Abstract
Background
The ratio of two nicotine metabolites, cotinine (COT) and trans-3′-hydroxycotinine (3-HC), has been validated as a method of phenotyping the activity of the liver enzyme cytochrome P450 (CYP) 2A6, and thus the rate of nicotine metabolism. Our objective was to evaluate the correlates and stability of the 3-HC:COT ratio in ad libitum and reducing smokers, using nicotine replacement therapy (NRT), over a period of months.
Methods
Smokers (N = 123, 94% Caucasian) participated in a smoking reduction study where one-third of the sample smoked ad libitum for 8 weeks (Waitlist phase), before joining the rest of the participants for 12 weeks of cigarette reduction (Reduction phase) using NRT. Urinary nicotine, cotinine, and 3-HC were measured at each visit.
Results
The baseline 3-HC:COT ratio was significantly but weakly correlated with cigarettes/day (r = .19), body mass index (r = -.27), and waking at night to smoke (r = .23). As assessed by repeated measures ANOVA, the 3-HC:COT ratio was stable in the Waitlist phase (coefficient of variation for 3-4 measurements, 38% [Range = 5%-110%]), while minor variation was noted in the Reduction phase (coefficient of variation for 3-5 measurements, 35% [Range = 10%-107%]).
Conclusions
In non-reducing ad libitum smokers, the 3-HC:COT ratio was generally stable, while during smoking reduction using NRT, some small variation was detected. While the current findings are suggestive of the stability of the 3-HC:COT ratio in a predominantly Caucasian sample smoking freely or reducing smoking with NRT, additional research is needed in more diverse populations.
Individual differences in nicotine metabolism have been shown to influence the developmental spectrum of nicotine dependence from acquisition to maintenance to cessation (1). Nicotine is primarily metabolized by the liver enzyme cytochrome P450 (CYP) 2A6 to cotinine. Accordingly, one approach to estimating the rate of nicotine metabolism has been to genotype CYP2A6. While genotypic data have been informative in explaining individual differences in smoking behavior, such data may be an imperfect index of enzyme activity, due to the presence of exogenous (e.g., menthol) or endogenous (e.g., female sex hormones) chemicals that can induce or inhibit CYP2A6 (2, 3). Thus, phenotypic assessment of CYP2A6 activity and nicotine clearance is also important.
Benowitz, Pomerleau, Pomerleau, and Jacob (4) suggested measuring the ratio of two nicotine metabolites, cotinine (COT) and trans-3′-hydroxycotinine (3-HC), as a measure of CYP2A6 activity. This ratio has been used to phenotype CYP2A6 activity, since CYP2A6 is the primary enzyme mediating nicotine metabolism, and CYP2A6 also catalyzes the conversion of cotinine to 3-HC. Hence, the 3-HC:COT ratio is a surrogate measure of CYP2A6 activity and nicotine clearance. A growing body of research has validated the 3-HC:COT ratio, showing it to be moderately correlated with both CYP2A6 genotype and the rate of nicotine metabolism (1, 3, 5-9).
While the 3-HC:COT ratio seems to correlate well with independent measures of nicotine clearance, limited data on its stability over time within individuals are available. Lea et al., (10) examined the stability of the 3-HC:COT ratio in 6 smokers, sampling morning and evening, over 7 consecutive days. The authors did not observe diurnal differences nor substantial variation over the sample week. However, the stability of the 3-HC:COT ratio has not been evaluated over a period of weeks or months, or during changes in nicotine intake. The purpose of this report was to evaluate the stability of the 3-HC:COT ratio over a period of 5 months in a well-defined population evaluated in the context of a within-subject study of nicotine replacement therapy (NRT) facilitated smoking reduction (11, 12). First, we examined convergent validity of the 3-HC:COT ratio in our sample, by assessing correlations between the ratio and baseline demographic, smoking, and smoking history variables. Second, we assessed whether the 3-HC:COT ratio was stable during extended ad libitum smoking and smoking reduction with NRT.
Methods
Subjects
The current analytic sample was drawn from a larger study of scheduled smoking reduction using NRT (11, 12). All subjects provided written, informed consent before beginning this study approved by the University of Minnesota Institutional Review Board. The sample was comprised of a total of 123 subjects (52% female; 94.3% Caucasian); the mean age was 45.6 years (SD = 10.4, Range = 20.0-68.0). The mean body mass index (BMI) was 27.0 kg/m2 (SD = 6.1). Among females (n = 64), 7.8% reported using hormone birth control (e.g., orthotricylcin). Baseline smoking characteristics were as follows: mean smoking rate, 26.4 cigarettes/day (SD = 7.2, Range = 15.0-50.0); and years of cigarette use, 16.6 (SD = 12.1, Range = 1.0-50.0). Most smoked regular or medium cigarettes (92.5%) that were not mentholated (92.5%). In terms of nicotine dependence, the average Fagerstrom Test of Nicotine Dependence (FTND)(13) score was 5.8 (SD = 1.5, Range = 2.0-9.0), with 45.5% of subjects smoking their first cigarette within 5 minutes of awakening. In past quit attempts, the mean number of DSM-IV nicotine withdrawal symptoms experienced in a previous quit attempt was 3.9 (SD = 1.9). The mean longest quit attempt was 348 days (Range = 1-4,015).
Procedures
The study consisted of two consecutive phases: (1) Waitlist ad libitum smoking (8 weeks); and (2) Reduction with NRT (12 weeks). One-third of subjects completed the 8-week, Waitlist ad libitum smoking phase, before joining the remaining sample in a 12-week Reduction phase that consisted of 6 weeks of scheduled reduction followed by 6 weeks of cigarette reduction maintenance. In the scheduled reduction phase, smokers were expected to reduce their daily cigarette use in three, consecutive two-week stages (as a % of baseline smoking, cigarettes/day): (1) weeks 1-2, 25% reduction; (2) weeks 3-4, 50% reduction; (3) weeks 5-6, 75% reduction; and (4) weeks 7-12, maintain 75% reduction. Evidence of reduction was seen in decreases in a number of tobacco-related biomarkers, including 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL)(11). To assist cigarette-smoking reduction, participants were provided brief behavioral treatment and NRT (i.e., 4 mg Nicorette® gum as well as 14 and 21 mg Nicoderm® CQ patches). Following scheduled reduction, subjects attempted to maintain their reduction levels or to further decrease cigarette consumption for an additional 6 weeks while continuing NRT use.
Biochemical and subjective measurements
At each clinic visit, urine samples were collected for assessment of nicotine, cotinine, and 3-HC levels. The large majority of samples were collected in the morning hours (Median = 10:15 AM); a recent reports suggests that sampling time during the day does not influence the 3-HC:COT ratio(10). The urinary levels of cotinine, nicotine and 3-HC were determined by GC/MS analysis as previously described (12). The reported drug or metabolite concentrations represent the totals of free and glucuronidated forms (c.f., 4). Urine concentrations were normalized to urinary creatinine concentrations (i.e., nmol compound/mg creatinine). Carbon monoxide (CO) was measured in expired breath samples at each clinic visit using a Bedfont Micro Smokerlyzer (Bedfont, Medford, NJ). The daily cigarette smoking rate (CPD) was determined by averaging self-recorded smoking on daily diary cards (which captured the date and time each cigarette was smoked). Self-reported demographic characteristics and smoking-history variables were collected at baseline by questionnaire(13).
Data analysis
All analyses were conducted with the Statistical Analysis System Version 9.1.3 (14). Available sample sizes differed because of attrition and occasional missing data (the maximum and minimum sample sizes were 123 at baseline and 47 in the Waitlist phase). Values of p < .05 were considered statistically significant, based on two-tailed tests, unless otherwise specified. Spearman rank-order correlations were used to assess univariate associations. Correlates of the 3-HC:COT ratio were subsequently evaluated in a full-rank multiple linear regression model, using log-normalized ratios as the dependent variable. Stability of the 3-HC:COT ratio was examined in two ways. First the coefficient of variation ([standard deviation/mean] × 100) was computed to indicate the relative percentage of variability around the mean (c.f., 10). Second, we used repeated-measures ANOVA models to assess for changes in log-normalized 3-HC:COT ratios over time. Type I error rate was controlled using a Tukey adjustment.
Results
Baseline biochemical levels
Median baseline biochemical levels were: expired air CO, 20 parts-per-million (Range = 5-55), total urine nicotine, 8.7 nmol/mg creatinine (Range = 1.1-46.8), total urine cotinine level, 24.6 nmol/mg creatinine (Range = 4.9-90.4); and total trans-3′-hydroxycotinine, 39.8 nmol/mg creatinine (Range = 1.8-167). The distribution of the 3-HC:COT ratio followed a log-normal distribution with a median of 2.0 (Range = 0.1-11.3).
Correlations between baseline sample characteristics and 3-HC:COT ratio
Spearman rank-order correlations were computed between demographic, metabolic, and nicotine dependence variables and the 3-HC:COT ratio (measured at baseline, before the Waitlist or Reduction phases). Available sample sizes ranged from 123 to 64 (for the birth control analysis restricted to females). The 3-HC:COT ratio was positively associated with CPD, r = .19, p <.05. Higher 3-HC:COT ratios were associated with lower BMI, r = -.27, p <.01. Those reporting waking at night to smoke tended to have higher 3-HC:COT ratios, r = .23, p <.05. The association between 3-HC and 3-HC:COT was strong, r = .68, p <.0001.
We further evaluated the average 3-HC:COT ratio during weeks 2, 6, and 8 of the Waitlist phase with the three baseline variables that were associated with the baseline 3-HC:COT ratio. We found that in this reduced sample (N = 47), that the correlations were similar in direction, although the magnitudes were somewhat larger for CPD, r = .38, p <.01; and waking at night to smoke, r = -.49, p<.001, but not BMI, r = -.21, n.s.
Based on the foregoing univariate analyses, we modeled log-normalized 3-HC:COT as a function of the predictors cigarettes/day, BMI, and waking to smoke, with age and sex included as covariates. All three predictors remained significant: BMI, β = -0.03, t = -2.27, P = .03; cigarettes/day, β = 0.02, t = 2.07, P = .04; and waking to smoke, β = .36, t = 2.14, P =.03. However, only 9% of the total variability in the 3-HC:COT ratios was accounted for by the model.
Stability of nicotine metabolism
The stability of nicotine metabolism was first examined through calculating the coefficient of variation for each subject, during the Waitlist and Reduction phases. In the 8-week Waitlist phase (3-4 observations), subjects showed an average within-subject variation as follows: total cotinine, 32% (Range = 2%-103%); 3-HC, 35% (Range = 5%-72%); and the 3-HC ratio, 38% (Range = 5%-110%). In the 12-week Reduction phase (3-5 observations), subjects showed an average within-subject variation as follows: total cotinine, 39% (Range = 8%-148%); 3-HC, 40% (Range = 1%-180%); and the 3-HC:COT ratio, 35% (Range = 10%-108%).
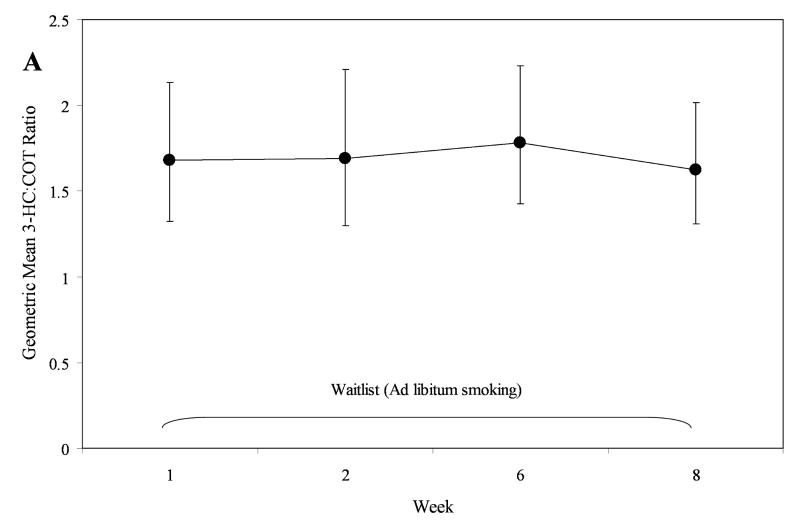
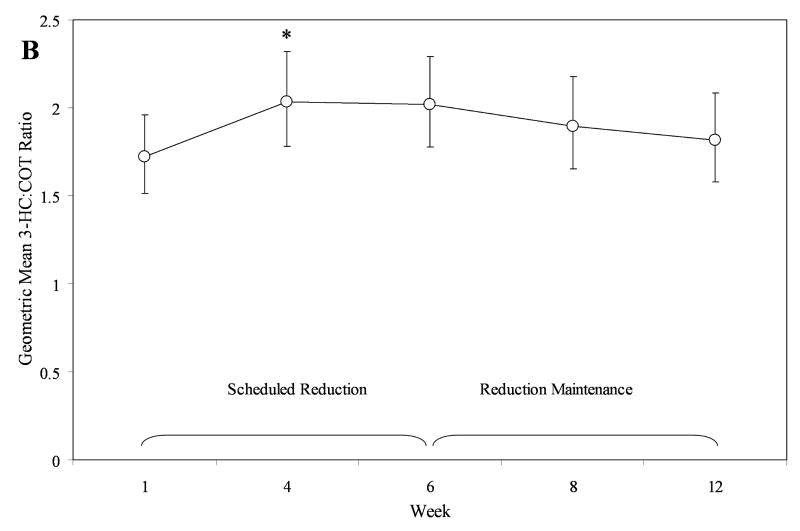
To formally assess the stability of the 3-HC:COT ratio, we evaluated repeated measures within subject models for the Reduction and Waitlist phases individually, using log-normalized 3-HC:COT ratios. From each model, we back-transformed estimated least square means and associated 95% confidence intervals to estimated geometric means (see Figure 1). In the Waitlist phase, the ratio remained stable, time, F = 0.73, P = 0.53 (see Figure 1, Plot A). In the Reduction Phase, slight variation was observed, time, F = 2.94, P = 0.02. This effect was primarily driven by a small increase in the metabolic ratio from week 1 to week 4, t = 2.93, P = 0.029 (see Figure 1, Plot B). No other significant differences between time points in the Reduction Phase were detected.
Figure 1.
Temporal stability of the 3-HC:COT ratio in those in the Reduction phase (Plot A) and Waitlist phase (Plot B). Values are geometric means and 95% CIs, back transformed from log 3-HC:COT. *Week 4 significantly greater than Week 1.
Discussion
In our univariate and multivariate analyses, cigarettes/day, waking to smoke at night, and body mass index were associated with the 3-HC:COT ratio. The association between faster nicotine metabolism and greater daily smoking has been noted before, e.g.,(4). This relationship may arise since smokers seek to maintain satisfactory blood concentrations, and therefore increased nicotine clearance leads to increased smoking. Concerning the association between faster nicotine metabolism and rising at night to smoke, Reider et al., (15) have described a clinical phenomenon, “nocturnal sleep-disturbing nicotine craving” (NSDNC), which involves the smoker waking with a need to smoke a cigarette in order to fall back to sleep. From sleep onset to awakening, plasma nicotine levels fall as a function of nicotine metabolism, although nicotine clearance is somewhat lower at night (16). Our present observation of faster nicotine metabolism in those rising to smoke is consistent with NSDNC. In the case of BMI, the 3-HC:COT ratio tended to decline with increased body mass, indicting slower nicotine metabolism. Relatively little work has been published on the effects of obesity on nicotine pharmacokinetics(17), and further pharmacokinetic studies are needed. These results lend some evidence of concurrent validity the 3-HC:COT ratio.
Given the increasing importance of characterizing nicotine metabolism in smokers for research as well as potentially for treatment, a rapid measure of CYP2A6 activity will be highly valuable. The 3-HC:COT ratio has shown good concordance with other measures of nicotine clearance, including the CYP2A6 genotype and controlled laboratory tests of nicotine clearance. Levi and coworkers (7, 8) have demonstrated that the predictive relationship of 3-HC:COT ratio to nicotine clearance remains consistent under different patterns and quantity of smoking, permitting spot sampling. An important question concerns the long-term stability of the 3-HC:COT ratio. Following smokers for 7 days, Lea et al. (10) observed stability in the 3-HC:COT ratio within time of day and across the week (CV = 26%). In our sample, we extended the work of Lea and colleagues (10) to evaluate the stability of the 3-HC:COT ratio in both ad libitum and NRT-assisted reducing smokers over a period of months. We observed similar CVs in both the ad libitum Waitlist smokers (CV = 38%) and in those undergoing NRT-assisted smoking reduction (CV = 35%), however with some range of variation. We found that the variation in cotinine and 3-HC were similar to the 3-HC:COT ratio. Some evidence of a small increase in the 3-HC:COT ratio in NRT-assisted reducing smokers in the first month of reduction, indicates higher nicotine clearance. However, factors related to this transient increased nicotine clearance are unknown. Overall, our findings further support the use of a single assessment of nicotine metabolism through the 3-HC:COT ratio.
One limitation of the current study is that it relied solely on urine to assess tobacco biomarkers. While the agreement between urine, saliva, and plasma matrices is generally good(18, 19), the current report would have been strengthened by including saliva or plasma measurements. A second limitation of this study was that 94.3% of participants were Caucasian. In addition, few participants were exposed to exogenous chemicals known to induce metabolism of nicotine (i.e., 7.5% smoked mentholated cigarettes, 7.8% of females used hormone birth control). The effects of racial and ethnic differences as well as exogenous chemicals on nicotine metabolism have been noted, and evaluation of the stability of the 3-HC:COT ratio in more diverse samples is still needed. A third limitation was that due to concurrent use of two forms of NRT in the reduction phase, we were unable to meaningfully evaluate the role of the 3-HC:COT ratio in smoking reduction, since participants were able to compensate for nicotine not obtained from cigarettes with medicinal nicotine. Given recent interest in reduced smoking as an intervention for smokers unwilling or unable to quit, further evaluation of the role of nicotine metabolism in smoking reduction is warranted.
In conclusion, the 3-HC:COT ratio appears to be stable under conditions of extended ad libitum smoking in a mostly Caucasian sample. Some modest variation in the 3-HC:COT ratio was seen during NRT-facilitated reduction. Although the evidence of the temporal stability of the 3-HC:COT ratio is accumulating, additional research in more racially diverse samples is needed to strongly recommend spot sampling of COT and 3-HC as an approach to quickly achieve a useful estimate of nicotine clearance.
Acknowledgments
This research was supported by the NIDA grants P50-DA13333. Dr. Mooney is supported by a NIDA Career Development award K01-DA-019446. GlaxoSmithKline provided the nicotine gum and patches, but had no control or oversight in the preparation of this manuscript. We wish to recognize Nathan Holtz for his help in coding additional data for this report. We thank the participants for taking part in this study.
References
- 1.Malaiyandi V, Sellers EM, Tyndale RF. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clin Pharmacol Ther. 2005;77:145–58. doi: 10.1016/j.clpt.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–15. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- 3.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–8. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–4. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- 5.Dempsey D, Tutka P, Jacob P, 3rd, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone E, Benowitz N, Cargill A, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80:319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Levi M, Dempsey DA, Benowitz NL, Sheiner LB. Prediction methods for nicotine clearance using cotinine and 3-hydroxy-cotinine spot saliva samples II. Model application. J Pharmacokinet Pharmacodyn. 2007;34:23–34. doi: 10.1007/s10928-006-9026-0. [DOI] [PubMed] [Google Scholar]
- 8.Levi M, Dempsey DA, Benowitz NL, Sheiner LB. Population pharmacokinetics of nicotine and its metabolites I. Model development. J Pharmacokinet Pharmacodyn. 2007;34:5–21. doi: 10.1007/s10928-006-9027-z. [DOI] [PubMed] [Google Scholar]
- 9.Malaiyandi V, Goodz SD, Sellers EM, Tyndale RF. CYP2A6 Genotype, Phenotype, and the Use of Nicotine Metabolites as Biomarkers during Ad libitum Smoking. Cancer Epidemiol Biomarkers Prev. 2006;15:1812–9. doi: 10.1158/1055-9965.EPI-05-0723. [DOI] [PubMed] [Google Scholar]
- 10.Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30:386–9. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]
- 11.Hecht SS, Murphy SE, Carmella SG, et al. Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. J Natl Cancer Inst. 2004;96:107–15. doi: 10.1093/jnci/djh016. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SE, Link CA, Jensen J, et al. A comparison of urinary biomarkers of tobacco and carcinogen exposure in smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:1617–23. [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.The SAS System for Windows Version 9.13. Cary, NC: SAS Institute Inc.; [Google Scholar]
- 15.Rieder A, Kunze U, Groman E, Kiefer I, Schoberberger R. Nocturnal sleep-disturbing nicotine craving: a newly described symptom of extreme nicotine dependence. Acta Med Austriaca. 2001;28:21–2. doi: 10.1046/j.1563-2571.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- 16.Gries JM, Benowitz N, Verotta D. Chronopharmacokinetics of nicotine. Clin Pharmacol Ther. 1996;60:385–95. doi: 10.1016/S0009-9236(96)90195-2. [DOI] [PubMed] [Google Scholar]
- 17.Prather RD, Tu TG, Rolf CN, Gorsline J. Nicotine pharmacokinetics of Nicoderm (nicotine transdermal system) in women and obese men compared with normal-sized men. J Clin Pharmacol. 1993;33:644–9. doi: 10.1002/j.1552-4604.1993.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 18.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health. 1988;78:696–8. doi: 10.2105/ajph.78.6.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vine MF, Hulka BS, Margolin BH, et al. Cotinine Concentrations in Semen, Urine, and Blood of Smokers and Nonsmokers. American Journal of Public Health. 1993;83:1335–1338. doi: 10.2105/ajph.83.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]