Abstract
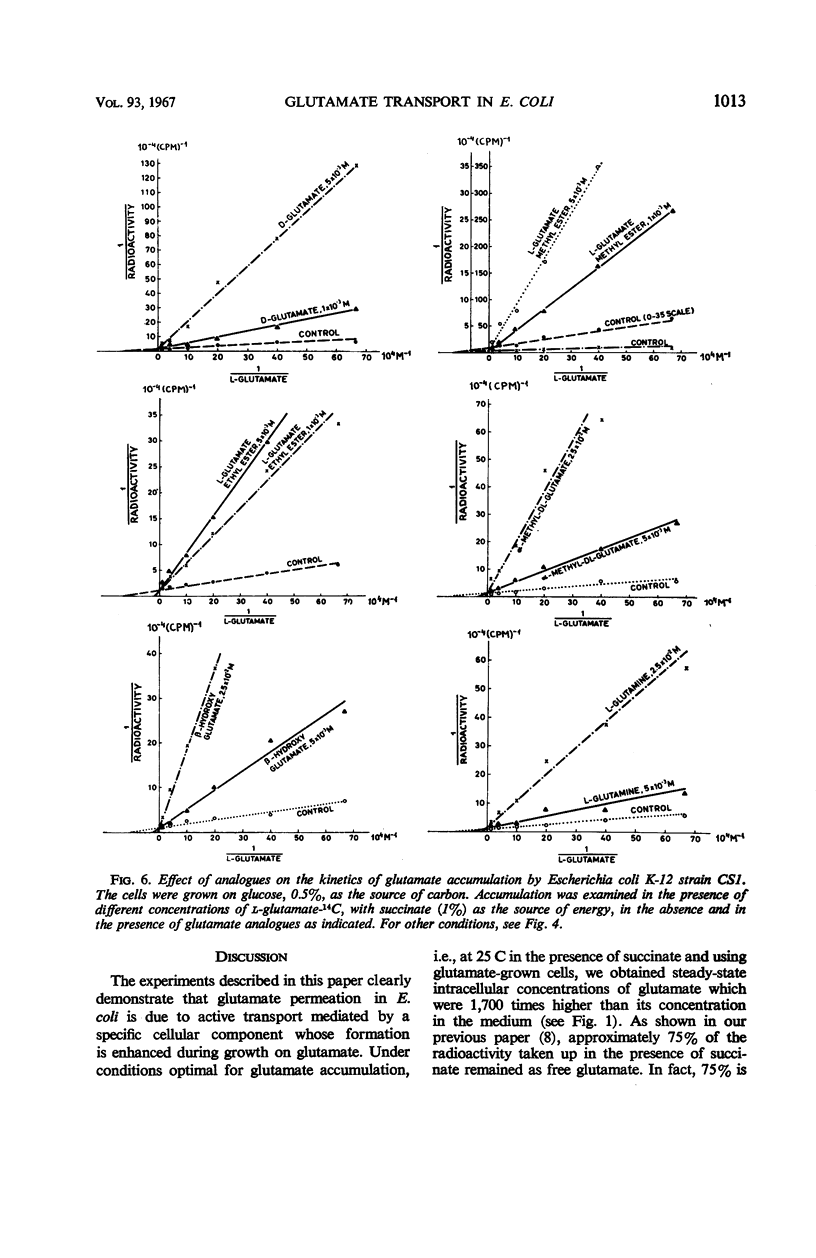
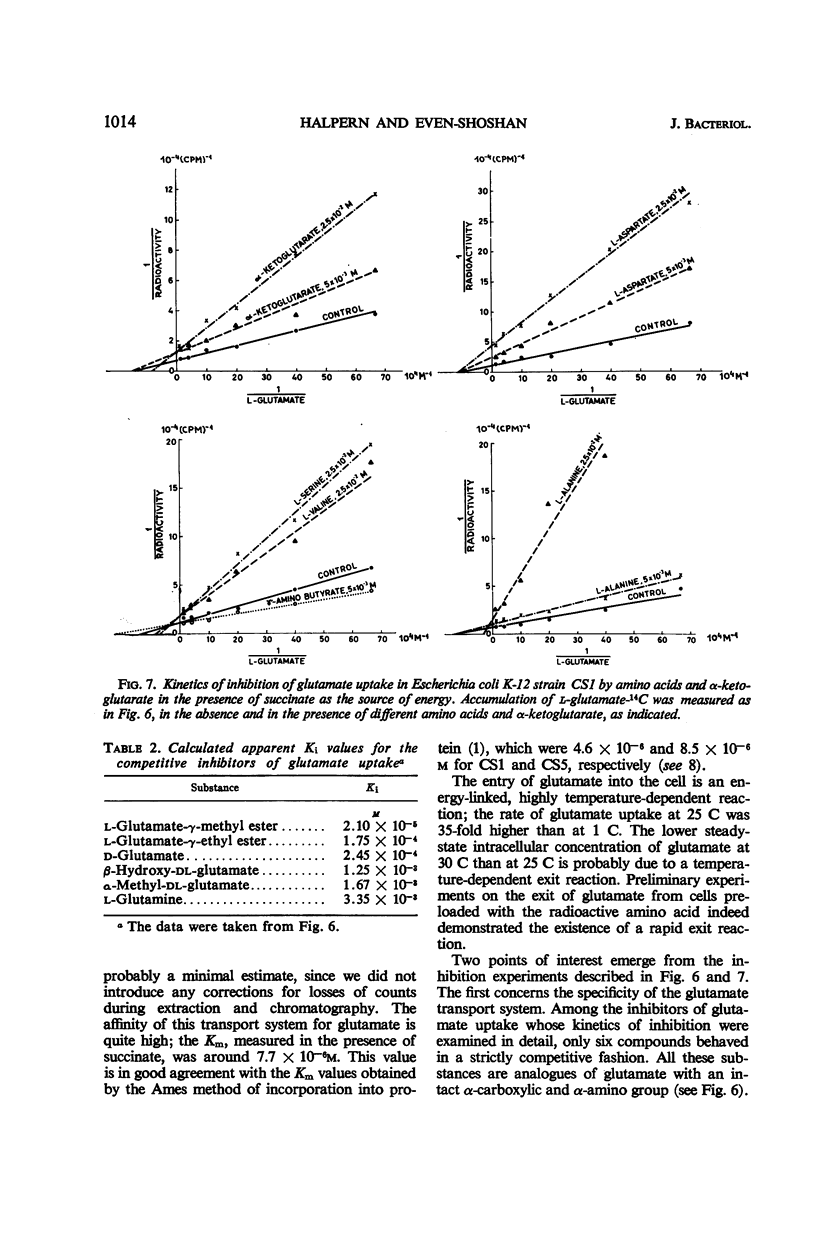
The properties of the glutamate transport system in two glutamate-utilizing mutants of Escherichia coli K-12 were investigated. Growth in the presence of glutamate enhanced the capacity of the bacteria for glutamate uptake. Accumulation of glutamate was found to be an energy-linked highly temperature-dependent process. Nonlinear double reciprocal plots of uptake were obtained in the absence of an exogenous energy source and in the presence of glucose or glycerol. Addition of γ-aminobutyrate, succinate, ketoglutarate, or aspartate accelerated glutamate uptake and brought about “normalization” of its kinetics. Straight-line kinetics of uptake was also observed when succinate served as the source of energy. Under these conditions, aspartate and α-ketoglutarate inhibited glutamate uptake in a noncompetitive fashion, whereas γ-aminobutyrate activated the system. A number of other amino acids were found to act as “noncompetitive” inhibitors. d-glutamate and some derivatives of glutamate with an unsubstituted α-carboxylic and α-amino group inhibited l-glutamate uptake in a strictly competitive fashion. An allosteric permease model, consistent with all of these findings, is proposed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES G. F. UPTAKE OF AMINO ACIDS BY SALMONELLA TYPHIMURIUM. Arch Biochem Biophys. 1964 Jan;104:1–18. doi: 10.1016/s0003-9861(64)80028-x. [DOI] [PubMed] [Google Scholar]
- BURROUS S. E., DEMOSS R. D. STUDIES ON TRYPTOPHAN PERMEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 6;73:623–637. doi: 10.1016/0006-3002(63)90332-9. [DOI] [PubMed] [Google Scholar]
- COHEN G. N., MONOD J. Bacterial permeases. Bacteriol Rev. 1957 Sep;21(3):169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBusk B. G., DeBusk A. G. Molecular transport in Neurospora crassa. I. Biochemical properties of a phenylalanine permease. Biochim Biophys Acta. 1965 Jun 15;104(1):139–150. doi: 10.1016/0304-4165(65)90229-1. [DOI] [PubMed] [Google Scholar]
- HOLDEN J. T., HOLMAN J. Accumulation of freely extractable glutamic acid by lactic acid bacteria. J Biol Chem. 1959 Apr;234(4):865–871. [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Glutamate transport in wild-type and mutant strains of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1288–1295. doi: 10.1128/jb.90.5.1288-1295.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J. T. Restoration of normal glutamic acid transport in vitamin B6-deficient Lactobacillus plantarum by acetate, ammonium, and vitamin B6. Biochim Biophys Acta. 1965 Jun 15;104(1):121–138. doi: 10.1016/0304-4165(65)90228-x. [DOI] [PubMed] [Google Scholar]
- Jacquez J. A., Sherman J. H. The effect of metabolic inhibitors on transport and exchange of amino acids in Ehrlich ascites cells. Biochim Biophys Acta. 1965 Sep 27;109(1):128–141. doi: 10.1016/0926-6585(65)90097-x. [DOI] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- Vogel H. J. REPRESSION OF AN ACETYLORNITHINE PERMEATION SYSTEM. Proc Natl Acad Sci U S A. 1960 Apr;46(4):488–494. doi: 10.1073/pnas.46.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]



