SUMMARY
Virus-induced IL-1β and IL-18 production in macrophages is mediated via a caspase-1 pathway. Multiple microbial components, including viral RNA, are thought to trigger assembly of the cryopyrin inflammasome and consequent caspase-1 activation. Here we demonstrate that cryopyrin−/− and caspase-1−/− mice are more susceptible than wildtype controls following infection with a pathogenic influenza A virus. This profile of enhanced morbidity correlates with decreased neutrophil and monocyte recruitment and reduced cytokine and chemokine production. Despite the effect on innate immunity, cryopyrin-deficiency was not associated with any obvious defect in virus control or on the later emergence of the adaptive response. Early epithelial necrosis was, however, more severe in the infected mutants, with extensive collagen deposition leading to later respiratory compromise. These findings reveal a novel function of the cryopyrin inflammasome in healing responses. Cryopyrin and caspase-1 are clearly central to both innate immunity and to moderating lung pathology in influenza pneumonia.
Keywords: Caspase-1, cryopyrin/Nalp3, NLR, Influenza A virus
INTRODUCTION
Seasonal influenza A virus epidemics are a major cause of morbidity and economic loss, with 35–45,000 annual deaths estimated in the USA alone (Kilbourne, 2006). The highly pathogenic (HP) H5N1 avian influenza A viruses have been causing continuing, sporadic mortality in (particularly) South Asia. Although they have not yet adapted to mediate human to human spread, understanding the severe pathology associated with these infections is a matter of urgency, particularly as the recently reconstructed 1918/19 pandemic virus shows similar pathogenicity in rhesus macacques (Kobasa et al., 2007). Recent analysis indicates that a very rapid onset, pro-inflammatory cytokine “storm” is a major contributor to the lethality induced by these HP viruses in humans, mice and macacques (de Jong et al., 2006; Hampton, 2007; Kobasa et al., 2007; Wareing et al., 2004). The mechanisms underlying this excessive innate response are only now being characterized, with the studies to date demonstrating roles for the pattern-recognition receptors TLR3, TLR7 and RIG-I (Diebold et al., 2004; Heer et al., 2007; Hornung et al., 2006; Kato et al., 2005; Kawai et al., 2005; Koyama et al., 2007; Le Goffic et al., 2006; Lopez et al., 2004; Lund et al., 2004; Meylan et al., 2005; Pichlmair et al., 2006; Seth et al., 2005; Xu et al., 2005; Yoneyama et al., 2004).
Even so, the situation with regard to innate immunity and the HP influenza viruses is not simple, as diminishing the magnitude of the initial, non-antigen specific response can also lead to later-onset pathology that ultimately makes the disease process more severe. For example, infection of monocytes and macrophages with a variety of pathogens is known to induce secretion of the proinflammatory cytokines IL-1β and IL-18, which respectively bind to the type 1 IL-1 receptor (IL-1R1) and the IL-18 receptor (IL-18R) to induce a plethora of genes and activities. Recent studies with a pathogenic influenza A virus demonstrated impaired neutrophil and CD4+ T cell recruitment to the infected respiratory tract of IL-1R1−/− mice, greatly diminished lung inflammatory infiltrates, reduced IgM levels in both serum and at mucosal sites and decreased activation of CD4+ T “helpers” in secondary lymphoid tissue (Schmitz et al., 2005). These changes were not, however, associated with protection: the IL-1R1−/− mice were ultimately more susceptible, though lung virus titers were only moderately increased (Schmitz et al., 2005). Also, IL-18−/− mice inoculated intranasally (i.n.) with the mouse-adapted influenza A/PR/8/34 (PR8) virus showed increased mortality with enhanced virus growth, massive infiltration of inflammatory cells, and elevated NO production over the first 3 days following respiratory challenge (Liu et al., 2004). Using a less virulent influenza challenge, IL-18−/− deficiency was associated with decreased CD8+ T cell cytokine production (Denton et al., 2007). Furthermore, the administration of IL-18 was shown to protect against herpes simplex virus and vaccinia virus (Liu et al., 2004). Thus, while both IL-1β and IL-18 are clearly involved in the innate response, they seem more associated with survival than with lethal immunopathology, at least when the challenge is with viruses of moderate pathogenicity.
Both IL-1β and IL-18 are synthesized as inactive cytoplasmic precursors that are processed into biologically active mature forms in response to various proinflammatory stimuli (including viruses) by the cysteine protease caspase-1 (Burns et al., 2003; Dinarello, 2005; Kanneganti et al., 2007b; Lamkanfi et al., 2007; Pirhonen et al., 2001; Pirhonen et al., 1999). Caspase-1 is synthesized as an inactive zymogen that becomes activated by cleavage at aspartic residues to generate an enzymatically active 10 kDa/20 kDa heterodimer (Martinon et al., 2002). Results from our laboratory and others have shown that a set of NOD-like receptors (NLRs), including ipaf and cryopyrin, induce caspase-1 activation and the release of the IL-1β and IL-18 through the assembly of large protein complexes called inflammasomes. Salmonella and Legionella flagellin are sensed by the ipaf inflammasome (Amer et al., 2006; Franchi et al., 2006; Miao et al., 2006), whereas cryopyrin mediates caspase-1 activation in response to a wide variety of microbial components including dsRNA and viral RNA or its analog poly(I:C) (Kanneganti et al., 2007a, Mariathasan et al., 2006; Mariathasan et al., 2006; Martinon et al., 2006; Sutterwala et al., 2006a; Sutterwala et al., 2006b).
Genetic disruption (−/−) of cryopyrin or the adaptor apoptosis-associated speck-like protein (ASC) abrogates caspase-1 activation in poly(I:C), dsRNA or viral RNA stimulated macrophages, although this process proceeds normally in cells lacking TLR3 or TLR7 (Kanneganti et al., 2007a). In addition, influenza A virus infection activates the cryopyrin inflammasome in cultured macrophages (Kanneganti et al., 2007a). Here, we show that in vivo activation of the cryopyrin inflammasome by a pathogenic influenza A virus controls the release of IL-1 β and IL-18 and modulates the extent of inflammatory lung pathology and bronchiolar necrosis. Purified influenza virus RNA was sufficient to induce enhanced levels of IL-1β in serum and bone marrow-derived dendritic cells in a cryopyrin- and caspase-1-dependent manner. These results suggest that recognition of influenza virus RNA by antigen presenting cells (APCs) triggers activation of the cryopyrin inflammasome, which in turn modulates the severity of influenza pneumonia.
RESULTS
Cryopyrin and caspase-1 protect against influenza A virus-induced mortality
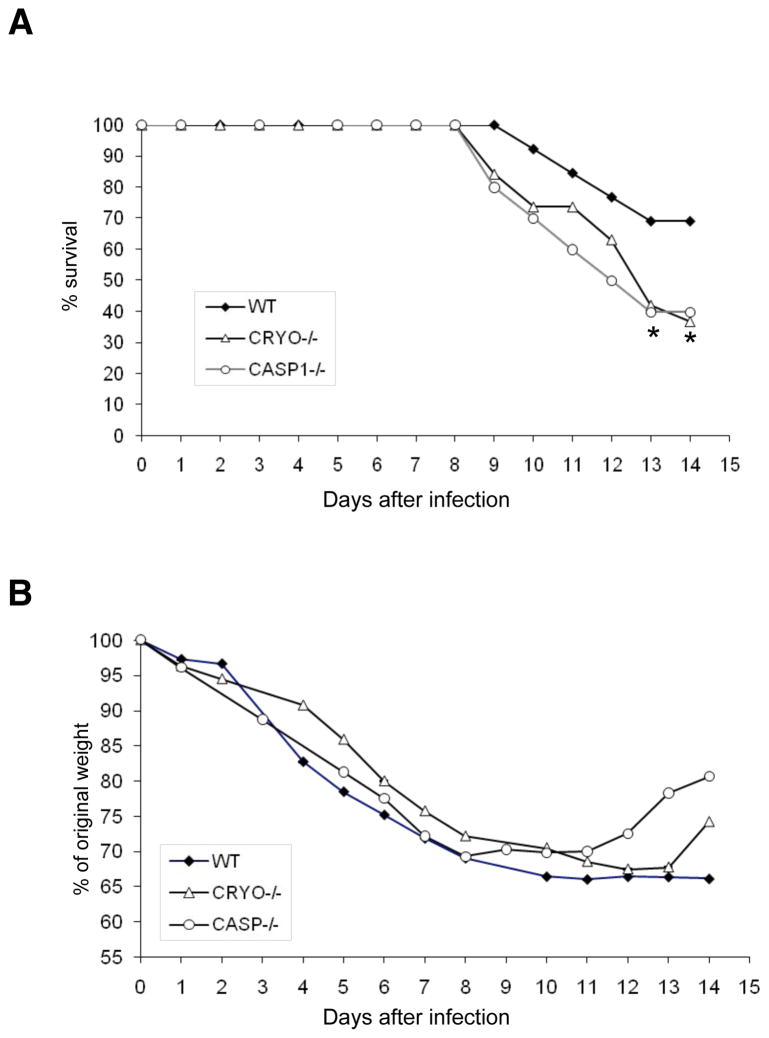
We first infected wildtype (WT+/+), cryopyrin−/− and caspase-1−/− B6 mice i.n. with a high virus dose (8000 EID50) of PR8 to determine the role of the cryopyrin inflammasome in influenza-induced lethality. The absence of cryopyrin and caspase-1, but not ipaf, was associated with greater mortality (Fig. 1A and Supplemental Figure 1), though, somewhat surprisingly (Schmitz et al., 2005), this was not preceded by enhanced weight loss (Fig. 1B). After obtaining this result, we sought to determine the mechanism of protection provided by caspase-1 and cryopyrin.
Figure 1. Cryopyrin and caspase-1 provide protection against influenza A virus-induced lethality.
Groups of 10 wildtype, cryopyrin−/− (CRYO−/−) and casapse-1 (CASP1−/−) mice were infected i.n. with 8×103 EID50 (= 1LD50) of PR8 and survival was monitored daily for 14d. Differences in group survival were analyzed with Cox proportional hazards test and p<0.05 was considered statistically significant.
In vivo cytokine and chemokine production depends on cryopyrin but not ipaf
The cyropyrin inflammasome has been shown in other systems to play a central role in cytokine production and regulation. Cytokines and chemokines are key promoters of inflammation and have been implicated in the host response to a wide range of infections. The levels and timing of inflammatory mediator production during the development of influenza A virus pneumonia were thus determined by collecting bronchoalveolar lavage (BAL) fluid at 0, 3 and 6 days (d) after i.n. challenge of WT mice with the pathogenic PR8 influenza A virus, then assaying for a set of 8 cytokines and chemokines (IL-1β, IL-18, TNF-α, IL-6, KC, MIP-2, IFN-γ and IL-12p40). All were significantly upregulated in the lung fluid recovered on d3 (Suppl. Fig. 2, d3), though they were undetectable in BAL samples from normal, uninfected mice (Suppl. Fig. 2, d0). The amounts of IL-1β, IL-18, TNF-α, IL-6, KC and MIP-2 fell significantly over the next 3 days, with only IFN-γ and IL-12p40 being higher at the d6 time point (Suppl. Fig 2, d6). The latter may also be regarded as measures of adaptive immunity, as IFN-γ produced by T cells further promotes IL-12 synthesis in monocytes. Similar cytokine patterns were detected in the serum (data not shown). These results demonstrate that proinflammatory cytokines and chemokines are produced during the early stages of influenza A virus infection and contribute to the innate host response.
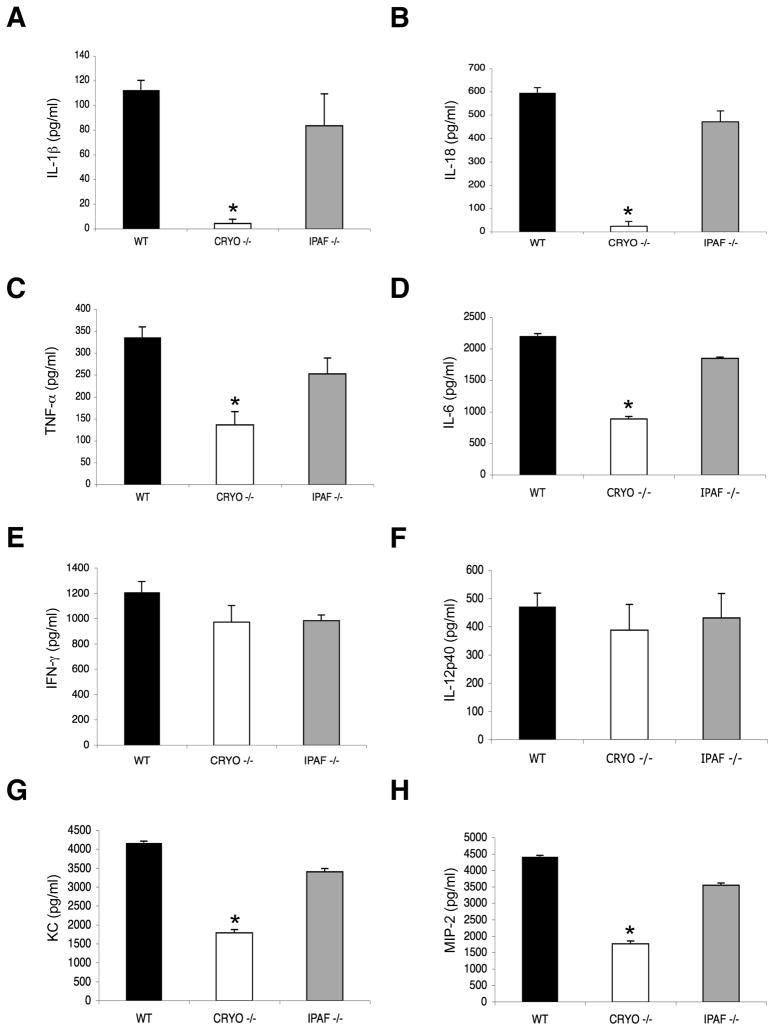
The fact that IL-1R−/− and IL-18−/− mice can respond sub-optimally to influenza A virus challenge (Liu et al., 2004; Schmitz et al., 2005) suggests that the caspase-1-dependent cytokines IL-1β and IL-18 are important mediators of innate immunity in this infection, although the upstream mechanisms remain unclear. The NLR proteins cryopyrin and ipaf have been implicated in caspase-1 activation and the production of IL-1β and IL-18 following in vitro stimulation of macrophages with a wide variety of bacterial and viral pathogen-associated molecular patterns (PAMPs) including viral RNA and DNA (Kanneganti et al., 2007b). However, the in vivo role of these NLRs in influenza immunity has not been determined. We thus infected WT, cryopyrin−/− and ipaf−/− mice i.n. with the PR8 influenza A virus and measured levels of inflammatory cytokines and chemokines on d3 after challenge. As expected (Fig. 2), IL-1β, IL-18, TNF-α and IL-6 were all detected in the BAL fluid and serum of wildtype hosts (Fig. 2A–D and data not shown). However, the concentrations of the caspase-1-dependent cytokines IL-1β and IL-18 were greatly reduced in the BAL and serum samples from the cryopyrin−/− (but not the ipaf−/−) mice (Fig. 2A, B and data not shown). Less predictably, the secretion of IL-6, TNF-α, IFN-α, KC and MIP-2 was also selectively diminished in the cryopyrin−/− group (Fig. 2C–H and Suppl. Fig. 3), although the decrease was less dramatic than with IL-1β and IL-18. In contrast, IL-12 and IFN-γ looked to be cryopyrin-independent following influenza virus infection (Fig. 2E, F). These results suggest that the cryopyrin-dependent production of IL-1β and IL-18 in influenza A-virus infected mice contributes to the secretion of IL-6, TNF-α, KC and MIP-2 through an autocrine or paracrine loop, whereas IFN-γ and IL-12p40 are generated independently of IL-1β and IL-18. Again, the situation for IFN-γ is intriguing, as this cytokine is produced by NK cells and T cells.
Figure 2. Influenza virus-induced cytokine and chemokine production depends on cryopyrin, but not ipaf.
Groups (n=4) of B6 (WT), cryopyrin−/− (CRYO−/−) and ipaf−/− (IPAF−/−) mice were infected i.n with 4×103 EID50 of PR8 influenza virus and sampled 3d later to determine levels of IL-1β (A), IL-18 (B), TNF-α (C), IL-6 (D), IFN-γ (E), IL-12p40 (F), KC (G), and MIP-2 (H), in BAL fluid. Results are mean ± S.D. and are representative of three independent experiments. Data were analyzed with Student’s t-test (*, p<0.05).
Requirement for caspase-1
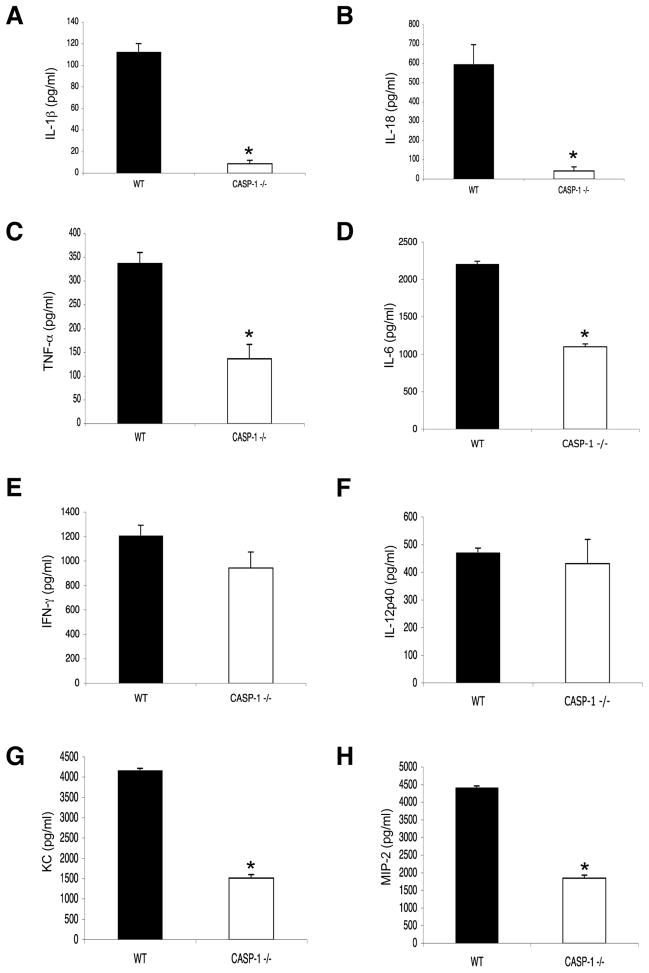
The analysis was repeated in caspase-1−/− mice to establish whether the effect of cryopyrin inactivation on IL-1β, IL-18, IL-6, TNF-α and the chemokines KC and MIP-2 is indeed mediated via a caspase-1 dependant pathway. Again, IL-1β and IL-18 could not be detected in the BAL fluid (Fig. 3A, B) or serum (Suppl. Fig. 4) of caspase-1−/− mice and the secretion of TNF-α, IL-6, IFN-α, KC and MIP-2 (Fig. 3C, D, G, H) was significantly reduced, while IFN-γ and IL-12p40 levels remained unaffected (Fig. 3E, F). Thus, in addition to promoting IL-1β and IL-18, the cryopyrin inflammasome influences the production of a broader subset of pro-inflammatory cytokines and chemokines during influenza A virus infection.
Figure 3. Caspase-1 is required for influenza virus-induced secretion of cryopyrin-dependent cytokines and chemokines.
Groups of 4 B6 (WT) and caspase-1−/− (CASP1−/−) mice were infected i.n with 4×103 EID50 of PR8 influenza virus and levels of IL-1β (A), IL-18 (B), TNF-α (C), IL-6 (D), IFN-γ (E), IL-12p40 (F), KC (G), and MIP-2 (H) were measured in BAL fluid 3d later. Results are mean ± S.D.. Data were analyzed with Student’s t-test (*, p<0.05) and are representative of three independent experiments.
Influenza RNA is sufficient for cryopyrin and caspase-1 mediated IL-1β secretion
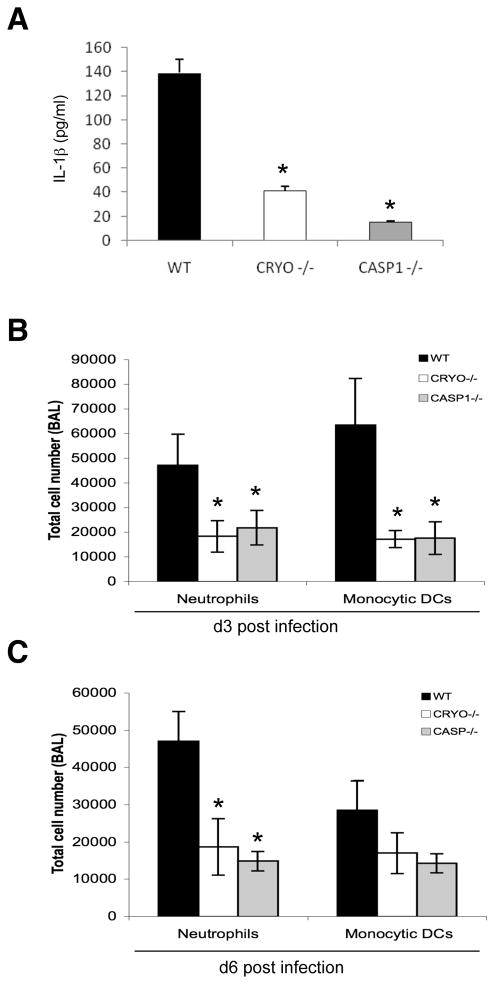
Inflammasome activation has been attributed to a diverse set of microbial products (Kanneganti et al., 2007b). Having established that cryopyrin and caspase-1 were required for the initial wave of IL-1β in vivo, we investigated whether influenza virus RNA alone induced this activity. Intraperitoneal administration of influenza virus RNA led to the presence of detectable IL-1β levels in the serum of WT mice, with the effect being highly dependent on cryopyrin (Supplementary Fig. 5). The ssRNA influenza virus genome has been shown to activate the TLR7 and RIG-I pathways in antigen-presenting cells (APCs) such as macrophages and dendritic cells (Diebold et al., 2004; Pichlmair et al., 2006). To test whether influenza virus RNA also activated the cryopyrin inflammasome in APCs, we transfected purified viral RNA into WT, cryopyrin−/−, and caspase-1−/− bone marrow derived dendritic cells (BMDCs) and measured IL-1β secretion (Figure 4A). Both cryopyrin−/− and caspase-1−/− BMDCs produced minimal levels of IL-1β after RNA stimulation, though WT BMDCs secreted robust amounts of the cytokine. Apparently influenza virus RNA can activate inflammasome recognition in the absence of virus replication and the involvement of other viral products.
Figure 4. Innate cellular responses to influenza are mediated by cryopyrin and caspase-1.
(A) Dendritic cells from WT, CRYO−/− and CASP1−/− mice were transfected with 2 μg purified influenza A viral RNA for 24 h and cell-free supernatants were analyzed by ELISA for production of IL-1β. Data are representative of two independent experiments. Groups of 4 C57BL/6 WT, CRYO−/− and CASP1−/− mice were infected i.n. with 2×103 EID50 of PR8 influenza virus and cells recovered by BAL on d3 (B) and d6 (C) were characterized by flow cytometry (see Experimental Procedures). Results are mean ± S.D. Data were analyzed with ANOVA (*, p<0.05) and are representative of three independent experiments.
Cryopyrin and caspase-1 mediate neutrophil and moncyte/DC recruitment
The pro-inflammatory cytokine IL-1β is known to be a potent pyrogen that increases the expression of adhesion factors on endothelial cells to facilitate the transmigration of leukocytes (Dinarello, 2002, 2005, 2006; Wewers, 2004; Wewers et al., 1997). In addition, KC is a chemoattractant for neutrophils (Lira et al., 1994) while MIP-2 draws polymorphonuclear leukocytes to sites of infection (Iida and Grotendorst, 1990). The decreased production of these cytokines and chemokines in cryopyrin−/− and caspase-1−/− mice might thus be expected to result in diminished leukocyte extravasation into the airways following influenza A virus-infection. This proved to be the case, with the numbers of neutrophils (Ly-6g+/MHC Class II-) and CD11cint/CD11bhi monocytic dendritic cells (DCs) being dramatically reduced in the BAL populations recovered on d3 after i.n. PR8 challenge of cryopyrin−/− and caspase-1−/− mice (Fig. 4B). The effect persisted until at least d6 (Fig. 4C) though, in contrast, the BAL cell numbers in PR8-infected ipaf−/− mice were comparable throughout to those found for the WT controls (data not shown).
Cryopyrin and caspase-1 are not required for the adaptive response
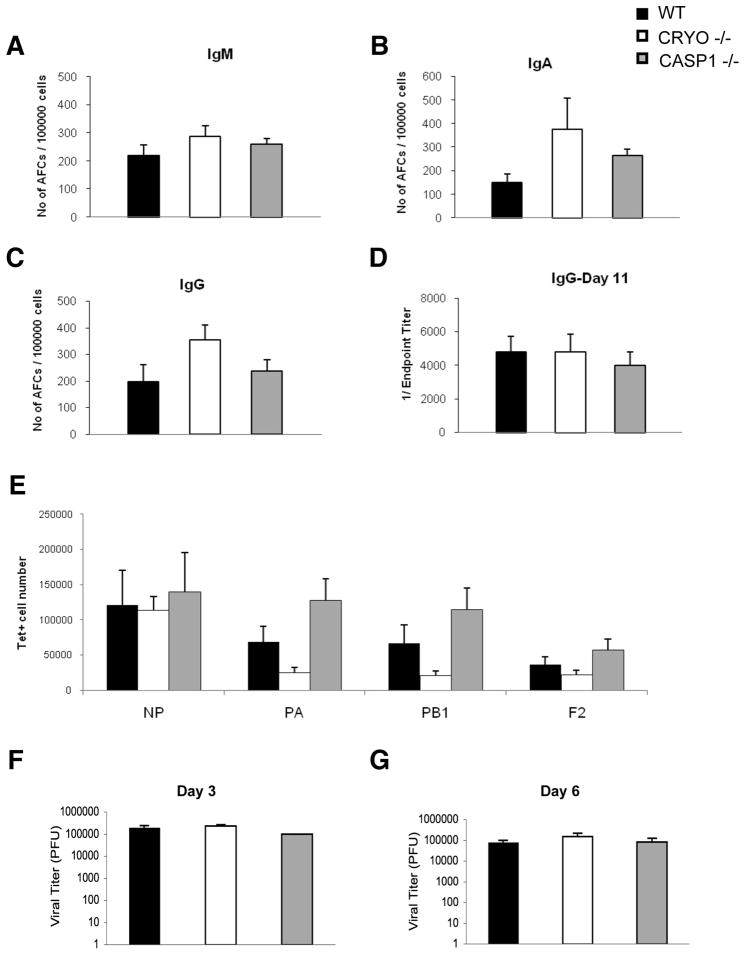
Influenza A virus-infected cryopryin−/− and caspase-1−/− mice thus generate lower levels of key cytokines and chemokines and recruit less inflammatory cells to the infected respiratory tract (Figs. 2, 3 and 4). Does this modify the ensuing adaptive responses? Naive wildtype, cryopyrin−/− and caspase-1−/− mice were challenged i.n. with a sublethal dose (4000 EID50) of PR8 and influenza-specific B cell activation was measured by antibody secreting cell ELISPOTs (Figs. 5 A–C, to measure the total number of anitibody secreting cells (ASs) and by serum antigen-specific ELISA (Fig. 5D, to measure antibody production). No differences were observed in antibody secreting cell counts for three isotypes at d7 after infection and the d11 serum IgG titers were equivalent, indicating that the cryopyrin inflammasome has no obvious effect on humoral immunity. Additionally, measurement of four dominant antigen-specific CD8+ T cell specificities showed comparable splenic responses at d11 in these mice (Fig. 5E). Overall, the cyropyrin−/− and caspase-1 −/− phenotypes manifest primarily as innate immune deficiencies. Furthermore, WT, cryopyrin−/− and caspase-1−/− mice had equivalent lung virus titers at d3 and d6 after infection (Fig. 5F,G), establishing that the innate responses mediated by cryopyrin and caspase-1 do not play an essential part in limiting virus production.
Figure 5. Cryopyrin and caspase-1 are not required for early virus clearance or for adaptive immunity.
Groups of 4 WT, CRYO −/− and CASP1−/− mice were infected i.n. with 4×103 EID50 of PR8 influenza. Seven days following infection, the frequency of antibody secreting cells in the draining mediastinal lymph node was determined by ELISPOT for the indicated isotype (A–C). Antigen-specific serum IgG was measured by ELISA on d11 following infection (D). Also on d11, antigen-specific CD8+ T cell numbers were enumerated from the BAL by tetramer staining (E). Virus titers in lungs were determined on d3 (F) and d6 (G) by standard MDCK plaque assay. Data were analyzed with ANOVA (*, p<0.05) and are representative of three independent experiments.
Enhanced virus-induced pathology and diminished respiratory function
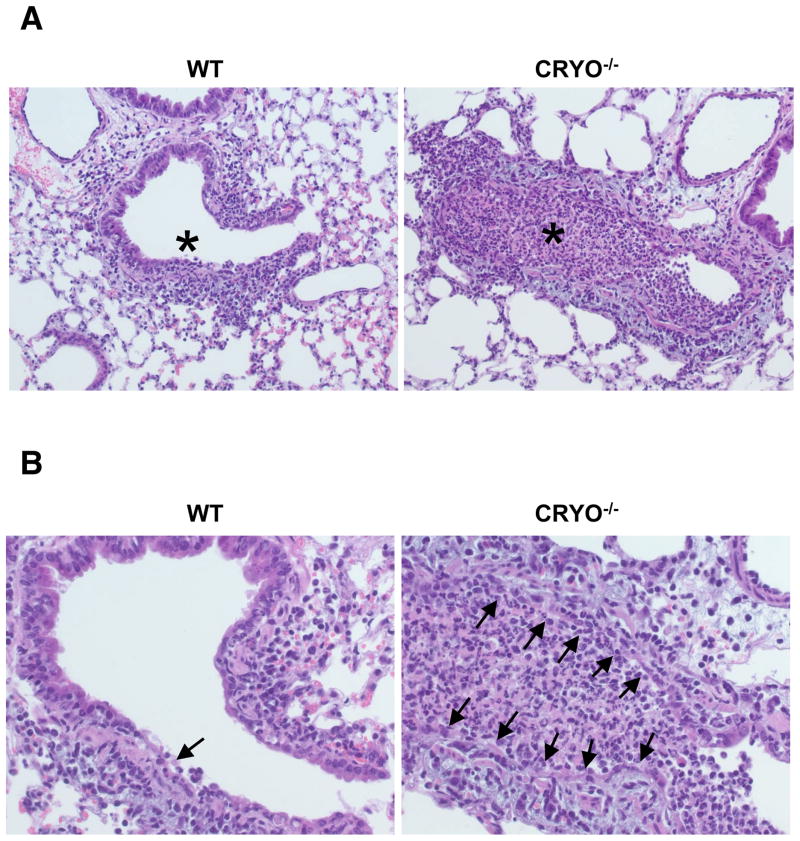
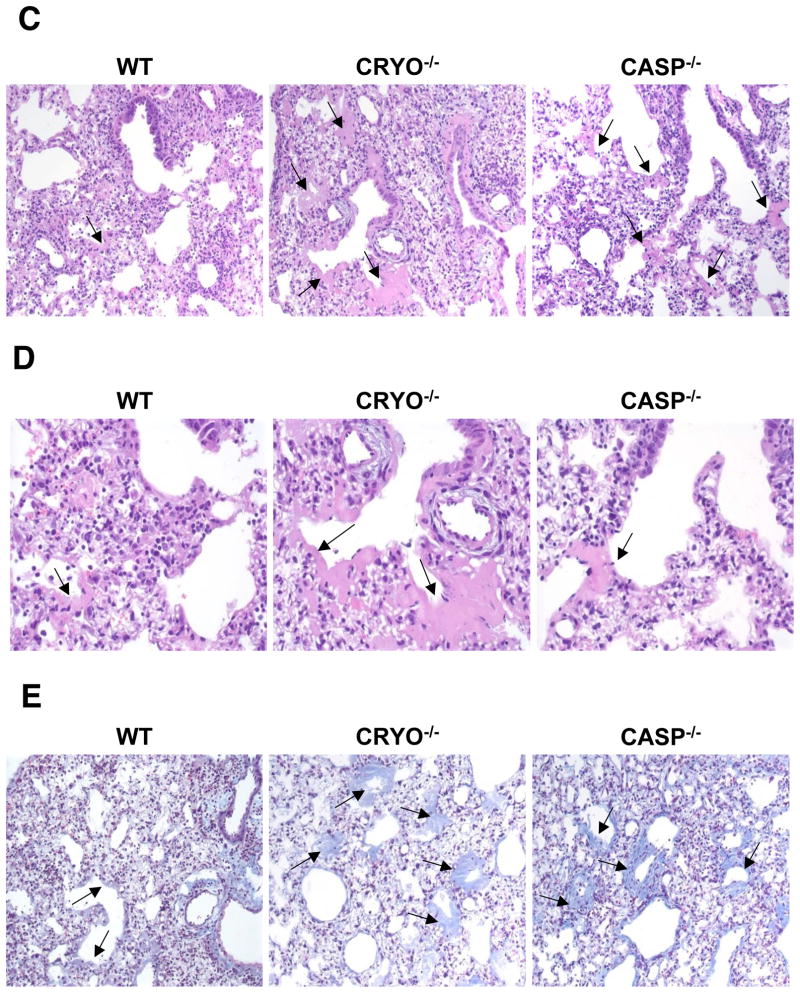
Does the diminished cytokine/chemokine production and reduced neutrophil/monocyte recruitment in cryopyrin−/− mice modify the extent of early damage to the virus-infected respiratory tract? Comparing lung H&E stained paraffin sections from virus-challenged WT and cryopyrin−/− mice on d3 established that the overall extent of pneumonia was mild for both experimental groups, though significant differences in histopathology were observed at the level of the small bronchi and bronchioles. The lungs of WT mice were only minimally affected, while the cryopyrin−/− animals showed focal lesions of epithelial necrosis, obstruction of the airway by fibrin, and degenerate neutrophils, macrophages and necrotic cell debris with mild edema around the adjacent vessels (Fig. 6A, B). We next asked whether this early necrotic phenotype led to increased pathology at later time points. Evidence of pneumonia was found on d11 in mice from all experimental groups. Foci of minimal collagen deposition were observed in the alveoli (and occasionally in the terminal airways) of WT mice (Fig. 6C, D arrows), and a thin layer of collagen was occasionally seen to line the alveolar spaces (Fig. 6E, blue). In stark contrast, abundant, concentric layers of collagen were prevalent in the distal airways and lung interstitium of the cryopyrin−/− and caspase-1−/− groups, to the extent that the alveoli were often occluded (Fig. 6D arrows).
Figure 6. Virus-induced pulmonary necrosis is enhanced in the absence of cryopyrin.
Groups of 4 WT and cryopyrin−/− mice were infected i.n. with 8×103 EID50 of PR8 influenza virus. Lung samples were collected on d3 and processed for routine H&E staining of 4μ paraffin sections. (A), top panels, ×200, WT airway epithelium is intact with only small foci of necrosis (arrow) and peribronchiolar inflammation; * shows unobstructed airway lumen. Cryopyrin−/−, the small bronchi and bronchioles show diffuse necrosis with loss of the airway epithelium and obstruction of the lumen by debris (*). (B), bottom panels ×400, Intact wildtype epithelium with limited foci of necrosis (arrow), and diffuse necrosis of CRYO−/− bronchiolar epithelium (arrows). Histologic evaluation was performed by an experienced veterinary pathologist (KLB). Groups of 4 wildtype and cryopyrin−/− mice were infected i.n. with 8×103 EID50 of PR8 influenza virus. Lung samples were collected on d11 and 4μm paraffin sections processed for routine H&E (C,D) or Masson’s Trichrome staining (E). (C) ×200, the WT mice show only focal and minimal collagen deposition in the alveoli and occasionally in the terminal airways (arrows). This collagen deposition is exuberant in the mutant groups and often occludes alveoli and terminal airways (arrows). (D) ×400, arrows indicate areas of collagen deposition. (E) ×200, bottom row Masson’s Trichrome stain confirms collagen deposition. In the WT groups the insult was sufficiently severe to cause basement membrane damage, evidenced by a thin layer of collagen lining the alveolar spaces. In the mutant lungs, deposition of collagen is abundant and forms concentric layers that occlude alveoli and terminal airways.
The next step was to measure blood gases in the control and virus-infected cryopyrin−/− and WT and mice, to determine whether there was a correlation between the extent of lung pathology and the integrity of respiratory function. Oxygen levels (measured as partial pressure of oxygen, pO2) were higher in the uninfected controls than in the infected WT and mutant mice, with the decrease being most apparent for the cryopyrin−/− group (60 mmHg vs. 110 mmHg, Supp Figure 6) that also showed the most severe alveolar damage. These data thus confirm the histological analyses and provide support for the view that the protective mechanism mediated via the cyropyrin inflammasome is the promotion of early healing and lung repair that minimizes respiratory compromise.
DISCUSSION
Both IL-1β and IL-18 are thought to play an important part in the pathogenesis of influenza A virus infections, but the underlying molecular mechanisms have remained largely obscure. Precursor forms of IL-18 and IL-1 β lack a signal peptide and require cleavage by caspase-1 (IL-1β-converting enzyme) for their maturation, and hence for biological activity (Kuida et al., 1995; Li et al., 1995; Thornberry et al., 1992). The activation of caspase-1 takes place upon the assembly of the “inflammasome”, a signaling platform scaffolded by proteins belonging to the NLR family of innate immune receptors (Martinon et al., 2002). However, the virus-induced signals that are involved in activating the inflammasome have not been completely characterized. Recently, we and others reported that both cytosolic poly(I:C) and viral RNA induce caspase-1 activation and the subsequent processing and secretion of IL-1β and IL-18 in vitro via a cryopyrin-dependent pathway (Kanneganti et al., 2006; Pirhonen et al., 2001; Pirhonen et al., 1999). However, prior to the present analysis, nothing was known about the in vivo role of the cryopyrin inflammasome during influenza virus infection.
The results presented here clearly demonstrate an in vivo requirement for cryopyrin and caspase-1 that in turn correlates with IL-1β and IL-18 production in the influenza A virus-infected respiratory tract. Furthermore, enhanced bronchial airway necrosis was evident as early as d3 after infection of the cyropyrin−/− mice, with significantly increased collagen deposition being apparent by d11. These findings were further supported by blood gas analysis showing severely compromised lung function in the absence of cryopyrin. This finding is significant, as it indicates that the inflammasome plays a hitherto unrecognized role in tissue repair following infectious injury.
Earlier evidence suggested that IL-1β is indeed involved in lung healing. The repair of mechanically injured rat type II alveolar epithelial cell monolayers was promoted by adding IL-1β to the in vitro cultures (Geiser et al., 2000). Similarly, edema fluid from patients with acute respiratory distress syndrome was found to stimulate repair of the human epithelial cell line A549 in an IL-1β dependent manner (Olman et al., 2004). Other studies have shown a time–dependant, pro-fibrotic, inflammatory role for IL-1β in wound healing (Thickett and Perkins, 2008).
Influenza viruses cause a highly inflammatory pneumonia characterized by the early recruitment of neutrophils and monocytes to sites of infection. The migration of neutrophils is critically mediated by the two CXC chemokines, MIP-2 and KC, both of which are significantly downregulated in cryopyrin−/− and caspase-1−/− mice. The present study establishes that a cryopyrin/caspase-1-dependent pathway is indeed involved in the extravasation of inflammatory cells into (and/or maintenance in) the influenza virus-infected mouse lung, a finding that is accord with the capacity of IL-1 to regulate MIP-2 and KC production (Shanley et al., 1997). The observed reduction in MIP-2 and KC levels in influenza A virus infected cryopyrin−/− and caspase-1−/− lungs may thus be considered to explain the diminished presence of neutrophils. Furthermore, recent studies of murine renal and hepatic ischemia reperfusion injury also indicated that IL-1 is involved in neutrophil mobilization (Haq et al., 1998). As in our influenza model, IL-1 could be thought to contribute directly or indirectly to neutrophil accumulation during acute ischemic and/or virus-induced injury, though it may be less important in the later neutrophilia characteristic of chronic conditions like tuberculosis. Even so, the part played by neutrophils in the pathophysiology of influenza virus infection is not fully resolved, though they do seem to be protective in the early phase of this pneumonia. Other reports have suggested that IL-1β functions to promote virus clearance (Schmitz et al., 2005). While this was not apparent in the present analysis, it is possible that the HP virus used here may have masked effects that would have been apparent in mice given a less virulent challenge.
A key mediator of the acute morbidity and mortality characteristic of infection with ultra-HP influenza A viruses, like the recent H5N1 strains and the reconstructed 1918 virus, is thought to be an early onset “cytokine storm”(Korteweg and Gu, 2008). However, diminishing the extent of cytokine/chemokine production following infection with the PR8 influenza A virus increased, rather than diminished, the severity of influenza pneumonia and the incidence of fatal disease. It thus seems that, even with a relatively pathogenic virus like PR8, the cropyrin/caspase-1-dependant cytokines/chemokines are broadly protective and function to contain the extent of lung damage and associated epithelial necrosis. Perhaps the difference from the extreme HP 1918 and HP H5N1 strains is that, while a measure of increased epithelial permeability early in the response may promote recovery, that same process is lethal if the resultant fluid transudation is so acute and massive that it compromises lung function. Clearly, any therapeutic protocols that might be designed to modulate the “cytokine storm” effect in HP influenza would need to be implemented with caution.
In conclusion, our results demonstrate that influenza A viruses trigger activation of the cryopyrin inflammasome, which controls production of the proinflammatory cytokines IL-1β and IL-18 and partially regulates MIP1 and KC production. These findings are in line with the demonstration that IL-1, the IL-1RI and IL-18 play a significant role in the innate immune response to influenza A viruses (Liu et al., 2004; Schmitz et al., 2005), though are less significant for adaptive responses (Denton et al., 2007; Schmitz et al., 2005). Finally, the augmented alveolar fibrosis and increased mortality characteristic of influenza A virus infection in cryopyrin−/− and caspase-1−/− mice underscores the importance of this inflammasome for maintaining respiratory integrity in the damaged lung.
EXPERIMENTAL PROCEDURES
Virus
Influenza virus A/Puerto Rico/8/34 (PR8) was generated by an eight-plasmid reverse genetics system (Hoffmann et al., 2002). Stocks were propagated no more than twice by allantoic inoculation of 10-day-old embryonated hen’s eggs with seed virus diluted 1:106. Virus titers were determined as 50% egg infectious dose (EID50).
Mice
The cryopyrin−/− (CRYO−/−) ipaf−/− (IPAF−/−) and caspase-1−/− (CASP1−/−) mice have been described (Franchi et al., 2006; Kanneganti et al., 2007a), the WT C57BL/6J mice were purchased from the Jackson Laboratories, Bar Harbor ME, and housed in an SPF facility. All experiments were conducted under protocols approved by the St. Jude Children’s Research Hospital Committee on Use and Care of Animals.
Virus infection and sampling
Mice were anaesthetized with Avertin (2,2,2-tribromoethanol) and infected i.n. with diluted virus (2–8×103 EID50) in 30μL of endotoxin-free phosphate-buffered saline (PBS). They were then either weighed and monitored for mortality daily for a period of 14d, or sacrificed at various intervals for sampling (Allan et al., 1990) the lung lumen by bonchoalveolar lavage (BAL). The BAL fluid was collected in 3×1 mL washes. Following light centrifugation, total cell numbers/BAL were determined using a Coulter Counter (IG Instrumenten Gesellschaft AG) and cells were processed for further analysis as indicated. Aliquots of BAL and spleen cell populations were stained with allophycocyanin-labeled anti-CD11b, phycoerythrin (PE)-labeled anti-Class II, anti-Ly6g FITC and anti-CD11c PE-Cy5 (eBioscience) MAbs, after blocking the Fc receptor with anti-CDAY 32/CD16 MAb (from BD Pharmingen unless indicated differently) at 4°C, and analyzed by flow cytometry. A 10% (w/v) lung homogenate was prepared for virus plaque assay in PBS using a Tissue Tearor homogenizer (Fisher Scientific). Blood was recovered via terminal retro-orbital bleed.
Viral titer determination
Lung homogenates were titered by plaque assay on Madin-Darby Canine Kidney (MDCK) cells. Near confluent 9.6 cm2 MDCK cell monolayers were infected with 1 mL aliquots of 6×10 fold dilutions of lung homogenate for 1 hr at 37°C, then washed in PBS before adding 3 ml MEM containing 1 mg/ml L-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin (Worthington) and 0.9% agarose. Cultures were incubated at 37°C, 5% CO2 for 72 h. Plaques were visualized with crystal violet.
Measurement of cytokines and chemokines
Mouse cytokines and chemokines in BAL fluid and serum were measured using the BioRad multiplex assay following the manufacturer’s instructions. Blood gases were measured from arterial blood obtained via cardiac puncture using an iStat analyzer (Heska), cartridge CG8+.
Elispot Assay
96-well nitrocellulose-bottomed multiscreen HA filtration plates (Millipore, Bedford, MA) were coated (1μg/well) with a concentrated, purified PR8 virus preparation that had been incubated with disruption buffer (0.5% Triton X-100, 0.6 M KCl and 0.05 M Tris-Hcl, pH 7.5) for 15 minutes at room temperature. After overnight incubation at 4 °C, the plates were washed with PBS then blocked. Next, different dilutions of cell suspensions were added in volumes of 100 μl/well. After 4 hours incubation at 37 °C in a humidified atmosphere containing 5% CO2, the plates were washed with PBS and Alkaline Phosphatase-conjugated goat anti-mouse IgM, IgG or IgA (Southern Biotechnology, Birmingham, AL) diluted in PBS containing 5% BSA were added (100μl/well) and incubated overnight at 4°C. Spots were developed by adding 100μl/well of BCIP/NBT Phosphatase substrate (KPL, Gaithersburg, MD), then counted with an Olympus SZX9 stereozoom microscope for analyisis by KS Elispot software (Zeiss, Hallbergmoos, Germany).
Antigen-specific ELISA
Briefly, microtiter plates (Corning) were coated overnight at 4°C with purified whole PR8 virus in PBS. Influenza-specific IgG1 was detected with a goat anti-mouse IgG alkaline-phosphatase conjugate (Southern Biotechnology Associates) diluted 1/1,000 in PBS with 1% BSA. A substrate (p-nitrophenyl phosphate; Sigma-Aldrich) was added, plates were incubated for 60 min at room temperature for color development, and OD values were determined at 405 nm in an ELISA reader (Molecular Devices). Titers were presented as the highest dilution that yielded an OD three times higher than that for a 1/100 dilution of pre-immune serum.
Measuring Virus-Specific CD8+ T Cells
Single-cell suspensions of spleen or BAL were incubated for 60 min at room temperature with APC or PE-conjugated tetramers corresponding to the epitopes of interest (DbNP366, DbPA224, KbPB1703 or DbPB1-F262), followed by 20-min incubation at 4°C with anti-CD8 α (BD Pharmingen) and anti-mouse CD16/CD32 (clone 2.4G2) mAb (BD Pharmingen) to block nonspecific Fc-receptor-mediated binding. All samples were washed in PBS and resuspended in 2%-PBS azide for detection of fluorochrome-labeled cells on a FACS Calibur (BD Biosciences). Data were analyzed using FlowJo software (Tree Star).
Transfection of BM-DC
Bone marrow derived murine dendritic cells (Lutz et al., 1999) were scraped off the culture dish, seeded on to 24 well plates, then cultured overnight (37°C and 10% CO2) in high glucose complete DMEM + 10 ng/mL GM-CSF. On the following day, the cells were washed once with serum-free DMEM media and fed fresh complete media. Viral RNA (2μg isolated from sucrose density gradient purified PR8) was then added to diluted TransIT-LT1 transfection reagent (Mirus) (50 μl serum free DMEM+1 μl of TransIT-LT1 per well of a 24 well plate). The RNA-transfection reagent mix was incubated at room temperature for 15–30 minutes, then the RNA complexes were added drop-wise to the DCs whilst mildly rocking. The cells were incubated (37°C, 10% CO2) for 24 hrs before collecting the media for cytokine analysis. Other DCs were mock transfected as a negative control.
Supplementary Material
Supplementary Figure 1: IPAF is not required for protection against Influenza A virus-induced lethality. Groups of 10 WT and ipaf−/− (IPAF−/−) mice were infected i.n. with 8×103 EID50 (= 1LD50) of PR8 and survival was monitored daily for 14d. Differences in group survival were analyzed with Cox proportional hazards test and p<0.05 was considered statistically significant.
Supplementary Figure 2: Induction of inflammatory cytokines and chemokines in the respiratory tract of mice with influenza. Levels of IL-1β (A), IL-18 (B), TNF-α (C), IL-6 (D), KC (E), MIP-2 (F), IFN-γ (G) and IL-12p40 (H) were determined for individual (n ≥ 4) lung BAL washes from naïve (d0) and day 3 and d6 infected (4×103 EID50 of PR8 i.n.) B6 WT mice. Bars represent the mean ± SD of triplicate wells. Results are representative of three independent experiments.
Supplementary Figure 3: Influenza virus-induced IFN-α production depends on cryopyrin and caspase-1. Groups (n=4) of B6 (WT), cryopyrin−/− (CRYO−/−) and casase-1−/− (CASP1−/−) mice were infected i.n with 4×103 EID50 of PR8 influenza virus and sampled 3d later to determine levels of IFN-α in BAL fluid. Results are mean ± SE and are representative of three independent experiments.
Supplementary Figure 4: Caspase-1 is required for influenza virus-induced secretion of cryopyrin-dependent cytokines. Groups of 4 B6 (WT) and caspase-1−/− (CASP1−/−) mice were infected i.n with 4×103 EID50 of PR8 influenza virus and levels of IL-1β (A), and IL-18 (B) were measured in serum 3d later. Results are mean ± SE and are representative of three independent experiments.
Supplementary Figure 5: Caspase-1 is required for influenza virus RNA induced secretion of cryopyrin-dependent cytokines. B6 (WT) and caspase-1−/− (CASP1−/−) mice were injected i.p. with 50 μg influenza virus RNA and levels of IL-1β were measured in serum 6 h later.
Supplementary Figure 6: Cryopyrin-deficiency results in severely compromised lung function following infection. WT and cryopyrin−/− animals were infected i.n. with 8×103 EID50 (= 1LD50) of PR8 and arterial blood was analyzed on d10 following infection for partial pressure of oxygen (mmHg 02). Uninfected controls were analyzed at the same time. Results are mean ± SE and were analyzed by Student’s t-test (* indicates p<0.05).
Acknowledgments
We thank Anthony Coyle, Ethan Grant, John Bertin (Millennium Pharmaceuticals), Gabriel Nuñez (University of Michigan) and Richard Flavell (Yale) for the generous supply of mutant mice and Jennifer Rogers from the Cellular Immunology Core Facility of St. Jude Children’s research hospital for technical support. This work was supported by NIH grant AR056296 to T-D.K, AI065097 to P.G.T, AI170251 to P.C.D, and NIAID Contract No. HHSN266200700005C.
The authors declare that they have no competing financial interests.
Abbreviations
- BAL
bronchoalveolar lavage
- DC
dendritic cell
- H
influenza virus hemagglutinin
- HP
high pathogenicity
- i.n
intranasal
- IL
interleukin
- N
influenza virus neuraminidase
- NLR
NOD-like receptor
- PFU
plaque-forming unit
- TLR
Toll-like receptor
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, et al. Regulation of Legionella phagosome maturation and infection through flagellin and host IPAF. J Biol Chem. 2006 doi: 10.1074/jbc.M604933200. [DOI] [PubMed] [Google Scholar]
- Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol. 2003;15:26–30. doi: 10.1016/s0952-7915(02)00017-1. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton AE, Doherty PC, Turner SJ, La Gruta NL. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur J Immunol. 2007;37:368–375. doi: 10.1002/eji.200636766. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. [PubMed] [Google Scholar]
- Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201:1355–1359. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr. 2006;83:447S–455S. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1beta augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1184–1190. doi: 10.1152/ajplung.2000.279.6.L1184. [DOI] [PubMed] [Google Scholar]
- Hampton T. Virulence of 1918 influenza virus linked to inflammatory innate immune response. JAMA. 2007;297:580. doi: 10.1001/jama.297.6.580. [DOI] [PubMed] [Google Scholar]
- Haq M, Norman J, Saba SR, Ramirez G, Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol. 1998;9:614–619. doi: 10.1681/ASN.V94614. [DOI] [PubMed] [Google Scholar]
- Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Krauss S, Perez D, Webby R, Webster RG. Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine. 2002;20:3165–3170. doi: 10.1016/s0264-410x(02)00268-2. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science (New York, NY) 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Iida N, Grotendorst GR. Cloning and sequencing of a new gro transcript from activated human monocytes: expression in leukocytes and wound tissue. Mol Cell Biol. 1990;10:5596–5599. doi: 10.1128/mcb.10.10.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007a;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007b;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12:9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. Am J Pathol. 2008;172:1155–1170. doi: 10.2353/ajpath.2008.070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science (New York, NY) 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kanneganti TD, Franchi L, Nunez G. Caspase-1 inflammasomes in infection and inflammation. Journal of leukocyte biology. 2007;82:220–225. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2:e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- Lira SA, Zalamea P, Heinrich JN, Fuentes ME, Carrasco D, Lewin AC, Barton DS, Durham S, Bravo R. Expression of the chemokine N51/KC in the thymus and epidermis of transgenic mice results in marked infiltration of a single class of inflammatory cells. J Exp Med. 1994;180:2039–2048. doi: 10.1084/jem.180.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Mori I, Hossain MJ, Dong L, Takeda K, Kimura Y. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J Gen Virol. 2004;85:423–428. doi: 10.1099/vir.0.19596-0. [DOI] [PubMed] [Google Scholar]
- Lopez CB, Moltedo B, Alexopoulou L, Bonifaz L, Flavell RA, Moran TM. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J Immunol. 2004;173:6882–6889. doi: 10.4049/jimmunol.173.11.6882. [DOI] [PubMed] [Google Scholar]
- Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- Olman MA, White KE, Ware LB, Simmons WL, Benveniste EN, Zhu S, Pugin J, Matthay MA. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 beta-induced IL-6 expression. J Immunol. 2004;172:2668–2677. doi: 10.4049/jimmunol.172.4.2668. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science (New York, NY) 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- Pirhonen J, Sareneva T, Julkunen I, Matikainen S. Virus infection induces proteolytic processing of IL-18 in human macrophages via caspase-1 and caspase-3 activation. Eur J Immunol. 2001;31:726–733. doi: 10.1002/1521-4141(200103)31:3<726::aid-immu726>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. Virus infection activates IL-1 beta and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322–7329. [PubMed] [Google Scholar]
- Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Shanley TP, Schmal H, Warner RL, Schmid E, Friedl HP, Ward PA. Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J Immunol. 1997;158:3439–3448. [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, et al. Critical Role for NALP3/CIAS1/Cryopyrin in Innate and Adaptive Immunity through Its Regulation of Caspase-1. Immunity. 2006a;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Zamboni DS, Roy CR, Flavell RA. NALP3: a key player in caspase-1 activation. J Endotoxin Res. 2006b;12:251–256. doi: 10.1177/09680519060120040701. [DOI] [PubMed] [Google Scholar]
- Thickett DR, Perkins GD. IL1 may be elevated but is it all bad in ARDS? Thorax. 2008;63:750–751. author reply 751. [PubMed] [Google Scholar]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. Journal of leukocyte biology. 2004;76:886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- Wewers MD. IL-1beta: an endosomal exit. Proc Natl Acad Sci U S A. 2004;101:10241–10242. doi: 10.1073/pnas.0403971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewers MD, Dare HA, Winnard AV, Parker JM, Miller DK. IL-1 beta-converting enzyme (ICE) is present and functional in human alveolar macrophages: macrophage IL-1 beta release limitation is ICE independent. J Immunol. 1997;159:5964–5972. [PubMed] [Google Scholar]
- Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: IPAF is not required for protection against Influenza A virus-induced lethality. Groups of 10 WT and ipaf−/− (IPAF−/−) mice were infected i.n. with 8×103 EID50 (= 1LD50) of PR8 and survival was monitored daily for 14d. Differences in group survival were analyzed with Cox proportional hazards test and p<0.05 was considered statistically significant.
Supplementary Figure 2: Induction of inflammatory cytokines and chemokines in the respiratory tract of mice with influenza. Levels of IL-1β (A), IL-18 (B), TNF-α (C), IL-6 (D), KC (E), MIP-2 (F), IFN-γ (G) and IL-12p40 (H) were determined for individual (n ≥ 4) lung BAL washes from naïve (d0) and day 3 and d6 infected (4×103 EID50 of PR8 i.n.) B6 WT mice. Bars represent the mean ± SD of triplicate wells. Results are representative of three independent experiments.
Supplementary Figure 3: Influenza virus-induced IFN-α production depends on cryopyrin and caspase-1. Groups (n=4) of B6 (WT), cryopyrin−/− (CRYO−/−) and casase-1−/− (CASP1−/−) mice were infected i.n with 4×103 EID50 of PR8 influenza virus and sampled 3d later to determine levels of IFN-α in BAL fluid. Results are mean ± SE and are representative of three independent experiments.
Supplementary Figure 4: Caspase-1 is required for influenza virus-induced secretion of cryopyrin-dependent cytokines. Groups of 4 B6 (WT) and caspase-1−/− (CASP1−/−) mice were infected i.n with 4×103 EID50 of PR8 influenza virus and levels of IL-1β (A), and IL-18 (B) were measured in serum 3d later. Results are mean ± SE and are representative of three independent experiments.
Supplementary Figure 5: Caspase-1 is required for influenza virus RNA induced secretion of cryopyrin-dependent cytokines. B6 (WT) and caspase-1−/− (CASP1−/−) mice were injected i.p. with 50 μg influenza virus RNA and levels of IL-1β were measured in serum 6 h later.
Supplementary Figure 6: Cryopyrin-deficiency results in severely compromised lung function following infection. WT and cryopyrin−/− animals were infected i.n. with 8×103 EID50 (= 1LD50) of PR8 and arterial blood was analyzed on d10 following infection for partial pressure of oxygen (mmHg 02). Uninfected controls were analyzed at the same time. Results are mean ± SE and were analyzed by Student’s t-test (* indicates p<0.05).