Abstract
The synthesiys and biological evaluation of a series of vancomycin aglycon analogues bearing alternate residue 1 N-methyl-d-amino acids are described. The analogues were prepared to define whether H-bonding d-amino acids could improve the affinity for the model ligands N,N′-Ac2-l-Lys-d-Ala-d-Ala (2) and N,N′-Ac2-l-Lys-d-Ala-d-Lac (3), and improve antimicrobial activity against vancomycin-sensitive or vancomycin-resistant bacteria. Additionally, a series of analogues with appended nucleophiles (hydrazines and amines) on the residue 1 d-amino acids are described that were examined for their ability to react with the C-terminal ester of 3, forming a covalent attachment of l-Lys-d-Ala to the natural product analogues.
Introduction
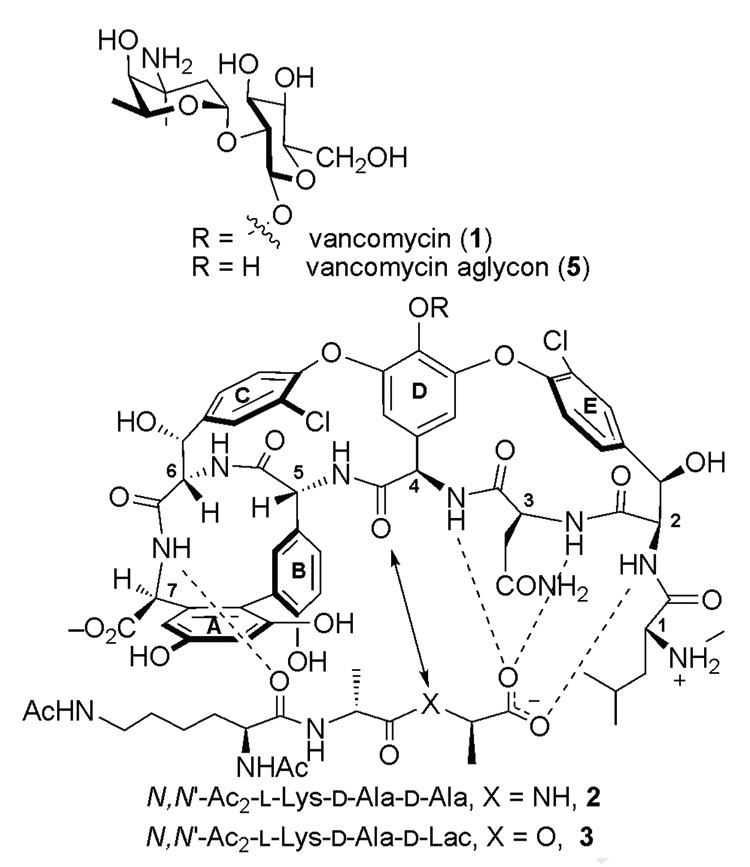
Vancomycin (1) is used as the antibiotic of last resort for the treatment of Gram-positive bacterial infections, especially those caused by methicillin-resistant Staphylococcus aureus, and for patients that are allergic to β-lactam antibiotics. Vancomycin binds to the peptide terminal portion of the bacterial cell wall peptidoglycan precursor, specifically to the sequence l-Lys-d-Ala-d-Ala, thereby inhibiting transpeptidase-catalyzed cross-linking, and maturation of the bacterial cell wall.1 However, Enterocococci bacterial strains, VanA and VanB, have developed an inducible vancomycin-resistance pathway, in which the C-terminal amino acid is modified from d-alanine to d-lactic acid (Figure 1).1,2 This modification (NH→O) reduces the affinity of 1 for the ligand 1000-fold and renders the antibiotic ineffective, with a corresponding 1000-fold drop in antimicrobial activity.1d,k This loss of affinity has been partitioned into the loss of a key H-bond and lone pair repulsive interactions between the residue 4 carbonyl of vancomycin and the ester oxygen of the d-Lac on the C-terminus of the ligand.3,4 The former contributes 10-fold and the latter 100-fold to the experimentally observed 1000-fold loss in affinity for the ligand.3,4
Figure 1.
Schematic representation of the interactions between vancomycin (1), vancomycin aglycon (5), and model ligands N,N′-Ac2-l-Lys-d-Ala-d-Ala (2) and N,N′-Ac2-l-Lys-d-Ala-d-Lac (3).
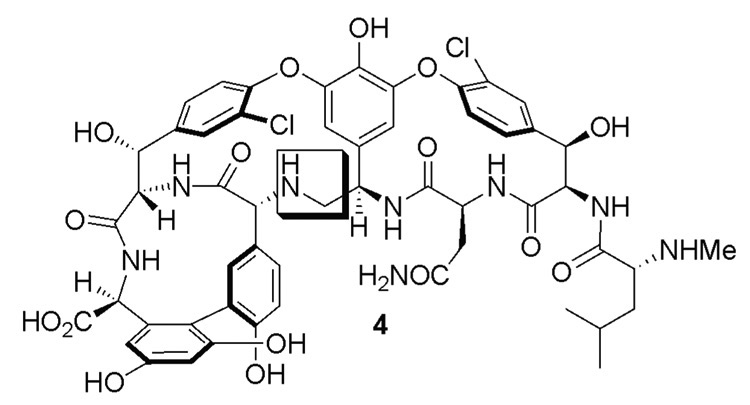
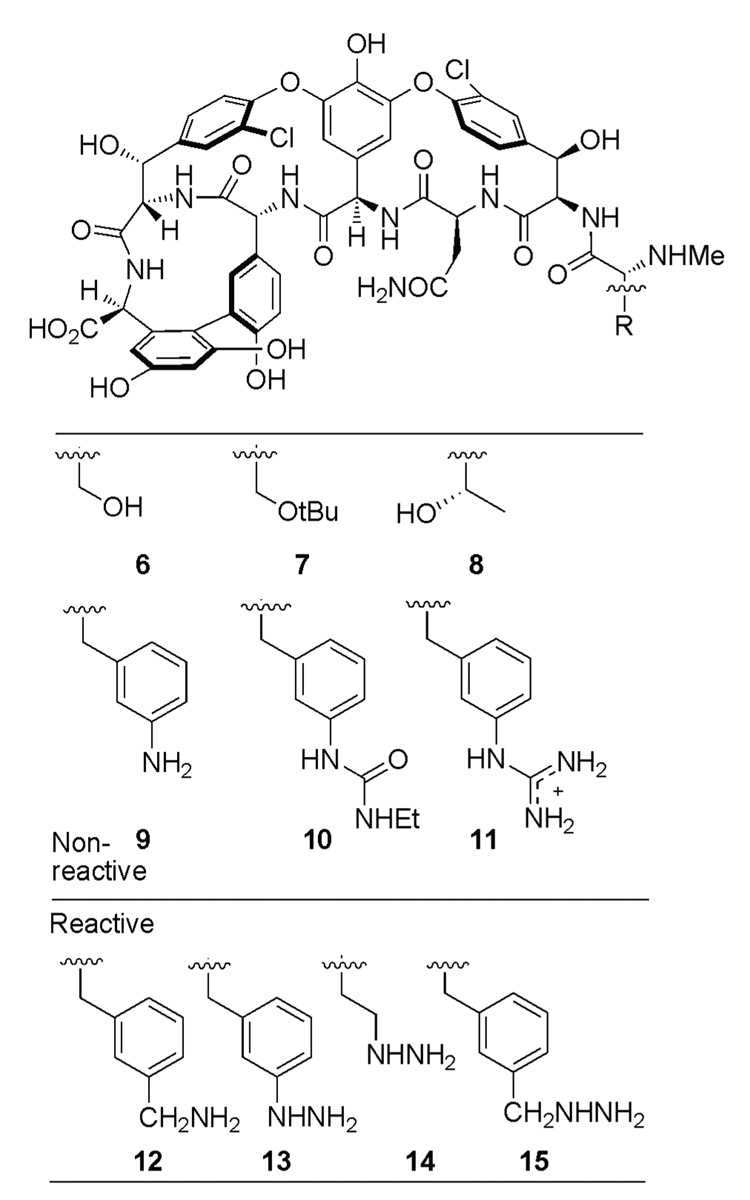
A significant amount of the lost affinity and antimicrobial activity can be restored by simply removing the offending residue 4 carbonyl of the natural product.4 The resulting methylene derivative Ψ[CH2NH]Tpg4]vancomycin aglycon (4) was recently prepared by total synthesis and was shown to exhibit significant activity against resistant VanA bacteria and dual binding properties for both N,N′-Ac2-l-Lys-d-Ala-d-Ala (2) and N,N′-Ac2-l-Lys-d-Ala-d-Lac (3) (Figure 2).4 In continuing efforts to improve on the properties of 4, we wished to establish whether further modification at the N-terminus of 4 with unnatural amino acids presenting appended H-bond donating groups that H-bond to the residue 2 carbonyl of the ligand, might further enhance its affinity for the model ligands 2 or 3, and thereby further improve the antimicrobial properties. However, rather than embark on a total synthesis of such analogues, the premise could be easily investigated first by preparing the analogues on the aglycon of vancomycin 5 (Figure 3), where semi-synthetic procedures are well-established.5,6 Thus, the analogues containing N-terminal d-serine (6), O-tert-butyl-d-serine (7), d-threonine (8), mono- or bi-dentate H-bond donating analogues of 3-amino-d-phenylalanine (9–11) and 3-aminomethyl-d-phenylalanine (12), and their corresponding hydrazine counter parts 13–15 were prepared herein for examination (Figure 4). The modifications at the N-terminus of 5 were expected to alter the binding pocket for the ligand (Figure 1SI and Figure 2SI), an effect which could be compensated for by the H-bond donating residues.
Figure 2.
[Ψ[CH2NH]Tpg4]Vancomycin aglycon.
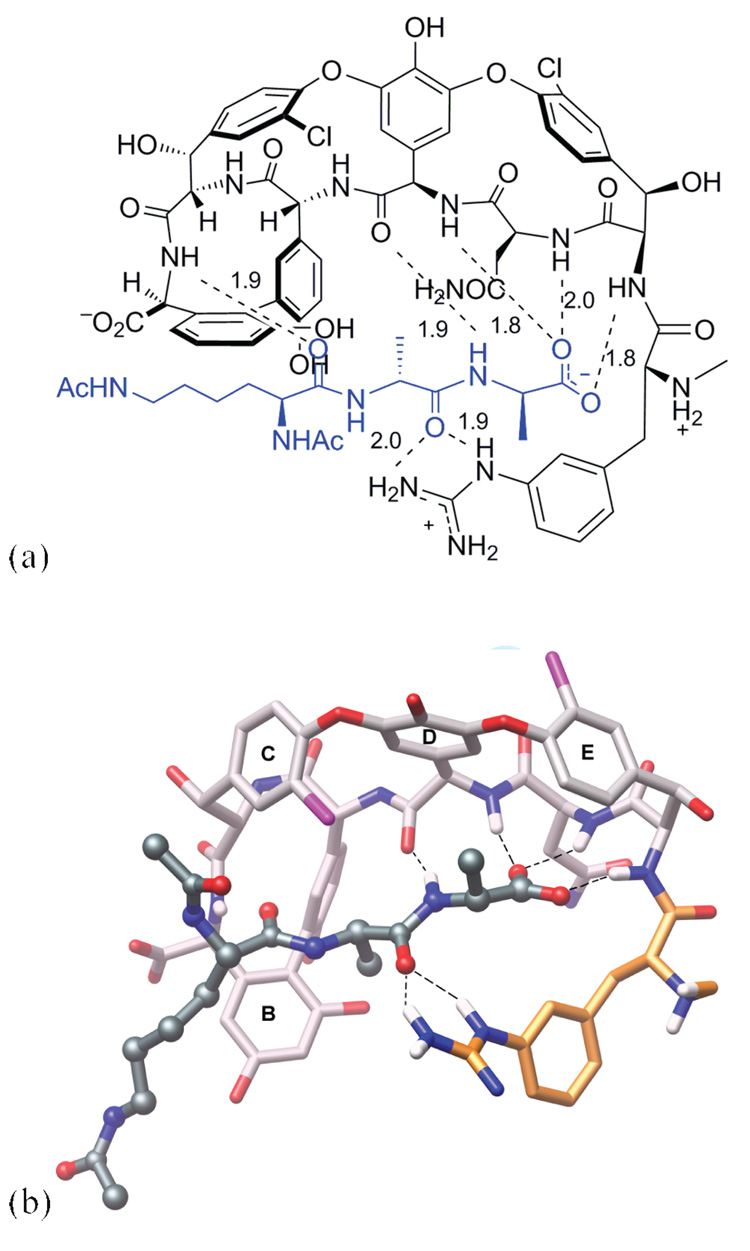
Figure 3.
Proposed binding of the N-terminally modified vancomycin analogue 11 with ligand 2 as determined by molecular modeling using the program MOLOC.7a,b Molecular modeling was preformed starting with the X-ray crystal structure of 5 co-crystallized with 2 determined to 1.8 Å-resolution (PDB Code: 1FVM).7c,d (a) Schematic view. (b) Ball and stick representation. Black dashed lines represent H-bonds. Distances between H-atom and heteroatom of H-bonding partner are given in angstroms. Color code: ligand skeleton: dark-grey; vancomycin skeleton: light-grey; N-terminal amino acid skeleton: orange; and O: red; N: blue; H: white. Figure was generated with the molecular graphics program Chimera.8
Figure 4.
Target molecules.
The effect of N-terminal modification on the antimicrobial activity for a series of chlorobiphenyl vancomycin derivatives has been explored by Kahne and co-workers.9 Chlorobiphenyl vancomycin and related analogues operate with a different or additional mode of action than vancomycin, and are ca. 100-fold more active against vancomycin-sensitive bacteria and vancomycin-resistant Enterococci (VRE). Moreover, and unlike vancomycin, this activity is maintained when the N-terminal d-leucyl residue is modified or completely removed.1a,10 Williams and co-workers5 probed the charge verses non-polar contributions of the N-methylammonium and d-leucyl moieties of 1, and Hunt and co-workers11 have examined the antimicrobial properties of N-demethylvancomycin (2-fold enhancement). In addition, Preobrazhenskaya and co-workers12 studied the effect on antimicrobial activity of N-acylation or nitrosylation at the N-terminus of vancomycin. Most recently, Williams and co-workers reported the effect of acylation at the N-terminus N-methylamine of 1 on the affinity for 2.13
Chemistry
Desleucylaglucovancomycin (16) was prepared in two steps according to literature procedures starting from vancomycin hydrochloride (1), which was deglycosylated to provided the intermediate vancomycin aglycon (5).14 Subsequent removal of the N-terminal d-leucyl residue of 5 by Edman degradation using the sequential action of phenylisothiocyante and trifluoroacetic acid, provided 16 as reported.6
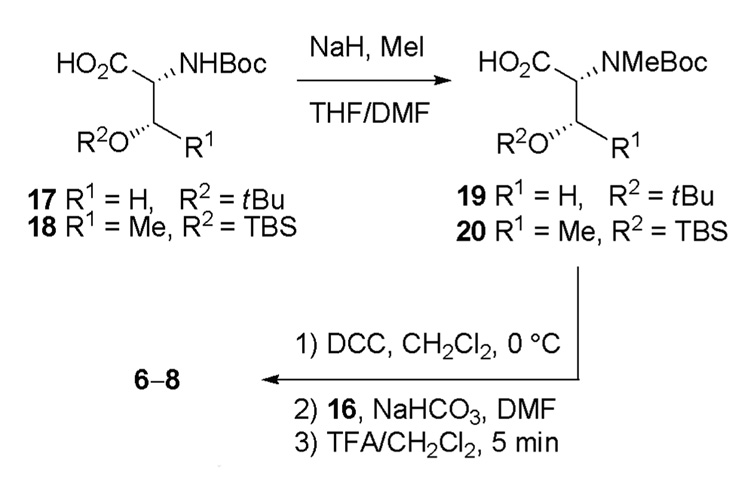
Target molecules 6 and 7 were prepared from commercially available N-Boc-O-tert-butyl-d-serine (17), and 8 was accessed using N-Boc-O-tert-butyldimethylsilyloxy-d-threonine (18). Selective N-methylation using the conditions of Cheung and Benoiton15 provided the corresponding N-Boc-N-methyl amino acids 19 and 20 in 92% and 60% yield, respectively. Dicyclohexylcarbodiimde (DCC) mediated symmetrical anhydride formation of 19 and 20 followed by coupling to the free amine of 16 in the presence of sodium bicarbonate (NaHCO3) provided the protected target molecules. Subsequent treatment of the resulting intermediates with trifluoroacetic acid (TFA) in dichloromethane provided the TFA salts of target molecules 6, 7, and 8 with yields for the two-step transformation of 24%, 47%, and 53%, respectively, after purification by reverse-phase HPLC (Scheme 2).
Scheme 2.
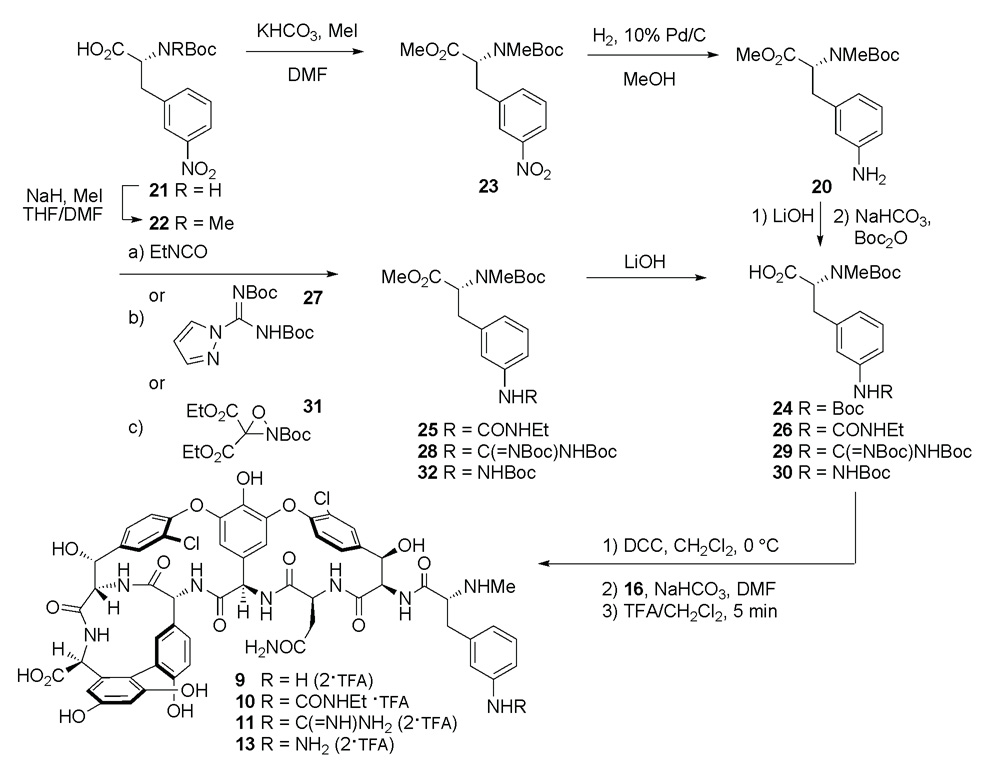
Compounds 9–11 were obtained from intermediate 20, which in turn was prepared from commercially available N-Boc-3-nitro-d-phenylalanine (21, Scheme 3). Selective N-methylation of 21 using the conditions of the Cheung and Benoiton15 provided 22 in 82% yield. Subsequent esterification with methyl iodide (MeI) and potassium carbonate (K2CO3) provided ester 23 in 94% yield, which was reduced to amine 20 (H2, 10% Pd/C, 98%). Intermediate 20 was hydrolyzed and protected to provide 24 in 75% yield by sequential action of LiOH and di-tert-butyl dicarbonate (Boc2O). DCC-mediated symmetrical anhydride formation of 24, and coupling with 16 in the presence of NaHCO3 provided the target molecule 9 as its bis-TFA salt in 51% yield (two-steps), after treatment with 1:1 TFA in dichloromethane and purification by reverse-phase HPLC (Scheme 3).
Scheme 3.
Intermediate 20 was treated with ethylisocyanate (EtNCO) to generate the ethylurea 25 in 97% yield. Hydrolysis of ester 25 with LiOH provided the carboxylic acid 26 (88%), which was coupled to 16 in the presence of NaHCO3 following preparation of its symmetrical anhydride using DCC. The target molecule 10 was isolated by reverse-phase HPLC as its TFA salt in a 44% yield (two-steps) after treatment of the protected intermediate with TFA in dichloromethane (Scheme 3).
The guanidine derivative 11 was also obtained from intermediate 20 (Scheme 3). Treatment of 20 with commercially available N,N′-di-Boc-1H-pyrazole-1-carboxamidine (27)16 provided the bis-protected guanidine derivative 28 in 47% yield. Subsequent hydrolysis of ester 28 with LiOH provided the carboxylic acid 29 (66%), and was followed by treatment of 29 with DCC to prepare the symmetrical anhydride, which was coupled with the free amine of 16 in the presence of NaHCO3. The target molecule 11 was isolated by reverse-phase HPLC as its bis-TFA salt in 27% yield (two-steps) after treatment of the protected intermediate with TFA in dichloromethane (Scheme 3).
The phenylhydrazine amino acid precursor 30 was obtained in two steps from intermediate 20. Amination of the aniline of 20 with oxaziridine 3117 proceeded in modest, but sufficient yield (23%) with both standard and extended reaction times (24–72 h) as the bis-aminated side-product predominated (not shown). Subsequent hydrolysis of the methyl ester 32 with LiOH provided the carboxylic acid 30 in 65%. The acid 30 was then transformed to its symmetrical anhydride with DCC and coupled with 16 in the presence of NaHCO3. The protected intermediate was treated with TFA in dichloromethane to provide the desired target molecule 13 (49%, two-steps) as its bis-TFA salt after purification by reverse-phase HPLC. Compound 13 was somewhat unstable and was used for all biological and UV-difference titration assays within one day after liberation of the hydrazine 13 (Scheme 3). Compounds 14 and 15 were subsequently prepared, as these analogues were envisioned to have improved stability (Figure 4).
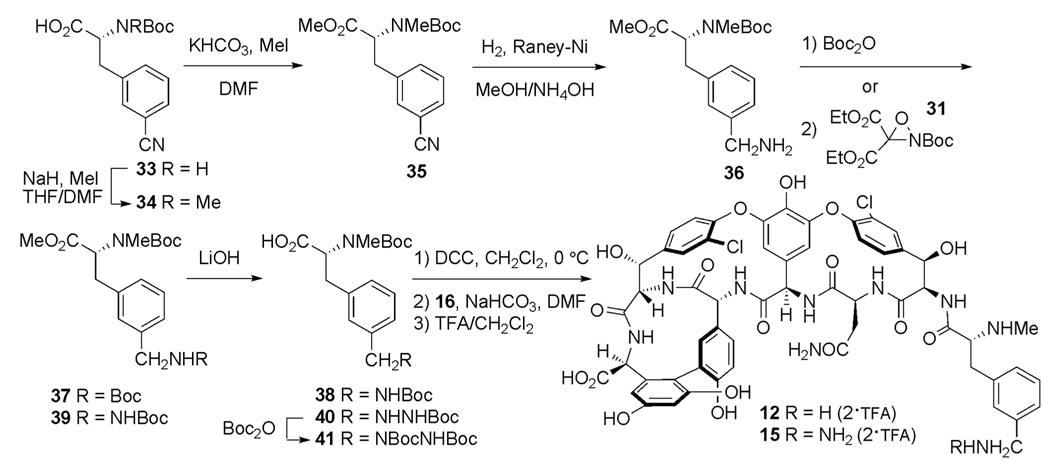
Target molecules 12 and 15 were prepared starting from commercially available N-Boc-3-cyano-d-phenylalanine (33, Scheme 4). Selective N-methylation following the conditions of Cheung and Benoiton15 provided carboxylic acid 34 in 97% yield, which was esterified with MeI/KHCO3, in DMF to provide ester 35 in 99% yield. Reduction of the nitrile to the corresponding methylamine with hydrogen gas (H2), Raney-Ni, and aqueous 28% ammonium hydroxide (NH4OH) in methanol provided the amine 36 in 97% yield. Alternative hydrogenation conditions employing H2 and 10% Pd/C at atmospheric pressure or 50 psi in methanol, tetrahydrofuran, or acetic acid provided only complex mixtures and reactions performed with Raney-Ni in the absence of NH4OH cleanly provided the bis-alkylamine by-product. Subsequent protection of the amine 36 with di-tert-butyl dicarbonate provided 37 (86%), and hydrolysis of the ester with LiOH in tetrahydrofuran/water provided the desired carboxylic acid 38 in 88%. DCC-mediated anhydride formation of 38 followed by coupling with 16 provided the target molecule 12 as its bis-TFA salt in 47% yield (two-steps) after treatment with 1:9 TFA in dichloromethane and purification by reverse-phase HPLC (Scheme 4).
Scheme 4.
Amination of the amine on 36 with oxaziridine 3117 provided the protected hydrazine 39 (58%) in anhydrous toluene (30 min), and the yield was comparable conducting the reaction in dichloromethane (52%) (Scheme 4). Extended reaction times of 24 h or 48 h in dichloromethane or toluene provided lower yields of the desired hydrazine (22% and 30–47%, respectively) and the bis-amination by-product dominated. Hydrolysis of the ester of 39 using LiOH in tetrahydrofuran/water provided the desired carboxylic acid 40 (62%). The hydrazine was then protected with di-tert-butyl dicarbonate in the presence of NaHCO3 to provide 41 in 87%. DCC-mediated anhydride formation of carboxylic acid 41 followed by coupling with 16 provided the target molecule 15 as its bis-TFA salt in 52% yield (two-steps) after treatment with TFA in dichloromethane (1:9) and purification by reverse-phase HPLC (Scheme 4). As observed with the derivative 13, the hydrazine function of compound 15 proved unstable, with a life time of 3 d when stored as a solid. Therefore, compound 15 was used for all biological and UV-difference titration assays within one day after liberation of the free hydrazine. For assay purposes, a crude sample, after deprotection and without purification by reverse-phase HPLC, was used for all assays as purification caused additional decomposition. However, a purified sample was prepared for characterization purposes.
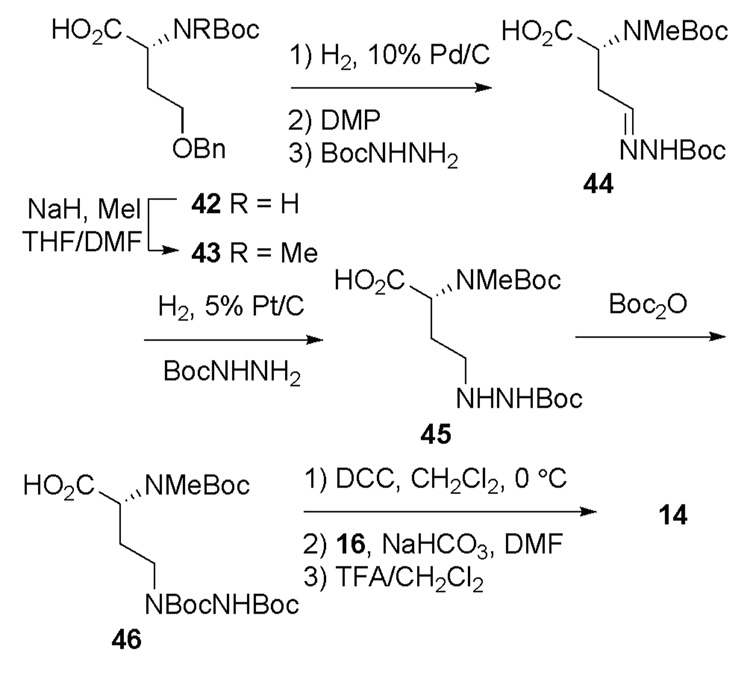
Compound 14 was prepared from commercially available N-Boc-O-benzyl-d-homoserine (42) initiated by selective N-methylation of 42 as described previously using the conditions of Cheung and Benoiton15 to provide intermediate 43 (96%, Scheme 5). Subsequent debenzylation under an atmosphere of H2 gas over 10% Pd/C provided the crude alcohol, which was immediately oxidized to the aldehyde using Dess–Martin periodinane, and condensed with tert-butyl carbazate to provide the hydrazone 44 (48%, three-steps). Reduction of the hydrazone was initially problematic, as bis-alkyl hydrazine was the major by-product (not shown). The hydrazone was cleanly reduced to the hydrazine 45 in 76% yield under an atmosphere of H2 gas over 5% Pt/C with the additive tert-butyl carbazate (2 equiv), which prevented bis-alkyl hydrazine formation. Similar conditions in the absence of tert-butyl carbazate provided a mixture of unreacted starting material and bis-alkyl hydrazine with only trace amounts of desired product. Other conditions employing sodium cyanoborohydride (NaBH3CN) or hydrogenation over 10% Pd/C in acetic acid, methanol, or tetrahydrofuran at atmospheric pressure or at 50 psi of pressure provided only complex mixtures. Mono-Boc protected hydrazine 45 was then bis-Boc protected with di-tert-butyl dicarbonate in a mixture of tetrahydrofuran/water to provide carboxylic acid 46 (49%). DCC-mediated anhydride formation with 46 and coupling with 16 provided the target molecule 14 as its bis-TFA salt in 49% yield (two-steps) after treatment with 1:9 TFA in dichloromethane and purification by reverse-phase HPLC (Scheme 5). As observed with hydrazine derivatives 13 and 15, derivative 14 was also unstable. It was therefore used for all biological and UV-difference titration assays within one day after liberation of the hydrazine, and stored as a solid. For assay purposes, a crude sample, isolated after deprotection and without purification by reverse-phase HPLC, was used for all assays, as additional purification caused further decomposition. However, a purified sample was prepared for the purposes of characterization.
Scheme 5.
Results and Discussion
The antimicrobial properties of derivatives 6–15 were determined against a vancomycin-sensitive Staphylococcus aureus (S. aureus, strain ATCC 25923) and a vancomycin-resistant (VanA) Enterococcus faecalis (E. faecalis, strain BM4166) in a microtiter plate-based antimicrobial assay (Table 1).4,18 The latter VanA strain maintains an inducible resistance pathway upon treatment with glycopeptide antibiotics, including vancomycin (1) and teicoplanin, and is a virulent strain that is difficult to treat compared to a VanB strain. When unchallenged it utilizes d-Ala-d-Ala peptidoglycan cell wall precursors, but upon treatment with a glycopeptide will switch to d-Ala-d-Lac peptidoglycan cell wall precursors. The binding properties of 6–15 for 2 and 3 were also determined and compared to 1 and 5 using a well-established UV-difference titration assay, which gives association constants (Ka) for the complexes with the model tripeptides 2 and 3 of the peptidoglycan cell wall precursors (Table 1).18,19 For an exemplary saturation binding curve with 2 or 3 and subsequent Scatchard analysis see the Supporting Information (Figures 3SI and 4SI).
Table 1.
Binding and Antimicrobial Properties
| Compound | MIC (µg/mL) | Association Constants | ||
|---|---|---|---|---|
| S. aureusa | E. faecalisb | 2c (×105 M−1) | 3d (×102 M−1) | |
| 1 | 1.25e | 2000e | 2.0f | 1.8f |
| 5 | 0.625e | 640e | 1.7f | 1.2f |
| 6 | 40 | 800 | 0.3 | 2.1 |
| 7 | 20 | 320 | 0.9 | 2.0 |
| 8 | 40 | 800 | 2.6 | 0.8 |
| 9 | 20 | 320 | 0.3 | 2.1 |
| 10 | 31 | 640 | 2.0 | 4.6 |
| 11 | 10 | 320 | 1.1 | 3.5 |
| 12 | >10 | 640 | 0.8 | 0.8 |
| 13 | 10 | 320 | 0.3 | 1.9 |
| 14 | 10 | >400 | 1.1 | 1.5 |
| 15 | 1.25 | 170 | 1.7 | 1.5 |
Compounds 6–14 all lost a significant amount of antimicrobial activity against a vancomycin-sensitive S. aureus (Table 1). Only the vancomycin derivative 15 (MIC = 1.25 µg/mL), which arose as the best of the series, displayed a MIC comparable to vancomycin (MIC = 1.25 µg/mL)4,18 and the vancomycin aglycon (MIC = 0.625 µg/mL)4,18 against vancomycin-sensitive S. aureus (Table 1). The reduced in vitro antimicrobial properties were paralleled by lower measured affinities (Ka values) of compounds 6, 7, 9, 12, and 13 for the model ligand 2, which lost anywhere from 2–10 fold in affinity. Interestingly, compounds 8, 10, 11, 14, and 15 displayed comparable affinities for 2, and with the exception of 15, this did not translate to comparable antimicrobial properties (Table 1).
The antimicrobial properties of 6–15 were also investigated against a vancomycin-resistant E. faecalis (VanA), and the results revealed that 6–14 did not display markedly different antimicrobial properties than the natural product. The antimicrobial activities of the N-terminal d-serine and d-threonine analogues 6 and 8 were slightly lower than 5, even though the former had a similar affinity for the model ligand 3 (Table 1). Analogue 7 bearing the O-tert-butyl ether showed improved potency over its hydroxy counterpart 6, and a comparable affinity for 3; a result that may be attributed to its enhanced hydrophobic character. There was no change in the antimicrobial activities of ethylureido derivative 10 and benzylamine derivative 12, despite the higher Ka of the former for the ligand 3, and the altered binding pocket (Figure 2SI). Aniline derivative 9, phenylhydrazine derivative 13, and alkylhydrazine derivative 14 displayed slightly enhanced antimicrobial properties which paralleled enhanced affinities for 3 (Table 1), again despite the potentially altered binding pocket (Figure 2SI). As observed in the assay against vancomycin-sensitive S. aureus, the only analogue that showed enhanced potency against the VanA strain was compound 15 (MIC = 170 µg/mL, Table 1). However, this increase (4-fold) was modest and overall the results did not reveal a unique behavior for the hydrazine series against the vancomycin-sensitive or vancomycin-resistant bacteria. Since, the binding affinity of 15 for 3 did not correlate directly with its antimicrobial potency, as 15 had the same affinity as natural product for 3, it is not clear what factors are contributing to its modestly enhanced potency.
For vancomycin analogues 6–8, any observed loss in affinity may be the result of a change in the overall non-polar surface area in this region of the natural product (Figure 5S1) as observed for the N-terminal d-alanine and d-glycine analogues of vancomycin reported by Williams and co-workers.5 These analogues, which in contrast maintained the vancosamine sugar, exhibited a loss in affinity for 2 (20-fold, 0.85 × 105 M−1 and 0.77 × 105 M−1, respectively).5 Further, analysis by molecular modeling7 of the conformation of the d-serine and d-threonine derivatives 6 and 7 revealed that the hydroxy groups may point away from the carboxylate when the ligand is bound, adopting a more favorable gauche conformation (Figure 5SI). Therefore, it might be expected that the serine derivative 6 would have a lower affinity for 2, and that the analogous gauche conformation for 7 directs the threonine methyl substituent toward the ligand, increasing both the non-polar surface area in this region of the ligand and its affinity for 2 when compared to 6 (Table 1). However, the reverse trend is observed for the binding results with the ester ligand 3 and the distinctions are modest enough that definitive trends are difficult to discern.
The d-phenylalanine derivatives 9 and 13, with the H-bond donating groups (aniline or hydrazine) attached directly to the aromatic ring lost significant affinity for 2, but gained affinity for 3. Extension of the H-bond donating system by one or two additional atoms from the aromatic ring, restores some of the lost affinity for 2 and 3 as demonstrated by compounds 10–11 and 14–15, in spite of the altered binding pocket in the N-terminal region of the natural product (Figure 2SI). However, analogue 12 with the single methylene extension still has a slightly lower Ka, in comparison to the natural product 5 (Table 1). The most significant positive impact on binding affinity was observed with 10 and 11 and their relative affinity for 3, which increased 3 to 4-fold potentially reflecting the bi-dentate H-bond represented in Figure 3.
A series of reaction conditions were explored to investigate the potential of a reaction occurring between the appended hydrazines of analogues 13–15 and the C-terminal ester of N,N′-Ac2-l-Lys-d-Ala-d-Lac (3) (Table 1SI). The hope being that the hydrazine would react with the ester of the bound ligand 3 generating a covalent adduct with the natural product analogue. However, varying the solvent, temperature, concentration, substrate, or pH did not influence the reaction outcome and the desired adduct product was never observed. This result was surprising, as the same intermolecular reaction between 3 and the commercially available hydrazines phenylhydrazine and 2-bromophenylhydrazine with triethylamine (Et3N) at 70 °C, did provide the desired acylated hydrazines (data not shown).
Conclusions
A series of N-terminal derivatives of the vancomycin aglycon were prepared replacing the residue 1 N-methyl d-leucyl amino acid with a series of N-methyl d-amino acids bearing H-bond donor or reactive nucleophilic substituents. An examination of their antimicrobial activity against vancomycin-sensitive and vancomycin-resistant bacteria and their affinity for model ligands 2 and 3 incorporating d-Ala-d-Ala and d-Ala-d-Lac did not reveal evidence of significant enhancements in activity or affinity, including those capable of formation of covalent adducts derived from their reaction with ligand 3 bearing an ester. Whether this reflects the limitations of our original designs or features of binding not yet recognized is unknown, but is a topic of our continuing studies.
Supplementary Material
Compounds 5 and 15 were prepared according to published procedures.6,14 Full experimental details and characterization for all new intermediates and final compounds are provided in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.
Scheme 1.
Acknowledgments
We gratefully acknowledge the financial support of the National Institutes of Health (CA 41101), Dr. G. Boldt for supplying gram quantities of vancomycin aglycon, and Professor K. D. Janda for the use of the HF apparatus.
Abbreviations
- VanA
vancomycin/teicoplanin type-A resistant
- VanB
teicoplanin-senstitive and vancomycin type-B resistant
- VRE
vancomycin-resistant Enterococci
- H-bond
Hydrogen bond
- d-Lac
d-Lactic acid
- DCC
dicyclohexylcarbodiimde
- TFA
trifluoroacetic acid
- Pd/C
Palladium on activated Carbon
- EtNCO
ethylisocyanate
- Pt/C
Platinum on activated Carbon
- NaBH3CN
sodium cyanoborohydride
- MIC
minimum inhibition concentration
- Et3N
triethylamine
References
- 1.For reviews see: Kahne D, Leimkuhler C, Lu W, Walsh CT. Glycopeptide and Lipoglycopeptide Antibiotics. Chem. Rev. 2005;105:425–428. doi: 10.1021/cr030103a. Hubbard BK, Walsh CT. Vancomycin Assembly: Nature’s Way. Angew. Chem., Int. Ed. 2003;42:730–765. doi: 10.1002/anie.200390202. Williams DH, Bardsley B. The Vancomycin Group of Antibiotics and the Fight Against Resistant Bacteria. Angew. Chem., Int. Ed. 1999;38:1172–1193. doi: 10.1002/(SICI)1521-3773(19990503)38:9<1172::AID-ANIE1172>3.0.CO;2-C. Malabarba A, Nicas TI, Thompson RC. Structural Modifications of Glycopeptide Antibiotics. Med. Res. Rev. 1997;17:69–137. doi: 10.1002/(sici)1098-1128(199701)17:1<69::aid-med3>3.0.co;2-r.
- 2.For reviews on glycopeptide resistance see: Malabarba A, Ciabatti R. Glycopeptide Derivatives. Curr. Med. Chem. 2001;8:1759–1773. doi: 10.2174/0929867013371716. Pootoolal J, Neu J, Wright GD. Glycopeptide Antibiotic Resistance. Annu. Rev. Pharmacol. Toxicol. 2002;42:381–408. doi: 10.1146/annurev.pharmtox.42.091601.142813. Van Bambeke FV, Laethem YV, Courvalin P, Tulkens PM. Glycopeptide Antibiotics: from Conventional to New Derivatives. Drugs. 2004;64:913–1028. doi: 10.2165/00003495-200464090-00001. Süssmuth RD. Vancomycin Resistance: Small Molecule Approaches Targeting the Bacterial Cell Wall Biosynthesis. ChemBioChem. 2002;3:295–298. doi: 10.1002/1439-7633(20020402)3:4<295::AID-CBIC295>3.0.CO;2-G. Gao Y. Glycopeptide Antibiotics and Development of Inhibitors to Overcome Vancomycin Resistance. Nat. Prod. Rep. 2002;19:100–107. doi: 10.1039/b100912p. Healy VL, Lessard IA, Roper DI, Knox JR, Walsh CT. Vancomycin Resistance in Enterococci: Reprogramming of the d-Ala-d-Ala Ligases in Bacterial Peptidoglycan Biosynthesis. Chem. Biol. 2000;7:R109–R119. doi: 10.1016/s1074-5521(00)00116-2.
- 3.McComas CC, Crowley BM, Boger DL. Partitioning the Loss in Vancomycin Binding Affinity for d-Ala-d-Lac into Lost H-Bond and Repulsive Lone Pair Contributions. J. Am. Chem. Soc. 2003;125:9314–9315. doi: 10.1021/ja035901x. [DOI] [PubMed] [Google Scholar]
- 4. Crowley BM, Boger DL. Total Synthesis and Evaluation of [Ψ[CH2NH]Tpg4]Vancomycin Aglycon: Reengineering Vancomycin for Dual d-Ala-d-Ala and d-Ala-d-Lac Binding. J. Am. Chem. Soc. 2006;128:2885–2892. doi: 10.1021/ja0572912. For related synthetic efforts, see: Boger DL, Miyazaki S, Kim SH, Wu JH, Loiseleur O, Castle SL. Diastereoselective Total Synthesis of the Vancomycin Aglycon with Ordered Atropisomer Equilibrations. J. Am. Chem. Soc. 1999;121:3226–3227. Boger DL, Miyazaki S, Kim SH, Wu JH, Castle SL, Loiseleur O, Jin Q. Total Synthesis of the Vancomycin Aglycon. J. Am. Chem. Soc. 1999;121:10004–10011. Boger DL, Kim SH, Miyazaki S, Strittmatter H, Weng J-H, Mori Y, Rogel O, Castle SL, McAtee JJ. Total Synthesis of the Teicoplanin Aglycon. J. Am. Chem. Soc. 2000;122:7416–7417. doi: 10.1021/ja003835i. Boger DL, Kim SH, Mori Y, Weng J-H, Rogel O, Castle SL, McAtee JJ. First and Second Generation Total Synthesis of the Teicoplanin Aglycon. J. Am. Chem. Soc. 2001;123:1862–1871. doi: 10.1021/ja003835i. Crowley BM, Mori Y, McComas CC, Tang D, Boger DL. Total Synthesis of the Ristocetin Aglycon. J. Am. Chem. Soc. 2004;126:4310–4317. doi: 10.1021/ja039795a. Boger DL. Vancomycin, Teicoplanin, and Ramoplanin: Synthetic and Mechanistic Studies. Med. Res. Rev. 2001;21:356–381. doi: 10.1002/med.1014.
- 5.Cristofaro MF, Beauregard DA, Yan H, Osborn NJ, Williams DH. Cooperativity between Non-polar and Ionic Forces in the Binding of Bacterial Cell Wall Analogues by Vancomycin in Aqueous Solution. J. Antibiot. 1995;48:805–810. doi: 10.7164/antibiotics.48.805. [DOI] [PubMed] [Google Scholar]
- 6.(a) Booth PM, Stone DJM, Williams DH. The Edman Degradation of Vancomycin: Preparation of Vancomycin Hexapeptide. J. Chem. Soc., Chem. Commun. 1987:1694–1695. [Google Scholar]; (b) Booth PM, Williams DH. Preparation and Conformational Analysis of Vancomycin Hexapeptide and Aglucovancomycin Hexapeptide. J. Chem. Soc., Perkin Trans. 1989;1:2335–2339. [Google Scholar]
- 7. Gerber PR, Müller K. MAB, a Generally Applicable Molecular Force Field for Structure Modelling in Medicinal Chemistry. J. Comput.-Aided Mol. Des. 1995;9:251–268. doi: 10.1007/BF00124456. (b) Gerber Molecular Design (http://www.moloc.ch). (c) Protein Databank (PDB code: 1FVM), 1.8 Å-resolution (http://www.rcsb.org). Nitanai Y, Kikuchi T, Kakoi K, Hanamaki S, Fujisawa I, Aoki K. Crystal Structures of the Complexes between Vancomycin and Cell-Wall Precursor Analogs. J. Mol. Biol. 2009 doi: 10.1016/j.jmb.2008.10.026. (in press).
- 8.(a) All molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081). For details see: Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera-A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. (c) Chimera home page can be found at http://www.cgl.ucsf.edu/chimera.
- 9.Kahne D, Walker S, Silva DJ. Patent WO 2000/59528 (US 2004110665) Desleucyl Glycopeptide Antibiotics and Methods of Making Same. 2000
- 10.Kim SJ, Matsuoka S, Patti GJ, Schaefer J. Vancomycin Derivative with Damaged d-Ala-d-Ala Binding Cleft Binds to Cross-linked Peptidoglycan in the Cell Wall of Staphylococcus aureus. Biochemistry. 2008;47:3822–3831. doi: 10.1021/bi702232a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt AH, Marconi GG, Elzey TK, Hoehn MM. N-Demethylvancomycin. J. Antibiot. 1984;37:917–919. doi: 10.7164/antibiotics.37.917. [DOI] [PubMed] [Google Scholar]
- 12.Pavlov AY, Berdnikova TF, Olsufyeva EN, Lazhko EI, Malkova IV, Preobrazhenskaya MN. Synthesis and Biological Activity of Derivatives of Glycopeptide Antibiotics Eremomycin and Vancomycin Nitrosated, Acylated, or Carbamoylated at the N-terminal. J. Antibiot. 1993;46:1731–1739. doi: 10.7164/antibiotics.46.1731. [DOI] [PubMed] [Google Scholar]
- 13.Gale TF, Görlitzer J, O’Brien SW, Williams DH. The Synthesis and Binding of N-terminal Derivatives of Vancomycin to a Bacterial Cell Wall Analogue. J. Chem. Soc., Perkin Trans. 1999;1:2267–2270. [Google Scholar]
- 14.Wanner J, Tang D, McComas CC, Crowley BM, Jiang W, Moss J, Boger DL. A New and Improved Method for Deglycosylation of Glycopeptide Antibiotics Exemplified with Vancomycin, Ristocetin, and Ramoplanin. Bioorg. Med. Chem. Lett. 2003;13:1169–1173. doi: 10.1016/s0960-894x(03)00051-9. [DOI] [PubMed] [Google Scholar]
- 15.Cheung ST, Benoiton NL. N-Methylamino Acids in Peptide Synthesis. V. The Synthesis of N-tert-Butyloxycarbonyl,N-Methylamino Acids by N-methylation. Can. J. Chem. 1997;55:906–910. [Google Scholar]
- 16.Drake B, Patek M, Lebl M. A Convenient Preparation of Monosubstituted N,N’-di(Boc)-Protected Guanidines. Synthesis. 1994;6:579–582. [Google Scholar]
- 17.Armstrong A, Jones LH, Knight JD, Kelsey RD. Oxaziridine-Mediated Amination of Primary Amines: Scope and Application to a One-Pot Pyrazole Synthesis. Org. Lett. 2005;7:713–716. doi: 10.1021/ol0474507. [DOI] [PubMed] [Google Scholar]
- 18.Antimicrobial assays were run as previously described: McComas CC, Crowley BM, Hwang I, Boger DL. Synthesis and Evaluation of Methyl Ether Derivatives of Vancomycin, Teicoplanin, and Ristocetin Aglycon Methyl Ethers. Bioorg. Med. Chem. Lett. 2003;13:2933–2936. doi: 10.1016/s0960-894x(03)00513-4. McAtee JJ, Castle SL, Jin Q, Boger DL. Synthesis and Evaluation of Vancomycin and Vancomycin Aglycon Analogues that Bear Modifications in the Residue 3 Asparagine. Bioorg. Med. Chem. Lett. 2002;12:1319–1322. doi: 10.1016/s0960-894x(02)00130-0.
- 19.UV-difference titration assays were run as previously described: Nieto M, Perkins HR. The Specificity of Combination between Ristocetins and Peptides Related to Bacterial Cell Wall Mucopeptide Precursors. Biochem. J. 1971;124:845–852. doi: 10.1042/bj1240845. Nieto M, Perkins HR. Physiochemical Properties of Vancomycin and Iodovancomycin and their Complexes with Diacetyl-l-lysyl-d-alanyl-d-alanine. Biochem. J. 1971;123:773–787. doi: 10.1042/bj1230773. Perkins HR. Specificity of Combination between Mucopeptide Precursors and Vancomycin or Ristocetin. Biochem. J. 1969;111:195–205. doi: 10.1042/bj1110195.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Compounds 5 and 15 were prepared according to published procedures.6,14 Full experimental details and characterization for all new intermediates and final compounds are provided in the supporting information. This material is available free of charge via the Internet at http://pubs.acs.org.