showing induction of CD200R expression by macrophages with IL-4 treatment. C: Bar chart showing results of real time PCR analyses for CD200R mRNA expression in human microglia treated with 10 ng/ml and 100 ng/ml of IL-4, 100 ng/ml IL-13, and 10 ng/ml of IL-12 and IL-18 for 24 hours. CD200R expression values were normalized for expression of β-actin mRNA. Results show significant induction of CD200R mRNA expression with IL-4 and IL-13 treatments. D: Representative gel image showing induction of CD200R expression by human microglia with IL-4 treatment. (***; P <0.001; ** P< 0.01 as compared to control groups).
IL-4 mRNA expression in brain
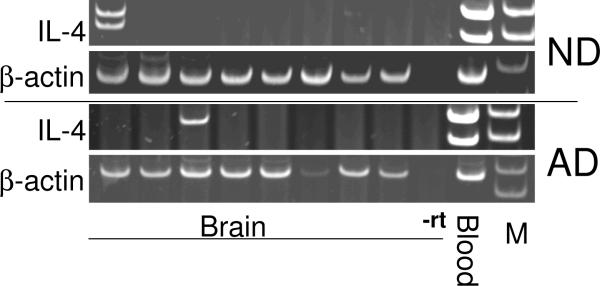
To assess whether there was a difference in IL-4 mRNA expression between brain and blood accounted for the difference in expression in CD200R between blood macrophages and brain microglia, PCR analyses of cDNA samples derived from ITG of ND and AD brain samples, and cDNA samples from blood-purified white blood cells was carried out for IL-4 mRNA expression. Data show that in elderly brain (ND or AD), IL-4 expression was not generally detectable (2 out of 16 samples) in brain, but could readily be detected in white blood cells (Fig. 12). Both transcript variants of IL-4 were detected in blood-derived cells but not in brain (Fig. 12). As there was no difference in expression in IL-4 mRNA between AD and ND samples, it is not possible to conclude whether there is a decline in expression associated with age, as suggested in other studies (Nolan et al., 2005), or with disease. The almost complete absence of IL-4 mRNA in elderly brain suggests that this cytokine is not present to induce expression of CD200R or CD200 in brain. We could not detect expression of IL-13 in brain, or in blood-derived cells under non-stimulated conditions (data not shown).
Figure 12.
Comparison of expression of interleukin-4 mRNA expression in AD and ND inferior temporal gyrus samples compared to blood cells. PCR analyses demonstrated that IL-4 mRNA expression was not detectable in most of the ND and AD samples tested (n = 8 in each group). Levels of expression in the positive expressing samples were low compared to sample derived from white blood cells (Blood) (representative image shown), which demonstrated strong expression of both transcript variants of IL-4. IL-4 PCR was carried out for 35 cycles; β-actin PCR was carried out for 24 cycles. (-rt: shows one representative brain sample that was processed without reverse transcriptase).
Discussion
In this report, we have focused on CD200 and CD200R expression in elderly human AD and ND brains, and also studied expression of CD200R in vitro in human neural-derived and macrophage cells. There have been many publications on potential roles for microglia and inflammation in AD pathogenesis (recent reviews (Craft, Watterson, and Van Eldik, 2005;Pereira et al., 2005)), but studies of cellular anti-inflammatory systems in the brain are more limited. These systems include CD200/CD200R, CD47/CD172a and CD22/CD45 (Barclay et al., 2002;Neumann, 2001;Mott et al., 2004;Griffiths, Neal, and Gasque, 2007). In this study, we have demonstrated that there is a deficit of not only CD200 but also CD200R in AD brains. From our studies, based on detection of mRNA by PCR or protein by western blot or immunohistochemistry, it is appears that CD200 is abundant in brain, even in cases that have reduced levels of expression, while CD200R appears to be present at lower levels.
There have been a number of studies of CD200 and CD200R in animal models of various diseases (Banerjee and Dick, 2004;Boudakov et al., 2007;Chitnis et al., 2007;Deckert et al., 2006;Gorczynski et al., 2001;Hoek et al., 2000;Rijkers et al., 2008), but few studies of these molecules in human brains (Koning et al., 2007;Wright et al., 2001). We show that there is a deficit in neuronal expression of CD200 in brain regions affected by AD pathology using quantitative measurements of protein and mRNA in brain extracts, as well as descriptively in antibody stained tissue sections. There was clearly a heterogeneous expression of CD200 by neurons with some having strong immunoreactivity, while others showing little staining. This is in contrast to animal studies where neuronal staining appeared to be ubiquitous (Lyons et al., 2007;Chitnis et al., 2007). Most immunohistochemistry studies were carried out with our custom antibody A2522 as it produced the most robust staining, but these heterogeneous neuronal staining patterns were confirmed with two commercially available monoclonal antibodies. It was observed that there was less immunoreactivity in neurons in AD brains (Fig. 5) in the hippocampal formation and inferior temporal gyrus (Fig. 5). Confocal microscopy showed CD200 expression primarily colocalized with neurons, though some reactive astrocytes were also immunoreactive. In this study, we did not detect CD200 staining of microglia or vascular endothelial cells. Protein fractionation studies indicated that the majority of CD200 protein in human inferior temporal gyrus gray matter was in the membrane fraction as it was extractable by non-ionic detergent treatment. The difference observed in total CD200 protein levels between ND and AD brains was confirmed in analyses of membrane fractions. We could not show the presence of a secreted C-terminal truncated form of CD200 in brain. Secreted forms of other members of the immunoglobulin superfamily have been identified, including receptor for advanced glycation endproducts (RAGE), intercellular adhesion molecule-1 (ICAM-1) and platelet cell adhesion molecule-1 (PECAM-1), all of which have significant immunoregulatory properties (Hudson et al., 2007;Cheng et al., 2005;Postadzhiyan et al., 2008;Figueras-Aloy et al., 2007;Kalinowska and Losy, 2006).
Correlation analyses between brain plaque and tangle scores and expression levels of CD200 mRNA expression showed weak but significant negative correlation between these parameters for the inferior temporal gyrus but not for the hippocampus. This correlation was significant using Pearson correlation analysis, but not for Spearman non-parametric analysis. These analyses may be suggestive, but not proof, that CD200 expression is decreasing as AD pathology develops but the mechanism is unclear. We also detected a weak negative correlation in the inferior temporal gyrus between CD200 protein levels and plaque score (data not shown). A larger series of brain cases will be analyzed using a CD200 ELISA to confirm whether this association holds at the protein level. The lack of association of AD pathology in the hippocampus and CD200 expression indicates that other factors are affecting its expression in this brain region. As CD200 expression is not restricted to neurons, with expression being detected in white matter tracts and certain astrocytes, it is likely that hippocampus samples are more heterogeneous than the inferior temporal gyrus samples, which can be more easily dissected to contain gray matter regions across all cortex layers. The hippocampus samples are cut from frozen blocks to include parts of corpus ammonus (CA) 1 and CA2 regions, but will include stratum radiatum.
Contrary to what was expected, expression of CD200R mRNA was also decreased in AD in brain regions affected by AD pathology compared with matched ND cases. It had been expected that expression would be increased in response to proinflammatory stimuli (Deckert et al., 2006), although in other studies, where a strong inflammatory response had occurred to tissue injury, CD200R expression was not upregulated (Chitnis et al., 2007;Koning et al., 2007). For example, in active and inactive MS lesions, CD200R mRNA expression was shown to be unaltered compared to unaffected white matter (Koning et al., 2007). Our data demonstrated weak but significant negative correlation between CD200R mRNA levels in the hippocampus and tangle scores, and between CD200R mRNA levels in inferior temporal gyurus and brain plaque scores using Pearson correlation analysis. CD200R expression by human microglia isolated from postmortem brains was significantly lower than by blood-derived macrophages. CD200R expression was also detected by human neuronal-like cells derived from the SH-SY5Y neuroblastoma cell line. This is the first indication that CD200R might be expressed by neurons. Although we have not been able to detect CD200R protein in microglia in human brain tissue sections by immunohistochemistry using our custom CD200R antibody or five additional commercial CD200R antibodies, preliminary findings with our peptide CD200R antibody and one commercial CD200R antibody have demonstrated potential neuronal CD200R immunoreactivity (unpublished data).
From these findings we can suggest that a deficiency of CD200 in AD brain could contribute to maintaining chronic inflammation, as has been hypothesized from animal studies, but also that a deficiency in CD200R might contribute to reduce efficiency of the CD200-CD200R system to control inflammation. These data suggest that CD200-CD200R interactions in elderly brains might not be functioning effectively due a combination of these two factors, but animal models are needed to prove this mechanism. It has been shown that inhibitory response to CD200 is dependent on the level of expression of CD200R in responding cells (Jenmalm et al., 2006). Increasing expression of CD200 and CD200R therapeutically may provide a method of enhancing the efficiency of the system. It has been reported that the cytokine IL-4 can increase CD200 expression by cultured neurons from mice, and that IL-4 knockout mice had lower levels of CD200 (Lyons et al., 2007). In this study, we showed that CD200R mRNA expression was strongly increased in microglia and macrophages by IL-4 treatment in a dose-dependent manner. We also showed that IL-13 treatment of microglia and macrophage had a similar stimulation of CD200R mRNA expression. These two cytokines are known to share common receptors, and both lead to activation of the signal transducer and activator of transcription (STAT)-6 transcription factor (Hebenstreit et al., 2006). Although IL-4 has a distinct role in promoting the development of Th2 T-helper cells, it has multiple anti-inflammatory effects on different immune cells; IL-13 shares many of the properties of IL-4. The feasibility of using STAT6 activation in a therapeutic manner to increase CD200R expression is unclear at present as IL-4 mediated STAT6 activation has been associated with disease processes (Kaplan et al., 2007;Nguyen et al., 2007). A significant age-related decline of IL-4 has been observed in the hippocampus of rats between 4 months and 22 months, which correlated with increased levels of the proinflammatory IL-1β and decreased levels of pSTAT6 activation (Nolan et al., 2005). This study also demonstrated that IL-4 used in a therapeutic manner could reverse the age-associated reduction in longterm potentiation. In human elderly brain, we showed that IL-4 mRNA expression was undetectable in most of the brain samples examined, with no apparent difference between ND and AD cases. These findings are suggestive that in elderly brain, IL-4 levels have decreased to very low levels, which are insufficient to induce expression of CD200R and CD200. From our results, we do not know if the reduction in IL-4 expression is solely associated with age or whether disease is also a factor. The data presented in this study show that CD200 and CD200R are expressed constitutively, but the apparent absence of IL-4 or IL-13 indicate that the stimuli to increase CD200 and CD200R expression when needed is not available.
In summary, we have described the reduced expression of CD200 and CD200R in human elderly brains affected by AD. This implies that this system may be deficient due to decreased levels of CD200 and CD200R in AD brains where chronic inflammation is an established feature, however functional studies are needed to support this conclusion. Augmentation or activation of this system has been shown to be protective in a number of animal models of inflammation; this suggests that enhancement of CD200-CD200R in AD may also be therapeutic. As we showed that IL-4 increases CD200R expression by microglia, and others have shown IL-4 to increase neuronal expression of CD200 (Lyons et al., 2007), this common molecular pathway may allow development of agents that can augment both arms of this anti-inflammatory system.
Acknowledgements
This work was supported by grants from the Alzheimer's Association and Michael J Fox Foundation for Parkinson's Research (DGW). The authors are indebted to Dr. Thomas G. Beach and Ms. Lucia Sue for providing tissue for these studies from the Sun Health Research Institute Tissue Bank.
References
- Banerjee D, Dick AD. Blocking CD200-CD200 receptor axis augments NOS-2 expression and aggravates experimental autoimmune uveoretinitis in Lewis rats. Ocul Immunol Inflamm. 2004;12:115–125. doi: 10.1080/09273940490895326. [DOI] [PubMed] [Google Scholar]
- Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- Boudakov I, Liu J, Fan N, Gulay P, Wong K, Gorczynski RM. Mice lacking CD200R1 show absence of suppression of lipopolysaccharide-induced tumor necrosis factor-alpha and mixed leukocyte culture responses by CD200. Transplantation. 2007;84:251–257. doi: 10.1097/01.tp.0000269795.04592.cc. [DOI] [PubMed] [Google Scholar]
- Chen DX, Gorczynski RM. Discrete monoclonal antibodies define functionally important epitopes in the CD200 molecule responsible for immunosuppression function. Transplantation. 2005;79:282–288. doi: 10.1097/01.tp.0000149506.61000.86. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J. Biol. Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Cheng C, Tsuneyama K, Kominami R, Shinohara H, Sakurai S, Yonekura H, Watanabe T, Takano Y, Yamamoto H, Yamamoto Y. Expression profiling of endogenous secretory receptor for advanced glycation end products in human organs. Mod Pathol. 2005;18:1385–1396. doi: 10.1038/modpathol.3800450. [DOI] [PubMed] [Google Scholar]
- Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT, Khoury SJ. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol. 2007;170:1695–1712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Chaouat G. Loss of surface CD200 on stored allogeneic leukocytes may impair anti-abortive effect in vivo. Am J Reprod Immunol. 2005;53:13–20. doi: 10.1111/j.1600-0897.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- Clark DA, Keil A, Chen Z, Markert U, Manuel J, Gorczynski RM. Placental trophoblast from successful human pregnancies expresses the tolerance signaling molecule, CD200 (OX-2) Am J Reprod Immunol. 2003;50:187–195. doi: 10.1034/j.1600-0897.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copland DA, Calder CJ, Raveney BJ, Nicholson LB, Phillips J, Cherwinski H, Jenmalm M, Sedgwick JD, Dick AD. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am J Pathol. 2007;171:580–588. doi: 10.2353/ajpath.2007.070272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft JM, Watterson DM, Van Eldik LJ. Neuroinflammation: a potential therapeutic target. Expert Opin Ther Targets. 2005;9:887–900. doi: 10.1517/14728222.9.5.887. [DOI] [PubMed] [Google Scholar]
- Deckert M, Sedgwick JD, Fischer E, Schluter D. Regulation of microglial cell responses in murine Toxoplasma encephalitis by CD200/CD200 receptor interaction. Acta Neuropathol. 2006;111:548–558. doi: 10.1007/s00401-006-0062-z. [DOI] [PubMed] [Google Scholar]
- Elward K, Gasque P. “Eat me” and “don't eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol. 2003;40:85–94. doi: 10.1016/s0161-5890(03)00109-3. [DOI] [PubMed] [Google Scholar]
- Figueras-Aloy J, Gomez-Lopez L, Rodriguez-Miguelez JM, Salvia-Roiges MD, Jordan-Garcia I, Ferrer-Codina I, Carbonell-Estrany X, Jimenez-Gonzalez R. Serum soluble ICAM-1, VCAM-1, L-selectin, and P-selectin levels as markers of infection and their relation to clinical severity in neonatal sepsis. Am J Perinatol. 2007;24:331–338. doi: 10.1055/s-2007-981851. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2005 doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Gan L, Ye S, Chu A, Anton K, Yi S, Vincent VA, Von Schack D, Chin D, Murray J, Lohr S, Patthy L, Gonzalez-Zulueta M, Nikolich K, Urfer R. Identification of Cathepsin B as a Mediator of Neuronal Death Induced by A{beta}-activated Microglial Cells Using a Functional Genomics Approach. J Biol Chem. 2004;279:5565–5572. doi: 10.1074/jbc.M306183200. [DOI] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Yu J, Karshin W, Tom D, Li J, Kazanskaia A, Kirkpatrick J, Roher AE. The HHQK domain of beta-amyloid provides a structural basis for the immunopathology of Alzheimer's disease. J Biol Chem. 1998;273:29719–29726. doi: 10.1074/jbc.273.45.29719. [DOI] [PubMed] [Google Scholar]
- Gorczynski R, Chen Z, Kai Y, Lee L, Wong S, Marsden PA. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J Immunol. 2004;172:7744–7749. doi: 10.4049/jimmunol.172.12.7744. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM, Chen Z, Lee L, Yu K, Hu J. Anti-CD200R ameliorates collagen-induced arthritis in mice. Clin Immunol. 2002a;104:256–264. doi: 10.1006/clim.2002.5232. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM, Chen Z, Yu K, Hu J. CD200 immunoadhesin suppresses collagen-induced arthritis in mice. Clin Immunol. 2001;101:328–334. doi: 10.1006/clim.2001.5117. [DOI] [PubMed] [Google Scholar]
- Gorczynski RM, Hu J, Chen Z, Kai Y, Lei J. A CD200FC immunoadhesin prolongs rat islet xenograft survival in mice. Transplantation. 2002b;73:1948–1953. doi: 10.1097/00007890-200206270-00018. [DOI] [PubMed] [Google Scholar]
- Griffiths M, Neal JW, Gasque P. Innate immunity and protective neuroinflammation: new emphasis on the role of neuroimmune regulatory proteins. Int Rev Neurobiol. 2007;82:29–55. doi: 10.1016/S0074-7742(07)82002-2. 29-55. [DOI] [PubMed] [Google Scholar]
- Hatherley D, Barclay AN. The CD200 and CD200 receptor cell surface proteins interact through their N-terminal immunoglobulin-like domains. Eur J Immunol. 2004;34:1688–1694. doi: 10.1002/eji.200425080. [DOI] [PubMed] [Google Scholar]
- Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A. Signaling mechanisms, interaction partners, and target genes of STAT6. Cytokine Growth Factor Rev. 2006;17:173–188. doi: 10.1016/j.cytogfr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2007 doi: 10.1096/fj.07-9909com. [DOI] [PubMed] [Google Scholar]
- Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J Immunol. 2006;176:191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- Kalinowska A, Losy J. PECAM-1, a key player in neuroinflammation. Eur J Neurol. 2006;13:1284–1290. doi: 10.1111/j.1468-1331.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Sehra S, Chang HC, O'Malley JT, Mathur AN, Bruns HA. Constitutively active STAT6 predisposes toward a lymphoproliferative disorder. Blood. 2007;110:4367–4369. doi: 10.1182/blood-2007-06-098244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning N, Bo L, Hoek RM, Huitinga I. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann Neurol. 2007;62:504–514. doi: 10.1002/ana.21220. [DOI] [PubMed] [Google Scholar]
- Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D, Frederickson S, Maruyama T, Wild MA, Nolan MJ, Wu D, Springhorn J, Bowdish KS. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol. 2007;178:5595–5605. doi: 10.4049/jimmunol.178.9.5595. [DOI] [PubMed] [Google Scholar]
- Launer L. Nonsteroidal anti-inflammatory drug use and the risk for Alzheimer's disease: dissecting the epidemiological evidence. Drugs. 2003;63:731–739. doi: 10.2165/00003495-200363080-00001. [DOI] [PubMed] [Google Scholar]
- Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, Stern D, Yan SD. Involvement of Microglial Receptor for Advanced Glycation Endproducts (RAGE) in Alzheimer's Disease: Identification of a Cellular Activation Mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie IRA, Munoz DG. Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology. 1998;50:986–990. doi: 10.1212/wnl.50.4.986. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, McGeer EG. Expression of the histocompatibility glycoprotein HLADR in neurological disease. Acta Neuropathol (Berl) 1988;76:550–557. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, Mohler T, De VJ, Rossi JF, Goldschmidt H, Klein B. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108:4194–4197. doi: 10.1182/blood-2006-06-029355. [DOI] [PubMed] [Google Scholar]
- Moreaux J, Veyrune JL, Reme T, De VJ, Klein B. CD200: A putative therapeutic target in cancer. Biochem Biophys Res Commun. 2008;366:117–122. doi: 10.1016/j.bbrc.2007.11.103. [DOI] [PubMed] [Google Scholar]
- Mott RT, it-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, Ehrhart J, Mullan M, Tan J. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46:369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- Neumann H. Control of glial immune function by neurons. Glia. 2001;36:191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- Neuroinflammation Working Group Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CQ, Gao JH, Kim H, Saban DR, Cornelius JG, Peck AB. IL-4-STAT6 signal transduction-dependent induction of the clinical phase of Sjogren's syndrome-like disease of the nonobese diabetic mouse. J Immunol. 2007;179:382–390. doi: 10.4049/jimmunol.179.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, Mills KH, Lynch MA. Role of Interleukin-4 in Regulation of Age-Related Inflammatory Changes in the Hippocampus. J J Biol Chem. 2005;280:9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- Pereira C, Agostinho P, Moreira PI, Cardoso SM, Oliveira CR. Alzheimer's disease-associated neurotoxic mechanisms and neuroprotective strategies. Curr Drug Targets CNS Neurol Disord. 2005;4:383–403. doi: 10.2174/1568007054546117. [DOI] [PubMed] [Google Scholar]
- Petermann KB, Rozenberg GI, Zedek D, Groben P, McKinnon K, Buehler C, Kim WY, Shields JM, Penland S, Bear JE, Thomas NE, Serody JS, Sharpless NE. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J Clin Invest. 2007;117:3922–3929. doi: 10.1172/JCI32163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postadzhiyan AS, Tzontcheva AV, Kehayov I, Finkov B. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and their association with clinical outcome, troponin T and C-reactive protein in patients with acute coronary syndromes. Clin Biochem. 2008;41:126–133. doi: 10.1016/j.clinbiochem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Rijkers ES, de RT, Baridi A, Veninga H, Hoek RM, Meyaard L. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol Immunol. 2008;45:1126–1135. doi: 10.1016/j.molimm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Rosenblum MD, Olasz EB, Yancey KB, Woodliff JE, Lazarova Z, Gerber KA, Truitt RL. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? J Invest Dermatol. 2004;123:880–887. doi: 10.1111/j.0022-202X.2004.23461.x. [DOI] [PubMed] [Google Scholar]
- Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer's disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2007 doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann P, Tieu K, Cohen O, Choi DK, Wu dC, Marks D, Vila M, Jackson-Lewis V, Przedborski S. Pathogenic role of glial cells in Parkinson's disease. Mov Disord. 2003;18:121–129. doi: 10.1002/mds.10332. [DOI] [PubMed] [Google Scholar]
- Vieites JM, de la TR, Ortega MA, Montero T, Peco JM, Sanchez-Pozo A, Gil A, Suarez A. Characterization of human cd200 glycoprotein receptor gene located on chromosome 3q12-13. Gene. 2003;311:99–104. doi: 10.1016/s0378-1119(03)00562-6. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Rosen DB, Lanier LL, Locksley RM. CD200 receptor family members represent novel DAP12-associated activating receptors on basophils and mast cells. J Biol Chem. 2004;279:54117–54123. doi: 10.1074/jbc.M406997200. [DOI] [PubMed] [Google Scholar]
- Walker DG, Link J, Lue LF, Dalsing-Hernandez JE, Boyes BE. Gene expression changes by amyloid {beta} peptide-stimulated human postmortem brain microglia identify activation of multiple inflammatory processes. J Leukoc Biol. 2006;79:596–610. doi: 10.1189/jlb.0705377. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH, Barclay AN. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102:173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GJ, Puklavec MJ, Willis AC, Hoek RM, Sedgwick JD, Brown MH, Barclay AN. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Haroutunian V, Ho L, Purohit D, Pasinetti GM. Microglia activation in the brain as inflammatory biomarker of Alzheimer's disease neuropathology and clinical dementia. Dis Markers. 2006;22:95–102. doi: 10.1155/2006/276239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J Immunol. 2004;173:6786–6793. doi: 10.4049/jimmunol.173.11.6786. [DOI] [PubMed] [Google Scholar]